I2-Promoted oxidative annulation of three different amines to access diverse biheteroaryls†
Guangping
Fan
,
Penghui
Cao
,
Fang
Bai
,
Yanyan
Yin
,
Xueyan
Hou
,
Yani
Liu
,
Zhenzhen
Xing
,
Yanhui
Wang
,
Tangqiang
Sun
* and
Qinghe
Gao
 *
*
School of Pharmacy, Xinxiang Medical University, Xinxiang, Henan 453003, P. R. China. E-mail: suntq@xxmu.edu.cn; gao_qinghe@xxmu.edu.cn
First published on 25th October 2024
Abstract
Utilizing tertiary alkylamines as reliable two-carbon building blocks, a concise and efficient route for the regioselective synthesis of biquinolines from 2-methylquinolines and arylamines has been established. This I2-promoted approach enables a [4π + 2σ] annulation of three nucleophilic species via two kinds of transient enamines. The C(sp3)–H annulation strategy is also extended to other methylazaarenes toward constructing nonsymmetrical benzo[f]quinoline-containing biheteroaryls. Notably, the applicability of this process for the late-stage functionalization of complex molecules highlights its considerable potential for application in biorelevant areas.
N-Heterocyclic frameworks found in natural products, pharmaceuticals, biologically active compounds, and functional materials frequently feature unsubstituted C
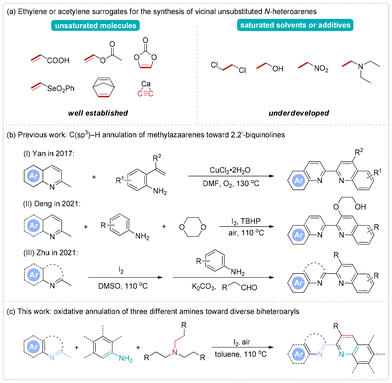
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.1 In fact, access to unsubstituted vinylene-embedded azaarenes could be accomplished by direct heteroannulations of ethylene or acetylene with suitable coupling partners. Nevertheless, the operational safety issues associated with these gaseous two-carbon reactants have made these processes extremely challenging.2 Fortunately, several breakthrough molecules such as acrylic acid,3 vinyl acetate,4 vinylene carbonate,5 vinyl selinene,6 norbornadiene,7 and calcium carbide8 have been identified as effective and practical equivalents to facilitate the construction of vicinal unsubstituted N-heteroarenes through transition-metal-catalyzed ortho C–H bond activation/annulation reactions. Recently, certain general and inert solvents or additives have emerged as unique vinylene candidates in dehydrogenative annulation strategies. For example, the You team made a groundbreaking advancement by using 1,2-dichloroethane to generate ring-fused pyridiniums through a rhodium-catalyzed annulation between N-vinylpyridinium and alkynes.9 Li and Majee independently reported an iron-catalyzed α,β-dehydrogenation of ethanol and nitroethane for the formation of 3,4-unsubstituted 2-arylquinolines, respectively.10 Our group realized an NH4I-mediated vinylation annulation of α-aminoazoles with triethylamine, establishing a versatile class of promising ethylene precursors for the synthesis of pharmaceutically significant azolo[1,5-a]pyrimidines (Scheme 1a).11 In this context, we hypothesize that an ethylene annulation building block derived from triethylamine could be exploited in the C(sp3)–H annulation of methylazaarenes to enable efficient preparation of valuable biheteroaryls. Herein, we introduce the first example of an I2-promoted oxidative annulation from three different and simple amines, providing a straightforward pathway to a variety of biheteroaryl molecules with good substrate and functional group compatibility.
C bonds.1 In fact, access to unsubstituted vinylene-embedded azaarenes could be accomplished by direct heteroannulations of ethylene or acetylene with suitable coupling partners. Nevertheless, the operational safety issues associated with these gaseous two-carbon reactants have made these processes extremely challenging.2 Fortunately, several breakthrough molecules such as acrylic acid,3 vinyl acetate,4 vinylene carbonate,5 vinyl selinene,6 norbornadiene,7 and calcium carbide8 have been identified as effective and practical equivalents to facilitate the construction of vicinal unsubstituted N-heteroarenes through transition-metal-catalyzed ortho C–H bond activation/annulation reactions. Recently, certain general and inert solvents or additives have emerged as unique vinylene candidates in dehydrogenative annulation strategies. For example, the You team made a groundbreaking advancement by using 1,2-dichloroethane to generate ring-fused pyridiniums through a rhodium-catalyzed annulation between N-vinylpyridinium and alkynes.9 Li and Majee independently reported an iron-catalyzed α,β-dehydrogenation of ethanol and nitroethane for the formation of 3,4-unsubstituted 2-arylquinolines, respectively.10 Our group realized an NH4I-mediated vinylation annulation of α-aminoazoles with triethylamine, establishing a versatile class of promising ethylene precursors for the synthesis of pharmaceutically significant azolo[1,5-a]pyrimidines (Scheme 1a).11 In this context, we hypothesize that an ethylene annulation building block derived from triethylamine could be exploited in the C(sp3)–H annulation of methylazaarenes to enable efficient preparation of valuable biheteroaryls. Herein, we introduce the first example of an I2-promoted oxidative annulation from three different and simple amines, providing a straightforward pathway to a variety of biheteroaryl molecules with good substrate and functional group compatibility.
Biheteroaryls with quinoline cores are an important class of structures in bioactive molecules, chemical sensors, ligands, and organic materials.12 Among these, 2,2′-biquinolines are associated with lead compounds and drug coordinates known for their anticancer, antimicrobial, and anti-neurodegenerative properties.13 However, current methods towards 2,2′-biquinolines have mostly been limited to nickel-catalyzed reductive couplings of 2-haloquinolines,14 metal-free deoxygenative dimerizations of quinoline N-oxides,15 and dipyridannulations of specific isocyanides16 and these traditional methods for accessing such biheteroaromatic compounds by homocoupling have a restricted substrate scope. Recently, C(sp3)–H bond heteroannulation of 2-methylquinolines has allowed for the development of novel approaches for enhancing functional and space diversity in the synthesis of challenging nonsymmetrical 2,2′-biquinoline derivatives. In 2017, the group of Yan first demonstrated a copper-catalyzed oxidative annulation of 2-methylquinolines and 2-vinylanilines to deliver 4-aryl-2,2′-biquinolines.17 Subsequently, Deng et al. skillfully employed 1,4-dioxane as a 2-(vinyloxy)ethan-1-ol source, achieving the successful implementation of oxidative annulation with 2-methylquinolines and arylamines.18 In the same year, Zhu and co-workers reported a one-pot tandem protocol for the synthesis of 2-heteroaryl-substituted quinolines, wherein 2-methylquinolines were converted into quinoline-2-carbaldehydes in the presence of iodine and DMSO (Scheme 1b).19 Driven by these achievements, we present a general strategy for the direct assembly of biomedically relevant nonsymmetrical 2,2′-biquinolines and related bis-azines such as isoquinoline, quinoxaline, pyridine, benzothiazole, benzo[d]oxazole, and 1H-benzo[d]imidazole-tethered benzo[f]quinolines in the presence of I2 (Scheme 1c). Moreover, this mild quinolylation protocol unveils a [4π + 2σ] annulation involving methylazaarenes and arylamines with tertiary alkylamines as two-carbon cycloaddition units, offering a convenient avenue for the late-stage functionalization of natural products and pharmaceuticals.
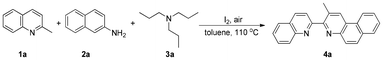
Initially, 2-methylquinoline (1a), naphthalen-2-amine (2a), and tripropylamine (3a) were selected as the model substrates (Table 1). Fortunately, the desired 2-methyl-3-(quinolin-2-yl)benzo[f]quinoline (4a) was isolated in 70% yield when the reaction was carried out in the presence of I2 and DTBP in toluene at 110 °C (entry 4). However, replacing the iodine source with KI, TBAI, NIS, or NH4I did not effectively promote the reaction and no Povarov product could be obtained without I2 (entries 2 and 3). Other common oxidants including CHP, TBHP, TBPB, DCP, K2S2O8, and DMSO also did not lead to any increase in the yield of 4a (entry 4). Surprisingly, the yield of such a biheteroaryl was improved to 85% in the absence of DTBP (entry 1). The C(sp3)–H bond functionalization of 2-methylquinolines often requires Brønsted acids to accelerate tautomerization,20 and this dehydrogenative annulation reaction thus used a variety of Brønsted acids as additives. Unfortunately, the target product was produced in diminished yields (entry 5). Afterward, a survey of solvents suggested that while toluene remained the preferred choice for the model substrate pair, o-DCB, PhCl, and o-xylene were also relatively effective (entries 6 and 7). Further evaluation of temperatures failed to show better results (entries 8–10). Control experiments demonstrated that aerial oxygen as the oxidant was essential for facilitating this quinolylation process (entries 11 and 12). Performing the reaction by adjusting the loading of 3a, 1a, or molecular iodine no longer had a positive effect on the yield of 4a (entries 13–17).
| Entry | Variation from standard conditions | Yieldb (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.2 mmol), 3a (0.2 mmol), [I] (0.2 mmol), and solvent (2.0 mL) at 110 °C for 20 h in a sealed tube. b Isolated yields. c Oxidant (0.6 mmol). d Acid (0.2 mmol). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | None | 85 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | KI, TBAI, NIS, NH4I instead of I2 | 0–52 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Without I2 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4c | Addition of CHP, TBHP, TBPB, DCP, K2S2O8, DMSO, or DTBP | 26–70 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5d | Addition of CF3SO3H, CH3SO3H, CH3COOH, or PhCOOH | 55–79 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | o-DCB, PhCl, o-xylene instead of toluene | 63–81 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | DCE, THF, DMSO, CH3CN, DMF, NMP instead of toluene | 18–43 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | 120 °C instead of 110 °C | 71 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | 100 °C instead of 110 °C | 78 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | 80 °C instead of 110 °C | 62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Under Ar | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Under O2 | 87 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | With 0.4 mmol 3a | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | With 0.4 mmol 1a | 86 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | With 0.2 mmol 1a | 64 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | With 0.3 mmol I2 | 77 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | With 0.1 mmol I2 | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
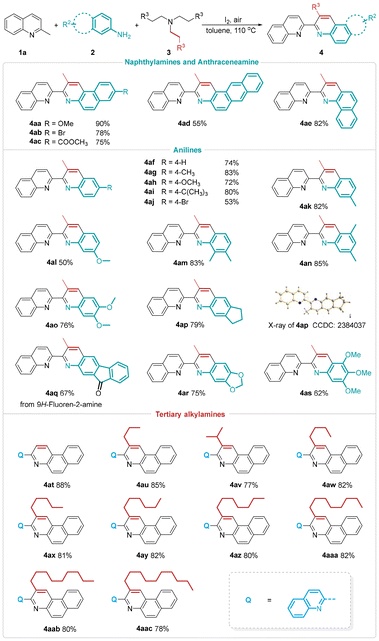
Having established the optimized reaction conditions, we subsequently investigated the scope of this I2-promoted [4π + 2σ] annulation with methylazaarenes (Scheme 2). A variety of substituents on the phenyl rings of 2-methylquinolines, regardless of whether they were electron-donating (Me and OMe), electron-withdrawing (NO2, CO2Et, and CO2iBu) or halo (F, Cl, and Br) groups, were well tolerated, affording the quinolylation products (4b–4f and 4h–4m) in 71–88% yields. Gratifyingly, cyclododecyl-containing 2-methylquinoline (1g) was also proven to be compatible with the reaction. In the case of 4-chloro-2-methylquinoline (1n), the relatively lower yield was presumably attributable to the reduced electron density on the pyridine ring, which had an adverse effect on the iodination process. To our delight, the π-system-expanded 3-methylbenzo[f]quinoline 1o was a suitable candidate to deliver the symmetrical 3,3′-bibenzo[f]quinoline skeleton in 85% yield. Meanwhile, 4-methylquinoline (1p) and 1-methylisoquinoline (1q) displayed reactivities similar to 2-methylquinoline. Further investigations revealed that mononuclear 2-methyl and 4-methyl feedstocks were excellent partners and gave rise to the corresponding pyridine–benzo[f]quinoline linked biheteroaryls (4s and 4t) in 85% and 87% yields, respectively. The C(sp3)–H annulation strategy was also extended to other 2-methylazaarenes featuring two heteroatoms, providing a straightforward access to various types of biheteroarene structural motifs (4r and 4u–4w). Given the intriguing versatility and practicality of this transformation, we conducted late-stage functionalization on several natural products and drug molecules. It is noteworthy that 2-methylquinolines derived from L-menthol, estrone, and vitamin E underwent successful modifications to yield the biheteroarene products. In addition, a larger scale experiment proceeded smoothly, with 1.0 mmol of starting material 2a under the standard conditions.
Next, we explored the generality of the dehydrogenative annulation strategy with respect to aromatic amines (Scheme 3). Naphthalen-2-amines with methoxyl, bromo, and formic ester groups at the C6 position exhibited good performance and generated the quinoline-substituted benzo[f]quinoline derivatives 4aa–4ac in good to excellent yields (75–90%). In comparison, the tetracyclic naphtho[2,3-f]quinoline 4ad was obtained in disappointing yields when the reaction was run with anthracen-2-amine. Instead of naphthalen-2-amine, when naphthalen-1-amine was used, 4ae was efficiently furnished in 82% yield. Remarkably, the versatility of this process was further demonstrated by the successful use of various anilines, which provided robust access to a series of nonsymmetrical 2,2′-biquinolines. Unsubstituted aniline, a challenging substrate in related research, reacted smoothly with 1a and 3a to form the methylated 2,2′-biquinoline 4af in 74% yield. Regarding monosubstituted anilines at the para position, the phenomenon wherein electron-rich groups such as methyl, methoxyl, and tertiary butyl favored the [4π + 2σ] cycloaddition step to give higher yields compared to the electron-poor substituent (2k) was observed. When meta-substituted arylamines were applied to this annulation procedure, only isomers 4ak and 4al were obtained successfully. Meanwhile, reactions using acyclic or cyclic disubstituted and trisubstituted anilines, characterized by enhanced nucleophilicity, also afforded the corresponding biquinoline derivatives in up to 85% yields (4am–4as). Unexpectedly, a methylene oxidation reaction occurred during such a quinoline annulation and permitted the integration of 9-fluorenone into the biheteroaryl structure (4aq). By utilizing this quinolylation of methylazaarenes, the unsubstituted N-biheterocycle 4at could be synthesized as well, wherein the unsubstituted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond fragment favorably incorporated into the pyridine ring formed from triethylamine (3b). Moreover, efforts focused on testing other tertiary alkylamines (3c–3k), including triamylamine, trihexylamine, triheptylamine, trioctylamine, trinonylamine, tridecylamine, triundecylamine, tridodecylamine, and the bulky triisopentylamine. Much to our satisfaction, all these substrates showed consistent results with triethylamine in the established system, affording alkylation heteroannulation products (4au–4aac) in yields ranging from 77% to 85%.
C bond fragment favorably incorporated into the pyridine ring formed from triethylamine (3b). Moreover, efforts focused on testing other tertiary alkylamines (3c–3k), including triamylamine, trihexylamine, triheptylamine, trioctylamine, trinonylamine, tridecylamine, triundecylamine, tridodecylamine, and the bulky triisopentylamine. Much to our satisfaction, all these substrates showed consistent results with triethylamine in the established system, affording alkylation heteroannulation products (4au–4aac) in yields ranging from 77% to 85%.
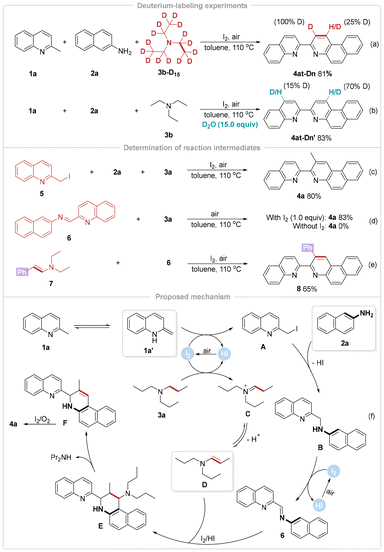
To elucidate the mechanism of this C(sp3)–H annulation reaction, a deuterium-labeling experiment with 3b-d15 as the substrate was carried out and the expected deuterated product 4at-Dn was obtained with 100% and 25% deuterium incorporation at the β-C and γ-carbon atoms of the newly formed pyridine ring, respectively (Scheme 4a). Furthermore, the H/D exchange experiment confirmed that γ-selective deuteration reactions of two pyridine rings proceeded simultaneously. These results indicated that the mechanism involved the cleavage of three C(sp3)–D bonds and one C–N bond, showing that only the two C(sp3)–D bonds in the α,β-carbon atoms of triethylamine were effectively integrated into the biquinoline skeleton (Scheme 4b). When 2-(iodomethyl)quinoline (5) with 2a and 3a was subjected to the standard reaction conditions, product 4a was also achieved in 80% yield (Scheme 4c). Meanwhile, the [4π + 2σ] annulation of imine (6) and 3a proceeded well in the current system. Obviously, both heterobenzyl iodides and imines were recognized as potential intermediates during the transformation. However, the lack of I2 rendered the reaction between 6 and 3a unsuccessful, highlighting the indispensability of I2 in the [4π + 2σ] annulation procedure (Scheme 4d). Undoubtedly, the formation of a phenyl-substituted biheteroaryl (8) from N,N-diethyl-2-phenylethenamine (7) supported the idea that enamines generated from tertiary alkylamines through dual inert C(sp3)–C(sp3) bond dehydrogenation served as the actual intermediates for this [4π + 2σ] annulation (Scheme 4e). Furthermore, radical inhibition experiments ruled out a radical pathway (Scheme S1†).
Based on the above observations and relevant reports,21 a plausible mechanism for the C(sp3)–H annulation of three nucleophiles has been proposed (Scheme 4f). Under suitable pH conditions, 2-methylquinoline (1a) can be transformed into its more nucleophilic transient enamine counterpart 1a′, followed by iodination with I2 to give the intermediate 2-(iodomethyl)quinoline (A). Subsequently, heterobenzyl iodide (A) undergoes nucleophilic amination directly with naphthalen-2-amine (2a) to generate the secondary amine intermediate B, which is further rapidly oxidized by I2 to form the key imine intermediate 6. At the same time, the dehydrogenation of tripropylamine (3a) with I2 and air easily results in the formation of a common imine-type intermediate (C). It is critical that I2 and HI enable the aza-Diels–Alder reaction of 6 and the transient enamine (D) derived from the hidden tautomerization of C to afford the Povarov product E. Finally, the sequential deamination and oxidative aromatization can deliver the desired product 4a.
Conclusions
In conclusion, we have developed a [4π + 2σ] annulation approach that offers a general and complementary solution to a wide array of nonsymmetrical biheteroaryls under mild and metal-free conditions. The key to the oxidative annulation of three different nucleophiles is that molecular iodine establishes efficient transformations of methylazaarenes and linear alkyl tertiary amines into heterobenzyl iodide species and transient enamines, respectively. This C(sp3)–H annulation strategy exhibits outstanding compatibility with functional groups and heterocycles, is applicable to the late-stage functionalization of complex quinolines, and paves the way for the discovery of bioactive molecules and functional materials.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Natural Science Foundation of Henan Province (242300421356), the Key Research and Promotion Special Projects of Henan Provincial Science and Technology Department (242102320053) and Open Grant from Pingyuan Laboratory (2023PY-OP-0205) for financial support.References
- (a) R. A. Jones, S. S. Panda and C. D. Hall, Quinine Conjugates and Quinine Analogues as Potential Antimalarial Agents, Eur. J. Med. Chem., 2015, 97, 335–355 CrossRef CAS PubMed; (b) P. Czobor, P. Skolnick, B. Beer and A. Lippa, A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study of Efficacy and Safety of Ocinaplon (DOV 273,547) in Generalized Anxiety Disorder, CNS Neurosci. Ther., 2010, 16, 63–75 CrossRef CAS PubMed; (c) L. Z. Bendjeddou, N. Loaëc, B. Villiers, E. Prina, G. F. Späth, H. Galons, L. Meijer and N. Oumata, Exploration of the Imidazo[1,2-b]pyridazine Scaffold as A Protein Kinase Inhibitor, Eur. J. Med. Chem., 2017, 125, 696–709 CrossRef CAS PubMed; (d) M. T. Rudd, J. A. McCauley, J. W. Butcher, J. J. Romano, C. J. McIntyre, K. T. Nguyen, K. F. Gilbert, K. J. Bush, M. K. Holloway, J. Swestock, B.-L. Wan, S. S. Carroll, J. M. DiMuzio, D. J. Graham, S. W. Ludmerer, M. W. Stahlhut, C. M. Fandozzi, N. Trainor, D. B. Olsen, J. P. Vacca and N. J. Liverton, Discovery of MK-1220: A Macrocyclic Inhibitor of Hepatitis C Virus NS3/4A Protease with Improved Preclinical Plasma Exposure, ACS Med. Chem. Lett., 2011, 2, 207–212 CrossRef CAS PubMed; (e) G. Yang and W. Zhang, Renaissance of Pyridine-Oxazolines as Chiral Ligands for Asymmetric Catalysis, Chem. Soc. Rev., 2018, 47, 1783–1810 RSC.
- (a) I.-T. Trotuş, T. Zimmermann and F. Schüth, Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited, Chem. Rev., 2014, 114, 1761–1782 CrossRef; (b) S. Tang, D. Wang, Y. Liu, L. Zeng and A. Lei, Cobalt-Catalyzed Electrooxidative C−H/N−H [4 + 2] Annulation with Ethylene or Ethyne, Nat. Commun., 2018, 9, 798 CrossRef.
- (a) X. Ji, H. Huang, Y. Li, H. Chen and H. Jiang, Palladium-Catalyzed Sequential Formation of C−C Bonds: Efficient Assembly of 2-Substituted and 2,3-Disubstituted Quinolines, Angew. Chem., Int. Ed., 2012, 51, 7292–7296 CrossRef CAS; (b) J. M. Neely and T. Rovis, Rh(III)-Catalyzed Decarboxylative Coupling of Acrylic Acids with Unsaturated Oxime Esters: Carboxylic Acids Serve as Traceless Activators, J. Am. Chem. Soc., 2014, 136, 2735–2738 CrossRef CAS PubMed.
- (a) N. J. Webb, S. P. Marsden and S. A. Raw, Rhodium(III)-Catalyzed C−H Activation/Annulation with Vinyl Esters as an Acetylene Equivalent, Org. Lett., 2014, 16, 4718–4721 CrossRef CAS PubMed; (b) H. Chu, S. Sun, J.-T. Yu and J. Cheng, Rh-Catalyzed Sequential Oxidative C−H Activation/Annulation with Geminal-Substituted Vinyl Acetates to Access Isoquinolines, Chem. Commun., 2015, 51, 13327–13329 RSC; (c) S. J. Murugan and M. Jeganmohan, Cp*Co(III)-Catalyzed Regioselective [4 + 2] Annulation of N-Chlorobenzamides with Vinyl Acetate/Vinyl Ketones, J. Org. Chem., 2023, 88, 1578–1589 CrossRef PubMed.
- (a) X. Cui, R. Chauvin, C. Pi, Y. Wu and X. Cui, Vinylene Carbonate as Synthon in Transition Metal-Catalyzed C−H Bond Activation/Annulation Reactions, Adv. Synth. Catal., 2023, 365, 3400–3412 CrossRef CAS; (b) Y. Ge, Q. Yan and J. Nan, Advancement of Vinylene Carbonate as a Coupling Partner in Metal-Catalyzed C−H Functionalization, Org. Chem. Front., 2023, 10, 5717–5734 RSC; (c) X. Gao, R. Zhai, X. Chen and S. Wang, Recent Progress in C−H Bond Activation Reaction with Vinylene Carbonate, Chin. J. Org. Chem., 2023, 43, 3119–3134 CrossRef CAS; (d) K. Ghosh, Y. Nishii and M. Miura, Rhodium-Catalyzed Annulative Coupling Using Vinylene Carbonate as an Oxidizing Acetylene Surrogate, ACS Catal., 2019, 9, 11455–11460 CrossRef CAS; (e) Y. Nishii and M. Miura, Cp*M-Catalyzed Direct Annulation with Terminal Alkynes and Their Surrogates for the Construction of Multi-Ring Systems, ACS Catal., 2020, 10, 9747–9757 CrossRef CAS.
- A. Inami, Y. Nishii, K. Hirano and M. Miura, Rhodium-Catalyzed Isoquinoline Synthesis Using Vinyl Selenone as Oxidizing Acetylene Surrogate, Org. Lett., 2023, 25, 3206–3209 CrossRef CAS PubMed.
- B.-S. Zhang, Y. Li, Z. Zhang, Y. An, Y.-H. Wen, X.-Y. Gou, S.-Q. Quan, X.-G. Wang and Y.-M. Liang, Synthesis of C4-Aminated Indoles via a Catellani and Retro-Diels−Alder Strategy, J. Am. Chem. Soc., 2019, 141, 9731–9738 CrossRef CAS PubMed.
- (a) H. Liu and Z. Li, Copper-Catalyzed Construction of Benzo[4,5]imidazo[2,1-a]isoquinolines Using Calcium Carbide as a Solid Alkyne Source, Org. Lett., 2021, 23, 8407–8412 CrossRef CAS; (b) Y. Yu, W. Huang, Y. Chen, B. Gao, W. Wu and H. Jiang, Calcium Carbide as the Acetylide Source: Transition-Metal-Free Synthesis of Substituted Pyrazoles via [1,5]-Sigmatropic Rearrangements, Green Chem., 2016, 18, 6445–6449 RSC; (c) X. You, B. Wang, F. Wen and Z. Li, Construction of Pyrazolo[1,5-a]pyrimidines and Pyrimido[1,2-b]indazoles with Calcium Carbide as an Alkyne Source, Org. Biomol. Chem., 2024, 22, 5822–5826 RSC; (d) M. S. Ledovskaya, K. S. Rodygin and V. P. Ananikov, Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation, Org. Chem. Front., 2018, 5, 226–231 RSC.
- (a) Z. Wang, J. Yin, F. Zhou, Y. Liu and J. You, Multicomponent Reactions of Pyridines To Give Ring-Fused Pyridiniums: In Situ Activation Strategy Using 1,2-Dichloroethane as a Vinyl Equivalent, Angew. Chem., Int. Ed., 2019, 58, 254–258 CrossRef CAS; (b) Z. Wang, L. Jiang, J. Ji, F. Zhou, J. Lan and J. You, Construction of Cationic Azahelicenes: Regioselective Three-Component Annulation Using In Situ Activation Strategy, Angew. Chem., Int. Ed., 2020, 59, 23532–23536 CrossRef CAS; (c) J. Ji, L. Jiang, Z. Wang, Z. Bin, J. You and Y. Yang, Copper-Catalyzed Oxidative C−H Annulation of Quinolines with Dichloroethane toward Benzoquinoliziniums Using an In Situ Activation Strategy, Org. Lett., 2022, 24, 6256–6260 CrossRef CAS PubMed.
- (a) S. Mahato, A. Mukherjee, S. Santra, G. V. Zyryanov and A. Majee, Facile Synthesis of Substituted Quinolines by Iron(III)-Catalyzed Cascade Reaction between Anilines, Aldehydes and Nitroalkanes, Org. Biomol. Chem., 2019, 17, 7907–7917 RSC; (b) X. Li, Q. Xing, P. Li, J. Zhao and F. Li, Three-Component Povarov Reaction with Alcohols as Alkene Precursors: Efficient Access to 2-Arylquinolines, Eur. J. Org. Chem., 2017, 618–625 CrossRef.
- (a) Q. Gao, X. Han, P. Tong, Z. Zhang, H. Shen, Y. Guo and S. Bai, Aerobic α,β-C(sp3)−H Bond Difunctionalization and C−N Bond Cleavage of Triethylamine: Difunctional Ammonium Iodide Enabling the Regioselective Synthesis of 4-Arylpyrimido[1,2-b]indazoles, Org. Lett., 2019, 21, 6074–6078 CrossRef CAS PubMed; (b) Q. Gao, Z. Sun, Q. Xia, R. Li, W. Wang, S. Ma, Y. Chai, M. Wu, W. Hu, P. Ábrányi-Balogh, G. M. Keserű and X. Han, Vinylation of α-Aminoazoles with Triethylamine: A General Strategy to Construct Azolo[1,5-a]pyrimidines with a Nonsubstituted Ethylidene Fragment, Org. Lett., 2021, 23, 2664–2669 CrossRef CAS PubMed.
- (a) E. Vitaku, D. T. Smith and J. T. Njardarson, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (b) J. D. Vasta, K. A. Andersen, K. M. Deck, C. P. Nizzi, R. S. Eisenstein and R. T. Raines, Selective Inhibition of Collagen Prolyl 4-Hydroxylase in Human Cells, ACS Chem. Biol., 2016, 11, 193–199 CrossRef CAS PubMed; (c) D.-L. Ma, S. Lin, W. Wang, C. Yang and C.-H. Leung, Luminescent Chemosensors by Using Cyclometalated Iridium(III) Complexes and Their Applications, Chem. Sci., 2017, 8, 878–889 RSC; (d) E. C. Hansen, D. J. Pedro, A. C. Wotal, N. J. Gower, J. D. Nelson, S. Caron and D. J. Weix, New Ligands for Nickel Catalysis from Diverse Pharmaceutical Heterocycle Libraries, Nat. Chem., 2016, 8, 1126–1130 CrossRef CAS; (e) Y. Mao, H. Du, X. Wang, M. Tian, Y. Wang, L. Liu, J. Wei, F. Xue, G. Liu, X. Zhang and T. Yi, A Ratiometric Fluorescent Probe for Rapidly Detecting Bio-thiols in Vitro and in Living Cells, Dyes Pigm., 2019, 171, 107688 CrossRef CAS.
- (a) S. J. Steinke, S. Gupta, E. J. Piechota, C. E. Moore, J. J. Kodanko and C. Turro, Photocytotoxicity and Photoinduced Phosphine Ligand Exchange in a Ru(II) Polypyridyl Complex, Chem. Sci., 2022, 13, 1933–1945 RSC; (b) C. Zhang, X. Guo, X. Da, Z. Wang, X. Wang and Q. Zhou, A Ru-Anthraquinone Dyad with Triple Functions of PACT, Photoredox Catalysis and PDT Upon Red Light Irradiation, Dalton Trans., 2021, 50, 10845–10852 RSC; (c) R. Starosta, A. Brzuszkiewicz, A. Bykowska, U. K. Komarnicka, B. Bażanów, M. Florek, Ł. Gadzała, N. Jackulak, J. Król and K. Marycz, A Novel Copper(I) Complex, [CuI(2,2′-biquinoline)P(CH2N(CH2CH2)2O)3]− Synthesis, Characterisation and Comparative Studies on Biological Activity, Polyhedron, 2013, 50, 481–489 CrossRef CAS; (d) I. Murakami-Kubo, K. Doh-ura, K. Ishikawa, S. Kawatake, K. Sasaki, J. Kira, S. Ohta and T. Iwaki, Quinoline Derivatives Are Therapeutic Candidates for Transmissible Spongiform Encephalopathies, J. Virol., 2004, 78, 1281–1288 CrossRef CAS.
- (a) L.-Y. Liao, X.-R. Kong and X.-F. Duan, Reductive Couplings of 2-Halopyridines without External Ligand: Phosphine-Free Nickel-Catalyzed Synthesis of Symmetrical and Unsymmetrical 2,2′-Bipyridines, J. Org. Chem., 2014, 79, 777–782 CrossRef CAS PubMed; (b) C. Janiak, S. Deblon and L. Uehlin, Synthesis of 6,6′-Diamino-2,2′-Biquinoline and 2,2′-Bi-1,6-naphthyridine, Synthesis, 1999, 959–964 CrossRef CAS.
- (a) D. E. Stephens, J. Lakey-Beitia, J. E. Burch, H. D. Armana and O. V. Larionov, Mechanistic Insights into the Potassium tert-Butoxide-Mediated Synthesis of N-Heterobiaryls, Chem. Commun., 2016, 52, 9945–9948 RSC; (b) K. Inamoto, Y. Araki, S. Kikkawa, M. Yonemoto, Y. Tanaka and Y. Kondo, Organocatalytic Functionalization of Heteroaromatic N-oxides with C-nucleophiles Using In Situ Generated Onium Amide Bases, Org. Biomol. Chem., 2013, 11, 4438–4441 RSC; (c) J. Everaert, M. Debruyne, F. V. Bussche, K. V. Hecke, T. S. A. Heugebaert, P. V. D. Voort, V. V. Speybroeck and C. V. Stevens, Synthesis of Nitrile-Functionalized Polydentate N-Heterocycles as Building Blocks for Covalent Triazine Frameworks, Synthesis, 2023, 333–340 CAS.
- (a) Y. Liu, Q. Tan, L. Bao, Y. Nie, L. Zhang, Z. Hu and X. Xu, De Novo Synthesis of 2,2′-Bipyridines and Related Bis-azines via Cascade Coupling and Double Pyridannulation of Isocyanides, Org. Lett., 2024, 26, 5043–5048 CrossRef CAS PubMed; (b) K. Kobayashi, J. Yonemori, A. Matsunaga, T. Kitamura, M. Tanmatsu, O. Morikawa and H. Konishi, Synthesis of 2,2′-Biquinolines from o-Isocyanostyrenes, Heterocycles, 2001, 55, 33–36 CrossRef CAS.
- X. Pang, M. Wu, J. Ni, F. Zhang, J. Lan, B. Chen and R. Yan, Copper-Catalyzed Tandem Aerobic Oxidative Cyclization for the Synthesis of Polysubstituted Quinolines via C(sp3)/C(sp2)−H Bond Functionalization, J. Org. Chem., 2017, 82, 10110–10120 CrossRef CAS PubMed.
- H. Qi, Y. Yan, Y. Liao, F. Jiang, H. Gao and G.-J. Deng, I2-Catalyzed Oxidative Dehydrogenative Tandem Cyclization of 2-Methylquinolines, Arylamines and 1,4-Dioxane, Org. Chem. Front., 2021, 8, 6108–6113 RSC.
- Q.-Q. Hu, Y.-T. Gao, J.-C. Sun, J.-J. Gao, H.-X. Mu, Y.-M. Li, Y.-N. Zheng, K.-R. Yang and Y.-P. Zhu, Iodine-Imine Synergistic Promoted Povarov-Type Multicomponent Reaction for the Synthesis of 2,2′-Biquinolines and Their Application to a Copper/Ligand Catalytic System, Org. Lett., 2021, 23, 9000–9005 CrossRef CAS PubMed.
- (a) F. Xiao, S. Chen, Y. Chen, H. Huang and G.-J. Deng, Efficient 2-Sulfolmethyl Quinoline Formation from 2-Methylquinolines and Sodium Sulfinates under Transition-Metal Free Conditions, Chem. Commun., 2015, 51, 652–654 RSC; (b) F.-F. Wang, C.-P. Luo, G. Deng and L. Yang, C(sp3)−C(sp3) Bond Formation via Copper/Brønsted Acid Co-catalyzed C(sp3)−H Bond Oxidative Cross-Dehydrogenative-Coupling (CDC) of Azaarenes, Green Chem., 2014, 16, 2428–2431 RSC; (c) D. Wang, F. Zhang, F. Xiao and G.-J. Deng, A Three-Component Approach to Isoxazolines and Isoxazoles under Metal-Free Conditions, Org. Biomol. Chem., 2019, 17, 9163–9168 RSC.
- (a) B. K. Malviya, A. K. Jassal, M. Karnatak, V. P. Verma and S. Sharma, Electro-Oxidative sp3 C−H Bond Functionalization and Annulation Cascade: Synthesis of Novel Heterocyclic Substituted Indolizines, J. Org. Chem., 2022, 87, 2898–2911 CrossRef CAS PubMed; (b) X.-J. Zhang, J.-K. Cao, J.-J. Ren, L. Hong, R.-J. Liang, K.-Y. Hao, K.-L. Wei, B.-J. Mi, Y. Liu and Y.-P. Zhu, Generation of Azaarene Nitrile Oxides from Methylazaarenes and t-BuONO Enabling the Synthesis of Furoxans and 1,2,4-Oxadiazoles, Org. Chem. Front., 2022, 9, 1121–1126 RSC; (c) Z.-H. Shang, Z.-X. Zhang, W.-Z. Weng, Y.-F. Wang, T.-W. Cheng, Q.-Y. Zhang, L.-Q. Song, T.-Q. Shao, K.-X. Liu and Y.-P. Zhu, A Metal- and Azide-Free Oxidative Coupling Reaction for the Synthesis of [1,2,3]Triazolo[1,5-a]quinolines and Their Application to Construct C−C and C−P Bonds, 2-Cyclopropylquinolines and Imidazo[1,5-a]quinolines, Adv. Synth. Catal., 2021, 363, 490–496 CrossRef; (d) X.-K. Zhang, X.-Y. Miao, H.-R. Jiang, F. Ge, J.-C. Sun, R.-Y. Zhang, Q. Ouyang, W.-Y. Fan, Y.-P. Zhu and Y.-Y. Sun, Iodine-Promoted Synthesis of Dipyrazolo/Diuracil-Fused Pyridines and o-Amino Diheteroaryl Ketones via Oxidative Domino Annulation of 2/4-Methylazaarenes, Adv. Synth. Catal., 2021, 363, 4632–4638 CrossRef CAS; (e) W.-Z. Weng, Y.-H. Gao, X. Zhang, Y.-H. Liu, Y.-J. Shen, Y.-P. Zhu, Y.-Y. Sun, Q.-G. Meng and A.-X. Wu, Oxidative C(sp3)−H Functionalization of Methyl-Azaheteroarenes: A Facile Route to 1,2,4-Triazolo[4,3-a]pyridines, Org. Biomol. Chem., 2019, 17, 2087–2091 RSC; (f) R.-J. Xie, J.-H. Liu, Q.-Y. Zhang, Y.-J. Yang, L.-Q. Song, T.-Q. Shao, K.-X. Liu and Y.-P. Zhu, Copper-Catalyzed Aerobic Oxidative Domino Cyclization of Methyl Azaarenes with 6-Amino-pyrimidine-2,4-diones and Pyrazol-5-amines: Access to Dipyrimidine/Dipyrazolo-fused Pyridines, Org. Chem. Front., 2021, 8, 2274–2279 RSC; (g) G. S. Mani, S. P. Shaik, Y. Tangella, S. Bale, C. Godugu and A. Kamal, A Facile I2-Catalyzed Synthesis of Imidazo[1,2-a]pyridines via sp3 C−H Functionalization of Azaarenes and Evaluation of Anticancer Activity, Org. Biomol. Chem., 2017, 15, 6780–6791 RSC; (h) Y.-J. Hu, Y. Zhou, J.-J. Gao, H. Zhang, K.-R. Yang, J.-J. Li, X.-X. Yan, Y.-L. Li and Y.-P. Zhu, I2-Mediated [3 + 2] Annulation of Methyl-Azaarenes with Alkyl 2-Isocyanoacetates or Amino Acid Ester Hydrochlorides: Selective Synthesis of Iodine-Functionalized and Non-Iodine-Functionalized Fused Imidazoles, Org. Chem. Front., 2022, 9, 1403–1409 RSC; (i) B. Sridevi, S. R. Kandimalla and B. V. S. Reddy, Oxidative sp3 C−H Functionalization of Methyl Substituted Aza-Aromatics: An Easy Access to N-Fused Polyheterocycles, Eur. J. Org. Chem., 2019, 6800–6806 CrossRef CAS; (j) X. Geng, X. Wu, P. Zhao, J. Zhang, Y.-D. Wu and A.-X. Wu, Synergistic I2/Amine Promoted Povarov-Type Reaction for the Synthesis of 2-Acyl-3-aryl(alkyl)quinolines Using Aryl(alkyl)acetaldehydes as Alkene Surrogates, Org. Lett., 2017, 19, 4179–4182 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2384037. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01801j |
| This journal is © the Partner Organisations 2025 |

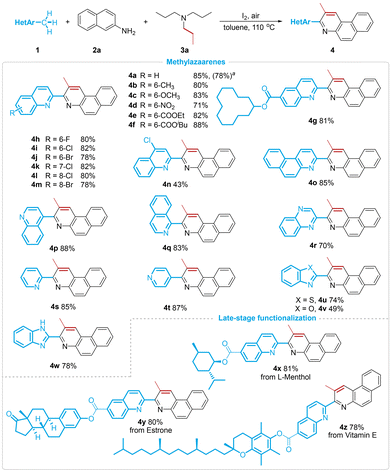
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mmol scale based on 2a.
1 mmol scale based on 2a.