Facile single-step synthesis of pentaaryl-substituted pyrano[3,2-b]pyrrol-5(1H)-ones showing aggregation-induced emission†
Yongfeng
Wang
,
Ruijun
Chu
,
Yanxiao
Wang
,
Mengyuan
Zhou
,
Yuan
Chen
,
Cheng
Xu
*,
Jingyu
Sun
and
Guodong
Yin
 *
*
Hubei Key Laboratory of Pollutant Analysis and Reuse Technology, College of Chemistry and Chemical Engineering, Hubei Normal University, Huangshi 435002, China. E-mail: chemxucheng@hbnu.edu.cn; gdyin@hbnu.edu.cn
First published on 29th October 2024
Abstract
The continuing desire for multifunctional AIE materials has stimulated intensified research efforts to develop both innovative synthetic techniques and novel scaffolds. Herein, we report a new class of heterocyclic AIEgens, 1,2,3,6,7-pentaaryl substituted pyrano[3,2-b]pyrrol-5(1H)-ones, which contain an intriguing fused pyrrole/α-pyrone core. These structurally novel molecules were efficiently constructed via multicomponent reactions of easily accessible isatins, 2,3-diaryl cyclopropenones, and alkyl bromides in a single step under metal-free conditions. Subsequent late-stage modification of the resulting products was performed via the Suzuki–Miyaura coupling and reduction reactions. Furthermore, the HOMO/LUMO gaps were calculated and the single crystal structural analyses were conducted in order to gain deeper insights into the photophysical properties of these propeller-like AIEgens.
Introduction
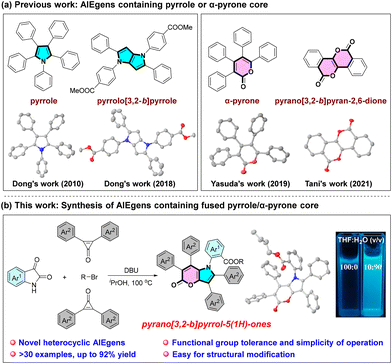
Aggregation-induced emission luminogens (AIEgens) represent a distinctive class of compounds that exhibit strong luminescence in both the aggregated and solid states.1,2 These molecules overcome the inherent limitation of traditional aggregation-caused quenching (ACQ) materials, which often display non-luminescence or low emission efficiency in their aggregated states.3 The fascinating AIE phenomenon has garnered substantial interest from the scientific community, prompting numerous research groups to pursue the development of novel AIEgens and investigate their potential applications in analytical chemistry,4 materials science,5 and life sciences.6 Typically, a propeller-like configuration is considered the optimal structural design for compounds endowed with favorable AIE properties. This configuration effectively precludes the phenomenon of fluorescence quenching attributed to π–π stacking in the aggregated state.7 Thanks to the dedicated efforts of numerous scientists, an array of interesting AIEgens, such as tetraphenylethene (TPE),8 9,10-distyrylanthracene (DSA),9 and cyanostilbene,10 have been successfully developed in accordance with the generally acknowledged mechanism of restricted intramolecular motion (RIM). Nevertheless, it is essential to recognize that some of these known AIEgens are more or less associated with intrinsic disadvantages. These include the complexity of preparation and purification, difficulties in structural modification, and instability under certain conditions, which restrict the potential applications of these compounds.11 The continuing desire for multifunctional AIE materials has stimulated intensified research efforts to develop both innovative synthetic techniques and novel scaffolds.12In comparison with pure hydrocarbon systems, heterocyclic AIEgens exhibit a considerable degree of structural diversity, rendering them comparatively simple to prepare and modify.13 Heteroatoms can influence the properties and aggregation structures of materials through electronic effects.14 The incorporation of heteroatoms into AIEgens significantly enriches the variety of AIE systems and expands the scope of AIE research. Pyrrole represents a fundamental five-membered nitrogen-containing heterocycle, featuring abundant electron density and pronounced aromaticity.15 Dong demonstrated that pentaphenyl pyrrole (PPP) shows an aggregation-induced emission enhancement phenomenon. This is attributed to the more twisted configuration which prevents parallel orientation of conjugated chromophores combined with the restricted intramolecular motion effect.16 By enlarging the conjugation and coplanarity of pyrrole to a pyrrolo[3,2-b]pyrrole core,17 it was also found that their polyaryl substituted compounds showed obvious AIE characteristics with versatile properties and applications in chloroform detection and acid response. Furthermore, α-pyrone is an important class of six-membered lactones, and Yasuda and Tani have disclosed that tetraaryl-substituted α-pyrone18a and a fully ring-fused benzopyrano[3,2-b]pyran-2,6-dione (PPD)18b exhibit AIE properties, respectively (Scheme 1a). Nevertheless, a fused pyrrole/α-pyrone core with polyaryl substituents has not yet been synthesized and investigated, and it is anticipated that this novel hybridized skeleton will be an intriguing candidate for AIEgens and might possess potential applications in diverse fields. As a continuation of our research efforts to develop new synthetic methods for constructing valuable heterocyclic skeletons,19 herein, we report a new class of propeller-like AIEgens, 1,2,3,6,7-pentaaryl substituted pyrano[3,2-b]pyrrol-5(1H)-ones, which could be readily synthesized from isatins, 2,3-diaryl cyclopropenones, and alkyl bromides through a single-step procedure under metal-free conditions with satisfactory yields (Scheme 1b). It is noteworthy that the bromo-substituted products can be readily functionalized via the Suzuki–Miyaura coupling reactions with aromatic boronic acids. The photoluminescence (PL) properties of these compounds were initially characterized, and then their AIE properties were elucidated through theoretical calculations and single-crystal analysis.
Results and discussion
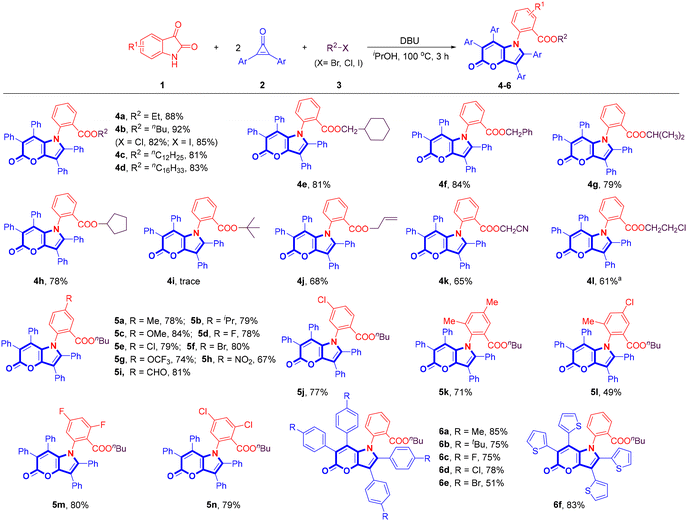
The multicomponent reaction of isatins (1), 2,3-diaryl cyclopropenones (2), and alkyl bromides (3) was conducted in the presence of DBU in iPrOH at 100 °C, which proved highly compatible and was demonstrated to be a versatile method for creating a diverse array of pyrano[3,2-b]pyrrol-5(1H)-ones (4–6), as illustrated in Scheme 2. Some representative primary bromoalkanes (–Et, –nBu, –nC12H25, –nC16H33, methylcyclohexyl, and –Bn) and secondary bromoalkanes (–iPr and cyclopentyl) gave the desired products in excellent yields (4a–4h, 78–92%). 4b was also obtained in good yields from 1-chlorobutane and 1-iodobutane. However, only a trace amount of the expected product 4i was observed using tert-butyl bromide as the substrate. Allyl bromide and bromo acetonitrile were also suitable for this reaction, furnishing 4j and 4k in 68% and 65% yields, respectively. Additionally, utilizing 1,2-dichloroethane as a substrate resulted in the formation of 4l in 61% yield. The substrate scope of isatins was subsequently investigated. Isatins bearing electron-donating groups (–Me, –iPr, and –OMe) and electron-deficient groups (–F, –Cl, –Br, –OCF3, –NO2, and –CHO) all delivered the corresponding products in moderate to good yields (5a–5j, 67–84%), and several di-substituted isatins were also found to be compatible with the reaction (5k–5n, 49–80%). Several 2,3-diaryl cyclopropenones bearing different substituents (–Me, –tBu, –F, –Cl, –Br) on the phenyl rings were tested to further extend the substrate scope. Fortunately, the reaction proceeded successfully and produced the desired products in satisfactory yields (6a–6e, 51–85%). 2,3-Di(thiophen-2-yl)cycloprop-2-en-1-one was also compatible for the conversion, furnishing 6f in 83% yield. Unsymmetrical 2-phenyl-3-(p-tolyl)cycloprop-2-en-1-one produced a complex mixture of inseparable regioisomers. All the synthesized compounds were characterized by 1H/13C NMR and IR spectroscopy, and HRMS analysis.Given that the incorporation of electron-donating amino groups into a luminogen typically results in electronic perturbation and modulation of its optoelectronic properties, further transformations of the obtained products were conducted. For example, the Suzuki–Miyaura coupling reactions20 of the bromo-substituted product 5f with two different aromatic boronic acids afforded its derivatives 7 and 8 in excellent yields (Scheme 3a). Compound 9 was easily synthesized through a reduction reaction21 of 5h using a SnCl2/HCl system (Scheme 3b). The synthesis of compound 10 commenced with the coupling reaction of 6e with four molecules of 4-(diphenylamino)phenyl boronic acid (Scheme 3c). It is worth noting that the reaction of isatin (1a) with 2,3-diphenyl cyclopropenone (2a) delivered 2-(5-oxo-2,3,6,7-tetraphenylpyrano[3,2-b]pyrrol-1(5H)-yl)benzoic acid (11) in 56% yield in the absence of alkyl halides, which could be converted into 1,2,3,6,7-pentaphenylpyrano[3,2-b]pyrrol-5(1H)-one (12) in 73% yield via a copper-catalyzed decarboxylation reaction (Scheme 3d). Furthermore, a scale-up experiment was conducted to provide further evidence of the synthetic viability and prospective applications of our methodology (Scheme 3e).
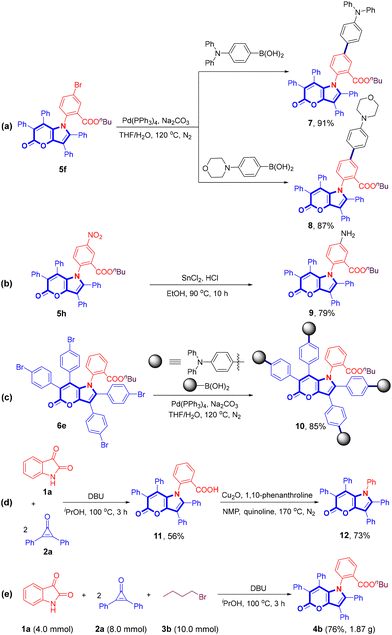 | ||
| Scheme 3 Structural modification of 1,2,3,6,7-pentaaryl pyrano[3,2-b]pyrrol-5(1H)-ones and gram-scale experiment. | ||
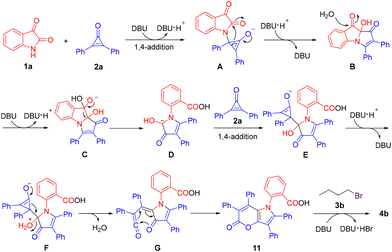
Based on the experimental results (see Scheme S1 in the ESI†) and previous reports,22 a possible mechanism for this novel multicomponent reaction to assemble pyrano[3,2-b]pyrrol-5(1H)-ones is depicted in Scheme 4. Firstly, the 1,4-addition of isatin (1a) to 2,3-diphenyl cyclopropenone (2a) in the presence of DBU affords intermediate A, which then progresses to give intermediate Bvia a ring-opening and intramolecular cyclization process.22a–c Subsequently, B is subjected to a nucleophilic attack by H2O, followed by an intramolecular rearrangement to produce intermediate D. Then, a 1,4-addition reaction between intermediate D and another molecule of 2,3-diphenyl cyclopropenone (2a) gives rise to intermediate E, which undergoes a ring-opening process to generate intermediate G. Further intramolecular electrocyclization of G furnishes intermediate 11.22d Finally, the DBU-mediated nucleophilic substitution reaction of 11 with 1-bromobutane (3b) furnishes the product 4b as desired.
To demonstrate the prospective applications of the synthesized products as optical materials, the photophysical properties of the selected pyrano[3,2-b]pyrrol-5(1H)-one derivatives 4b, 6c, 7, 9 and 10 were investigated and compared with those of previously documented AIEgens PPP and TPPP-C1.16b,17b The normalized UV-vis absorption and photoluminescence (PL) emission spectra in THF solutions (10−5 M) are shown in Fig. 1, and the detailed data are presented in Table 1. Broad absorption bands of four pyrano[3,2-b]pyrrol-5(1H)-one derivatives 4b, 6c, 7 and 9 were observed between 361 and 378 nm with molar extinction coefficients of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440–95
440–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407 M−1 cm−1. Compound 10 showed two distinct absorption bands, and the higher peak at 343 nm was identified as π–π* transition of the conjugated backbone, while the additional weaker shoulder peak at 396 nm should be attributed to intramolecular charge transfer (ICT) transition.23 The emission spectra of these compounds all showed peaks at 448–500 nm with different Stokes shifts ranging from 4716 to 5379 cm−1. Among them, compound 10 displays the longest emission wavelength with the highest fluorescence quantum yield up to 52.4%. Enlarging the conjugated system and introducing D–A structures by incorporating four triphenylamine groups have red-shifted both its maximum absorption and emission. The calculated HOMO and LUMO energy levels of these selected compounds are also summarized in Table 1. The results reveal that their HOMO–LUMO gaps range from 3.12 to 3.64 eV.
407 M−1 cm−1. Compound 10 showed two distinct absorption bands, and the higher peak at 343 nm was identified as π–π* transition of the conjugated backbone, while the additional weaker shoulder peak at 396 nm should be attributed to intramolecular charge transfer (ICT) transition.23 The emission spectra of these compounds all showed peaks at 448–500 nm with different Stokes shifts ranging from 4716 to 5379 cm−1. Among them, compound 10 displays the longest emission wavelength with the highest fluorescence quantum yield up to 52.4%. Enlarging the conjugated system and introducing D–A structures by incorporating four triphenylamine groups have red-shifted both its maximum absorption and emission. The calculated HOMO and LUMO energy levels of these selected compounds are also summarized in Table 1. The results reveal that their HOMO–LUMO gaps range from 3.12 to 3.64 eV.
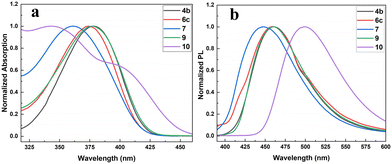 | ||
| Fig. 1 The normalized absorption (a) and emission (b) spectra of representative aryl-substituted pyran[3,2-b]pyrrole derivatives in THF (10−5 M). | ||
| Compounds |
λ
abs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a nm a nm |
λ
em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a nm a nm |
Stokes shift cm−1 (nm) | Molar abs coeff. εmax M−1·cm−1 |
Φ
F/THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b % b % |
HOMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c eV c eV |
LUMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c eV c eV |
E g eV |
|---|---|---|---|---|---|---|---|---|
| a In THF solution at RT (10−5 mol L−1). b Quinine sulfate (Φ = 0.55) used as a standard. c DFT calculations were performed at the B3LYP/6-31G(d) level using the Gaussian 16 package. | ||||||||
| 4b | 378 | 460 | 4716 (82) | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 639 |
0.93 | −5.17 | −1.56 | 3.61 |
| 6c | 374 | 460 | 4999 (86) | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
3.14 | −5.39 | −1.75 | 3.64 |
| 7 | 361 | 448 | 5379 (87) | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407 407 |
1.52 | −5.13 | −1.56 | 3.57 |
| 9 | 377 | 462 | 4880 (85) | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 426 |
1.38 | −5.06 | −1.42 | 3.64 |
| 10 | 343, 396 | 500 | 5253 (104) | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
52.4 | −4.72 | −1.60 | 3.12 |
| PPP | 306 | 386 | 6773 (80) | — | 2.2 | — | — | 4.45 |
| TPPP-C1 | 321, 372 | 505 | 7078 (133) | — | 1.11 | −5.18 | −1.80 | 3.38 |
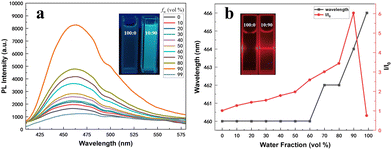
Upon examination of the structures of these newly synthesized compounds, it becomes evident that five rotatable phenyl rings are linked to the central stator, a fused pyrrole/α-pyridone core, through C–C and C–N single bonds. As postulated by the RIM mechanism,24 these molecules are expected to show an AIE effect. To substantiate this hypothesis, the photoluminescence (PL) behaviors were investigated in mixed solvents of THF/H2O with different water fractions (fw). For compound 4b, a weak emission peak at 460 nm was observed in a dilute THF solution (Fig. 2a). The fluorescence intensity of the THF/H2O mixture was enhanced in conjunction with an increase in the water fraction (fw). Additionally, a red-shifted as well as 6-fold emission enhancement was detected when fw increased to 90%. Nevertheless, the fluorescence intensity exhibited a decline as fw reached 99%. This phenomenon could be attributed to the tendency of luminogen molecules to rapidly agglomerate in a random manner, leading to the formation of less emissive particles.25 The Tyndall effect of 4b in THF/H2O illustrates the formation of aggregates (Fig. 2b). The quantum yield of 4b in THF is 0.93%. Other selected pyrano[3,2-b]pyrrol-5(1H)-one derivatives also behaved similarly (Fig. S3†). These results clearly demonstrate that the fluorescence emissions of the selected compounds are enhanced with the formation of aggregates and they exhibit AIE properties.
To gain deeper insights into the relationship between the molecular structure and the photophysical properties of these selected aryl-substituted pyrano[3,2-b]pyrrol-5(1H)-one derivatives, density functional theory (DFT) calculations were performed at the B3LYP/6-31G(d) level using the Gaussian 16 package (Fig. 3). The highest occupied molecular orbitals (HOMOs) of three AIE-active compounds 4b, 6c, and 7 were found to be localized on the central pyrano[3,2-b]pyrrol-5(1H)-one core and the adjacent phenyl groups at the 2,3,6-positions, while their lowest unoccupied molecular orbitals (LUMOs) were primarily distributed on N-substituted phenyl rings which contain an ester group. Apparently, the electron cloud distributions in HOMOs and LUMOs were spatially separated. A comparable distribution of the HOMO–LUMO and energy gap of compound 9 resulted in a similar absorption band at 377 nm. The HOMO of 10 was mainly localized at the benzene rings and the conjugated triphenylamine moiety at the 3,6-positions. This exhibited a higher LUMO energy level but a lower energy gap in comparison with other compounds.
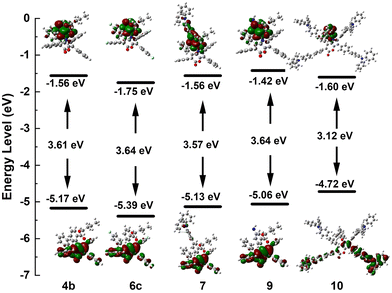 | ||
| Fig. 3 Molecular orbitals and energy levels of the selected aryl-substituted pyran[3,2-b]pyrroles 4b, 6c, 7, 9 and 10. | ||
The accurate crystal structures provided the most direct evidence for elucidating the emission behaviors of these AIEgens. Single crystals of 4b (CCDC 2327902†) and 6c (CCDC 2298102†) were obtained by the slow solvent evaporation of a DCM–EtOAc–hexane mixture. Their structures were subsequently elucidated through X-ray single-crystal diffraction (Fig. 4). In the crystal, 4b exhibits a markedly twisted conformation, and the dihedral angles between its central pyrano[3,2-b]pyrrol-5(1H)-one core and the attached phenyl rings were 73.3°, 51.3°, 44.8°, 50.7°, and 68.6°, respectively (Fig. 4a). It showed a layer-by-layer staggered arrangement stacking pattern, and the distance between adjacent pyrano[3,2-b]pyrrol-5(1H)-one cores was 5.992 Å, which was larger than the distance of π–π interaction (3.6 Å) (Fig. 4b). The crystal structure of 6c also adopts a propeller-like conformation, displaying similarities in dihedral angles and stacking patterns (Fig. 4d and e). In addition, multiple inter- and intramolecular C–H⋯π and C–H⋯O interactions were identified with distances in the range of 2.356 Å–2.803 Å for 4b and 2.379 Å–2.985 Å for 6c in their single crystals (Fig. 4c and f). Such non-covalent interactions constrained the rotational motion of phenyl rings in the aggregated state, which blocked the non-radiative channel and boosted the radiative pathway, thereby enabling them to exhibit significant AIE characteristics.
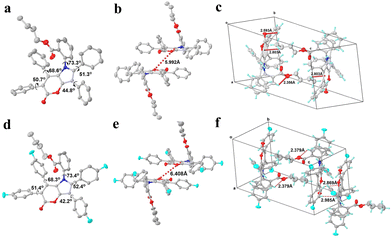 | ||
| Fig. 4 The crystal structures, packing patterns, and inter- and intramolecular interactions of 4b (a, b, and c) and 6c (d, e, and f). | ||
Conclusions
In summary, a novel class of heterocyclic AIEgens, 1,2,3,6,7-pentaaryl substituted pyrano[3,2-b]pyrrol-5(1H)-ones, has been developed. These new compounds could be readily synthesized from isatins, 2,3-diaryl cyclopropenones and alkyl bromides through a single step process under metal-free conditions, with favorable yields. Subsequent functionalization of the resulting products was conducted via the Suzuki–Miyaura coupling and reduction reactions, allowing for the construction of their derivatives 7–10. The photophysical properties of representative pyran[3,2-b]pyrrole derivatives 4b, 6c, 7, 9 and 10 were investigated using UV-vis absorption spectra, PL emission spectra, DFT calculations, and analyses of single crystal structures. A spatial separation of the electron cloud distributions in HOMOs and LUMOs was observed for the selected compounds, with energy gaps ranging from 3.12 to 3.64 eV. In the crystal structures, 4b and 6c adopted a propeller-like conformation that lacks intermolecular π–π interactions but was capable of forming multiple inter- and intramolecular C–H⋯π and C–H⋯O interactions. This behavior was consistent with the typical AIE characteristics associated with the RIM mechanism. These results not only facilitate the molecular design of substituted pyrano[3,2-b]pyrrol-5(1H)-ones but also enrich the family of heterocyclic AIEgens. Efforts toward developing new multifunctional AIEgens are currently under investigation in our laboratory.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Natural Science Foundation of Hubei Province (No. 2023AFB477) and the Science and Technology Research Project of Education Department of Hubei Province (No. B2022164) is gratefully acknowledged.References
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced Emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC.
- (a) J. Yang, Z. Chi, W. Zhu, B. Z. Tang and Z. Li, Aggregation-induced Emission: a Coming-of-age Ceremony at the Age of Eighteen, Sci. China: Chem., 2019, 62, 1090–1098 CrossRef CAS; (b) J. Yang, M. Fang and Z. Li, Organic Luminescent Materials: The Concentration on Aggregates from Aggregation–induced Emission, Aggregate, 2020, 1, 6–18 CrossRef; (c) F. Würthner, Aggregation–Induced Emission (AIE): A Historical Perspective, Angew. Chem., Int. Ed., 2020, 59, 14192–14196 CrossRef PubMed.
- (a) Y. Liu, L. Wang, L. Xu and Y. Song, From Aggregation-caused Quenching to Aggregation-induced Delayed Fluorescence: the Impact of the Effect of Substituents, J. Mater. Chem. C, 2023, 11, 13403–13417 RSC; (b) Y. Huang, J. Xing, Q. Gong, L. C. Chen, G. Liu, C. Yao, Z. Wang, H. L. Zhang, Z. Chen and Q. Zhang, Reducing Aggregation Caused Quenching Effect Through co-assembly of PAH Chromophores and Molecular Barriers, Nat. Commun., 2019, 10, 169 CrossRef PubMed.
- (a) X. Dong, H. Ouyang, X. Lou, F. Xia, L. Jin, S. Wang and J. Dai, Dual-Activated H2O2-Responsive AIE Probes for Oocyte Quality Assessment, Anal. Chem., 2024, 96, 5960–5967 CrossRef CAS PubMed; (b) W. Qiao, T. Ma, S. Wang, L. Li, M. Liu, H. Jiang, Y. Wu, J. Zhu and Z. Li, Designing Squaraine Dyes with Bright Deep–Red Aggregation–Induced Emission for Specific and Ratiometric Fluorescent Detection of Hypochlorite, Adv. Funct. Mater., 2021, 31, 2105452 CrossRef CAS.
- (a) T. Han, D. Yan, Q. Wu, N. Song, H. Zhang and D. Wang, Aggregation–Induced Emission: A Rising Star in Chemistry and Materials Science, Chin. J. Chem., 2021, 39, 677–689 CrossRef CAS; (b) X. Cai and B. Liu, Aggregation–Induced Emission: Recent Advances in Materials and Biomedical Applications, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS; (c) M. T. Chen, Y. Zhang, M. O. Vysotsky, J. O. Lindner, M. H. Li, M. J. Lin and F. Würthner, 1,1′-Bi(2-naphthol-4,5-dicarboximide)s: Blue Emissive Axially Chiral Scaffolds with Aggregation-enhanced Emission Properties, Org. Chem. Front., 2019, 6, 3731–3740 RSC.
- (a) J. Q. Zhang, X. Y. Xu, F. S. Liu, S. Q. Cao, Y. X. Gui, Y. W. Su, X. Y. He, J. Y. Liang and Y. Q. Zou, Molecular Docking-aided AIEgen Design: Concept, Synthesis and Applications, Sci. China: Chem., 2024, 67, 2614–2628 CrossRef CAS; (b) S. Cao, J. Shao, H. Wu, S. Song, M. T. De Martino, I. A. B. Pijpers, H. Friedrich, L. K. E. A. Abdelmohsen, D. S. Williams and J. C. M. van Hest, Photoactivated Nanomotors via Aggregation Induced Emission for Enhanced Phototherapy, Nat. Commun., 2021, 12, 2077 CrossRef CAS PubMed.
- (a) L. Zong, Y. Xie, C. Wang, J. R. Li, Q. Li and Z. Li, From ACQ to AIE: the Suppression of the Strong π−π Interaction of Naphthalene Diimide Derivatives Through the Adjustment of Their Flexible Chains, Chem. Commun., 2016, 52, 11496–11499 RSC; (b) G. Jiang, J. Liu and P. Zhou, Unraveling the Mechanism of ACQ-to-AIE Transformation of Fluorescein Derivatives, J. Phys. Chem. A, 2023, 127, 5193–5201 CrossRef CAS.
- (a) B. Xu, J. He, Y. Mu, Q. Zhu, S. Wu, Y. Wang, Y. Zhang, C. Jin, C. Lo, Z. Chi, A. Lien, S. Liu and J. Xu, Very Bright Mechanoluminescence and Remarkable Mechanochromism Using a Tetraphenylethene Derivative with Aggregation-induced Emission, Chem. Sci., 2015, 6, 3236–3241 RSC; (b) M. Wu, Z. Tan, J. Zhao, H. Zhang, Y. Xu, T. Long, S. Zhao, X. Cheng and C. Zhou, Tetraphenylethene-modified Polysiloxanes: Synthesis, AIE Properties and Multi-stimuli Responsive Fluorescence, Talanta, 2024, 272, 125767 CrossRef CAS PubMed.
- (a) J. Zhao, Z. Chi, Z. Yang, Z. Mao, Y. Zhang, E. Ubba and Z. Chi, Recent Progress in the Mechanofluorochromism of Distyrylanthracene Derivatives with Aggregation-induced Emission, Mater. Chem. Front., 2018, 2, 1595–1608 RSC; (b) X. Zhang, Z. Chi, B. Xu, L. Jiang, X. Zhou, Y. Zhang, S. Liu and J. Xu, Multifunctional Organic Fluorescent Materials Derived from 9,10-distyrylanthracene with Alkoxyl Endgroups of Various Lengths, Chem. Commun., 2012, 48, 10895–10897 RSC.
- (a) L. Zhu and Y. Zhao, Cyanostilbene-based Intelligent Organic Optoelectronic Materials, J. Mater. Chem. C, 2013, 1, 1059–1065 RSC; (b) A. Gao, Q. Wang, H. Wu, J. W. Zhao and X. Cao, Research Progress on AIE Cyanostilbene-based Self-assembly Gels: Design, Regulation and Applications, Coord. Chem. Rev., 2022, 471, 214753 CrossRef CAS.
- (a) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (b) D. Yan, Q. Wu, D. Wang and B. Z. Tang, Innovative Synthetic Procedures for Luminogens Showing Aggregation-Induced Emission, Angew. Chem., Int. Ed., 2021, 60, 15724–15742 CrossRef CAS.
- (a) Q. Yu, Y. L. Wang, Z. Q. Chen, P. J. Zhao, C. Fan, C. Li and M. Q. Zhu, Geminal Cross Coupling (GCC) Reaction for AIE Materials, Chin. J. Polym. Sci., 2019, 37, 327–339 CrossRef CAS; (b) G. R. Suman, M. Pandey and A. S. J. Chakravarthy, Review on New Horizons of Aggregation Induced Emission: from Design to Development, Mater. Chem. Front., 2021, 5, 1541–1584 RSC; (c) G. Zbancioc, I. I. Mangalagiu and C. Moldoveanu, A Review on the Synthesis of Fluorescent Five- and Six-Membered Ring Azaheterocycles, Molecules, 2022, 27, 6321 CrossRef CAS.
- (a) Y. Tani and T. Ogawa, Palladium-Catalyzed Double Carbonylative Cyclization of Benzoins: Synthesis and Photoluminescence of Bis-Ester-Bridged Stilbenes, Org. Lett., 2018, 20, 7442–7446 CrossRef CAS; (b) B. W. Wang, K. Jiang, J. X. Li, S. H. Luo, Z. Y. Wang and H. F. Jiang, 1,1−Diphenylvinylsulfide as a Functional AIEgen Derived from the Aggregation–Caused–Quenching Molecule 1,1−Diphenylethene Through Simple Thioetherification, Angew. Chem., Int. Ed., 2020, 59, 2338–2343 CrossRef CAS PubMed; (c) F. Sun, S. Tan, H. J. Cao, J. Xu, V. I. Bregadze, D. Tu, C. Lu and H. Yan, Palladium–Catalyzed Hydroboration of Alkynes with Carboranes: Facile Construction of a Library of Boron Cluster–Based AIE–Active Luminogens, Angew. Chem., Int. Ed., 2022, 61, e202207125 CrossRef CAS; (d) J. Xie, W. Li, Y. Lu, Y. Zheng, Y. Huang, S. Chen and Q. Song, Unlocking Diverse π-Bond Enrichment Frameworks by the Synthesis and Conversion of Boronated Phenyldiethynylethylenes, J. Am. Chem. Soc., 2024, 146, 10167–10176 CrossRef CAS PubMed.
- (a) H. Yang, C. Cui and Y. Li, Effects of Heteroatom Substitution on the Photovoltaic Performance of Donor Materials in Organic Solar Cells, Acc. Mater. Res., 2021, 2, 986–997 CrossRef CAS; (b) A. Ekramipooya, F. M. Valadi, A. Farisabadi and M. R. Gholami, Effect of the Heteroatom Presence in Different Positions of the Model Asphaltene Structure on the Self-aggregation: MD and DFT Study, J. Mol. Liq., 2021, 334, 116109 CrossRef CAS; (c) Y. Xiao, X. Zhang, Y. Wang, K. Li, G. Wang, X. Kong, J. Wang and S. Li, Sequential Annulation of Bidentate Diamines for Modular Access to N-fused/helical/spiro-carbazole Scaffolds, Org. Chem. Front., 2024, 11, 401–406 RSC.
- (a) V. Estévez, M. Villacampa and J. C. Menéndez, Recent Advances in the Synthesis of Pyrroles by Multicomponent Reactions, Chem. Soc. Rev., 2014, 43, 4633–4657 RSC; (b) N. Singh, S. Singh, S. Kohli, A. Singh, H. Asiki, G. Rathee, R. Chandra and E. A. Anderson, Recent Progress in the Total Synthesis of Pyrrole-containing Natural Products (2011–2020), Org. Chem. Front., 2021, 8, 5550–5573 RSC.
- (a) X. Feng, B. Tong, J. Shen, J. Shi, T. Han, L. Chen, J. Zhi, P. Lu, Y. Ma and Y. Dong, Aggregation-Induced Emission Enhancement of Aryl-Substituted Pyrrole Derivatives, J. Phys. Chem. B, 2010, 114, 16731–16736 CrossRef CAS PubMed; (b) Y. Lei, Q. Liu, L. Dong, Z. Cai, J. Shi, J. Zhi, B. Tong and Y. Dong, The Dual–State Luminescent Mechanism of 2,3,4,5−Tetraphenyl–1H–pyrrole, Chem. – Eur. J., 2018, 24, 14269–14274 CrossRef CAS.
- (a) Z. Peng, Y. Ji, Z. Huang, B. Tong, J. Shi and Y. Dong, A Strategy for the Molecular Design of Aggregation-induced Emission Units Further Modified by Substituents, Mater. Chem. Front., 2018, 2, 1175–1183 RSC; (b) S. Dai, Z. Cai, Z. Peng, Z. Wang, B. Tong, J. Shi, S. Gan, Q. He, W. Chen and Y. Dong, A Stabilized Lamellar Liquid Crystalline Phase with Aggregation-induced Emission Features Based on Pyrrolopyrrole Derivatives, Mater. Chem. Front., 2019, 3, 1105–1112 RSC; (c) H. Wang, J. Huo, H. Tong, X. Wei, Y. Zhang, Y. Li, S. Chen, H. Shi and B. Z. Tang, Synthesis, Crystal Structure, Aggregation-induced Emission (AIE) and Electroluminescence Properties of a Novel Emitting Material Based on Pyrrolo[3,2-b]pyrrole, J. Mater. Chem. C, 2020, 8, 14208–14218 RSC.
- (a) T. Yata, Y. Kita, Y. Nishimoto and M. Yasuda, Regioselective Synthesis of 5-Metalated 2-Pyrones by Intramolecular Oxymetalation of Carbonyl-ene-yne Compounds Using Indium Trihalide, J. Org. Chem., 2019, 84, 14330–14341 CrossRef CAS; (b) Y. Tani and T. Ogawa, Structure–property Relationship in Contrasting Aggregation-induced Enhancement/quenching of Emission in Rigid Aromatic Molecules, J. Mater. Chem. C, 2021, 9, 4281–4288 RSC.
- (a) H. Li, Y. Wang, C. Xu, J. Zou, Y. Wu and G. Yin, TBHP-promoted Multicomponent Reaction to Access 2-aminobenzoxazinones Using Sodium Chlorodifluoroacetate as the C1 Synthon, Org. Chem. Front., 2023, 10, 1988–1993 RSC; (b) M. Luo, N. Dong, M. Zhu, Y. Wang, C. Xu and G. Yin, Transition-Metal-Free Approach to Acridone Derivatives by TBHP-Promoted Oxidative Annulation of Isatins with Arynes, J. Org. Chem., 2023, 88, 9419–9423 CrossRef CAS; (c) L. Fan, M. Liu, Y. Ye and G. Yin, Synthesis of 6-Substituted 6H-Indolo[2,3-b]quinolines from Isoindigos, Org. Lett., 2017, 19, 186–189 CrossRef CAS.
- (a) C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Palladium–Catalyzed Cross–Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS PubMed; (b) L. Fu, X. Cao, J. Wan and Y. Liu, Synthesis of Enaminone–Pd(II) Complexes and Their Application in Catalysing Aqueous Suzuki–Miyaura Cross Coupling Reaction, Chin. J. Chem., 2020, 38, 254–258 CrossRef CAS.
- K. Schwickert, M. Andrzejewski, S. Grabowsky and T. Schirmeister, Synthesis, X-ray Structure Determination, and Comprehensive Photochemical Characterization of (Trifluoromethyl)diazirine-Containing TRPML1 Ligands, J. Org. Chem., 2021, 86, 6169–6183 CrossRef CAS PubMed.
- (a) X. Li, C. Han, H. Yao and A. Lin, Organocatalyzed [3 + 2] Annulation of Cyclopropenones and β-Ketoesters: An Approach to Substituted Butenolides with a Quaternary Center, Org. Lett., 2017, 19, 778–781 CrossRef CAS PubMed; (b) J. Xu, J. Cao, C. Fang, T. Lu and D. Du, Organocatalytic C–C Bond Activation of Cyclopropenones for Ring-opening Formal [3 + 2] Cycloaddition with Isatins, Org. Chem. Front., 2017, 4, 560–564 RSC; (c) F. Jamshaid, V. V. Kondakal, C. D. Newman, R. Dobson, H. João, C. R. Rice, J. M. Mwansa, B. Thapa and K. Hemming, Cyclopropenones in the Synthesis of Indolizidine, Pyrrolo[2,1-a]isoquinoline and Indolizino[8,7-b]indole Alkaloids, Tetrahedron, 2020, 76, 131570 CrossRef CAS; (d) Q. Chen, Y. Teng and F. Xu, Lanthanide Silylamide-Catalyzed Synthesis of Pyrano[2,3-b]indol-2-ones, Org. Lett., 2021, 23, 4785–4790 CrossRef CAS PubMed.
- A. Pal, M. Karmakar, S. R. Bhatta and A. Thakur, A Detailed Insight into Anion Sensing Based on Intramolecular Charge Transfer (ICT) Mechanism: A Comprehensive Review of the Years 2016 to 2021, Coord. Chem. Rev., 2021, 448, 214167 CrossRef CAS.
- (a) J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Aggregation-Induced Emission: The Whole Is More Brilliant Than the Parts, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS; (b) Q. Li and Z. Li, The Strong Light–Emission Materials in the Aggregated State: What Happens from a Single Molecule to the Collective Group, Adv. Sci., 2017, 4, 1600484 CrossRef PubMed.
- M. Chen, L. Li, H. Nie, J. Tong, L. Yan, B. Xu, J. Z. Sun, W. Tian, Z. Zhao, A. Qin and B. Z. Tang, Tetraphenylpyrazine-based AIEgens: Facile Preparation and Tunable Light Emission, Chem. Sci., 2015, 6, 1932–1937 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2298102 and 2327902. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01600a |
| This journal is © the Partner Organisations 2025 |