Stereoselective access to furan-fused [5.5.0] bicyclic heterocycles enabled by gold-catalyzed asymmetric [8 + 4] cycloaddition†
Xunhua
Wang
a,
Jianhua
Wang
*b and
Xiaoxun
Li
 *ac
*ac
aDepartment of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), NMPA Key Laboratory for Technology Research and Evaluation of Drug Products, School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, Shandong 250012, China. E-mail: xli@sdu.edu.cn
bTranslational Pharmaceutical Laboratory, Jining First People's Hospital, Shandong First Medical University, Jining, 272000, China. E-mail: jianhua.wang_8710@163.com
cSuzhou Research Institute of Shandong University, No. 388 Ruoshui Road, SIP, Suzhou, Jiangsu 215123, China
First published on 29th October 2024
Abstract
The construction of fused [5.5.0] bicyclic heterocycles with precise regio-, stereo-, and enantioselective control remains a significant challenge in asymmetric catalysis. In this work, we introduce a novel gold-catalyzed asymmetric [8 + 4] cycloaddition reaction of 1-(1-alkynyl)cyclopropyl ketones with simple tropones, yielding highly functionalized cyclohepta[b]furo[3,4-d]oxepine derivatives with excellent diastereo- and enantioselectivity (38 examples, all >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, up to 95% ee). Additionally, an efficient kinetic resolution (KR) process is achieved (s factor up to 104). The gram-scale synthesis and subsequent synthetic transformations of the cycloadducts further demonstrate the synthetic potential of this method. Furthermore, the cycloadduct can undergo a [1,5]-H shift under acid catalysis, adding another dimension to the structural diversity.
1 dr, up to 95% ee). Additionally, an efficient kinetic resolution (KR) process is achieved (s factor up to 104). The gram-scale synthesis and subsequent synthetic transformations of the cycloadducts further demonstrate the synthetic potential of this method. Furthermore, the cycloadduct can undergo a [1,5]-H shift under acid catalysis, adding another dimension to the structural diversity.
Introduction
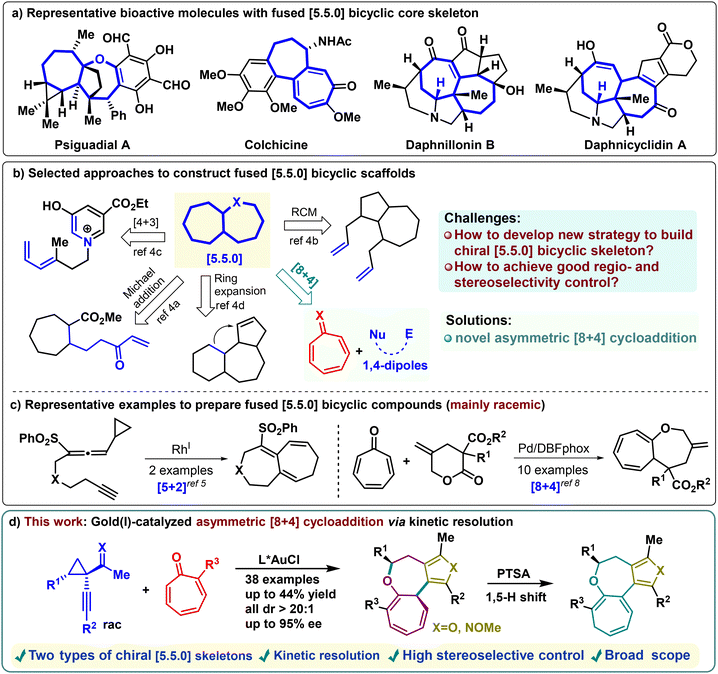
Medium-sized rings are privileged structural motifs in various natural products and exhibit wide applications in chemical synthesis and the pharmaceutical industry.1 Fused [5.5.0] bicyclic heterocycles, in particular, are prevalent in bioactive molecules, such as psiguadial A (anti-HIV), colchicine (antitubulin), daphnillonin B (anti-cancer), and daphnicyclidin A (Scheme 1a).2 Despite the structural diversity, intriguing architectures, and potential utility of these core scaffolds, general synthetic strategies from simple precursors with high efficiency and excellent stereocontrol remain elusive.3 To date, two principal approaches have been adopted for constructing this scaffold: intramolecular and intermolecular reactions (Scheme 1b). For instance, intramolecular reactions include methods such as intramolecular Michael addition,4a ring-closing metathesis,4b [4 + 3] cycloaddition,4c ring expansion, etc.4d These reactions often require meticulous substrate design, typically involving extensive synthetic procedures, and usually result in racemic mixtures. In 2009, the Mukai group disclosed rhodium(I)-catalyzed intramolecular [5 + 2] cycloaddition of alkyne-allenylcyclopropanes, reporting two elegant examples of bicyclo[5.5.0]dodecatrienes in racemic form (Scheme 1c, left).5 To resolve the above problems, intermolecular cycloaddition reactions offer a significant advantage for the efficient construction of such frameworks from simple building blocks. Most of these elegant examples have focused on the use of commercially available tropones through asymmetric [8 + n] cycloaddition approaches.6,7 Nevertheless, progress in intermolecular asymmetric high-order cycloadditions in this field remains significantly limited. In 2020, the pioneering work by Zhao, Shao, and colleagues made a notable advance by demonstrating a palladium-catalyzed [8 + 4] cycloaddition of γ-methylidene-δ-valerolactones with tropones, enabling the assembly of fused [5.5.0] bicyclic structures (Scheme 1c, right).8 While this work represents a significant breakthrough, only one chiral example was achieved (49% yield, 82% ee), highlighting the challenges in asymmetric synthesis of these frameworks. Thus, there is an urgent need to develop novel asymmetric intermolecular strategies that can achieve facile construction of enantioenriched fused [5.5.0] bicyclic skeletons, thereby enhancing their potential applications in drug discovery and development.Given the intricate challenge of preparing chiral fused [5.5.0] bicyclic heterocycles and the significance of fury structures9 in medicinal chemistry, we explored whether gold-catalyzed stereoselective [8 + 4] cycloadditions of tropones with Au-fury 1,4-dipoles could provide a convenient route to synthesize these valuable skeletons.10,11 However, this strategy faces several challenges. First, it is uncertain whether tropone and 1,4-dipolar species are sufficiently reactive to match, and there is a potential for competitive [6 + 4] cycloaddition reactions to occur. Additionally, for the unique [5.5.0] bicyclic heterocyclic core, it remains unclear whether diastereoselectivity and enantioselectivity can be effectively controlled during the cycloaddition process to overcome the inherent unfavorable entropy and enthalpy factors. Building on this concept, we report the first gold-catalyzed, highly diastereo- and enantioselective [8 + 4] cycloaddition of 1-(1-alkynyl)cyclopropyl ketones with tropones. This approach provides a straightforward method to synthesize polycyclic functionalized cyclohepta[b]furo[3,4-d]oxepine derivatives bearing furan/pyrrole-fused moieties (38 examples, all >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, up to 95% ee), enabled by a chiral gold catalyst (Scheme 1d). This method also facilitates the efficient kinetic resolution12 of 1-(1-alkynyl)-cyclopropyl ketones, leading to the synthesis of two optically active furan/pyrrole-fused ring systems with high enantioselectivity. Moreover, the cycloadduct undergoes a [1,5]-H shift under acid catalysis, further enhancing structural diversity.
1 dr, up to 95% ee), enabled by a chiral gold catalyst (Scheme 1d). This method also facilitates the efficient kinetic resolution12 of 1-(1-alkynyl)-cyclopropyl ketones, leading to the synthesis of two optically active furan/pyrrole-fused ring systems with high enantioselectivity. Moreover, the cycloadduct undergoes a [1,5]-H shift under acid catalysis, further enhancing structural diversity.
Results and discussion
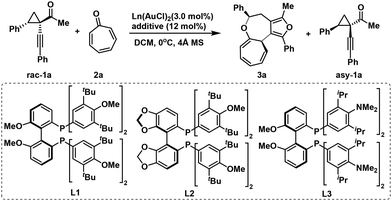
Initially, racemic cyclopropyl ketone 1a and tropone 2a were selected as model compounds to investigate the asymmetric [8 + 4] cycloaddition, where both cycloaddition products and chiral cyclopropyl ketones were obtained concurrently via the kinetic resolution process (Table 1). Preliminary screening of various reaction parameters revealed that chiral bidentate phosphine ligands were crucial in enhancing the efficiency of this cycloaddition (see the ESI† for details). Focusing our optimization efforts on chiral gold complexes, we were pleased to observe that the asymmetric [8 + 4] cycloaddition proceeded smoothly with L1(AuCl)2, yielding the desired cycloadduct 3a in 35% yield with 73% ee (entry 1). When (S)-DTBM-SEGPHOS was employed, the enantioselectivity increased to 85% ee, although the yield decreased significantly to just 15% (entry 2). Fortunately, switching to the L3 ligand improved the yield of product 3a to 31% while maintaining high enantioselectivity (entry 3).13 In addition, the choice of additive also had a substantial impact on the reaction's reactivity. Replacing the silver salt with NaBArF resulted in a marked improvement, yielding the desired product 3a in 40% yield with 81% ee (entries 4 and 5). Further temperature optimization (entries 6 and 7) identified −20 °C as the optimal reaction temperature, allowing cycloadduct 3a to be obtained in 40% yield with 89% ee (s factor is 51).| Entry | Ligand | Additive | Yieldb | eec | s |
|---|---|---|---|---|---|
a Unless otherwise indicated, all reactions were performed with 1a (0.1 mmol) and 2a (0.08 mmol) in the presence of Ln(AuCl)2 (3.0 mol%) and an additive (12.0 mol%) in a solvent (1.0 mL) at 0 °C or −10 °C or −20 °C under a nitrogen atmosphere.
b Isolated yields.
c Determined by HPLC analysis.
d Selectivity (s) values were calculated using the equation s = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [(1 − C) (1 − ee1a)]/ln [(1 − C) (1 − ee1a)]/ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [(1 − C) (1 + ee1a)], where C is the conversion; C = ee1a/(ee1a + ee3a).
e The reaction temperature was −10 °C.
f The reaction temperature was −20 °C. NaBArF: sodium tetrakis[3,5-bis(trifluoromethyl)-phenyl]borate. [(1 − C) (1 + ee1a)], where C is the conversion; C = ee1a/(ee1a + ee3a).
e The reaction temperature was −10 °C.
f The reaction temperature was −20 °C. NaBArF: sodium tetrakis[3,5-bis(trifluoromethyl)-phenyl]borate.
|
|||||
| 1 | L1 | AgPF6 | 35% | 73% | 17 |
| 2 | L2 | AgPF6 | 15% | 85% | 11 |
| 3 | L3 | AgPF6 | 31% | 85% | 20 |
| 4 | L3 | AgOTf | 36% | 81% | 18 |
| 5 | L3 | NaBArF | 40% | 81% | 37 |
| 6e | L3 | NaBArF | 41% | 83% | 38 |
| 7f | L3 | NaBArF | 40% | 89% | 51 |
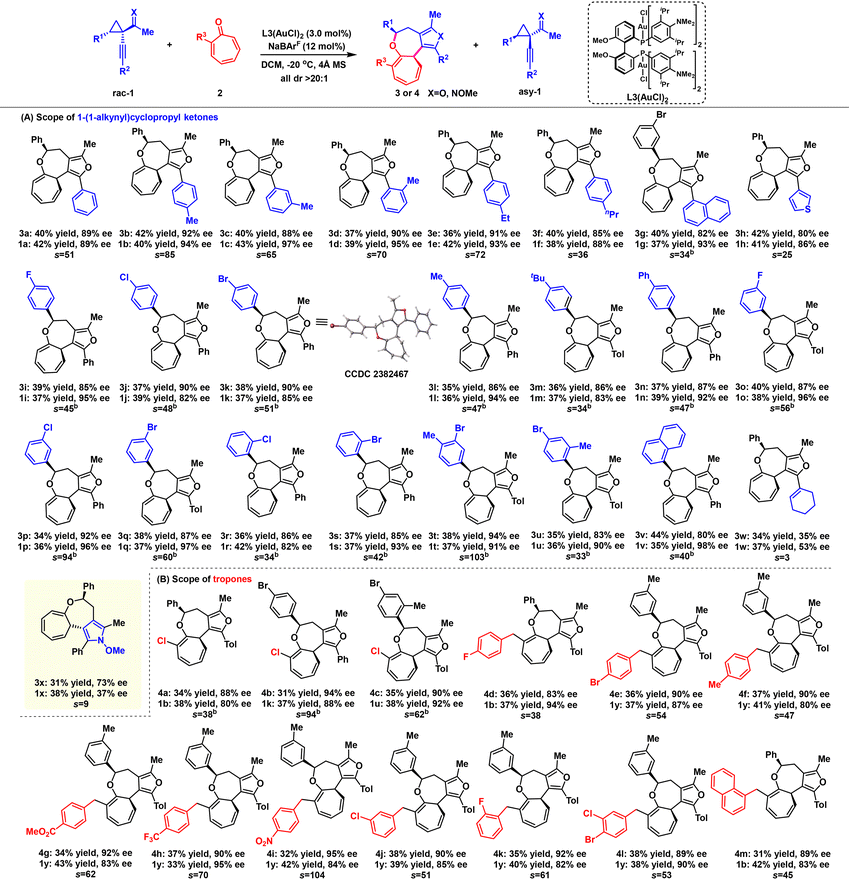
With the optimal reaction conditions in hand, we examined the substrate scope of the gold-catalyzed asymmetric [8 + 4] cycloaddition (Table 2). Encouragingly, all cycloadducts exhibited excellent exo-diastereoselectivity (all dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). First, we investigated the scope of cyclopropyl ketones 1 with commercially available tropone 2a as the model substrate (Table 2A). For the R2 substituent of cyclopropyl ketones 1, a range of racemic substrates (1a–1f) with different substituents at various positions of the benzene ring were well-tolerated, delivering the desired products 3a–3f in 36–42% yields with 85–92% ee, along with the corresponding chiral cyclopropyl ketones 1a–1f in 38–43% yields with 88–97% ee. Cyclopropyl ketones bearing 3-thiophenyl (1g) and 1-naphthalyl (1h) groups also performed well, affording products 3g and 3h in 42% yield with 80% ee and 42% yield with 82% ee, respectively. In addition, the asymmetric [8 + 4] cycloadditions proceeded smoothly regardless of the electronic and steric properties of the R1 substituents on the phenyl ring, whether at the ortho-, meta- or para-position. These reactions provided a series of furan-fused chiral [5.5.0] bicyclic heterocycles 3i–3u with good yields and stereoselectivities (s factor up to 103, up to 40% yield, up to 94% ee, all >20
1). First, we investigated the scope of cyclopropyl ketones 1 with commercially available tropone 2a as the model substrate (Table 2A). For the R2 substituent of cyclopropyl ketones 1, a range of racemic substrates (1a–1f) with different substituents at various positions of the benzene ring were well-tolerated, delivering the desired products 3a–3f in 36–42% yields with 85–92% ee, along with the corresponding chiral cyclopropyl ketones 1a–1f in 38–43% yields with 88–97% ee. Cyclopropyl ketones bearing 3-thiophenyl (1g) and 1-naphthalyl (1h) groups also performed well, affording products 3g and 3h in 42% yield with 80% ee and 42% yield with 82% ee, respectively. In addition, the asymmetric [8 + 4] cycloadditions proceeded smoothly regardless of the electronic and steric properties of the R1 substituents on the phenyl ring, whether at the ortho-, meta- or para-position. These reactions provided a series of furan-fused chiral [5.5.0] bicyclic heterocycles 3i–3u with good yields and stereoselectivities (s factor up to 103, up to 40% yield, up to 94% ee, all >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Substrates bearing 1-naphthyl groups (1v) led to the cycloadduct 3v in 44% yield with 80% ee and the corresponding chiral cyclopropyl ketone 1v in 35% yield with 98% ee. Notably, substrate 1w, containing a cyclohexenyl group, also reacted smoothly, yielding cycloadduct 3w in 34% yield with >20
1 dr). Substrates bearing 1-naphthyl groups (1v) led to the cycloadduct 3v in 44% yield with 80% ee and the corresponding chiral cyclopropyl ketone 1v in 35% yield with 98% ee. Notably, substrate 1w, containing a cyclohexenyl group, also reacted smoothly, yielding cycloadduct 3w in 34% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 35% ee. Pyrroles, being ubiquitous in natural products and displaying diverse biological activities, have garnered considerable interest for the construction of complex molecules incorporating these heterocyclic scaffolds, which are essential for advancing biological research.14 We were particularly pleased to observe that the oxime-containing substrate 1x performed well under the optimized conditions, generating the chiral [5.5.0] bicyclic compound with a pyrrole moiety (3x) in 31% yield and 73% ee. Next, the scope of various tropones 2 was explored (Table 2B). When 2-chloro-substituted tropone 2b was employed, the reaction proceeded smoothly, affording the desired cycloadducts 4a–4c in good yields and enantioselectivities with high stereoselectivity (31%–35% yields, dr > 20
1 dr and 35% ee. Pyrroles, being ubiquitous in natural products and displaying diverse biological activities, have garnered considerable interest for the construction of complex molecules incorporating these heterocyclic scaffolds, which are essential for advancing biological research.14 We were particularly pleased to observe that the oxime-containing substrate 1x performed well under the optimized conditions, generating the chiral [5.5.0] bicyclic compound with a pyrrole moiety (3x) in 31% yield and 73% ee. Next, the scope of various tropones 2 was explored (Table 2B). When 2-chloro-substituted tropone 2b was employed, the reaction proceeded smoothly, affording the desired cycloadducts 4a–4c in good yields and enantioselectivities with high stereoselectivity (31%–35% yields, dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 88–94% ee), irrespective of the substituents’ electronic nature. For 2-benzyl-substituted tropones 2c–2k, the cycloaddition displayed good versatility, performing well with both electron-poor and electron-rich groups at different positions, yielding chiral bicyclic heterocycles (4d–4l) with good results (s factor up to 104, up to 38% yield, up to 95% ee). Notably, a variety of functional groups, such as F, Cl, Br, Me, ester, CF3, and nitro moieties, showed good compatibility. Additionally, the substrate with 1-naphthyl (1h) substitution reacted well, providing the desired product 4m in 31% yield with 89% ee. The absolute configuration of 3k was determined as 5R, 11S by X-ray crystallographic analysis.15
1, 88–94% ee), irrespective of the substituents’ electronic nature. For 2-benzyl-substituted tropones 2c–2k, the cycloaddition displayed good versatility, performing well with both electron-poor and electron-rich groups at different positions, yielding chiral bicyclic heterocycles (4d–4l) with good results (s factor up to 104, up to 38% yield, up to 95% ee). Notably, a variety of functional groups, such as F, Cl, Br, Me, ester, CF3, and nitro moieties, showed good compatibility. Additionally, the substrate with 1-naphthyl (1h) substitution reacted well, providing the desired product 4m in 31% yield with 89% ee. The absolute configuration of 3k was determined as 5R, 11S by X-ray crystallographic analysis.15
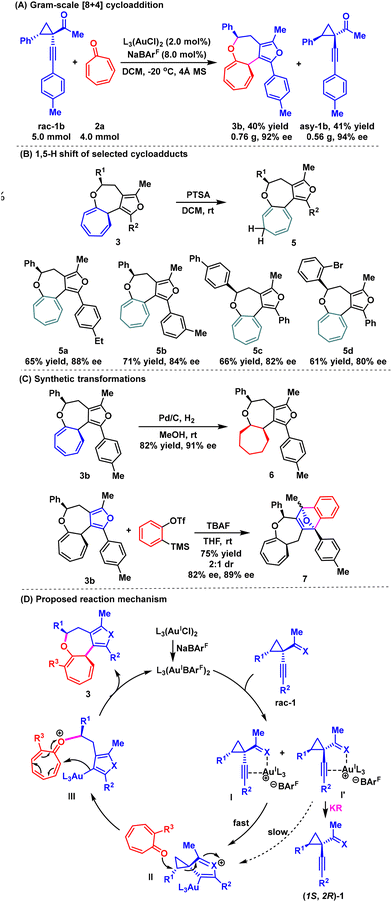
To further demonstrate the synthetic utility of this methodology, a gram-scale synthesis of chiral cyclohepta[b]furo[3,4-d]oxepine 3b was successfully achieved (Scheme 2A), maintaining both high efficiency and excellent stereochemical control (40% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 92% ee). Several synthetic transformations were then explored. Interestingly, the product underwent a 1,5-H shift process with p-toluenesulfonic acid (PTSA) at room temperature, yielding a more stable isomer (Scheme 2B).16 Using different substituents, we obtained products 5a–5d in 61%–71% yields with 80%–88% ee. Additionally, the treatment of 4b with Pd/C under a hydrogen atmosphere (H2 balloon) afforded the corresponding hydrogenated product 6 in 82% yield with 91% ee (Scheme 2C). Moreover, product 4b participated in a stereoselective [4 + 2] cycloaddition with in situ generated benzyne, resulting in a 75% yield of the O-bridged polycyclic compound 7.
1 dr, 92% ee). Several synthetic transformations were then explored. Interestingly, the product underwent a 1,5-H shift process with p-toluenesulfonic acid (PTSA) at room temperature, yielding a more stable isomer (Scheme 2B).16 Using different substituents, we obtained products 5a–5d in 61%–71% yields with 80%–88% ee. Additionally, the treatment of 4b with Pd/C under a hydrogen atmosphere (H2 balloon) afforded the corresponding hydrogenated product 6 in 82% yield with 91% ee (Scheme 2C). Moreover, product 4b participated in a stereoselective [4 + 2] cycloaddition with in situ generated benzyne, resulting in a 75% yield of the O-bridged polycyclic compound 7.
Based on previous studies,10 a plausible reaction pathway for the gold(I)-catalyzed asymmetric [8 + 4] cycloadditions is shown in Scheme 2D. Initially, the chiral gold complex facilitates the interaction between the alkyne and the carbonyl group of racemic 1-(1-alkynyl)cyclopropyl ketone 1, leading to the formation of intermediates I and I′. These intermediates undergo intramolecular ring closure at different rates due to the varying reactivities of the diastereomeric complexes. Specifically, the more reactive diastereomer I reacts faster, primarily forming intermediate II while preserving the enantiomeric purity of (1S,2R)-1 with good enantioselectivity. Guided by the chiral environment of the gold catalyst, tropone 2 undergoes SN2 addition with intermediate II to generate intermediate III. Finally, this is followed by intramolecular cyclization, yielding the desired cycloadduct 3, while regenerating the chiral gold catalyst.
Conclusions
In conclusion, we have developed a chiral gold-catalyzed stereoselective [8 + 4] cycloaddition of cyclopropyl ketones with simple tropones under mild reaction conditions. This method provides straightforward access to highly functionalized polycyclic cyclohepta[b]furo[3,4-d]oxepine derivatives featuring furan- and pyrrole-fused moieties from readily available starting materials, with good yields and excellent stereoselectivities. These derivatives can be readily transformed into another class of chiral fused [5.5.0] skeletons via a [1,5]-H shift process. This novel strategy not only offers a powerful tool for the asymmetric synthesis of synthetically challenging fused [5.5.0] bicyclic scaffolds, but also expands the repertoire of asymmetric intermolecular higher-order cycloaddition methodologies, with potential applications in drug discovery.Author contributions
X. Li designed the project. X. Wang performed the experiments. X. Wang and J. Wang analyzed the data. X. Li, J. Wang and X. Wang wrote the paper.Data availability
The data supporting this article have been included as part of the ESI.† Data for this work, including optimization tables, general experimental procedures, characterization data (NMR and HPLC spectra) for all new compounds and X-ray data, are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support for this work from the National Natural Science Foundation of China (21901142), the Natural Science Foundation of Jiangsu Province (BK20220097), the Natural Science Foundation of Shandong Province (ZR2024MB004), and the Fundamental Research Funds of Shandong University (2020QNQT007 and 2020QNQT009).References
- (a) S. A. M. W. Van Den Broek, S. A. Meeuwissen, F. L. Van Delft and F. P. J. T. Rutjes, Natural Products Containing Medium-Sized Nitrogen Heterocycles Synthesized by Ring-Closing Alkene Metathesis, in Metathesis in Natural Product Synthesis, ed. J. Cossy, S. Arseniyadis and C. Meyer, Wiley-VCH, Weinheim, Germany, 2010, vol. 45 Search PubMed; (b) A. Gradillas and J. Pérez-Castells, Macrocyclization by Ring-Closing Metathesis in the Total Synthesis of Natural Products: Reaction Conditions and Limitations, Angew. Chem., Int. Ed., 2006, 45, 6086 CrossRef CAS; (c) I. Shiina, Total Synthesis of Natural 8- and 9-Membered Lactones: Recent Advancements in Medium-Sized Ring Formation, Chem. Rev., 2007, 107, 239 CrossRef CAS PubMed; (d) A. Hussain, S. K. Yousuf and D. Mukherjee, Importance and Synthesis of Benzannulated Medium-Sized and Macrocyclic Rings (BMRs), RSC Adv., 2014, 4, 43241 RSC; (e) J. R. Donald and W. P. Unsworth, Ring-Expansion Reactions in the Synthesis of Macrocycles and Medium-Sized Rings, Chem. – Eur. J., 2017, 23, 8780 CrossRef CAS; (f) K. T. Mortensen, T. J. Osberger, T. A. King, H. F. Sore and D. R. Spring, Strategies for the Diversity-Oriented Synthesis of Macrocycles, Chem. Rev., 2019, 119, 10288 CrossRef CAS; (g) Y.-J. Hu, L.-X. Li, J.-C. Han, L. Min and C.-C. Li, Recent Advances in the Total Synthesis of Natural Products Containing Eight-Membered Carbocycles (2009–2019), Chem. Rev., 2020, 120, 5910 CrossRef CAS; (h) L. Min, Y.-J. Hu, J.-H. Fan, W. Zhang and C.-C. Li, Synthetic Applications of Type II Intramolecular Cycloadditions, Chem. Soc. Rev., 2020, 49, 7015 RSC; (i) R. L. Reyes, T. Iwai and M. Sawamura, Construction of Medium-Sized Rings by Gold Catalysis, Chem. Rev., 2021, 121, 8926 CrossRef CAS; (j) W. Tan, J.-Y. Zhang, C.-H. Gao and F. Shi, Progress in Organocatalytic Asymmetric (4+3) Cycloadditions for the Enantioselective Construction of Seven-membered Rings, Sci. China: Chem., 2023, 66, 966 CrossRef CAS.
- (a) D. N. Tran and N. Cramer, Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (-)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene, Chem. – Eur. J., 2014, 20, 10654 CrossRef CAS PubMed; (b) B. G. R. Ravelli, B. Gigant, P. A. Curmi, I. Jourdain, S. Lachkar, A. Sobel and M. Knossow, Insight into Tubulin Regulation from a Complex with Colchicine and a Stathmin-like Domain, Nature, 2004, 428, 198 CrossRef; (c) J. I. Kobayashi, Y. Inaba, M. Shiro, N. Yoshida and H. Morita, Daphnicyclidins A-H, Novel Hexa- or Pentacyclic Alkaloids from Two Species of Daphniphyllum, J. Am. Chem. Soc., 2001, 123, 11402 CrossRef CAS PubMed; (d) J.-H. Zhang, P. Cao, Y.-L. Ma, X. Fang, J. Yang, Y. Zhang, D.-Z. Chen, Y.-C. Gu, Y.-T. Di and X.-J. Hao, Structural Revision of Macropodumine A and Structure of 2-Deoxymacropodu-mine A, Daphniphyllum Alkaloids with 11-Membered Macrolactone Rings, J. Nat. Prod., 2019, 82, 427 CrossRef CAS PubMed; (e) D.-D. Zhang, J.-B. Xu, Y.-Y. Fan, L.-S. Gan, H. Zhang and J.-M. Yue, Daphnillonins A and B: Alkaloids Representing Two Unknown Carbon Skeletons from Daphniphyllum Longeracemosum, J. Org. Chem., 2020, 85, 3742 CrossRef CAS PubMed.
- (a) L. A. Paquette, A. R. Browne, E. Chamot and J. F. Blount, Twofold Cyclopropylidene C-H Insertion as a Route to Hexacyclic Bis(bicyclo[1.1.0]butanes) and Their Silver(I) Ion Promoted Rearrangement. A Direct Synthesis of Heptalene from Naphthalene, J. Am. Chem. Soc., 1980, 102, 644 Search PubMed; (b) P. Dowd and W. Zhang, Free-Radical Ring Expansion of Fused Cyclobutanones: Stereospecific Construction of 5,7-, 6,7-, 7,7-, 8,7-, and 5,8-Cis-Fused Bicyclic Systems, J. Org. Chem., 1992, 57, 7163 CrossRef CAS; (c) W. Zhang and P. Paul Dowd, Double Ring Expansions: A New Method for Making Medium and Large Cyclic Ketones, Tetrahedron Lett., 1996, 36, 957 CrossRef; (d) E. Aeissen, A. R. Seggern, M. Schmidtmann and J. Christoffers, Enantioselective Synthesis of [b]-Annulated Azepane Scaffolds, Eur. J. Org. Chem., 2023, e202300180 CrossRef CAS.
- (a) J. Christoffers and H. Oertling, Intramolecular Michael Reactions by Iron(III) Catalysis, Tetrahedron, 2000, 56, 1339 CrossRef CAS; (b) T. B. Dunn, J. M. Ellis, C. C. Kofink, J. R. Manning and L. E. Overman, Asymmetric Construction of Rings A-D of Daphnicyclidin-Type Alkaloids, Org. Lett., 2009, 11, 5658 CrossRef CAS PubMed; (c) J. Tu, M. M. Clark and M. Harmata, First Synthesis of an ABCE Ring Substructure of Daphnicyclidin A, Org. Biomol. Chem., 2022, 20, 6547 RSC; (d) Y.-P. Zou, Z.-L. Lai, M.-W. Zhang, J.-Z. Peng, S. Ning and C.-C. Li, Total Synthesis of (±)- and (-)-Daphnillonin B, J. Am. Chem. Soc., 2023, 145, 10998 CrossRef CAS PubMed.
- F. Inagaki, K. Sugikubo, Y. Miyashita and C. Mukai, Rhodium(I)-catalyzed Intramolecular [5+2] Cycloaddition Reactions of Alkynes and Allenylcyclopropanes: Construction of Bicyclo[5.4.0] undecatrienes and Bicyclo[5.5.0]-dodecatrienes, Angew. Chem., Int. Ed., 2010, 49, 2206 CrossRef CAS.
- For selected reviews of tropones, see: (a) D. McLeod, Expanding the Frontiers of Higher-Order Cycloadditions, Acc. Chem. Res., 2019, 52, 3488 CrossRef CAS PubMed; (b) N. I. Jessen, D. McLeod and K. A. Jørgensen, Higher-order Cycloadditions in the Age of Catalysis, Chem, 2022, 8, 20 CrossRef CAS; (c) M.-M. Zhang, B.-L. Qu, B. Shi, W.-J. Xiao and L.-Q. Lu, High-Order Dipolar Annulations with Metal Containing Reactive Dipoles, Chem. Soc. Rev., 2022, 51, 4146 RSC.
- For [8+n] cycloaddition reactions of tropones, see: (a) T. S. Cantrell, Photochemical 8 + 2 Cycloadditions of Tropone, J. Am. Chem. Soc., 1971, 93, 2540 CrossRef CAS; (b) R. Tejero, A. Ponce, J. Adrio and J. C. Carretero, Ni-Catalyzed [8+3] Cycloaddition of Tropones with 1,1-Cyclopropanediesters, Chem. Commun., 2013, 49, 10406 RSC; (c) R. Mose, G. Preegel, J. Larsen, S. Jakobsen, E. H. Iversen and K. A. Jørgensen, Organocatalytic Stereoselective [8+2] and [6+4] Cycloadditions, Nat. Chem., 2017, 9, 487 CrossRef CAS PubMed; (d) J. Zhang, W. Xiao, H. Hu, L. Lin, X. Liu and X. Feng, Catalytic Asymmetric [8+3] Annulation Reactions of Tropones or Azaheptafulvenes with Meso-Aziridines, Chem. – Eur. J., 2018, 24, 13428 CrossRef CAS PubMed; (e) H. Liu, H. Jia, W. Shi, C. Wang, C. Zhang and H. Guo, Nickel(II)-Catalyzed [8+3]-Cycloaddition of 2-Aryl-N-tosylaziridines with Tropone, Org. Lett., 2018, 20, 3570 CrossRef CAS; (f) S. Wang, C. Rodríguez-Escrich, M. Fianchini, F. Maseras and M. A. Pericàs, Diastereodivergent Enantioselective [8+2] Annulation of Tropones and Enals Catalyzed by N-Heterocyclic Carbenes, Org. Lett., 2019, 21, 3187 CrossRef CAS; (g) F. Xia, Z.-H. Gao, C.-L. Zhang and S. Ye, Oxidative N-Heterocyclic Carbene-Catalyzed [8+2] Annulation of Tropone and Aldehydes: Synthesis of Cycloheptatriene-Fused Furanones, Adv. Synth. Catal., 2019, 361, 2291 CrossRef CAS; (h) S. Zhang, R. Xie, A. Tang, P. Chen, Z. Zhao, M. Miao and H. Ren, Gold(I)-Catalyzed [8+4] Cycloaddition of 1,4-All-Carbon Dipoles with Tropone, Org. Lett., 2020, 22, 3056 CrossRef CAS PubMed; (i) K. Hong, J. Shu, S. Dong, Z. Zhang, Y. He, M. Liu, J. Huang, W. Hu and X. Xu, Asymmetric Three-Component Reaction of Enynal with Alcohol and Imine as An Expeditious Track to Afford Chiral α-Furyl-β-amino Carboxylate Derivatives, ACS Catal., 2022, 12, 14185 CrossRef CAS; (j) Z. Xu, J. Lu, H. Jiang, R. Wu and S. Zhu, Dirhodium(II)-catalyzed Regio- and Stereoselective Cycloisomerization towards 6,5,3-tricyclic Skeletons Containing Vicinal All-carbon Quaternary Stereocenters, Org. Chem. Front., 2024, 11, 5151 RSC; (k) C. Wang, D. Zhu, R. Wu and S. Zhu, Dirhodium-Catalyzed Enantioselective Synthesis of Difluoromethylated Cyclopropanes via Enyne Cycloisomerization, Adv. Sci., 2024, 11, 2306404 CrossRef CAS PubMed; (l) M. Bao, Y. Zhou, H. Yuan, G. Dong, C. Li, X. Xie, K. Chen, K. Hong, Z.-X. Yu and X. Xu, Catalytic (4+2) Annulation via Regio- and Enantioselective Interception of in situ Generated Alkylgold Intermediate, Angew. Chem., Int. Ed., 2024, 63, e202401557 CrossRef CAS.
- L.-C. Yang, Y.-N. Wang, R. Liu, Y. Luo, X. Q. Ng, B. Yang, Z.-Q. Rong, Y. Lan, Z. Shao and Y. Zhao, Stereoselective Access to [5.5.0] and [4.4.1] Bicyclic Compounds through Pd-Catalysed Divergent Higher-order Cycloadditions, Nat. Chem., 2020, 12, 860 CrossRef CAS.
- (a) R. A. Craig and B. M. Stoltz, Polycyclic Furanobutenolide-Derived Cembranoid and Norcembranoid Natural Products: Biosynthetic Connections and Synthetic Efforts, Chem. Rev., 2017, 117, 7878 CrossRef CAS; (b) M. D. Delost, D. T. Smith, B. J. Anderson and J. T. Njardarson, From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles, J. Med. Chem., 2018, 61, 10996 CrossRef CAS PubMed; (c) D. Xu, X. Zhang, X. Li, Y. Li, Z. Chen, B. Li and L. Li, A Review on Furan: Formation, Analysis, Occurrence, Carcinogenicity, Genotoxicity and Reduction Methods, Crit. Rev. Food Sci., 2021, 61, 395 CrossRef.
- (a) X. Wang, R. Lv and X. Li, Kinetic Resolution of 1-(1-alkynyl)cyclopropyl Ketones via Gold-catalyzed Divergent (4+4) Cycloadditions: Stereoselective Access to Furan Fused Eight-membered Heterocycles, Chem. Sci., 2024, 15, 9361 RSC; (b) K. Hong, M. Liu, L. Qian, M. Bao, G. Chen, X. Jiang, J. Huang and X. Xu, Catalytic [4+2]- and [4+4]-Cycloaddition using Furan-fused Cyclobutanone as a Privileged C4 Synthon, Nat. Commun., 2024, 15, 5407 CrossRef CAS.
- For selected reviews of asymmetric gold catalysis, see: (a) D. J. Gorin, B. D. Sherry and F. D. Toste, Ligand Effects in Homogeneous Au Catalysis, Chem. Rev., 2008, 108, 3351 CrossRef CAS; (b) Z. Li, C. Brouwer and C. He, Gold-Catalyzed Organic Transformations, Chem. Rev., 2008, 108, 3239 CrossRef CAS; (c) S. P. Nolan, The Development and Catalytic Uses of N-Heterocyclic Carbene Gold Complexes, Acc. Chem. Res., 2011, 44, 91 CrossRef CAS PubMed; (d) M. Rudolph and A. S. K. Hashmi, Gold Catalysis in Total Synthesis-an Update, Chem. Soc. Rev., 2012, 41, 2448 RSC; (e) C. C. J. Loh and D. Enders, Merging Organocatalysis and Gold Catalysis-A Critical Evaluation of the Underlying Concepts, Chem. – Eur. J., 2012, 18, 10212 CrossRef CAS; (f) Y.-M. Wang, A. D. Lackner and F. D. Toste, Development of Catalysts and Ligands for Enantioselective Gold Catalysis, Acc. Chem. Res., 2014, 47, 889 CrossRef CAS; (g) S. M. Inamdar, A. Konala and N. T. Patil, When Gold Meets Chiral Brønsted Acid Catalysts: Extending the Boundaries of Enantioselective Gold Catalysis, Chem. Commun., 2014, 50, 15124 RSC; (h) D.-F. Chen, Z.-Y. Han, X.-L. Zhou and L.-Z. Gong, Asymmetric Organocatalysis Combined with Metal Catalysis: Concept, Proof of Concept, and Beyond, Acc. Chem. Res., 2014, 47, 2365 CrossRef CAS PubMed; (i) W. Zi and F. D. Toste, Recent Advances in Enantioselective Gold Catalysis, Chem. Soc. Rev., 2016, 45, 4567 RSC; (j) Y. Li, W. Li and J. Zhang, Gold-Catalyzed Enantioselective Annulations, Chem. – Eur. J., 2017, 23, 467 CrossRef CAS PubMed; (k) A. Collado, D. J. Nelson and S. P. Nolan, Optimizing Catalyst and Reaction Conditions in Gold(I) Catalysis-Ligand Development, Chem. Rev., 2021, 121, 8559 CrossRef CAS PubMed; (l) M. Mato, A. Franchino, C. García-Morales and A. M. Echavarren, Gold-Catalyzed Synthesis of Small Rings, Chem. Rev., 2021, 121, 8613 CrossRef CAS PubMed; (m) L.-W. Ye, X.-Q. Zhu, R. L. Sahani, Y. Xu, P.-C. Qian and R.-S. Liu, Nitrene Transfer and Carbene Transfer in Gold Catalysis, Chem. Rev., 2021, 121, 9039 CrossRef CAS; (n) A. Das and N. T. Patil, Enantioselective C-H Functionalization Reactions Under Gold Catalysis, Chem. – Eur. J., 2022, 28, e202104371 CrossRef CAS; (o) G. Zuccarello, I. Escofet, U. Caniparoli and A. M. Echavarren, New-Generation Ligand Design for the Gold-Catalyzed Asymmetric Activation of Alkynes, ChemPlusChem, 2021, 86, 1283 CrossRef CAS PubMed; (p) Z. Zheng, X. Ma, X. Cheng, K. Zhao, K. Gutman, T. Li and L. Zhang, Homogeneous Gold-Catalyzed Oxidation Reactions, Chem. Rev., 2021, 121, 8979 CrossRef CAS PubMed; (q) J.-J. Jiang and M.-K. Wong, Recent Advances in the Development of Chiral Gold Complexes for Catalytic Asymmetric Catalysis, Chem. – Asian J., 2021, 16, 364 CrossRef CAS PubMed; (r) W. Wang, C.-L. Ji, K. Liu, C.-G. Zhao, W. Li and J. Xie, Dinuclear Gold Catalysis, Chem. Soc. Rev., 2021, 50, 1874 RSC; (s) S. Mishra, Urvashi and N. T. Patil, Chiral Ligands for Au(I), Au(III), and Au(I)/Au(III) Redox Catalysis, Isr. J. Chem., 2023, 63, e202200039 CrossRef CAS; (t) A. B. Gade, Urvashi and N. T. Patil, Asymmetric Gold Catalysis Enabled by Specially Designed Ligands, Org. Chem. Front., 2024, 11, 1858 RSC; (u) C. Pegu, B. Paroi and N. T. Patil, Enantioselective Merged Gold/Organocatalysis, Chem. Commun., 2024, 60, 3607 RSC.
- (a) E. Vedejs and M. Jure, Efficiency in Nonenzymatic Kinetic Resolution, Angew. Chem., Int. Ed., 2005, 44, 3974 CrossRef CAS; (b) H. Pellissier, Catalytic Non-enzymatic Kinetic Resolution, Adv. Synth. Catal., 2011, 353, 1613 CrossRef CAS; (c) H.-H. Liu, C. Shen, X. Chang and C.-J. Wang, Recent Advances in Catalytic Asymmetric 1,3-Dipolar Cycloaddition Reactions with Kinetic Resolution, Chin. J. Org. Chem., 2022, 42, 3322 CrossRef CAS; (d) T. Peng, S. Li, D. Yang and L. Wang, Recent Advances in Intramolecular Kinetic Resolution Reactions, Org. Chem. Front., 2023, 10, 3401 RSC.
- See the ESI† for details.
- (a) S. Thirumalairajan, B. M. Pearce and A. Thompson, Chiral Molecules Containing the Pyrrole Framework, Chem. Commun., 2010, 46, 1797 RSC; (b) I. S. Young, P. D. Thornton and A. Thompson, Synthesis of Natural Products Containing the Pyrrolic Ring, Nat. Prod. Rep., 2010, 27, 1801 RSC; (c) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles, Chem. Rev., 2013, 113, 3084 CrossRef CAS; (d) V. Estévez, M. Villacampa and J. C. Menéndez, Recent Advances in the Synthesis of Pyrroles by Multicomponent Reactions, Chem. Soc. Rev., 2014, 43, 4633 RSC; (e) V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman and P. Sharma, Pyrrole: A Resourceful Small Molecule in Key Medicinal Heteroaromatics, RSC Adv., 2015, 5, 15233 RSC; (f) G. Li Petri, V. Spanò, R. Spatola, R. Holl, M. V. Raimondi, P. Barraja and A. Montalbano, Bioactive Pyrrole-based Compounds with Target Selectivity, Eur. J. Med. Chem., 2020, 208, 112783 CrossRef CAS PubMed; (g) Y.-B. Chen, Y.-N. Yan, X.-Z. Hu, L.-W. Ye and B. Zhou, Recent Advances in the Construction of Axially Chiral Arylpyrroles, Sci. China: Chem., 2023, 66, 2480 CrossRef CAS.
- Deposition number CCDC 2382467 (3k) contains the supplementary crystallographic data for this paper.†.
- (a) G. Deslongchamps and P. Deslongchamps, Bent Bonds and the Antiperiplanar Hypothesis. A Model to Account for Sigmatropic [1, n]-Hydrogen Shifts, J. Org. Chem., 2018, 83, 10383 CrossRef CAS; (b) D. V. Vidhani, J. R. Gillett, Y. Cusido and I. V. Alabugin, [1,5]-Sigmatropic Shifts Regulated by Built-in Frustration, J. Phys. Chem. A, 2020, 124, 6016 CrossRef CAS PubMed; (c) J. Li, Y. Mo, L. Yan, X. Feng, Z. Su and X. Liu, Organocatalytic Stereoselective [8+2] Cycloaddition of Tropones with Azlactones, CCS Chem., 2022, 4, 650 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2382467. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01841a |
| This journal is © the Partner Organisations 2025 |