Open Access Article
Open Access ArticleAI-driven innovations in pharmaceuticals: optimizing drug discovery and industry operations
Jaskaran Preet Singh
Saini
 ,
Ankita
Thakur
,
Ankita
Thakur
 and
Deepak
Yadav
and
Deepak
Yadav
 *
*
Chitkara University School of Pharmacy, Chitkara University-Himachal Pradesh, HIMUDA Education Hub-Atal Shiksha Kunj, District: Solan, Himachal Pradesh, India-174103. E-mail: deepakyadavphd@gmail.com; Tel: +91-9878310858
First published on 3rd February 2025
Abstract
The integration of artificial intelligence into the pharmaceutical industry has led to significant transformation in the process of drug discovery and development and management of the pharmaceutical sector. Artificial intelligence has accelerated the process of drug discovery by several folds owing to its ability to analyse large datasets and predict drug–target(receptor) interactions, which effectively reduces the time and expenditure. AI enables clinical trial design and patient recruitment through predictive analytics during the trial. It also allows for real-time tracking of patient outcomes and predicts the effectiveness of a trial. Artificial intelligence-driven automation also assists in manufacturing and supply chain processes, enabling inventory optimization and predictive maintenance and thereby improving the productivity as well as affordability of these processes. The current review discusses various key applications, prospects, and challenges of AI in the pharmaceutical industry, focussing on its transformative potential while addressing the need for ethical and regulatory frameworks to ensure equitable and safe AI adoption.
1. Introduction
The development of artificial intelligence (AI) is changing the drug discovery, development, and management paradigm in the pharmaceutical industry. Thus far, drug discovery and development have been conventionally long and expensive processes that have taken as long as 10–15 years, and billions of dollars need to be spent for one molecule to come to the market (Fig. 1).1 Artificial intelligence has emerged as a powerful tool that can steer this process by streamlining each stage, including drug discovery and clinical trials, manufacturing, and even supply chain management. Its ability to analyse big data and find patterns to offer efficient and precise predictions points towards even more innovations in the industry.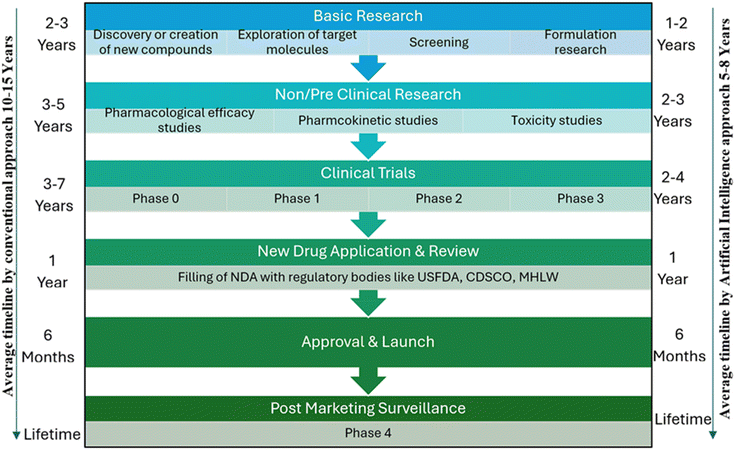 | ||
| Fig. 1 Comparative representation of how AI can impact drug discovery and approval processes in terms of timelines for different key steps. | ||
AI-based technologies, which include machine learning, natural language processing, and deep learning, are being applied to address the critical issues in pharmaceuticals. For instance, artificial intelligence-based drug discovery platforms accelerate the identification of likely candidates by modelling complex systems of biology and estimating the drug–target interactions with greater precision. Further, AI is making clinical trials faster and more efficient by streamlining the processes of recruitment, trial design, and real-time monitoring, thereby shortening the cycles of drug development while cutting costs.
In the pharmaceutical manufacturing field, AI holds potential for revolutionizing processes through predictive maintenance, process automation, and quality control, and it is used in the supply chain management that optimizes logistics, predicts demand, and manages inventory effectively. Improving patients’ results using AI is be the second focus area. AI, upon analysing the genetic and clinical data, tailors the treatments to individual patients, hence increasing the efficacy and decreasing the adverse effects.2,3
Although AI adoption has significant advantages, it faces certain challenges in the pharmaceutical sector. While many positive outcomes have resulted from AI integration in this sector, there are always challenges that accompany these advancements. Critical areas that need to be addressed to fully realize the potential of AI include data privacy issues, issues with bias in algorithms, and regulatory frameworks governing AI-driven processes. However, the combinations of breakthroughs in quantum computing may open new avenues by which AI would become significantly more powerful and beneficial to drug discovery and development activities.3–5
This article will delve deep into the influence of AI on the pharmaceutical industry, from discovery through phases of development, manufacturing, and personalized medicine (Fig. 2), with regard to the ways and mechanisms through which such impacts may differ at various levels. Most probably, it will discuss the challenges and ethical considerations when AI is embraced and look forward to future developments in the succeeding decades that will shape the industry.
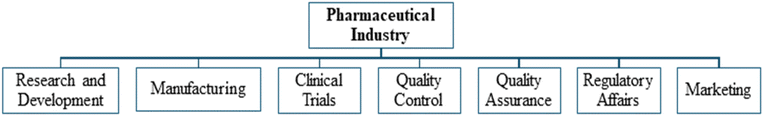 | ||
| Fig. 2 Different processes involved in the pharmaceutical industry where AI can be potentially applied. | ||
2. How AI works?
AI works by artificial neural networks (ANNs), machine learning (ML) and deep learning (DL) methods through data analysis, learning and application. Text, graphics, sound, and other data types that can be encoded can all be included in the input data format. Inspired by the networks of biological neurons, an artificial neural network (ANN) is a computational model in which inputs are converted into output values by the neurons. Even in a complicated, noisy, and highly nonlinear environment, the ANN can learn and self-correct. The ANN is commonly used in scenarios where the dataset is extensive and cannot be addressed using linear functions. Multilayers are structured as concealed layers of neurons that have a diverse range of neuron counts. Input variables are chosen from the existing information (for example, drug discovery and drug parameters) such as concentration, compounds, and ligands, and are refined through several training stages to achieve the output. The anticipated value is examined and contrasted with the known value. The disparity between predicted and actual values is traced back until the difference is minimal to obtain the estimated drug values. This is an extremely intricate simultaneous procedure. Thus, ANN is a computational or mathematical model that relies on a machine learning (ML) technique.6Like the human learning model, machine learning (ML) and deep learning (DL) may progressively identify various data features, deduce the patterns contained within, and continuously update their model parameters until a reliable model is created. These techniques do not need direct programming. ML creates a unique algorithm that concentrates on the data's characteristics and turns them into machine-readable knowledge that gives researchers fresh perspectives. Based on the application contexts, the models can be divided into regression models and classification models. The distinction between regression and classification tasks primarily depends on whether the output variable is continuous or discrete. Based on the type of learning algorithm needed to address the issue, models are classified into three groups: supervised learning, unsupervised learning, and reinforcement learning. Supervised learning is a process that utilizes labelled data to train a model on the connection between input and its predefined output, enabling it to forecast the categories or continuous variables of future inputs. In contrast, unsupervised techniques are employed to detect patterns in datasets without labels and to examine a dataset's possible structures, facilitating the clustering of data for additional analysis. Reinforcement learning builds models by continually engaging in interactive learning, using punishments for setbacks and incentives for achievements. There are several popular algorithms available for researchers to select from. A Random Forest algorithm creates an entire hierarchical structure from a collection of unconnected decision trees, each of which is in charge of a distinct challenge during model development.7 The decision trees’ majority vote determines the ultimate choice. One popular machine learning approach is support vector machines (SVMs). A number of additional machine learning models including logistic regression (LR), linear discriminant analysis (LDA), principal component analysis (PCA), and partial least-squares (PLS) have been used in pharmaceutical and biological data processing.8,9
Deep learning (DL) has gained popularity because of its impressive capabilities in generalization and feature extraction; its process for learning and making predictions is conducted in a seamless, end-to-end manner. In contrast to the conventional machine learning (ML) approach, which often involves several separate modules, DL produces output data (output-end) directly from input data (input-end) during the training of the model and continuously refines and enhances the model based on the discrepancies between the output and the actual value, until the desired outcome is achieved. A deep neural network (DNN) is a type of feed-forward neural network comprising closely connected input, hidden, and output layers. A recurrent neural network (RNN) is a type of artificial neural network, where connected nodes create a directed or undirected graph over a time sequence. An RNN features a feedback element that enables signals from one layer to be sent back to the prior layer. It is the sole neural network equipped with internal memory, aiding in the challenge of learning and retaining long-term information.10
An autoencoder (AE), which comprises an encoder and a decoder, is used to learn effective representations of input data. The encoder produces an encoding by processing the input, which the decoder then uses to reconstruct the original input. Typically, an AE is employed for purposes such as data compression and dimensionality reduction by utilizing the representation methods (i.e., the encoding) of a given dataset.11 A dataset intended for a model is generally separated into a training set, a validation set, and a test set. These sets serve the specific functions of training the model, fine-tuning the model, and assessing the model's performance, respectively.
3. Success and failure rate of AI
It is obvious that models are only a rough depiction of reality and are only useful in specific situations. Complex experimental techniques must be used to determine the materials and process parameters. Each model and simulation technique must be proven to be accurate, dependable, and capable of making predictions. For the majority of engineering applications, an agreement between simulation and experiment in the range of 10% is deemed sufficiently accurate. ANNs were employed by Ebube et al. to forecast the physiochemical characteristics of amorphous polymers.12 The model was able to predict accurately (with an error of 0–8%) the correlations between glass transition temperatures, viscosity, and water absorption patterns and the chemical composition of the polymer. For IVIVC, using in vitro data and parameters derived from the physiological traits of human volunteers, DeMatas et al. created an ANN model that forecasted the in vivo effectiveness of dry powder inhaler formulations. The ANN model was a highly successful IVIVC tool for inhaled medications, according to the results (R2 ≈ 80%), although substantial enhancements to the model can be achieved by incorporating important input variables, using larger datasets, and increasing the number of subjects.13 Multiple ANNs were created and evaluated in clinical environments for the detection, treatment, and prognosis of cancer. Colorectal cancer was identified through the evaluation of fluorescence (from biological fluorophores) with the use of ANNs. The sensitivity and specificity were determined to be 99.2% and 99.4%, respectively.14 In gastric cancer, the prediction of cancer metastasis was conducted through lymph node biopsy. The precision of ANN-1 was 79%, the sensitivity stood at 88%, and the specificity was 55%.15 In pancreatic cancer, the neural computing method achieved a training accuracy of 91.14% and a testing accuracy of 84.27%, along with a positive predictive value of 96.25% and a negative predictive value of 57.22%.16 Wessel, Jurs, Tolan, and Muskal used an artificial neural network (ANN) to predict the intestinal absorption of new compounds, resulting in an error rate of 16%. This result is viewed as acceptable, especially given the varied structure within the dataset.6,174. AI and its applications in drug discovery
The discovery of drugs is typically slow and costly, taking years and vast amounts of resources before there is a glimmer of hope in the form of an identified candidate that might make it to clinical trials. With AI, one could, in theory shave years off timelines, reduce costs, and give drug candidates a better chance of success. AI can handle massive data to predict how chemical compounds could interact and work to model biological systems, which positions it to change the drug-discovery game.4.1 Accelerating drug discovery
Drug discovery is a laborious process that searches chemical compounds in an effort to find those that may well function therapeutically against some diseases. It takes years if carried out classically since researchers will more often than not rely on trial and error. AI is a game-changer. ML algorithms are used in order to analyse large datasets of chemical compounds for predicting potential biological activity (Table 1). AI models are especially identified to be applicable in SAR modelling, which is the technique for predicting the impact of molecular structure on biological activity.18| AI Model/method | Application | Case study | Impact |
|---|---|---|---|
| Machine learning | Screening chemical compounds | IBM Watson for Drug Discovery | Accelerated candidate identification |
| Deep learning | Predicting drug–target interactions | AlphaFold by Deep Mind (Google) | Accurate protein structure predictions |
| Molecular dynamics | Simulating drug efficacy in silico | BenevolentAI for COVID-19 drug repurposing | Faster identification of drug candidates |
4.2 AI Models for drug–target interactions
Perhaps the most difficult step of drug discovery is to understand the drug–target interaction, which simply put, means predicting the chances of a drug binding to a biological target, such as a protein. In this, AI excels, particularly through its deep learning and molecular dynamics simulation tools.4.3 Examples and case studies
4.4 Impact of AI on drug discovery efficiency
There are myriads of advantages of including AI in drug discovery. AI saves enormous time, money, and effort put into a discovery by sieving the promising candidates with resultant focus resources on those candidates. It deals with vast quantities of data much faster than a human can, whereby researchers can quickly identify likely drug candidates and filter out unlikely ones. This leads to the potential for a more targeted approach with less expenditure in lab testing and clinical trials. Besides this, the predictability of AI in drug–target interactions and molecular dynamic simulation improves the accuracy of predictions, and hence, the success rate of drug development.5. AI in drug development and clinical trials
Since its inception AI has been readily studied for its ability to enhance the conduction of clinical trials, and certain modifications to the current AI models can make them more compatible with clinical trials.5.1 Conventional drug development and clinical trial phases
Drug development and its associated clinical trials are now faster with more resources as compared to the old-fashioned, time-consuming, and resource-consumptive procedures. The ability to use machine learning models, predictive analytics, and AI-based monitoring tools facilitates pharmaceutical companies accelerating the end-to-end clinical trial design, patient recruitment, and monitoring, resulting in new therapies hitting the markets faster (Table 2).38| Clinical trial stage | AI tools/method | Function | Example |
|---|---|---|---|
| Patient recruitment | Predictive analytics, EHR analysis | Identify ideal candidates based on genetic and clinical data | Tempus for cancer trials |
| Trial design optimization | In silico simulation. ML models | Optimize trial structure (e.g. dosage duration) | Pfizer AI-optimized trial for vaccines |
| Real time monitoring | NLP, image recognition | Monitor patient outcomes and adverse reactions in real time | AI powered wearable devices |
5.2 Optimizing clinical trial design
These are also the costliest and most time-consuming activities of drug development, taking years and millions of dollars. The new applications of AI provide a solution to both problems. It can optimize the design of a clinical trial, predict patient outcomes, and even conduct virtual trials through in silico simulations. ML models driven by AI can analyse huge datasets from previous trials, electronic health records, and genomic data to identify patterns that researchers can use in their research for developing more effective trials.395.3 Predictive modelling for patient recruitment and outcomes
Recruitment of patients to clinical trials has been identified as one of the major bottlenecks in the process of drug development. One of the areas where AI has proven itself to be valuable is in hastening and more accurately identifying potential candidates for a particular clinical trial. Different data sources would be used through the AI models such as electronic health records (EHRs), genetic profiles, and social determinants of health to select candidates that may most probably respond to the treatment being investigated.43 In a current oncology trial, the application of Mendel AI has resulted in a 24% to 50% increase in the accurate identification of patients who may be eligible, in contrast to conventional methods. Additionally, the process of assessing potential eligibility with Mendel AI is completed in mere minutes, whereas the standard prescreening duration averages 19 days for breast cancer patients and 263 days for those with lung cancer.44 The implementation of automated electronic health record (EHR) text-mining for participant identification in cardiovascular trials could lead to a significant 79.9% decrease in the number of patients requiring screening.40,45 A study involving pediatric oncology patients demonstrated that the use of an AI system achieved a 90% reduction in the workload associated with trial recruitment.46 Furthermore, the ACTES system was found to decrease patient screening time by 34% by minimizing the time allocated to administrative duties, discussions, and unstructured collaboration.47 Additionally, research conducted by Whitty indicated that AI systems, when utilized in two completed oncology studies focused on breast and lung cancer at the Comprehensive Blood and Cancer Center, resulted in a 24% to 50% increase in the identification of eligible patients compared to traditional practices.39,44,485.4 AI-driven monitoring tools
Monitoring of data on patients once the trial starts is necessary to ensure safety and efficacy. This real-time tracking of patient progress can now be done easier with the advent of AI tools, particularly through the use of NLP and image recognition technologies, which can discover adverse effects more rapidly than traditional means.5.5 Case studies
6. AI in manufacturing and supply chain management
Artificial intelligence is nowadays applied more significantly in the optimization of pharmaceutical production and in managing the pharmaceutical supply chain. Pharmaceutical companies can facilitate smoother, cost-effective, and efficient operations with the support of AI technology. AI enhances predictive maintenance, process automation, inventory management, and quality control, thus enabling consistent and high-quality pharmaceutical production. Such examples are those in the sector wherein AI has the potential to transform the industry's prospects for better-market-driven responses, compliance, and reduced risk.6.1 Role of AI in pharmaceutical manufacturing industry
Manufacturing in the pharmaceutical industry is highly regulated for quality, safety, and efficiency. AI's role in manufacturing revolves around improving predictive maintenance, process optimization, and quality control. The aim here is predictive maintenance:The collaboration of PAT, process data science, and AI establishes an effective monitoring system for the continuous manufacturing line. It provides several benefits that will result in decreased production expenses. This will involve decreased final product testing due to continuous monitoring and testing, which allows for the application of PAT principles. This leads to the capacity to release products with less final product testing, facilitating lower inventory and quicker recovery of production costs. The benefit is most significant for pharmaceutical companies planning to manufacture a product for an extended period. Thoughtful planning paired with informed leadership, eager to collaborate with regulators, will be advantageous for shareholders and patients.
AI tools that are beneficial in Process Analytical Technology (PAT) and continuous manufacturing encompass expert systems, fuzzy logic approaches, neural networks, case-based reasoning, genetic algorithms, and ambient intelligence. These methodologies, combined with computational power, emulate human-like reasoning and decision-making in uncertain situations. They find extensive applications in process control, optimization, monitoring, parameter estimation, prediction, chemometrics, process scale-up and scale-down, as well as the development of soft sensors. Fuzzy control systems offer real-time decision support to operators and facilitate automatic control. Current applications include fault diagnosis and control, as well as pattern recognition in various processes. Moreover, Model Predictive Control (MPC) enables real-time management of quality attributes.65
Although ANNs have already undergone testing for a number of uses in the most typical manufacturing processes, there are currently few real-time applications for PAT. The first stage in the production of the API in industrial pharmaceutical manufacturing is the synthesis of organic compounds. ANN-based synthesis optimization has been the subject of numerous studies.66–70 Information from data-rich PAT tools such as in-line microscopic images has been extracted using ANNs. When used for contamination classification, a ResNet CNN demonstrated >98% accuracy in classifying crystals found in PVM images.71 Additionally, CNN-based in-line image analysis could be used to measure the particle size distribution and predict the crystal growth rate.72 For a continuous wet granulation process, the use of AI has greatly decreased noise, improved process monitoring, and enhanced process understanding.73 Therefore, AI may play a significant role in the future development of intelligent, self-sufficient pharmaceutical production lines. In addition to lowering the environmental load through faster, more economic production or waste reduction, automated systems may also reduce human exposure to hazardous processes or pharmaceutical ingredients such as hormones or cytostatics.74
For pharmaceutical applications, the US FDA categorizes AI software as medical devices that must adhere to Good Manufacturing Practice (GMP)75 and the requirements for validating software.76–78 This indicates that an AI system must handle the information required for PAT and continuous manufacturing processing systems,79 all while maintaining its validated status. As indicated in the previous discussion, the regulatory endorsement of this system necessitates close collaboration among experienced manufacturing regulators, software regulators, and company personnel. Consequently, this implies that gaining regulatory approval will take longer than normal and the volume of data submitted to regulators will be substantial.65
6.2 Process automation
The pharmaceutical production lines morph into smart factories with AI-driven automation, where machines and systems can adjust processes autonomously based on live data. These AI-armed factories use sensor data, robotics, and IoT devices to fully automate the production of drugs, thereby making them easier to improve in terms of efficiency, precision, and scalability.6.3 Inventory and supply chain optimization
Pharmaceutical supply chains include numerous layers and levels, making them complex. It requires smooth management for the delivery of raw materials as well as final products on time. AI is critical in inventory management and demand forecasting and optimization of the overall supply chain:6.4 Reducing costs and improving efficiency
AI integration into pharmaceutical manufacturing would then imply the following huge opportunities for cost savings through enhancing production accuracy, minimizing waste, and optimizing use of resources.6.5 Case study examples: Pfizer and Novartis
7. AI in personalized medicine and pharmacovigilance
AI is revolutionizing both personalized medicine and pharmacovigilance. This is how AI can tailor a patient's medical treatments while further enhancing post-market drug safety surveillance. The introduction of AI based on machine learning, natural language processing, and big data analytics to facilitate real-time, data-driven decision-making has further helped to increase the benefits from the improvements in patient outcomes, early adverse drug reaction detection, or efficient monitoring of drug safety.84,857.1 Personalized medicine
Adjusting health care to the specific needs of every individual in the context of personalized medicine involves tailoring decisions regarding medical care, treatment, practice, or product to each patient. Furthermore, AI is particularly crucial for realizing personalized medicine through the use of genomics and other biological data.7.2 Pharmacovigilance and safety monitoring
Pharmacovigilance is monitoring the effects of licensed medicinal drugs after they have been marketed, especially to identify, assess, understand, and prevent adverse effects or any other drug-related problems. AI actually is important to enhance pharmacovigilance as it is a method through which data collection and analysis can be automatically done.7.3 Real-time monitoring of adverse drug reactions
One of the most promising applications of AI in pharmacovigilance is the real-time monitoring of adverse drug reactions. The systems of pharmacovigilance are basically manual reporting by healthcare professionals or patients, which results in delays far too great to identify dangerous side effects. AI, instead, can analyse data streams through various sources in real time and raise an alert on potential ADRs as soon as they appear.| AI platform | Application | Data sources used | Outcome |
|---|---|---|---|
| IBM Watson Health | Personalized treatment recommendation | Genomic data, EHRs | Tailored treatment plans based on patient data |
| NLP models | Real time adverse event detection | Patient feedback, medical records | Early detection of adverse drug reactions |
| Aetion | Pharmacovigilance | Post-market surveillance data | Continuous monitoring and faster ADR identification |
7.4 Case study examples
8. AI in pharmaceutical management
Artificial Intelligence, also known as AI, has changed drug development and, ultimately, clinical trials as well as the wider scope of operational and management functions in pharmaceutical companies. By deploying AI into management functions, pharmaceutical companies can drive greater operational efficiency, increase data-driven decision-making, and ensure compliance with regulatory requirements for bottom-line improvement in performance and reduction of risks.8.1 Operational efficiency
Efficiency in operations is a must factor across the different departments of the pharmaceutical industry, such as R&D, financial operation, supply chain, and HR. In current times, AI is being used to automate and streamline such operations:8.2 Data-driven decision-making
Data-driven decision-making is a key area in pharmaceutical management that AI has come to highly impact on. Coupled with the capacity of AI, which can process huge amounts of data, pharmaceutical managers will be able to utilize predictive analytics in terms of insights for the boosting of decision-making. These AI tools analyse types of internal and external data, whether they are market trends, sales figures, or research data. From these insights, the AI tools help in strategizing and planning as well as in the allocation of resources.8.3 Regulatory compliance and risk management
One of the issues tackled by the regulatory is ensuring that the practices implemented by those pharmaceutical companies comply with specific guidelines laid out by organizations such as FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency). Hence, compliance with these regulations also rests significantly on AI as it is helping the pharmaceutical companies by which you are staying up to the mark of norms and standards.8.4 AI tools in pharmaceutical management
Pharmaceutical companies have various AI tools and platforms, especially designed for their operations management. What also makes these platforms powerful is they automate mundane tasks and delivering analytics for decision-making and compliance management.9. Challenges and ethical considerations
The pharmaceutical world is witnessing significant changes with the development of Artificial Intelligence. However, it also brings several challenges and ethical issues. Pharmaceutical companies are facing numerous data privacy-related concerns, regulatory compliance, algorithmic biases, and many such factors in their efforts to utilize AI suitably and responsibly.9.1 Data privacy and security
Pharmaceutical companies deal with bulk sensitive information regarding patients during drug discovery, clinical trials, and personalized medicine applications. The use of AI, especially machine learning models that take considerable amounts of data for training, raises concerns over data privacy, breaches, and misuse. Robust data security is thus a critical issue, and the companies must follow various strategies for the protection of patient information.9.2 Regulatory hurdles
The pharma industry is still in the infancy stage of AI adoption, while regulators try to catch up with the rapid technological advancement. Each of the three principal areas, namely drug discovery based on AI, clinical trials, and patient care, is taking the regulatory evaluation into new territories, unlike the traditional approaches that involved drug development with well-defined regulatory guidelines.9.3 Ethical implications
Using AI within the pharmaceutical industry opens a particularly wide and weighty door concerning ethical issues, especially that of bias in algorithms and machine-driven dependency.Research indicates that a significant number of AI models used in healthcare, particularly in clinical trials, are prone to substantial bias due to the use of non-representative datasets. This bias often originates from the initial selection of patient populations for clinical trials, which frequently lack demographic diversity, potentially resulting in distorted outcomes when these models are trained.95 Even with efforts to construct diverse datasets, unconscious biases may still affect both data collection and interpretation. Various factors contribute to data bias, including limited patient demographics and gender bias, as historical trends in clinical trials have often favored male participants, which can skew drug efficacy evaluations for female patients, as well as racial and ethnic disparities.96 In the realm of pharmaceutical manufacturing, even a minor degree of bias in AI systems can lead to significant repercussions, affecting drug efficacy, safety, and patient outcomes. More intricate AI models may be particularly vulnerable to bias if they are not meticulously designed and validated. To address such data bias, it is essential to implement diverse data collection strategies, conduct thorough data quality assessments, make algorithmic adjustments such as employing weighting algorithms to rectify data imbalances, and ensure transparency and comprehensive reporting.
Data bias of this nature can result in significant ethical consequences such as misdiagnosis, biased treatment options, ineffective medications for specific demographic groups, and potential harm to the company's reputation if not managed appropriately. Ethical obligations encompass the proactive reduction of bias through the collection of diverse datasets, thorough evaluation of models, and clear communication of limitations.97 Consequently, pharmaceutical companies using AI must engage in practices that promote diverse data collection, implement quality assurance measures, employ techniques for bias detection, ensure transparent reporting, and conduct regular monitoring while seeking expert evaluations from ethics committees to examine the possible ethical ramifications of AI applications.98,99 Failure to address data bias may lead to legal challenges, cause damage to reputation, and result in increased health disparities.
9.4 The way for regulatory agencies to adopt AI
The FDA and EMEA have the opportunity to integrate artificial intelligence into manufacturing and clinical trials by developing comprehensive guidelines that encompass the creation, validation, and continuous oversight of AI-driven systems. These guidelines should prioritize transparency, data integrity, and patient safety, while also offering a structured approach for manufacturers to submit applications that incorporate AI technologies for evaluation and approval.101 Regulatory bodies can set forth criteria to assess the accuracy, sensitivity, and specificity of AI models used in manufacturing and clinical trials, taking into account potential biases present in the data sets.102 Furthermore, agencies should mandate stringent data collection protocols, appropriate data annotation, and thorough documentation of data origins to ensure the dependability of AI models trained on such information. The guidelines must emphasize the establishment of rigorous validation techniques to verify the performance and reliability of AI systems in practical applications including ongoing performance assessment after market introduction.103 Additionally, there should be a concerted effort to identify and address potential risks linked to AI deployment, such as algorithmic bias, concerns regarding data privacy, and vulnerabilities related to cybersecurity.104Specific guidelines may be established for the utilization of artificial intelligence in the analysis of real-time sensor data to detect and forecast potential challenges within the manufacturing process, thereby facilitating proactive modifications and enhancing quality control.105 The implementation of AI-driven systems can enable automatic adjustments to manufacturing parameters based on real-time data, ensuring consistent product quality. Additionally, AI guidelines can be issued to anticipate equipment failures in manufacturing environments, allowing for preventative maintenance and reducing production downtime.106 In the context of clinical trials, guidelines can be developed for patient recruitment and stratification, real-time monitoring of clinical data, and the application of AI in the analysis of medical images such as X-rays and MRI scans to aid in diagnosis and track disease progression. Close collaboration with industry stakeholder, including pharmaceutical companies, medical device manufacturers, and AI developers will be essential to ensure that these guidelines are both practical and feasible. Furthermore, it is important to design guidelines that can adapt to advancements in AI technology.107
9.5 Case study
10. Training needs of pharmaceutical and clinical trial professionals to adopt AI
The integration of artificial intelligence in pharmaceutical manufacturing and clinical trials necessitates comprehensive training for industrial personnel across several domains. This includes foundational knowledge in data science, familiarity with specific AI algorithms pertinent to their roles, understanding of regulatory compliance associated with AI, data management techniques, critical thinking abilities for interpreting AI-generated results, and awareness of ethical implications related to AI applications in healthcare.108,109In the context of pharmaceutical manufacturing, personnel will require training focused on process monitoring and control through AI. This involves leveraging AI models to forecast and optimize manufacturing parameters such as temperature, pressure, and ingredient ratios in real time. Additionally, quality control will benefit from AI applications, including AI-driven image analysis for defect detection and quality assurance in both drug substances and products. Training will also be essential for predictive maintenance, enabling personnel to use AI for anticipating equipment failures and scheduling preventive maintenance. Furthermore, data integration and analysis training will be necessary to connect manufacturing data from various sources for the training and deployment of AI models.110
Training will be essential for clinical trial personnel to effectively recruit and stratify patients using artificial intelligence, which will assist in identifying appropriate candidates for clinical trials based on their demographics and medical histories.40 Subsequently, personnel will need to undergo training focused on optimizing clinical trial design, utilizing AI to create more efficient trials that require fewer participants and can be completed within shorter timeframes.111 Additional training will encompass real-time patient monitoring, enabling the analysis of patient data from clinical therapies and other sources through AI to identify potential adverse events at an early stage. Furthermore, training in data analysis and interpretation will be necessary, where AI algorithms will be employed to analyze intricate clinical trial data and derive significant insights.112 Similarly, IT personnel and data scientists will require training in advanced AI methodologies, gaining practical experience in constructing, validating, and deploying AI models within a production setting, as well as understanding cloud computing for data storage and processing to facilitate large-scale AI applications, alongside data security and privacy considerations.39 These training programs can be delivered through a combination of online courses, tutorials, in-person workshops, seminars, mentorship initiatives, simulation exercises, customized training sessions, and ongoing learning opportunities.
11. Conclusion and future prospects
This future of AI in the pharmaceutical industry is full of potential changes that will reshape many aspects of developing a drug, conducting clinical trials of that drug, its manufacturing, and the care that comes after. Growth in AI technologies will bring further discoveries in quantum computing, reinforcement learning, and personalized medicine.The most notable development area will be the incorporation of quantum computers into drug discovery. Quantum computers will enable an AI algorithm to execute complex molecular simulations at an unprecedented speed and accuracy that a classical computer could not, solving certain problems in oncology and neurodegenerative diseases. AI applications in personalized medicine will expand further with the use of multi-omics data to predict specific responses by patients to personalized therapies.
From manufacturing to supply chain, smart factories would incorporate AI-driven adjustments of the given processes using real-time data for efficient resource utilization with reduced waste. Similarly, by predictive demand and prevention of shortage scenarios, resilience in supply chains will also increase.
However, with these come problems. For example, data privacy, bias of algorithms, and transparency of AI models raise ethical issues. There would be a need for the FDA and EMA among others to align their frameworks in new settings with that objective to assess the safety and efficacy of AI-driven tools in pharmaceuticals.
In summary, AI already showed transformative power across the pharmaceutical landscape through solutions related to drug development and patient safety that are faster, more efficient, and personalized. As AI technologies continue to evolve, it is necessary that the industry collaborates with regulatory bodies and answers ethical questions to actually tap into the deeper resources of AI for improved benefit to healthcare outcomes. With the capacity to streamline operations, accelerate innovation, and improve the care of patients, AI will be the core cornerstone of the future pharmaceutical industry.
Abbreviations
| AI | Artificial intelligence |
| CNN | Convolutional neural network |
| GANs | Generative adversarial networks |
| ML | Machine learning |
| NLP | Natural language processing |
| DL | Deep learning |
| SAR | Structural activity relationship |
| HTS | High-throughput screening |
| SVM | Support vector machine |
| RNNs | Recurrent neural networks |
| NDA | New drug application |
| USFDA | United States Food and Drug Administration |
| CDSCO | Central Drug Standards and Control Organization |
| MHLW | Ministry of Health and Labour Welfare |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| EHR | Electronic health record |
| ADR | Adverse drug reaction |
| R&D | Research and development |
| HR | Human resources |
| EMA | European Medicines Agency |
| GDPR | General data protection regulation |
| HIPPA | Health insurance portability and accountability act |
| IoT | Internet of things |
| RL | Reinforcement learning |
| NIBR | Novartis Institutes for BioMedical Research |
| CT | Clinical trials |
| CTMS | Clinical trial management system |
| CRO | Contract research organization |
| PMS | Post-marketing surveillance |
Data availability
No primary research results or new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts of interest to declare.References
- K.-K. Mak and M. R. Pichika, Drug Discovery Today, 2019, 24, 773–780 Search PubMed.
- P. Schneider, W. P. Walters, A. T. Plowright, N. Sieroka, J. Listgarten, R. A. Goodnow, J. Fisher, J. M. Jansen, J. S. Duca, T. S. Rush, M. Zentgraf, J. E. Hill, E. Krutoholow, M. Kohler, J. Blaney, K. Funatsu, C. Luebkemann and G. Schneider, Nat. Rev. Drug Discovery, 2020, 19, 353–364 Search PubMed.
- E. J. Topol, Nat. Med., 2019, 25, 44–56 Search PubMed.
- J. He, S. L. Baxter, J. Xu, J. Xu, X. Zhou and K. Zhang, Nat. Med., 2019, 25, 30–36 Search PubMed.
- K. Kourou, T. P. Exarchos, K. P. Exarchos, M. V. Karamouzis and D. I. Fotiadis, Comput. Struct. Biotechnol. J., 2015, 13, 8–17 Search PubMed.
- M. Puri, Y. Pathak, V. K. Sutariya, S. Tipparaju and W. Moreno, Artificial neural network for drug design, delivery and disposition, Academic Press, 2015 Search PubMed.
- J. Ubels, T. Schaefers, C. Punt, H.-J. Guchelaar and J. de Ridder, Bioinformatics, 2020, 36, i601–i609 Search PubMed.
- B. Liu, H. He, H. Luo, T. Zhang and J. Jiang, Stroke Vasc. Neurol., 2019, 4, 206–213 Search PubMed.
- D. Cirillo and A. Valencia, Curr. Opin. Biotechnol., 2019, 58, 161–167 Search PubMed.
- B.-J. Hou and Z.-H. Zhou, IEEE Trans. Neural Netw. Learn. Syst., 2020, 31, 2267–2279 Search PubMed.
- C. Sun, Y. Cao, J.-M. Wei and J. Liu, Bioinformatics, 2021, 37, 3618–3625 Search PubMed.
- N. K. Ebube, G. Owusu-Ababio and C. M. Adeyeye, Int. J. Pharm., 2000, 196, 27–35 Search PubMed.
- M. De Matas, Q. Shao, C. H. Richardson and H. Chrystyn, Eur. J. Pharm. Sci., 2008, 33, 80–90 Search PubMed.
- L. Kwek, S. Fu, T. Chia, C. Diong, C. Tang and S. Krishnan, Appl. Opt., 2005, 44, 4004–4008 Search PubMed.
- E. H. Bollschweiler, S. P. Mönig, K. Hensler, S. E. Baldus, K. Maruyama and A. H. Hölscher, Ann. Surg. Oncol., 2004, 11, 506–511 Search PubMed.
- A. Săftoiu, P. Vilmann, F. Gorunescu, J. Janssen, M. Hocke, M. Larsen, J. Iglesias-Garcia, P. Arcidiacono, U. Will and M. Giovannini, Clin. Gastroenterol. Hepatol., 2012, 10, 84–90 Search PubMed.
- M. D. Wessel, P. C. Jurs, J. W. Tolan and S. M. Muskal, J. Chem. Inf. Comput. Sci., 1998, 38, 726–735 Search PubMed.
- T. Cova, C. Vitorino, M. Ferreira, S. Nunes, P. Rondon-Villarreal and A. Pais, in Artificial Intelligence in Drug Design, ed. A. Heifetz, Springer US, New York, NY, 2022, pp. 321–347, DOI:10.1007/978-1-0716-1787-8_14.
- M. Takenaka, T. Kakue, T. Shimobaba and T. Ito, IEEE Access, 2021, 9, 36766–36774 Search PubMed.
- D. Paul, G. Sanap, S. Shenoy, D. Kalyane, K. Kalia and R. K. Tekade, Drug Discovery Today, 2021, 26, 80–93 Search PubMed.
- J. M. Stokes, K. Yang, K. Swanson, W. Jin, A. Cubillos-Ruiz, N. M. Donghia, C. R. MacNair, S. French, L. A. Carfrae, Z. Bloom-Ackermann, V. M. Tran, A. Chiappino-Pepe, A. H. Badran, I. W. Andrews, E. J. Chory, G. M. Church, E. D. Brown, T. S. Jaakkola, R. Barzilay and J. J. Collins, Cell, 2020, 180, 688–702 Search PubMed.
- J. Fang, L. Wang, Y. Li, W. Lian, X. Pang, H. Wang, D. Yuan, Q. Wang, A.-L. Liu and G.-H. Du, PLoS One, 2017, 12, e0178347 Search PubMed.
- A. Bender, H. Y. Mussa and R. C. Glen, SLAS Discovery, 2005, 10, 658–666 Search PubMed.
- A. Abdo, B. Chen, C. Mueller, N. Salim and P. Willett, J. Chem. Inf. Model., 2010, 50, 1012–1020 Search PubMed.
- Y. Li, L. Wang, Z. Liu, C. Li, J. Xu, Q. Gu and J. Xu, Mol. BioSyst., 2015, 11, 1241–1250 Search PubMed.
- M. Lu, J. Yin, Q. Zhu, G. Lin, M. Mou, F. Liu, Z. Pan, N. You, X. Lian and F. Li, Engineering, 2023, 27, 37–69 Search PubMed.
- M. Fernández, A. Tundidor-Camba and J. Caballero, J. Chem. Inf. Model., 2005, 45, 1884–1895 Search PubMed.
- M. Fernández, J. Caballero and A. Tundidor-Camba, Bioorg. Med. Chem., 2006, 14, 4137–4150 Search PubMed.
- H. González-Díaz, I. Bonet, C. Terán, E. De Clercq, R. Bello, M. M. García, L. Santana and E. Uriarte, Eur. J. Med. Chem., 2007, 42, 580–585 Search PubMed.
- M. Fernández, M. C. Carreiras, J. L. Marco and J. Caballero, J. Enzyme Inhib. Med. Chem., 2006, 21, 647–661 Search PubMed.
- M. Fernández and J. Caballero, Bioorg. Med. Chem., 2007, 15, 6298–6310 Search PubMed.
- Y.-P. Pang, P. Quiram, T. Jelacic, F. Hong and S. Brimijoin, J. Biol. Chem., 1996, 271, 23646–23649 Search PubMed.
- M. Fernández and J. Caballero, Bioorg. Med. Chem., 2006, 14, 280–294 Search PubMed.
- M. Fernández and J. Caballero, Chem. Biol. Drug Des., 2006, 68, 201–212 Search PubMed.
- D. A. Filimonov, A. A. Lagunin, T. A. Gloriozova, A. V. Rudik, D. S. Druzhilovskii, P. V. Pogodin and V. V. Poroikov, Chem. Heterocycl. Compd., 2014, 50, 444–457 Search PubMed.
- J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov, O. Ronneberger, K. Tunyasuvunakool, R. Bates, A. Žídek, A. Potapenko, A. Bridgland, C. Meyer, S. A. A. Kohl, A. J. Ballard, A. Cowie, B. Romera-Paredes, S. Nikolov, R. Jain, J. Adler, T. Back, S. Petersen, D. Reiman, E. Clancy, M. Zielinski, M. Steinegger, M. Pacholska, T. Berghammer, S. Bodenstein, D. Silver, O. Vinyals, A. W. Senior, K. Kavukcuoglu, P. Kohli and D. Hassabis, Nature, 2021, 596, 583–589 Search PubMed.
- T. Burki, The Lancet Digital Health, 2020, 2, e226–e227 Search PubMed.
- F. US , The Drug Development Process, https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process, (accessed 26.01.2025).
- S. Harrer, P. Shah, B. Antony and J. Hu, Trends Pharmacol. Sci., 2019, 40, 577–591 Search PubMed.
- X. Lu, C. Yang, L. Liang, G. Hu, Z. Zhong and Z. Jiang, J. Am. Med. Inform. Assoc., 2024, 31, 2749–2759 Search PubMed.
- X. Liu, C. Shi, U. Deore, Y. Wang, M. Tran, I. Khalil and M. Devarakonda, A Scalable AI approach for clinical trial cohort optimization, Paper presented at: Joint European Conference on Machine Learning and Knowledge Discovery in Databases, 2021.
- B. D. Dubois, J. Bioinf. Artif. Intell., 2024, 4, 72–83 Search PubMed.
- R. Harpaz, K. Haerian, H. S. Chase and C. Friedman, presented in part at the Proceedings of the 1st ACM International Health Informatics Symposium, 2010, 2010.
- D. Calaprice-Whitty, K. Galil, W. Salloum, A. Zariv and B. Jimenez, Ther. Innov. Regul. Sci., 2020, 54, 69–74 Search PubMed.
- W. B. van Dijk, A. T. Fiolet, E. Schuit, A. Sammani, T. K. J. Groenhof, R. van der Graaf, M. C. de Vries, M. Alings, J. Schaap and F. W. Asselbergs, J. Clin. Epidemiol., 2021, 132, 97–105 Search PubMed.
- Y. Ni, J. Wright, J. Perentesis, T. Lingren, L. Deleger, M. Kaiser, I. Kohane and I. Solti, BMC Med. Inf. Decis. Making, 2015, 15, 1–10 Search PubMed.
- Y. Ni, M. Bermudez, S. Kennebeck, S. Liddy-Hicks and J. Dexheimer, JMIR Med. Inform., 2019, 7, e14185 Search PubMed.
- A. Ismail, T. Al-Zoubi, I. El Naqa and H. Saeed, BJR Open, 2023, 5, 1–5 Search PubMed.
- D. AI , AI powered patient recruitment & RWD, https://deep6.ai/, (accessed 26.01.2025).
- A. Blanco-González, A. Cabezón, A. Seco-González, D. Conde-Torres, P. Antelo-Riveiro, Á. Piñeiro and R. Garcia-Fandino, Pharmaceuticals, 2023, 16, 891 Search PubMed.
- Benevolent , COVID-19 research, https://www.benevolent.com/about-us/sustainability/covid-19/, (accessed 26.01.2025).
- Exscientia , Precision Medicine, https://www.exscientia.com/precision-medicine/, (accessed 26.01.2025).
- G. Calzavara, E. Oliosi and G. Ferrari, A Time-aware Data Clustering Approach to Predictive Maintenance of a Pharmaceutical Industrial Plant, Paper presented at: 2021 International Conference on Artificial Intelligence in Information and Communication (ICAIIC); 13–16 April, 2021, 2021.
- I. Kavasidis, E. Lallas, V. C. Gerogiannis, T. Charitou and A. Karageorgos, Procedia Comput. Sci., 2023, 220, 576–583 Search PubMed.
- M. Puri, S. Manwatkar, P. Karpe and S. Kulkarni, in Biosystems, Biomedical & Drug Delivery Systems: Characterization, Restoration and Optimization, ed. S. Kulkarni, A. K. Haghi and S. Manwatkar, Springer Nature Singapore, Singapore, 2024, pp. 179–196, DOI:10.1007/978-981-97-2596-0_9.
- D. Yadav, K. Sonkar, J. Kumar and A. Chaudhary, Eur. J. Parenter. Pharm. Sci., 2023, 28(4), 1–20 Search PubMed.
- M. Vaghela, S. Rathi, R. Shirole, J. Verma, Shaheen, S. Panigrahi and S. Singh, Chin. J. Appl. Physiol., 2024, 40, e20240005 Search PubMed.
- F. M. Talaat and E. Hassan, Artificial intelligence in 3D printing, in Enabling Machine Learning Applications in Data Science, Algorithms for Intelligent Systems, Singapore: Springer Nature, 2021, pp. 77–88 Search PubMed.
- Z. Zhu, D. W. H. Ng, H. S. Park and M. C. McAlpine, Nat. Rev. Mater., 2021, 6, 27–47 Search PubMed.
- B. R. Hunde and A. D. Woldeyohannes, Results Eng., 2022, 14, 100478 Search PubMed.
- I. Rojek, D. Mikołajewski, E. Dostatni and M. Macko, Materials, 2020, 13, 5437 Search PubMed.
- M. Elbadawi, L. E. McCoubrey, F. K. Gavins, J. J. Ong, A. Goyanes, S. Gaisford and A. W. Basit, Adv. Drug Delivery Rev., 2021, 175, 113805 Search PubMed.
- A. Banerjee, H. K. Haridas, A. SenGupta and N. Jabalia, Emerging Applications of 3D Printing During CoVID 19 Pandemic, 2022, pp. 57–79 Search PubMed.
- Z. Grof and F. Štěpánek, Comput. Chem. Eng., 2021, 154, 107492 Search PubMed.
- G. Subramanian, Process control, intensification, and digitalisation in continuous biomanufacturing, John Wiley & Sons, 2021 Search PubMed.
- H. Valizadeh, M. Pourmahmood, J. S. Mojarrad, M. Nemati and P. Zakeri-Milani, Drug Dev. Ind. Pharm., 2009, 35, 396–407 Search PubMed.
- Z. Zhou, X. Li and R. N. Zare, ACS Cent. Sci., 2017, 3, 1337–1344 Search PubMed.
- D. Q. Gbadago, J. Moon, M. Kim and S. Hwang, Chem. Eng. J., 2021, 409, 128163 Search PubMed.
- W. C. Wong, E. Chee, J. Li and X. Wang, Mathematics, 2018, 6, 242 Search PubMed.
- I. Baranilingesan, Curr. Sci., 2021, 120(8), 1324–1333 Search PubMed.
- H. Salami, M. A. McDonald, A. S. Bommarius, R. W. Rousseau and M. A. Grover, Org. Process Res. Dev., 2021, 25, 1670–1679 Search PubMed.
- S. Chen, T. Liu, D. Xu, Y. Huo and Y. Yang, Image based measurement of population growth rate for l-glutamic acid crystallization, Paper presented at: 2019 Chinese Control Conference (CCC), 2019.
- Y. Roggo, M. Jelsch, P. Heger, S. Ensslin and M. Krumme, Eur. J. Pharm. Biopharm., 2020, 153, 95–105 Search PubMed.
- B. Nagy, D. L. Galata, A. Farkas and Z. K. Nagy, AAPS J., 2022, 24, 74 Search PubMed.
- S. Ramakrishna, L. Tian, C. Wang, S. Liao and W. E. Teo, Medical devices: regulations, standards and practices, Woodhead Publishing, 2015 Search PubMed.
- US FDA, Guidance for Industry Part 11, Electronic Records; Electronic Signatures – Scope and Application, 2003, FDA-2003-D-0143.
- US FDA, Considerations for the Use of Artificial Intelligence to Support Regulatory Decision-Making for Drug and Biological Products Guidance for Industry and Other Interested Parties, 2025, FDA-2024-D-4689.
- US FDA, Center for Drug Evaluation and Research, Artificial Intelligence in Drug Manufacturing, 2023, FDA-2023-N-0487.
- S. S. Kuwahara, Continuous Biomanufacturing–Innovative Technologies and Methods: Innovative Technologies and Methods, 2017, 533–548 Search PubMed.
- N. Bhatnagar, Role of Robotic Process Automation in Pharmaceutical Industries, Paper presented at: The International Conference on Advanced Machine Learning Technologies and Applications (AMLTA2019), 2020, https://link.springer.com/chapter/10.1007/978-3-030-14118-9_50.
- O. Joel, A. Oyewole, O. Odunaiya and O. Soyombo, Int. J. Manag. Entrep. Res., 2024, 6, 707–721 Search PubMed.
- M. DeMello , Artificial Intelligence at Pfizer – 3 Use Cases, https://emerj.com/artificial-intelligence-at-pfizer/, (accessed 26.01.2025).
- N. Shah, Comput. Chem. Eng., 2004, 28, 929–941 Search PubMed.
- M. Salas, J. Petracek, P. Yalamanchili, O. Aimer, D. Kasthuril, S. Dhingra, T. Junaid and T. Bostic, Pharm. Med., 2022, 36, 295–306 Search PubMed.
- L. Liang, J. Hu, G. Sun, N. Hong, G. Wu, Y. He, Y. Li, T. Hao, L. Liu and M. Gong, Drug Saf., 2022, 45, 511–519 Search PubMed.
- Y. Chen, J. D. Elenee Argentinis and G. Weber, Clin. Ther., 2016, 38, 688–701 Search PubMed.
- F. H.-L. R. Ltd, A Year in Review 2020, Basel, Switzerland, 2021 Search PubMed.
- I. Singh, J. Kaur, S. Kaur, B. R. Barik and R. Pahwa, Braz. Arch. Biol. Technol., 2023, 66, 1–16 Search PubMed.
- S. Singh, P. Shakdwipee and D. Shrimali, Harnessing Artificial Intelligence in Financial Operations: Opportunities and Challenges Conference paper First Online: 31 October 2024, pp. 547–560, https://link.springer.com/chapter/10.1007/978-981-97-6678-9_48?fromPaywallRec=false.
- P. Ullagaddi, J. Adv. Med. Pharm. Sci., 2024, 26, 75–86 Search PubMed.
- F. Pasquale, New Laws of Robotics, Defending Human Expertise in the Age of AI, Harvard University Press, 2020 Search PubMed.
- Z. Obermeyer, B. Powers, C. Vogeli and S. Mullainathan, Science, 2019, 366, 447–453 Search PubMed.
- N. Nwebonyi and F. McKay, BMC Med. Ethics, 2024, 25, 113 Search PubMed.
- A. S. Albahri, A. M. Duhaim, M. A. Fadhel, A. Alnoor, N. S. Baqer, L. Alzubaidi, O. S. Albahri, A. H. Alamoodi, J. Bai and A. Salhi, Inf. Fusion, 2023, 96, 156–191 Search PubMed.
- R. Challen, J. Denny, M. Pitt, L. Gompels, T. Edwards and K. Tsaneva-Atanasova, BMJ Qual. Saf., 2019, 28, 231–237 Search PubMed.
- J. Zhang and Z.-m. Zhang, BMC Med. Inf. Decis. Making, 2023, 23, 7 Search PubMed.
- R. B. Parikh, S. Teeple and A. S. Navathe, J. Am. Med. Assoc., 2019, 322, 2377–2378 Search PubMed.
- L. Belenguer, AI Ethics, 2022, 2, 771–787 Search PubMed.
- R. M. Ratwani, K. Sutton and J. E. Galarraga, J. Am. Med. Assoc., 2024, 332, 1051–1052 Search PubMed.
- D. F. Dariush and Z. Shaghayegh, Iran. J. Public Health, 2021, 50, i–v Search PubMed.
- H. B. Harvey and V. Gowda, Acad. Radiol., 2020, 27, 58–61 Search PubMed.
- R. S. Patil, S. B. Kulkarni and V. L. Gaikwad, Drug Discovery Today, 2023, 103700 Search PubMed.
- H. B. Harvey and V. Gowda, Radiol. Clin., 2021, 59, 1075–1083 Search PubMed.
- A. Romagnoli, F. Ferrara, R. Langella and A. Zovi, Pharm. Res., 2024, 41, 721–730 Search PubMed.
- M. Aboy, T. Minssen and E. Vayena, npj Digit. Med., 2024, 7, 237 Search PubMed.
- D. W. Opderbeck, Fordham L. Rev., 2019, 88, 553 Search PubMed.
- L. Nene, B. T. Flepisi, S. J. Brand, C. Basson and M. Balmith, Clin. Ther., 2024, e6–e14 Search PubMed.
- L. K. Vora, A. D. Gholap, K. Jetha, R. R. S. Thakur, H. K. Solanki and V. P. Chavda, Pharmaceutics, 2023, 15, 1916 Search PubMed.
- Z. Jan, F. Ahamed, W. Mayer, N. Patel, G. Grossmann, M. Stumptner and A. Kuusk, Expert Syst. Appl., 2023, 216, 119456 Search PubMed.
- P. Bhatt, S. Singh, V. Kumar, K. Nagarajan, S. K. Mishra, P. K. Dixit, V. Kumar and S. Kumar, Curr. Artif. Intell., 2024, 2, E051223224198 Search PubMed.
- S. Anuyah, M. K. Singh and H. Nyavor, arXiv, 2024, preprint, arXiv:2412.07050, DOI:10.30574/wjarr.2024.24.3.3671.
- R. E. Hariry, R. V. Barenji and A. Paradkar, Handbook of Smart Materials, Technologies, and Devices: Applications of Industry 4.0, 2020, pp. 1–22 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |