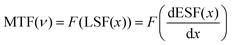Modulation of halogens in organic manganese halides for high-resolution and large-area flexible X-ray imaging†
Ying
Sun
a,
Qian
Ma
 b,
Dongheng
Zhao
b,
Pan
Gao
a,
Qi
Wang
a,
Zeyu
Guo
a and
Xiaomei
Jiang
b,
Dongheng
Zhao
b,
Pan
Gao
a,
Qi
Wang
a,
Zeyu
Guo
a and
Xiaomei
Jiang
 *a
*a
aSchool of Preventive Medicine Sciences (Institute of Radiation Medicine), Shandong First Medical University & Shandong Academy of Medical Sciences, No. 6699 Qingdao Road, Jinan 250117, People's Republic of China. E-mail: jiangxiaomei@sdfmu.edu.cn
bSchool of Material Science and Engineering, University of Jinan, No. 336 Nanxinzhuang West Road, Jinan 250022, People's Republic of China
First published on 19th November 2024
Abstract
Manganese-based organic metal halides, due to their excellent optoelectronic properties and stable chemical nature, have been widely used in optoelectronic devices and sensors. In this study, through rational modulation of halogens, three novel zero-dimensional organic manganese halides [(C6H8N)2MnX4 (X = Cl, Br, and I)] were obtained by changing the halogen atom via the solvent evaporation method. Due to the d–d electronic transition of Mn2+ in the tetrahedrally coordinated [MnX4]2− polyhedron, all samples exhibit strong green emission. Notably,(C6H8N)2MnBr4 exhibited a remarkable light yield of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 photons per MeV and a low detection limit of 1.9 μGy s−1, which is below the X-ray diagnostic limit and even superior to that of commercial BGO scintillators. Moreover, a 9 cm × 9 cm flexible scintillator film was successfully fabricated by mixing (C6H8N)2MnBr4 crystalline powder with polymethyl methacrylate, and this film manifested superior radiation stability and an X-ray imaging resolution of 12.1 lp mm−1. Notably, the X-ray images captured by the flexible film demonstrate distinguished clarity when adhered perfectly to curved metal sheets. The combination of excellent performance and facile solution processing opens new opportunities for low-cost, high-performance organic metal halide-based large-area flexible scintillators for X-ray detection and imaging.
224 photons per MeV and a low detection limit of 1.9 μGy s−1, which is below the X-ray diagnostic limit and even superior to that of commercial BGO scintillators. Moreover, a 9 cm × 9 cm flexible scintillator film was successfully fabricated by mixing (C6H8N)2MnBr4 crystalline powder with polymethyl methacrylate, and this film manifested superior radiation stability and an X-ray imaging resolution of 12.1 lp mm−1. Notably, the X-ray images captured by the flexible film demonstrate distinguished clarity when adhered perfectly to curved metal sheets. The combination of excellent performance and facile solution processing opens new opportunities for low-cost, high-performance organic metal halide-based large-area flexible scintillators for X-ray detection and imaging.
Introduction
As crucial elements in indirect X-ray imaging, X-ray scintillators have garnered significant attention in the realms of medical imaging, non-destructive testing, industrial flaw detection, and scientific research.1–4 Scintillators can absorb X-rays and convert them into visible light, which can then be transformed into electrical signals using photoelectric sensors like CCD cameras or photomultiplier tubes. These signals are ultimately transmitted to an intelligent display terminal for image processing, resulting in the final imaged picture.5,6 A series of representative traditional inorganic scintillators, such as Bi4Ge3O12 (BGO), NaI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tl, and CsI
Tl, and CsI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tl, have been successfully applied to X-ray imaging due to their high X-ray absorption capacity.7 However, the high-temperature conditions required for growing these crystals make the production process complex and expensive.8,9 Therefore, research is imperative for achieving low-cost and high-performance scintillators.
Tl, have been successfully applied to X-ray imaging due to their high X-ray absorption capacity.7 However, the high-temperature conditions required for growing these crystals make the production process complex and expensive.8,9 Therefore, research is imperative for achieving low-cost and high-performance scintillators.
In recent years, organic metal halide materials have become a focal point in the research of new-generation scintillator materials. These materials are favored for their cost-effective production, adjustable band gaps, and excellent luminescence properties, presenting vast opportunities in light-emitting diodes and detectors.10–13 Among them, organic lead halide perovskite materials demonstrate suitability for X-ray imaging, benefiting from their high X-ray attenuation ability, and have achieved notable advancements.14,15 However, the high toxicity and poor stability of lead-based perovskites limit their further practical application. With reports of scintillator materials based on the metals of tin (Sn), copper (Cu), antimony (Sb) and manganese (Mn), such as crystals of (C8H17NH3)2SnBr4,16 (4-bzpy)4Cu4I4,17 C50H44P2SbCl5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18,19 and (C38H34P2)MnBr4,20 new environmentally friendly lead-free counterparts have emerged.21 It is noteworthy that organic manganese halides stand out among other materials due to their unique luminous mechanism, high luminous efficiency, facile synthesis, and simple structure design, sparking waves of research enthusiasm.22
18,19 and (C38H34P2)MnBr4,20 new environmentally friendly lead-free counterparts have emerged.21 It is noteworthy that organic manganese halides stand out among other materials due to their unique luminous mechanism, high luminous efficiency, facile synthesis, and simple structure design, sparking waves of research enthusiasm.22
Compared with other lead-free halide materials, the luminescence of organic manganese halide scintillators originates from the energy transition emission of the Mn2+ luminescence center.23 It can be excited in the UV or blue region, exhibiting a larger Stokes shift and a high PLQY.24 The luminescence intensity is influenced by the crystal field intensity. Depending on the coordination environment of Mn2+ ions, octahedral Mn2+ halides exhibit red emission, while tetrahedral Mn2+ halides exhibit green emission.25 In addition, their low-dimensional crystal structure endows them with enhanced electron localization, which promotes radiative recombination and results in higher PLQY.22 For example, Jiang et al.24 reported that the C4H12NMnCl3 crystal with Mn2+ octahedral coordination exhibits red emission at 635 nm, with a PLQY as high as 91.8%. This scintillator demonstrates good radiation hardness and can achieve X-ray imaging resolutions of up to 5 lp mm−1. In addition, previously reported crystals such as TEA2MnI4,26 TPP2MnBr4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 and (C38H34P2)MnBr4,23 which all have tetrahedrally coordinated Mn2+, exhibit green light emission, and possess excellent photophysical properties. Their light yield (LY) can even rival that of commercial scintillators, showcasing immense potential in the field of X-ray imaging.
27 and (C38H34P2)MnBr4,23 which all have tetrahedrally coordinated Mn2+, exhibit green light emission, and possess excellent photophysical properties. Their light yield (LY) can even rival that of commercial scintillators, showcasing immense potential in the field of X-ray imaging.
The photophysical properties of organic metal halides are significantly influenced by various halogen elements.28 Each halogen not only affects the chemical stability of these materials, but also alters their optical characteristics. For instance, the previous literature indicates that owing to the smaller radius and higher electronegativity of chlorine, MAPbCl3 exhibits stronger ionic bond stability in thermal environments compared to MAPbBr3 and MAPbI3.29,30 In contrast, the incorporation of bromine often yields a favorable balance between light absorption and luminescence efficiency,31 demonstrating considerable potential for applications. Additionally, iodine, with its larger atomic radius and atomic number, enhances the adaptability of the crystal structure and increases X-ray attenuation.26 Thus, the careful selection and manipulation of different halogens are crucial for optimizing the optoelectronic properties of organic metal halides, providing valuable insights for the development of new and efficient optoelectronic devices.
For X-ray imaging equipment, such as commercial computed X-ray tomography scanners, the internal X-ray detection component is typically designed in a ring shape to achieve comprehensive scanning of the target object. Unfortunately, most available scintillators are rigid single crystals (e.g., NaI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tl and CsI
Tl and CsI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tl) that are assembled into rings using multiple scintillators,32,33 resulting in loss of image information at the joints. Therefore, there arises a demand for a large-area flexible film that can adapt to detectors of various shapes and configurations. Additionally, for irregularly shaped imaging objects, a flexible scintillation film can conform to the object's surface, reducing scattering lines and optical crosstalk, consequently improving image quality and spatial resolution.34
Tl) that are assembled into rings using multiple scintillators,32,33 resulting in loss of image information at the joints. Therefore, there arises a demand for a large-area flexible film that can adapt to detectors of various shapes and configurations. Additionally, for irregularly shaped imaging objects, a flexible scintillation film can conform to the object's surface, reducing scattering lines and optical crosstalk, consequently improving image quality and spatial resolution.34
Herein, three types of 0D manganese-based organic metal halides [(C6H8N)2MnX4 (X = Cl, Br, and I)] were rationally designed and prepared via solvent evaporation. 2-Methylpyridine was selected as the organic ligand for its ability to effectively integrate into [MnX4]2−, consequently increasing the Mn–Mn distance. Furthermore, its strong interactions with halides can enhance structural rigidity and reduce non-radiative transitions from thermal vibrations.24,25,35 Based on the d–d transition of the [MnX4]2− tetrahedron, the three compounds exhibit efficient green luminescence, with (C6H8N)2MnBr4 achieving a high PLQY of 64.7% that significantly surpasses the 17.18% of (C6H8N)2MnCl4 and the 15.31% of (C6H8N)2MnI4. Furthermore, the single crystals of (C6H8N)2MnBr4 display extraordinary scintillation properties, exhibiting an LY of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 photons per MeV and a low detection limit of 1.9 μGy s−1. By mixing the crystal powder with PMMA, a large-area flexible scintillation film was successfully fabricated with size of 9 cm × 9 cm. This film was utilized for imaging objects, achieving a spatial resolution of 12.1 lp mm−1. Additionally, the images of bent metal sheets further validated the superior performance of the flexible film in X-ray imaging. The film demonstrates its capability to conform to various complex and irregular surfaces, highlighting its vast range of application prospects in medical, industrial, security, and other fields.
224 photons per MeV and a low detection limit of 1.9 μGy s−1. By mixing the crystal powder with PMMA, a large-area flexible scintillation film was successfully fabricated with size of 9 cm × 9 cm. This film was utilized for imaging objects, achieving a spatial resolution of 12.1 lp mm−1. Additionally, the images of bent metal sheets further validated the superior performance of the flexible film in X-ray imaging. The film demonstrates its capability to conform to various complex and irregular surfaces, highlighting its vast range of application prospects in medical, industrial, security, and other fields.
Results and discussion
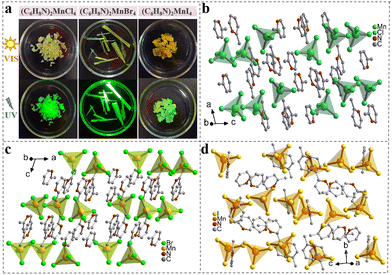
We selected 2-methylpyridine as the organic molecule and prepared single crystals (SCs) of (C6H8N)2MnX4 (X = Cl, Br, and I) using the solvent evaporation method (Fig. S1†). As shown in Fig. 1(a), the three different halide crystals vary in appearance from green to yellow under natural light. However, they all exhibit green luminescence when irradiated with a 365 nm UV lamp. The single-crystal X-ray diffraction (SCXRD) results indicate that the crystals of (C6H8N)2MnCl4 and (C6H8N)2MnBr4 crystallize in monoclinic space groups of I2/a and C2/c, respectively, while the single crystal of (C6H8N)2MnI4 belongs to the orthorhombic space group of Pbca (Table S1†). As illustrated in Fig. 1(b–d), within these organic manganese halides, each Mn2+ ion forms a distorted [MnX4]2− (X = Cl, Br, and I) tetrahedron by coordinating with four halogen ions, which is surrounded by C6H8N+ cations, creating a 0D structure at the molecular level. These tetrahedra exhibit varying degrees of distortion, with their Mn–X bond lengths ranging from 2.3421 to 2.3739 Å, 2.4807 to 2.5193 Å, and 2.6839 to 2.7199 Å, respectively. The X–Mn–X bond angles vary from 106.06° to 112.86°, 105.96° to 112.69°, and 105.50° to 112.24° (Table S2†). Referring to the previous literature,36 we calculated the bond length distortions and bond angle variances for the three crystals. The distortions in the bond lengths of [MnX4]2− (X = Cl, Br, and I) were found to be 3.37 × 10−5, 3.30 × 10−5, and 3.83 × 10−5, while the corresponding bond angle variances were 5.98, 5.51, and 6.69. Among these, [MnBr4]2− showed the least distortion and highest symmetry, making it the closest to an ideal tetrahedron, which can enhance the photoluminescence performance of the crystal.37 Additionally, the shortest Mn–Mn distances in (C6H8N)2MnX4 (X = Cl, Br, and I) SCs are 7.62 Å, 7.81 Å, and 8.27 Å, respectively (Fig. S2†). This change results from the differing electronegativities of Cl−, Br−, and I−, leading to variations in hydrogen bond strength between halides and organic molecules, which in turn affects the separation of Mn tetrahedra.36 Detailed crystal parameters and refinement data for each SC are provided in the ESI.†The powder X-ray diffraction (PXRD) results of the three crystals align with the SCXRD simulation results, confirming that the synthesized SCs possess a high-purity phase (Fig. 2(a)). The Fourier transform infrared (FTIR) spectra show notable peaks, including those for C–N stretching at 1099 cm−1 and N–H vibration at 1565 cm−1. These observations determine the presence of organic molecules (Fig. 2(b)). Additionally, the high-resolution X-ray photoelectron spectroscopy (XPS) spectra also demonstrate the presence of C, N, Mn, and X (X = Cl, Br, and I) elements in the three crystals (Fig. 2(c)). As shown in Fig. S3–S5,† there are two distinct Mn 2p characteristic peaks in the (C6H8N)2MnX4 (X = Cl, Br, and I) crystals, corresponding to Mn 2p3/2 and Mn 2p1/2 respectively. These peaks are observed at 641.7 and 653.5 eV for (C6H8N)2MnCl4, 641.8 and 653.8 eV for (C6H8N)2MnBr4, and 640.6 and 652.6 eV for (C6H8N)2MnI4. Additionally, the two peaks at 198.1 and 199.7 eV in Fig. S3(b)† can be attributed to Cl 2p3/2 and Cl 2p1/2 in the (C6H8N)2MnCl4 crystals. The peaks at 68.4 and 69.4 eV correspond to Br 3d5/2 and Br 3d3/2 in the (C6H8N)2MnBr4 crystals (Fig. S4(b)†), while the peaks at 617.6 and 629 eV are assigned to I 3d2/5 and I 3d3/2 in the (C6H8N)2MnI4 crystals (Fig. S5(b)†), respectively. The results of scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) of the (C6H8N)2MnX4 (X = Cl, Br, and I) SCs in Fig. S6–S8† further verify that elements such as C, N, Mn and X (X = Cl, Br, and I) are present and evenly distributed in the crystals. Furthermore, the thermal stability of the three halide crystals was evaluated through thermogravimetric analysis (TGA), showing that the (C6H8N)2MnX4 (X = Cl, Br, and I) crystals began to decompose at 156 °C, 185 °C, and 247 °C, respectively (Fig. 2(d)).
 | ||
| Fig. 2 (a) Experimental PXRD and simulated PXRD patterns, (b) FTIR spectra, (c) survey XPS spectra and (d) TG curves of (C6H8N)2MnX4 (X = Cl, Br, and I). | ||
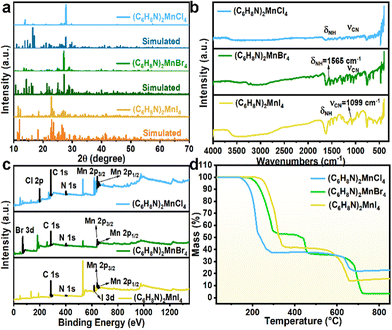
The steady-state photoluminescence (PL) and excitation (PLE) spectra reveal that all three organic manganese halide crystals exhibit narrow-band green emission in the visible light range (Fig. 3(a–c)). Notably, the PL and PLE spectra are red-shifted with increasing halogen atomic number. Specifically, the peak emission for the crystals based on Cl−, Br−, and I− are centered at 520, 525, and 548 nm, respectively. The emission wavelength is influenced by several factors, particularly the strength of the ligand field and the splitting of the d-orbitals in a tetrahedral environment. For Mn(II) ions, a weaker ligand field results in smaller d-orbital splitting.38 Consequently, samples incorporating Br− exhibit a slight red shift compared to those with Cl−. Additionally, the covalent bond strength of the Mn–I interaction affects the electronic environment of the Mn ions and their d-orbital splitting. Strong covalent bonds can lower the energy required for electronic transitions, leading to further red shift in the emission wavelength.39 UV-vis absorption spectra are used to study the absorption characteristics of the three manganese halide crystals (Fig. 3(d–f)). The three samples exhibit multiple absorption peaks at 263, 298, 364, 377, 434, 455, and 476 nm, corresponding to the 6A1 → 4A2(F), 6A1 → 4T1(F), 6A1 → 4Tg(4p), 6A1 → 4Eg(4D), 6A1 → 4Ag, 4Eg(4G), 6A1 → 4T2(4G) and 6A1 → 4T1(4G) transitions, respectively. After excitation, the excited electrons relax to the lowest excited state 4T1 through several non-radiative processes. Subsequently, they emit bright light via the 4T1 → 6A1 radiative transition (Fig. S9†). Additionally, the direct band gaps of (C6H8N)2MnX4 (X = Cl, Br, and I) were calculated using the Tauc method, yielding values of 2.50, 2.45, and 2.33 eV, respectively (inset of Fig. 3(d–f)). In the tetrahedral coordinated environment of manganese halides, the weak crystal field strength results in a high separation level of 4T1 → 6A1, contributing to the narrow-band green emission of the crystals.24 Afterwards, we recorded the PL and PLE spectra of the three samples at various excitation and emission wavelengths. The contour plots clearly show that the emission centers of the three samples do not shift (Fig. S10†). This consistency further indicates that the green light emission characteristics of (C6H8N)2MnX4 (X = Cl, Br, and I) arise solely from the tetrahedrally coordinated manganese center and are the result of relaxation from the same excited state. Additionally, the decay curves of time-resolved photoluminescence (TRPL) spectra for the (C6H8N)2MnX4 (X = Cl, Br, and I) crystals were fitted using a single exponential function. The calculated lifetimes are 509.4, 262.6 and 15.8 μs, respectively, showcasing a discernible trend of faster attenuation from Cl to Br to I (Fig. 3(g–i)). This trend is mainly due to the heavy-atom-enhanced reverse intersystem crossing (RISC) process.40,41
 | ||
| Fig. 3 (a–c) PLE and PL spectra, (d–f) UV-vis absorption spectra, and (g–i) PL decay curves of (C6H8N)2MnX4 (X = Cl, Br, and I). | ||
To evaluate the photophysical properties of the title samples, their PLQYs were measured as 17.18%, 64.7%, and 15.31%, respectively (Fig. S11†). Studies have shown that the Mn–Mn distance significantly influences the luminescence properties of the crystals. Generally, a larger Mn–Mn distance can reduce non-radiative transitions between manganese ions, thereby improving the excitation and recombination efficiency of the photo-generated carriers, which can effectively enhance the PLQY.42,43 Moreover, lower symmetry can accelerate PL decay, which is unfavorable for enhancing the PLQY.43 Consequently, (C6H8N)2MnBr4 exhibits a higher PLQY compared to that of (C6H8N)2MnCl4, which may arise from a longer Mn–Mn distance and a higher symmetry as suggested from the single-crystal structure data. It is also noteworthy that the lower PLQY of (C6H8N)2MnI4 may be ascribed to the significant structural distortion of the inorganic unit, as well as the enhanced spin–orbit coupling effects and strong self-absorption.28,42,43 Specifically, the larger atomic radius and lower electronegativity may lead to a stronger spin–orbit coupling effect, increasing the probability of non-radiative recombination. On the other hand, the substantial overlap between the absorption and emission peaks can enhance the self-absorption of the crystal.28,42 This results in some of the excitation light being absorbed by the material rather than being effectively converted to light emission, ultimately leading to a lower PLQY.
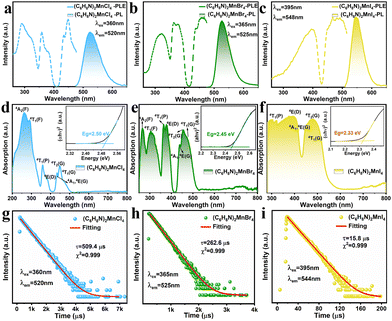
Using density functional theory (DFT), we performed the calculation and investigation of the electronic properties of (C6H8N)2MnBr4, as illustrated in Fig. 4(a). Our analysis reveals that the valence band maximum (VBM) and the conduction band minimum (CBM) are situated at the same high-symmetry point, resulting in a calculated direct bandgap of 2.4 eV, which closely aligns with the experimental values. Further examination indicates that both the VBM and CBM exhibit a significant degree of flatness, suggesting that electrons are in a highly localized state.44 Through partial density of states (PDOS) analysis, we observed that the VBM is primarily composed of Br-p and Mn-d orbitals, while the CBM is predominantly contributed to by the organic components (Fig. 4(b)). According to previous reports, this result indicates that organic molecules not only play an isolating role within the structure, but also transfer energy to the luminescent [MnX4]2− units during the luminescence process.28,45,46
 | ||
| Fig. 4 (a) Electronic energy band structures of (C6H8N)2MnBr4. (b) Orbital projection of the partial density of states of (C6H8N)2MnBr4. | ||
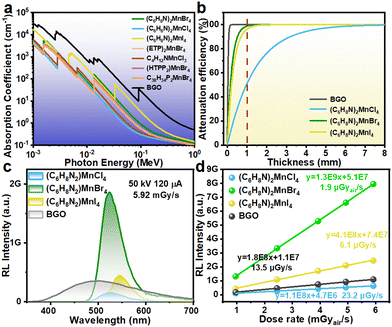
To further assess the scintillation performance of the (C6H8N)2MnX4 (X = Cl, Br, and I) crystals, we first calculated the relationship between the X-ray absorption coefficient and the photon energy for the three different samples based on the XCOM database. The results are shown in Fig. 5(a). It is evident that the X-ray absorption coefficients of (C6H8N)2MnX4 (X = Cl, Br, and I) exhibit a progressive increase with the halogen atomic number. This is attributed to their high atomic number, which can effectively interact with X-rays, leading to significant increases in their absorption cross-section for X-rays.47 Subsequently, we plotted the thickness-dependent attenuation efficiency at a photon energy of 22 keV (the average photon energy under 50 kV X-ray irradiation). It is noteworthy that despite the absorption coefficient and attenuation efficiency of these three samples being lower than those of commercial BGO scintillators, the absorption coefficient of (C6H8N)2MnI4 and (C6H8N)2MnBr4 is significantly superior to those of previously reported scintillators such as (C38H34P2)MnBr4,23 (HTPP2)MnBr4,48 C4H12NMnCl3,24 and (ETP)2MnBr4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in the range of 40–120 keV. As shown in Fig. 5(b), the attenuation efficiencies of the 1 mm-thick (C6H8N)2MnX4 (X = Cl, Br, and I) scintillator materials for 22 keV X-ray photons are 51.5%, 98.8% and 96.2%, respectively. Notably, at this incident photon energy, the attenuation efficiency of the Br−-based sample is significantly higher than that of the I−-based sample. This phenomenon can be attributed to the fact that the incident photon energy is located near the K absorption edge of (C6H8N)2MnBr4. In this energy range, the photoelectric effect is significantly enhanced, resulting in a notable increase in the absorption capacity of X-rays as they pass through the material.49
7 in the range of 40–120 keV. As shown in Fig. 5(b), the attenuation efficiencies of the 1 mm-thick (C6H8N)2MnX4 (X = Cl, Br, and I) scintillator materials for 22 keV X-ray photons are 51.5%, 98.8% and 96.2%, respectively. Notably, at this incident photon energy, the attenuation efficiency of the Br−-based sample is significantly higher than that of the I−-based sample. This phenomenon can be attributed to the fact that the incident photon energy is located near the K absorption edge of (C6H8N)2MnBr4. In this energy range, the photoelectric effect is significantly enhanced, resulting in a notable increase in the absorption capacity of X-rays as they pass through the material.49
The light yield (LY) is another critical parameter for evaluating scintillator performance, as it affects the sensitivity and detection limits of the X-ray detector.1 For comparison, a commercial BGO scintillator serves as a standard reference, along with a mini X-ray generator equipped with a tungsten target and a spectrometer to obtain radioluminescence (RL) spectra. When the scintillator material interacts with incident high-energy X-rays, numerous electron–hole pairs are generated. These electron–hole pairs are then transferred to the tetrahedrally coordinated manganese luminescent centers. Ultimately, the excited Mn2+ ions relax from the 4T1 state to the 6A1 state, emitting scintillation light.26 As depicted in Fig. 5(c), the RL spectra of (C6H8N)2MnX4 (X = Cl, Br, and I) share similar characteristics with their PL spectra, indicating identical radiative recombination pathways for both forms of excitation. At equivalent dose rates, the RL intensity of (C6H8N)2MnBr4 dramatically surpasses that of (C6H8N)2MnCl4, (C6H8N)2MnI4 and the commercial BGO scintillator. The BGO reference sample has an LY of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 photons per MeV.50 In comparison, the calculated LY values for (C6H8N)2MnX4 (X = Cl, Br, and I) are 3009, 18
600 photons per MeV.50 In comparison, the calculated LY values for (C6H8N)2MnX4 (X = Cl, Br, and I) are 3009, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 and 3770 photons per MeV, respectively. Additionally, we collected RL spectra of the samples at different dose rates (Fig. 5(d) and Fig. S12†). The (C6H8N)2MnX4 (X = Cl, Br, and I) crystals exhibit perfectly linear responses in the dose rate range of 0.96–5.92 mGy s−1. By calculation, the detection limit for (C6H8N)2MnX4 (X = Cl, Br, and I) is 23.2, 1.9, and 6.1 μGy s−1, respectively. Notably, under the same measurement conditions, the detection limit of (C6H8N)2MnBr4 is only a seventh of that of the BGO scintillator and is under the critical threshold for the X-ray diagnostic limit (5.5 μGy s−1).51–53
224 and 3770 photons per MeV, respectively. Additionally, we collected RL spectra of the samples at different dose rates (Fig. 5(d) and Fig. S12†). The (C6H8N)2MnX4 (X = Cl, Br, and I) crystals exhibit perfectly linear responses in the dose rate range of 0.96–5.92 mGy s−1. By calculation, the detection limit for (C6H8N)2MnX4 (X = Cl, Br, and I) is 23.2, 1.9, and 6.1 μGy s−1, respectively. Notably, under the same measurement conditions, the detection limit of (C6H8N)2MnBr4 is only a seventh of that of the BGO scintillator and is under the critical threshold for the X-ray diagnostic limit (5.5 μGy s−1).51–53
To further investigate the temperature-dependent radiation stability of the three samples, their RL spectra were recorded over the temperature range of 300–380 K (Fig. S13†). The results indicate that under the same conditions, (C6H8N)2MnBr4 exhibits lower attenuation and higher stability of RL intensity. At 380 K, its strength reduces to 59% of the initial value, which is significantly better than the 12% recorded for (C6H8N)2MnCl4 and the 24% for (C6H8N)2MnI4. This phenomenon underscores the fact that (C6H8N)2MnBr4 not only performs excellently at ambient temperature, but also maintains excellent stability and effectiveness in high-temperature environments. The above-mentioned exceptional performance of (C6H8N)2MnBr4 establishes it as a strong contender for use in future efficient and reliable X-ray imaging scintillators.
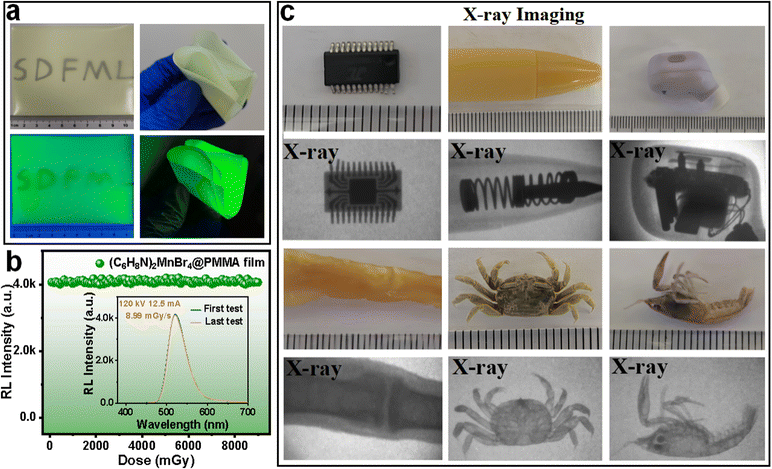
Considering the high X-ray attenuation efficiency and LY of (C6H8N)2MnBr4 crystals, we further investigated their potential as high-resolution X-ray imaging scintillators. Powdered (C6H8N)2MnBr4 crystals were mixed with PMMA to prepare large-area flexible scintillation screens, as shown in Fig. 6(a) and Fig. S14.† The (C6H8N)2MnBr4@PMMA film was approximately 9 cm × 9 cm with a thickness of 79 μm, emitting uniform green light under 365 nm UV irradiation and exhibiting high transparency under daylight, with clearly discernible letters beneath the film (Fig. 6(a) and Fig. S15†). Additionally, the film demonstrated remarkable flexibility, capable of withstanding multiple folds (Fig. 6(a)). Moreover, the SEM-EDS images confirm the uniform distribution of elements within the film (Fig. S16†). Furthermore, the X-ray-induced RL characteristics of the film and crystal remained consistent, indicating no alteration in the luminescent centers (Fig. S17†). To further assess the stability of the scintillating film, we performed continuous irradiation at a dose rate of 8.99 mGy s−1. As illustrated in Fig. 6(b), when the cumulative dose reached 9 Gy, the RL intensity showed no significant changes, indicating that the film possesses excellent radiation stability.
The X-ray imaging quality of (C6H8N)2MnBr4@PMMA flexible films has been systematically studied using a self-constructed imaging system, as depicted in Fig. S18.† Due to the varying X-ray absorption capacities of materials with different densities, the differential RL intensity of the scintillator films captures these variations, allowing optical cameras to obtain internal images of objects.54 At a tube voltage of 50 kV, the internal information of a microchip, a spring-loaded pen, and an earphone was clearly visible, which was imperceptible to the naked eye in daylight (Fig. 6c). To delve deeper into the potential of (C6H8N)2MnBr4 in medical imaging, we selected a shrimp, chicken feet, and a small crab as subjects for experimentation. The X-ray images revealed the internal bones and other tissue structures of these organisms, highlighting the promising application of (C6H8N)2MnBr4@PMMA scintillator films in medical diagnostics.
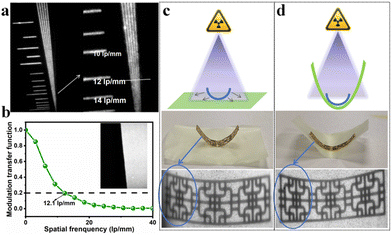
As shown in Fig. 7(a), the spatial resolution of a standard X-ray image is approximately 12 lp mm−1. By calculating the modulation transfer function (MTF) of the tilting edge X-ray image of the scintillator film (Fig. 7(b)), the spatial resolution was quantified as being 12.1 lp mm−1. This demonstrates its excellent performance in high-resolution imaging applications.
The flexible film can adjust to different shapes and sizes of objects, offering superior fit and coverage. This adaptability makes X-ray imaging of complex structures or irregular surfaces more efficient and precise.55 Moreover, the flexible film can minimize artifacts and distortions that occur when a rigid detector cannot fully conform to the object's surface.56 This enhancement boosts the resolution and clarity of X-ray imaging, resulting in more accurate diagnoses and analytical outcomes. As displayed in Fig. 7(c and d), (C6H8N)2MnBr4@PMMA exhibits a significant difference in X-ray imaging quality between images of a metal sheet in its curved and flat states. When the scintillation film is pressed against the irregular metal sheet, the image becomes clearer and the overall image quality is significantly improved. Therefore, the scintillation film not only enhances the resolution and clarity of the image, but also demonstrates its substantial application potential in flexible X-ray imaging.
Conclusions
In summary, by selecting 2-methylpyridine as the organic cation, we obtained three non-toxic 0D manganese-based organic metal halides (C6H8N)2MnX4 (X = Cl, Br, and I). Under UV and blue light excitation, these halides displayed efficient green emission stemming from the d–d transitions of Mn2+. With its advantages of a high PLQY value and robust stability, (C6H8N)2MnBr4 demonstrated potential for X-ray detection with excellent scintillation performance, featuring a high LY of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 photons per MeV and a low detection limit of 1.9 μGy s−1, outperforming commercial BGO scintillators and showcasing extensive application prospects. More importantly, the flexible film presented clearer imaging on bent metal sheets in contrast to planar imaging, making it more effective for detecting irregularly shaped objects or covering large areas. This work enriches the excellence of organic manganese halides and demonstrates their latent application prospects in medical imaging, industrial inspection, and security screening.
224 photons per MeV and a low detection limit of 1.9 μGy s−1, outperforming commercial BGO scintillators and showcasing extensive application prospects. More importantly, the flexible film presented clearer imaging on bent metal sheets in contrast to planar imaging, making it more effective for detecting irregularly shaped objects or covering large areas. This work enriches the excellence of organic manganese halides and demonstrates their latent application prospects in medical imaging, industrial inspection, and security screening.
Experimental section
Materials and crystal growth
2-Methylpyridine (C6H7N, 99%), manganese(II) bromide tetrahydrate (MnBr2·4H2O, 98%), manganese(II) chloride tetrahydrate (MnCl2·4H2O, 99.99%), manganese (Mn, 99.95%), hydrobromic acid (HBr, 40%), hydroiodic acid (HI, 55.0%–58.0%), methanol (CH3OH, 99.5%), hypophosphorous acid (H3PO2, 50 wt% in water), and polymethyl methacrylate ((C5H8O2)n) were all purchased from Aladdin. Hydrochloric acid (HCl, 36.0%–38.0%) and toluene (C7H8, 99.5%) were obtained from Tieta Reagent, and dioctyl phthalate (C24H38O4, AR) was obtained from Macklin. All reagents were used as received without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were first dissolved in a mixed solution of HCl and CH3OH (1/3, v/v) and the mixture was then heated and stirred at 90 °C to form a transparent precursor solution. Subsequently, the solution was then slowly evaporated on a heating plate at 60 °C in an atmospheric environment. After a few days, light green crystals of (C6H8N)2MnCl4 can be obtained.
1 were first dissolved in a mixed solution of HCl and CH3OH (1/3, v/v) and the mixture was then heated and stirred at 90 °C to form a transparent precursor solution. Subsequently, the solution was then slowly evaporated on a heating plate at 60 °C in an atmospheric environment. After a few days, light green crystals of (C6H8N)2MnCl4 can be obtained.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were dissolved in a mixed solution of HBr and CH3OH (1/3, v/v) and the mixture was then heated and stirred at 90 °C to form a transparent precursor solution. Subsequently, the solution was then slowly evaporated on a heating plate at 60 °C in an atmospheric environment. After a few days, green crystals of (C6H8N)2MnBr4 can be obtained.
1 were dissolved in a mixed solution of HBr and CH3OH (1/3, v/v) and the mixture was then heated and stirred at 90 °C to form a transparent precursor solution. Subsequently, the solution was then slowly evaporated on a heating plate at 60 °C in an atmospheric environment. After a few days, green crystals of (C6H8N)2MnBr4 can be obtained.
Preparation of (C6H8N)2MnBr4@PMMA flexible thin films
First, PMMA particles were dissolved in toluene at a concentration of 0.25 g mL−1 by stirring at 45 °C. Then, 0.9 g of finely ground (C6H8N)2MnBr4 crystal powder was added to 2.5 g of the mixed solution, followed by the addition of 0.2 g of the plasticizer dioctyl phthalate. The mixture was stirred continuously for several hours until it reached a uniform consistency. A scraper was then employed to apply the mixture onto a glass substrate, with adjustments made to control the film thickness. After the solvent had completely evaporated and the film had dried, it was carefully peeled off the glass substrate, resulting in a large-area flexible film.Measurements and characterization
Single-crystal X-ray diffraction (SCXRD) data were obtained using a Bruker SMART APEX-II diffractometer, which features a CCD detector (Mo Kα, λ = 0.71073 Å), and processed using APEX2 software. Structural analysis and refinement were conducted using direct methods with the SHELXTL97 software package. Powder X-ray diffraction (PXRD) was conducted using Cu Kα1 radiation (λ = 1.54186 Å) on a Bruker AXS D8 Advance diffractometer, with diffraction patterns collected over a 2θ range of 10–70° at a step size of 0.02°. Simulated PXRD data were derived from the single-crystal structure. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo Fisher Scientific ESCALAB 250 instrument. Thermogravimetric analysis (TG) was conducted using a Netzsch STA 449C analyzer, covering a temperature range from 35 to 800 °C at a heating rate of 5 °C min−1 under a nitrogen atmosphere. The UV-vis absorption spectral data were collected at room temperature using a Shimadzu UV2550 spectrophotometer equipped with an integrating sphere (200–1500 nm). Using an Edinburgh FS5 full-function fluorescence spectrometer equipped with an integrating sphere, steady-state photoluminescence excitation/emission (PLE/PL) spectra, time-resolved photoluminescence (TRPL) spectra, and PLQY values were recorded. The RL spectrum and detection limit were recorded using the FS5 fluorescence spectrometer, employing a mini X-ray tube (Moxtek Inc.) with a tungsten target as the X-ray source. X-ray imaging was carried out with a Nikon D7200 camera and an AF-S VR 105 mm f/2.8G lens.Calculation of X-ray attenuation efficiency
The X-ray attenuation efficiency (AE) was calculated using the following formula:| AE = [1 − exp(−tρd)] × 100% |
Calculation of light yield
Using a commercial BGO scintillator (LY = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 photons per MeV) as a reference, we calculated the LYs of (C6H8N)2MnX4 (X = Cl, Br, and I). The experiments were conducted at a tube voltage of 50 kV, with the sample powder compressed to a uniform thickness of approximately 1 mm. The attenuation efficiencies (AE(d)) for (C6H8N)2MnX4 (X = Cl, Br, and I) and BGO can be extracted from Fig. 5(b). The photon counting results (PCmeasured) were obtained by integrating the RL spectrum. Finally, the relative LY was calculated using the following eqn (1) and (2):
600 photons per MeV) as a reference, we calculated the LYs of (C6H8N)2MnX4 (X = Cl, Br, and I). The experiments were conducted at a tube voltage of 50 kV, with the sample powder compressed to a uniform thickness of approximately 1 mm. The attenuation efficiencies (AE(d)) for (C6H8N)2MnX4 (X = Cl, Br, and I) and BGO can be extracted from Fig. 5(b). The photon counting results (PCmeasured) were obtained by integrating the RL spectrum. Finally, the relative LY was calculated using the following eqn (1) and (2):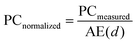 | (1) |
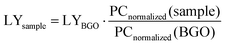 | (2) |
Calculation of detection limit
Within the dose rate range of 0.96–5.92 mGy s−1, we measured the RL spectra of (C6H8N)2MnX4 (X = Cl, Br, and I) and BGO scintillators. By employing linear fitting, the relationship was established between the RL intensity and the dose rate for each sample, resulting in the slope (k), which represents the sensitivity of the X-ray detector. Additionally, noise measurements were conducted in the absence of samples, and Gaussian statistical analysis of the noise intensity data provided the FWHM as the average noise level. Finally, the detection limit (DL) was calculated based on the detector sensitivity (k) and the average noise FWHM using the formula DL = 3 × FWHM/k.Calculation of modulation transfer function (MTF)
The modulation transfer function (MTF) curve of the scintillator film was determined using the slanted edge method. Initially, a piece of lead with a sharp edge, approximately 1 mm thick, was positioned on the scintillator for X-ray imaging, and the edge image was obtained. Subsequently, the edge spread function (ESF) was extracted from the image, followed by acquisition of the line spread function (LSF). Finally, the MTF value was computed using the Fourier transform of the LSF, as follows:where ν represents the spatial frequency and x denotes the pixel position. The spatial resolution is obtained at MTF = 0.2.
Author contributions
Xiaomei Jiang conceived the idea, designed the experiments, revised the manuscript and obtained the research grants; Ying Sun conducted the materials synthesis, characterization, and wrote the manuscript; Pan Gao, Qi Wang and Zeyu Guo conceived the discussion and conducted the device performance measurements. Qian Ma and Dongheng Zhao conducted the DFT calculations. All authors contributed to the general discussion.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 2381212, 2270667 and 2381207 have been deposited at the CCDC.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely acknowledge financial support from the National Natural Science Foundation of China (Grant No. 62305195), the projects ZR2024ME223 and ZR2022QF036 supported by the Shandong Provincial Natural Science Foundation, and the Opening Project of State Key Laboratory of Crystal Materials (Shandong University, KF2105).References
- H. Wu, Y. Ge, G. Niu and J. Tang, Metal Halide Perovskites for X-Ray Detection and Imaging, Matter, 2021, 4(1), 144–163 CrossRef CAS.
- Y. Wang, M. Li, Z. Chai, Y. Wang and S. Wang, Perovskite Scintillators for Improved X-ray Detection and Imaging, Angew. Chem., Int. Ed., 2023, 62(38), e202304638 CrossRef CAS PubMed.
- Z. Li, F. Zhou, H. Yao, Z. Ci, Z. Yang and Z. Jin, Halide perovskites for high-performance X-ray detector, Mater. Today, 2021, 48, 155–175 CrossRef.
- Q. Zhou, W. Li, J. Xiao, A. Li and X. Han, Low–Dimensional Metal Halide for High Performance Scintillators, Adv. Funct. Mater., 2024, 34(38), 2402902 CrossRef CAS.
- A. Jana, S. Cho, S. A. Patil, A. Meena, Y. Jo, V. G. Sree, Y. Park, H. Kim, H. Im and R. A. Taylor, Perovskite: Scintillators, direct detectors, and X-ray imagers, Mater. Today, 2022, 55, 110–136 CrossRef CAS.
- L. Lu, M. Sun, Q. Lu, T. Wu and B. Huang, High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy, Nano Energy, 2021, 79, 105437 CrossRef CAS.
- B. Li, Y. Xu, X. Zhang, K. Han, J. Jin and Z. Xia, Zero–Dimensional Luminescent Metal Halide Hybrids Enabling Bulk Transparent Medium as Large–Area X–Ray Scintillators, Adv. Opt. Mater., 2022, 10(10), 2102793 CrossRef CAS.
- J.-X. Zheng, Z.-A. Zhou, T. Feng, H. Li, C.-H. Sun, N. Wang, Y. Tian, Y. Zhao and S.-Y. Zhou, Hydrophobic long-chain two-dimensional perovskite scintillators for underwater X-ray imaging, Rare Met., 2023, 43, 175–185 CrossRef.
- Y. Takizawa, K. Kamada, N. Kutsuzawa, M. Yoshino, S. Yamamoto, K. Jin Kim, R. Murakami, V. V. Kochurikhin and A. Yoshikawa, The scintillation performance of one-inch diameter CsI/CsCl/NaCl eutectics grown by the Czochralski method, J. Cryst. Growth, 2021, 572, 126266 CrossRef CAS.
- Y. Han, S. Yue and B. B. Cui, Low–Dimensional Metal Halide Perovskite Crystal Materials: Structure Strategies and Luminescence Applications, Adv. Sci., 2021, 8(15), 2004805 CrossRef CAS PubMed.
- H. Lin, C. Zhou, Y. Tian, T. Siegrist and B. Ma, Low-Dimensional Organometal Halide Perovskites, ACS Energy Lett., 2017, 3(1), 54–62 CrossRef.
- Y. Zhan, P. Cai, X. Pu, Q. Ai, J. Si, X. Yao, G. Bai and Z. Liu, Exceptional optical performance of the zero-dimensional hybrid cuprous halide ETPA2Cu2I4 as an X-ray scintillator, Inorg. Chem. Front., 2024, 11(2), 579–588 RSC.
- X. Jiang, X. Fu, D. Ju, S. Yang, Z. Chen and X. Tao, Designing Large-Area Single-Crystal Perovskite Solar Cells, ACS Energy Lett., 2020, 5(6), 1797–1803 CrossRef CAS.
- B. Jia, D. Chu, N. Li, Y. Zhang, Z. Yang, Y. Hu, Z. Zhao, J. Feng, X. Ren, H. Zhang, G. Zhao, H. Sun, N. Yuan, J. Ding, Y. Liu and S. F. Liu, Airflow-Controlled Crystallization for a Multi-Inch 2D Halide Perovskite Single-Crystal Scintillator for Fast High-Resolution X-ray Imaging, ACS Energy Lett., 2022, 8(1), 590–599 CrossRef.
- M. D. Birowosuto, D. Cortecchia, W. Drozdowski, K. Brylew, W. Lachmanski, A. Bruno and C. Soci, X-ray Scintillation in Lead Halide Perovskite Crystals, Sci. Rep., 2016, 6(1), 37254 CrossRef CAS PubMed.
- J. Cao, Z. Guo, S. Zhu, Y. Fu, H. Zhang, Q. Wang and Z. Gu, Preparation of Lead-free Two-Dimensional-Layered (C8H17NH3)2SnBr4 Perovskite Scintillators and Their Application in X-ray Imaging, ACS Appl. Mater. Interfaces, 2020, 12(17), 19797–19804 CrossRef CAS PubMed.
- Q. Kong, X. Jiang, Y. Sun, J. Zhu and X. Tao, Yellow-emissive organic copper(I) halide single crystals with [Cu4I4] cubane unit as efficient X-ray scintillators, Inorg. Chem. Front., 2024, 11(10), 3028–3035 RSC.
- H. Meng, B. Chen, W. Zhu, Z. Zhou, T. Jiang, X. Xu, S. Liu and Q. Zhao, Stable Organic Antimony Halides with Near–Unity Photoluminescence Quantum Yield for X–Ray Imaging, Laser Photonics Rev., 2023, 17(7), 2201007 CrossRef CAS.
- Y.-Y. Wang, G.-H. Jia, M.-R. Huo, M.-T. Cheng, X.-Y. Chen, A.-R. Chang, J. Zhang, Y.-Y. Li and G.-M. Lin, A lead-free zero-dimensional hybrid antimony halide perovskite X-ray scintillator with exceptional emission efficiency and excellent stability as a highly sensitive fluorescent probe, Inorg. Chem. Front., 2024, 11(16), 5034–5042 RSC.
- X. Jiang, S. Xia, J. Zhang, D. Ju, Y. Liu, X. Hu, L. Wang, Z. Chen and X. Tao, Exploring Organic Metal Halides with Reversible Temperature–Responsive Dual–Emissive Photoluminescence, ChemSusChem, 2019, 12(24), 5228–5232 CrossRef CAS PubMed.
- H. Cui, W. Zhu, Y. Deng, T. Jiang, A. Yu, H. Chen, S. Liu and Q. Zhao, Lead–free organic–inorganic hybrid scintillators for X–ray detection, Aggregate, 2023, 5(2), e454 CrossRef.
- D. Liang, H. Xiao, W. Cai, S. Lu, S. Zhao, Z. Zang and L. Xie, Mn2+–Based Luminescent Metal Halides: Syntheses, Properties, and Applications, Adv. Opt. Mater., 2023, 11(15), 2202997 CrossRef CAS.
- L. J. Xu, X. Lin, Q. He, M. Worku and B. Ma, Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide, Nat. Commun., 2020, 11(1), 4329 CrossRef CAS PubMed.
- T. Jiang, W. Ma, H. Zhang, Y. Tian, G. Lin, W. Xiao, X. Yu, J. Qiu, X. Xu, Y. Yang and D. Ju, Highly Efficient and Tunable Emission of Lead–Free Manganese Halides toward White Light–Emitting Diode and X–Ray Scintillation Applications, Adv. Funct. Mater., 2021, 31(14), 2009973 CrossRef CAS.
- G. Hu, B. Xu, A. Wang, Y. Guo, J. Wu, F. Muhammad, W. Meng, C. Wang, S. Sui, Y. Liu, Y. Li, Y. Zhang, Y. Zhou and Z. Deng, Stable and Bright Pyridine Manganese Halides for Efficient White Light–Emitting Diodes, Adv. Funct. Mater., 2021, 31(19), 2011191 CrossRef.
- Z. Z. Zhang, J. H. Wei, J. B. Luo, X. D. Wang, Z. L. He and D. B. Kuang, Large-Area Laminar TEA2MnI4 Single-Crystal Scintillator for X-ray Imaging with Impressive High Resolution, ACS Appl. Mater. Interfaces, 2022, 14(42), 47913–47921 CrossRef CAS PubMed.
- K. Han, K. Sakhatskyi, J. Jin, Q. Zhang, M. V. Kovalenko and Z. Xia, Seed-Crystal-Induced Cold Sintering Toward Metal Halide Transparent Ceramic Scintillators, Adv. Mater., 2022, 34(17), 2110420 CrossRef CAS PubMed.
- Z. L. He, J. H. Wei, Z. Z. Zhang, J. B. Luo and D. B. Kuang, Manganese–Halide Single–Crystal Scintillator Toward High–Performance X–Ray Detection and Imaging: Influences of Halogen and Thickness, Adv. Opt. Mater., 2023, 11(18), 2300449 CrossRef CAS.
- A. Senocrate, G. Y. Kim, M. Grätzel and J. Maier, Thermochemical Stability of Hybrid Halide Perovskites, ACS Energy Lett., 2019, 4(12), 2859–2870 CrossRef CAS.
- G. P. Nagabhushana, R. Shivaramaiah and A. Navrotsky, Direct calorimetric verification of thermodynamic instability of lead halide hybrid perovskites, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(28), 7717–7721 CrossRef CAS PubMed.
- Z. Z. Zhang, Z. L. He, J. B. Luo, J. H. Wei, X. X. Guo, J. H. Chen and D. B. Kuang, Organic–Inorganic Hybrid Mn–Based Transparent Glass for Curved X–Ray Scintillation Imaging, Adv. Opt. Mater., 2023, 12(11), 2302434 CrossRef.
- Z. Luo, Y. Zhuang, W. Li, Y. Du, J. Sun, Z. Liu, Y. Wu, H. Jiang and J. Jiang, A new promising new choice for modern medical CT scanners: Cerium-doped gadolinium yttrium gallium aluminum garnet ceramic scintillator, Appl. Mater. Today, 2023, 35, 101986 CrossRef.
- P.-K. Wang, W.-F. Wang, B.-Y. Li, M.-J. Xie, H.-Y. Bian, S.-H. Wang, F.-K. Zheng and G.-C. Guo, Flexible strontium-based metal–organic framework scintillation screens for high-resolution X-ray imaging, Inorg. Chem. Front., 2023, 10(19), 5710–5718 RSC.
- F. Zhang, Y. Zhou, Z. Chen, M. Wang, Z. Ma, X. Chen, M. Jia, D. Wu, J. Xiao, X. Li, Y. Zhang, Z. Shi and C. Shan, Thermally Activated Delayed Fluorescence Zirconium–Based Perovskites for Large–Area and Ultraflexible X–ray Scintillator Screens, Adv. Mater., 2022, 34(43), 2204801 CrossRef CAS PubMed.
- L. Mao, P. Guo, S. Wang, A. K. Cheetham and R. Seshadri, Design Principles for Enhancing Photoluminescence Quantum Yield in Hybrid Manganese Bromides, J. Am. Chem. Soc., 2020, 142(31), 13582–13589 CrossRef CAS PubMed.
- X. Cheng, X. Chang, Y. Lin, L. Lv, L. Cong, Y. Jia, J. Yin, J. Li and B. B. Cui, Centimeter–Sized Single Crystals of Tetrahedral Manganese(II) Halide Hybrids for Wide–Color Gamut Backlighting Displays, Small, 2023, 20(18), 2307216 CrossRef PubMed.
- Z. Gong, J. Zhang, X. Deng, M. P. Ren, W. Q. Wang, Y. J. Wang, H. Cao, L. Wang, Y. C. He and X. W. Lei, Near-unity broadband emissive hybrid manganese bromides as highly–efficient radiation scintillators, Aggregate, 2024, 5(5), e574 CrossRef CAS.
- V. Morad, I. Cherniukh, L. Pottschacher, Y. Shynkarenko, S. Yakunin and M. V. Kovalenko, Manganese(II) in Tetrahedral Halide Environment: Factors Governing Bright Green Luminescence, Chem. Mater., 2019, 31(24), 10161–10169 CrossRef CAS PubMed.
- R. Babu, A. Samanta, S. Prasanthkumar and L. Polavarapu, Zn(II) Alloying Improves the Luminescence Efficiency of Hybrid Tetrahedral Mn(II) Halides ((DMAPH)2MnX4; X = Cl, Br, and I) to Near-Unity, ACS Mater. Lett., 2023, 5(8), 2131–2138 CrossRef CAS.
- J.-X. Wang, L. Gutiérrez-Arzaluz, X. Wang, T. He, Y. Zhang, M. Eddaoudi, O. M. Bakr and O. F. Mohammed, Heavy-atom engineering of thermally activated delayed fluorophores for high-performance X-ray imaging scintillators, Nat. Photonics, 2022, 16(12), 869–875 CrossRef CAS.
- W. F. Wang, M. J. Xie, P. K. Wang, J. Lu, B. Y. Li, M. S. Wang, S. H. Wang, F. K. Zheng and G. C. Guo, Thermally Activated Delayed Fluorescence (TADF)–active Coinage–metal Sulfide Clusters for High–resolution X–ray Imaging, Angew. Chem., Int. Ed., 2024, 63(7), e202318026 CrossRef CAS PubMed.
- W. Li, Y. Li, Y. Wang, Z. Zhou, C. Wang, Y. Sun, J. Sheng, J. Xiao, Q. Wang, S. Kurosawa, M. Buryi, D. John, K. Paurová, M. Nikl, X. OuYang and Y. Wu, Highly Efficient and Flexible Scintillation Screen Based on Organic Mn(II) Halide Hybrids toward Planar and Nonplanar X–Ray Imaging, Laser Photonics Rev., 2023, 18(2), 2300860 CrossRef.
- W. Liu, H. Xie, W. Cai, R. Zhang, B. Xu and C. Yang, Rapid synthesis of two photoluminescent pyridinium manganese-based halides by an anti-solvent method, J. Alloys Compd., 2023, 968, 172173 CrossRef CAS.
- C. Peng, J. Wei, L. Duan, Y. Tian and Q. Wei, Mn(II)-Activated Zero-Dimensional Zinc(II)-Based Metal Halide Hybrids with Near-Unity Photoluminescence Quantum Yield, Materials, 2024, 17(3), 562 CrossRef CAS PubMed.
- M. Zhou, H. Jiang, T. Hou, S. Hou, J. Li, X. Chen, C. Di, J. Xiao, H. Li and D. Ju, Inch-size and thickness-adjustable hybrid manganese halide single-crystalline films for high resolution X-ray imaging, Chem. Eng. J., 2024, 490, 151823 CrossRef CAS.
- J. Lu, R. X. Qian, S. F. Lu, S. H. Wang, F. K. Zheng and G. C. Guo, High–Resolution X–Ray Circular Polarization Imaging Enabled by Luminescent Photopolymerized Chiral Metal-Organic Polymers, Adv. Funct. Mater., 2024, 2410219 CrossRef.
- G. Kakavelakis, M. Gedda, A. Panagiotopoulos, E. Kymakis, T. D. Anthopoulos and K. Petridis, Metal Halide Perovskites for High–Energy Radiation Detection, Adv. Sci., 2020, 7(22), 2002098 CrossRef CAS PubMed.
- J. B. Luo, J. H. Wei, Z. Z. Zhang, Z. L. He and D. B. Kuang, A Melt-Quenched Luminescent Glass of an Organic-Inorganic Manganese Halide as a Large-Area Scintillator for Radiation Detection, Angew. Chem., Int. Ed., 2023, 62(7), e202216504 CrossRef CAS PubMed.
- W. Shao, T. He, L. Wang, J. X. Wang, Y. Zhou, B. Shao, E. Ugur, W. Wu, Z. Zhang, H. Liang, S. De Wolf, O. M. Bakr and O. F. Mohammed, Capillary Manganese Halide Needle–Like Array Scintillator with Isolated Light Crosstalk for Micro–X–Ray Imaging, Adv. Mater., 2024, 36(21), 2312053 CrossRef CAS PubMed.
- J. H. Wei, Y. W. Yu, J. B. Luo, Z. Z. Zhang and D. B. Kuang, Bright Cyan–Emissive Copper(I)–Halide Single Crystals for Multi–Functional Applications, Adv. Opt. Mater., 2022, 10(16), 2200724 CrossRef CAS.
- L. Liu, H. Hu, W. Pan, H. Gao, J. Song, X. Feng, W. Qu, W. Wei, B. Yang and H. Wei, Robust Organogel Scintillator for Self–healing and Ultra–flexible X–ray Imaging, Adv. Mater., 2023, 36(13), 2311206 CrossRef PubMed.
- P.-K. Wang, W.-F. Wang, K.-B. Jiang, B.-Y. Li, S.-H. Wang, F.-K. Zheng and G.-C. Guo, Efficient X-ray Detection of Polyoxometalates@Metal-Organic Frameworks Based on Host–Guest Electron Transfer, ACS Mater. Lett., 2024, 6(4), 1086–1093 CrossRef CAS.
- B. Y. Li, M. J. Xie, J. Lu, W. F. Wang, R. Li, J. R. Mi, S. H. Wang, F. K. Zheng and G. C. Guo, Highly Sensitive Direct X–Ray Detector Based on Copper(II) Coordination Polymer Single Crystal with Anisotropic Charge Transport, Small, 2023, 19(42), 2302492 CrossRef CAS PubMed.
- H. Meng, W. Zhu, Z. Zhou, R. Zhou, D. Yan, Q. Zhao and S. Liu, High-efficiency luminescent organic–inorganic hybrid manganese(II) halides applied to X-ray imaging, J. Mater. Chem. C, 2022, 10(34), 12286–12291 RSC.
- S. Zhao, Z. Jia, Y. Huang, Q. Qian, Q. Lin and Z. Zang, Solvent–Free Synthesis of Inorganic Rubidium Copper Halides for Efficient Wireless Light Communication and X–Ray Imaging, Adv. Funct. Mater., 2023, 33(47), 2305858 CrossRef CAS.
- W. Chen, T. Wang, T. Wang, J. Yu, S. Yao, W. Feng, Q. Wang, L. Huang, X. Xu and X. Yu, Customizable Scintillator of Cs3Cu2I5:2% In+@Paper for Large–Area X–Ray Imaging, Adv. Sci., 2023, 10(34), 2304957 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Single-crystal X-ray diffraction data, XPS spectra, excitation wavelength-dependent PL and PLE spectra, X-ray dose rate-dependent RL spectra, RL spectrum of the scintillation film and X-ray images. CCDC 2270667, 2381207 and 2381212. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02522a |
| This journal is © the Partner Organisations 2025 |