Open Access Article
Open Access ArticleDirect observation of the interface reaction dynamics of the NdCeFeB phase via in situ annealing & quenching STEM†
Xiangyu Zhu*a,
Qingxiao Wangac,
Li Shand,
Byung Oh Jungb,
Myungshin Choib,
Sunyong Songb,
Seok Namkungb,
Namseok Kangb,
Hui-Youn Shinb,
Minho Joob,
Xianming Dai d and
M. J. Kim
d and
M. J. Kim *a
*a
aDepartment of Materials Science and Engineering, University of Texas Dallas, 800 W Campbell Rd, Richardson, TX 75080, USA. E-mail: moonkim@utdallas.edu; X.Y_Zhu@outlook.com
bMaterials & Devices Advanced Research Institute, LG Electronics, LG Science Park, 10, Magokjungang 10-ro, Gangseo-gu, Seoul, 07796, Korea
cImaging and Characterization Core Lab, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia
dDepartment of Mechanical Engineering, University of Texas at Dallas, 800 W Campbell Rd, Richardson, TX 75080, USA
First published on 7th January 2025
Abstract
Although the Rare Earth (RE)2Fe14B type magnets were invented in the 1980s and are widely used worldwide. Yet, the phase formation and dissolution mechanisms are still not crystal clear. The reaction dynamics between rare earth elements (REE) and the iron-enriched matrix are essential to understanding the formation of hard magnetic REE–Fe–B phase or, conversely, phase dissociation and performance degeneration. Developing a reaction mechanism is fundamentally important for process engineering and performance manipulation. This work investigates the interface reaction dynamics between REE and an iron enriched matrix via in situ scanning transmission electron microscopy (STEM). The focused ion beam (FIB) procedure and in situ STEM experiments are specifically designed to achieve both oxygen-involved and oxygen-free reaction mechanisms within one specimen. The high-temperature reaction dynamics are frozen to room temperature (RT) by rapid quenching, preserving the solid–liquid interface dynamics between Fe23B6 and liquid phases. Serial atomic resolution STEM images depict lattice evolution while REE atoms embed into the Fe23B6 lattice. The presented work also demonstrates that combining an advanced FIB procedure with in situ annealing & quenching STEM is a powerful tool for investigating the complex system's high-temperature reaction mechanisms and interface phenomena.
1. Introduction
The ever-increasing market demand for REE–Fe–B type hard magnetic material in auto, energy, and electric device fields raises up-and-coming challenges on material engineering at cost and performance optimization.1–3 In particular, Ce-substituted NdFeB magnets have attracted broad research interest in both industrial and academic prospects owing to decent magnetic properties (coercivity, energy density) and unique cost vs. performance ratio.4–7 However, current studies are mainly focused on phase identification, microstructural features, and macroscopic magnetic properties with less insight at the nanoscale and reaction mechanism, which is also fundamental to explaining phase transformation and morphology evolution mechanisms. Even though the reaction of the Ce–Nd–Fe–B quaternary system is complex (even more complex if considering oxygen8,9), a study on the thermally induced evolution dynamics is a principal that facilitates the manufacturing of high-quality magnets.10,11 In particular, the reaction behavior between REE and the iron enriched matrix is still unclear, which may hinder thermal treatment design and microstructure optimization. Metastable iron boride phases (Fe23B6 and Fe3B) are considered the precursors that generate a RE–Fe–B type hard magnetic phase via dissolving the REE atoms within the lattice. Several hypotheses have been proposed accordingly.12–14 Still, direct experimental evidence is missing so far.Direct observation of the crystal interface reaction during growth is of paramount significance to establish the understanding of boundary phase-change kinetics and further govern the magnetic performance, material reliability, and dissociation behavior of magnet materials. To this end, in situ STEM is the desired characterization technique combing the external stimuli (thermal, electrical, and atmosphere) with atomic resolution imaging capability in real time. Our recent in situ annealing STEM study pushed the insights on elemental diffusion and phase evolution behavior of (Nd0.75Ce0.25)2Fe14B material down to the sub-nanometer scale.15 The result shows the REE tends to react with oxygen starting from 200 °C and diffuse into the grain boundary area. It has been widely demonstrated that the REE-enriched grain boundary phase is essential for performance optimization. It acts as a domain boundary and enhances the coercivity by lowering the short-range exchange coupling between adjacent grains.16–18 At the same time, the reaction between the REE-enriched phase and the iron boride phase is considered the initial reaction that forms the Ce–Nd–Fe–B phase and generates the hard magnetic properties.12–14 Yet, the oxygen-involved phase dissociation at high-temperature behavior seems avoidable or reversible in the real world or under in situ STEM experiment conditions. The REE atoms are mostly oxygen-deprived and transformed to the oxide phase under thermal stimuli. Thus, unfortunately, the dissolving process of the NdCe enriched phase into the iron boride phase remains a black box to researchers, which is critical for the crystallization of the NdCeFeB hard magnet phase.
This research aims to directly observe the dissolving process of REE atoms into an iron boride matrix via in-site annealing and quenching STEM experiment. A two-step FIB sample preparation procedure is specially designed to achieve the observation of oxidized and oxygen-free areas in sequence. First, annealing at 700 °C produces the metastable stable phase Fe23B6 and NdCe enriched liquid phase system, then rapid quenching interrupts the reaction in between and fixes the interface dynamics. In particular, the lattice structure of the solid–liquid interface and short-range order are analyzed at an atomic scale. Our results reveal the crystallization interface morphology of the REE atoms embedded into the Fe23B6 phase and shade lights on the formation mechanism of NdCeFeB magnet phases.
2. Experiment
2.1 Synthesis of NdCeFeB alloy
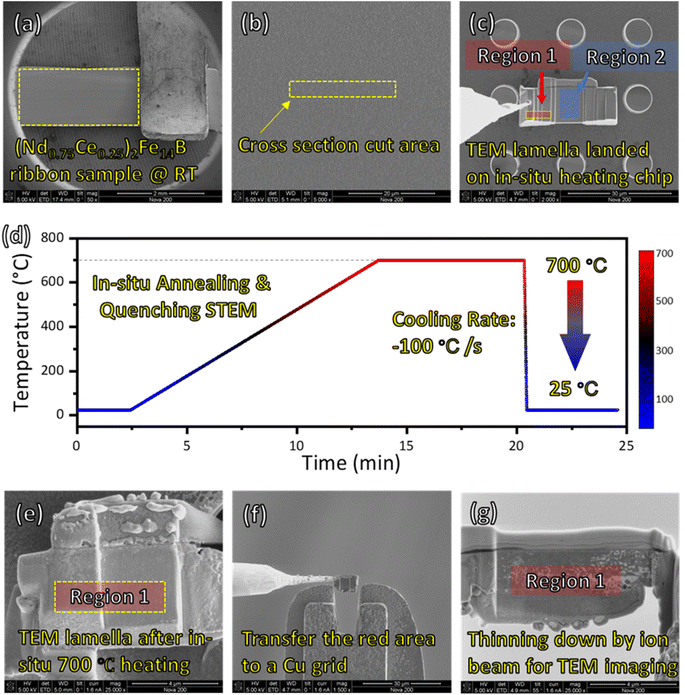
The RE2Fe14B type (tetragonal, P42/mnm) with nominal compositions of (Nd0.75Ce0.25)12.9Fe76.47Co4.5Ga0.53B5.6 (at%) polycrystalline samples were used in this study.15 Alloy ingots were made by induction melting furnace with nominal constituent composition under an argon atmosphere. The as-cast ingots were melt-spun onto a molybdenum wheel from a calcium oxide (CaO) crucible with a surface speed of about 30 m s−1 to obtain nanocrystalline ribbons. The physical dimensions of synthesized ribbons are 1.5 mm in width, 5 mm in length, and 0.1 mm in thickness. These ribbons exhibit uniform and smooth surface morphology, as confirmed by SEM image (Fig. 1a and b), with defects-free surfaces benefiting from the rapid cooling process. The rapid solidification results in a nanocrystalline structure with grain sizes typically tens of nanometers. This fine microstructure promotes the magnetic properties of the ribbons particularly through enhanced coercivity and energy density.2.2 FIB sample preparation
The FIB technique was used to create a cross-sectional lamella from the bulk melt-spun ribbon. First, 2 μm protection deposition (carbon and Pt layer) is reacted on the material surface to avoid ion-beam damage. Then, two rough millings are performed to create a deep hole and isolate the target area. A U-cut milling approach was employed, followed by a lift-out using a nanoprobe to transfer the lamella onto an in situ heating chip. The region 2 of lamella was then thinned to a thickness of approximately 100 nm to ensure electron transparency for STEM analysis. The 3D modeling of the FIB sample for in situ annealing is shown in ESI S1.†To achieve regions with distinct oxygen concentrations, the sample was strategically prepared using the following steps Fig. 1(c–g):
• Region 1 (oxygen-free): this region was left un-thinned (∼3 μm thickness) before annealing, ensuring it remained encapsulated by the surrounding bulk material and isolated from ambient oxygen during transfer and annealing. After annealing, another FIB cut is performed on the un-thinned region 1 area and lifted out.
• Region 2 (oxygen-exposed): this region was thinned to 100 nm before annealing, exposing it to trace levels of surface-absorbed oxygen during transfer and the in situ annealing process.
2.3 In situ STEM annealing and quenching experiment
The in situ microelectromechanical system is based on Protochips Aduro TEM holder and heating E-chips. In situ STEM samples were prepared by a focus ion beam (FEI Nova 200) instrument equipped with a nanoprobe (Omniprobe 200) for lift-out operation. The structural and elemental analysis was done by cs-corrected scanning transmission electron microscope (JEOL ARM 200F) operated at 200 kV equipped with Energy Dispersive X-ray Spectroscopy (EDS) (Oxford X-MaxN100TLE with 100 mm2 silicon drift detector). The acquisition semi-angle for high angle annular dark field (HAADF) detector and annular bright field (ABF) detector was 90–370 mrad and 12–24 mrad, respectively.3. Results
The in situ experiment and two-step FIB procedure are specifically designed, aiming to eliminate the oxidation effect before observation (Fig. 1). The oxygen concentration is closely related to the thermally induced evolution behavior of REE, which could lead to the formation of Nd–Ce–Fe–B phase with oxygen-free surrounding, or conversely phase dissociation and hard magnetic properties degeneration caused by the loss of main phase. Here we describe the experiment in three parts: (I) sample preparation for the in situ experiment using FIB; (II) in situ annealing and quenching STEM experiment; (III) second FIB sample preparation and STEM observation after the in situ experiment. The crystal–liquid interface is observed in region 1 after quenching, which is oxygen free whereas region 2 is set as a control group because the reaction is affected by oxygen (Fig. 1).(I) Fig. 1a–c illustrates the sample preparation and transfer operation via FIB. A melt-spun (Nd0.75Ce0.25)2Fe14B ribbon is fixed on a stage by a Cu tip (Fig. 1a), and even contrast indicates the uniformity of the sample. The cross-section cut area is selected randomly as indicated by Fig. 1b yellow marker (15 × 2 μm2). After ion-beam milling and U-cut, the sample is transferred on an in situ heating E-chip (Fig. 1c). Here, two areas are selected for STEM observation with different reaction mechanisms due to oxygen concentration differences. Region 1, marked by red color, is not thinned (∼3 μm) before annealing. Thus the area is covered by the material and isolated to oxygen before annealing. By comparison, region 2 is thinned down to 100 nm before annealing and is exposed to the atmosphere during the transfer process from FIB to the STEM chamber. Although the in situ annealing is operated under pressure as low as 10−6 torr, the tiny amount of surface-absorbed oxygen does affect the reaction mechanism of region 2.
(II) The temperature vs. time diagram of the in situ annealing & quenching experiment is indicated by Fig. 1d. Strat from room temperature 25 °C, the sample is heated up to 700 °C for 7 min with a 1 °C s−1 ramping rate. Then the sample was quenched down to 25 °C with a 100 °C s−1 cooling rate that frozen the reaction inside. The detailed temperature recording and thermal condition of the in situ sample are discussed in the ESI (S2†). At stage II, the STEM imaging and EDS mapping are focused on region 2 (i.e., oxygen-involved reaction).
(III) After the in situ quenching experiment, a cross-section FIB cut was made to the left thick region of the quenched sample (Fig. 1e) and transferred on a Cu grid (Fig. 1f). Then, the sample was thinned down to 100 nm for STEM observation (Fig. 1g). At stage III, the STEM imaging and elemental mapping are focused on region 1 (i.e., the oxygen-free reaction region).
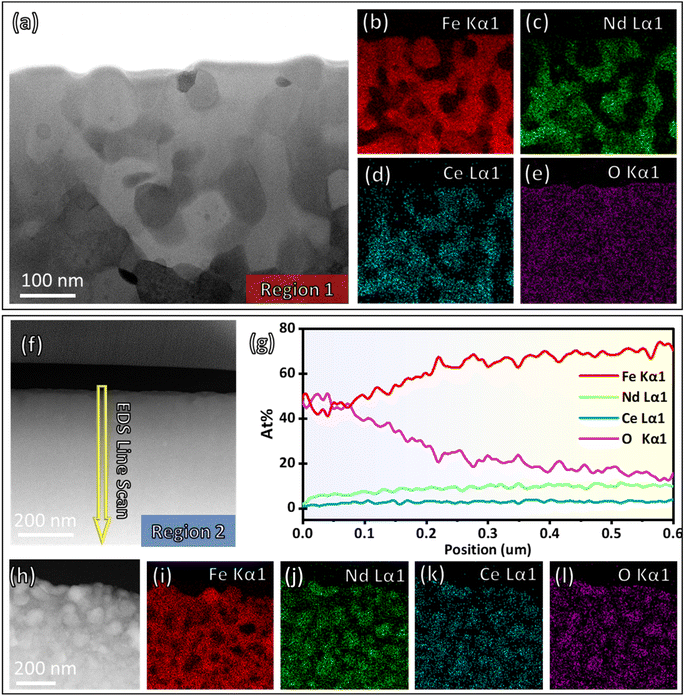
The morphology and elemental distribution at regions 1 and 2 are shown in Fig. 2a–e and f–l respectively. Before in situ annealing, the (Nd0.75Ce0.25)2Fe14B sample exhibits uniform morphology (Fig. 2f). EDS line scan (Fig. 2g) shows a gradient decreasing oxygen concentration from the top surface to inside, and the concentration of Fe, Nd, and Ce increase accordingly with a specific ratio. The EDS line scan result confirms the existence of absorption oxygen at the bulk surface. The atomic concentration of oxygen is significantly high near the surface (0–500 nm) compared with the area inside the sample (ESI S3†). (Accordingly, the second FIB cut at region 1 is selected at around 5 μm depth under the surface to minimize oxygen absorption.) The observed trend arises from the preparation method—melt spun. The molten alloy is rapidly cooled during melt spinning as it contacts a rotating chilled substrate. The rapid cooling rate limits the diffusion of oxygen into the bulk but allows oxygen to interact with the exposed surface. As a result, the surface of ribbons is enriched with oxygen element.19,20 After annealing and quenching, the phase decomposition reaction is found in both regions. The Fe-enriched phase is separated from the Nd & Ce enriched phase at region 1 (Fig. 2b–d) and region 2 (Fig. 2i–k). The characteristic X-ray oxygen signal (Fig. 2l) of region 2 overlaps with Nd and Ce signals, indicating the formation of the rare earth oxide phase. By comparison, the oxygen signal (Fig. 2e) of region 1 is uniformly distributed, meaning oxygen is not involved in the reaction. We believe oxygen concentration is the major factor determining the different reaction mechanisms between region 1 and region 2. The presented work mainly focuses on the phase evolution and interface dynamics in region 1, which is an oxygen-free reaction. The detailed sample structure at room temperature and oxygen-involved reaction dynamics of the Nd–Ce–Fe–B system were introduced in our recent publication with a similar experiment setup and phenomenon observed in region 2.15
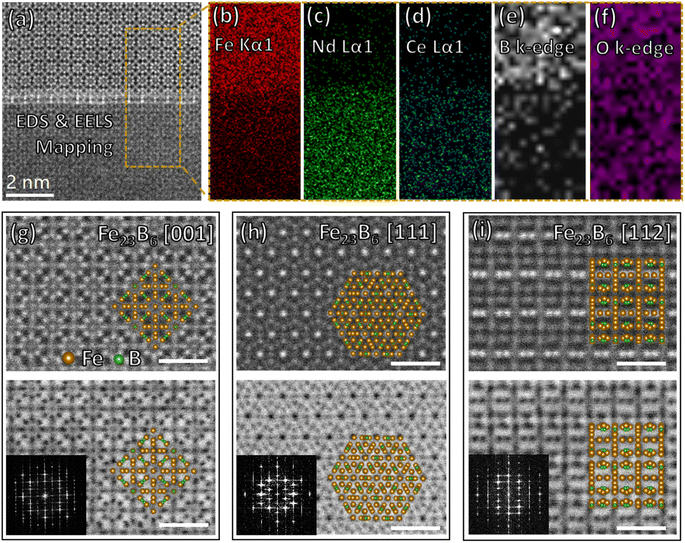
Three phases are found in region 1 after in situ annealing & quenching, α-Fe, Fe23B6, and REE-enriched amorphous phases. The typical crystal–liquid interface between Fe23B6 and REE-enriched amorphous phases is shown in Fig. 3a. The top area is a metastable phase Fe23B6 lattice, and the bottom area is an amorphous phase considered a liquid phase at high temperature. EDS (Fig. 3b–d) and EELS (Fig. 3e) mappings show the Fe and B elements constitute the metastable phase Fe23B6, and rare earth elements are enriched in the liquid phase. Still, the oxygen signal (Fig. 3f) is uniformly distributed among the interface at the nanoscale, proving that oxygen was not involved in the reaction of region 1. The intensity gradient of Fe, Nd, Ce, and B elemental mapping at the phase interface exhibits a moderate variation, indicating the tendency of elemental interdiffusion behavior. The metastable phase Fe23B6 is crystallized as a face-centered cubic (FCC) lattice structure, Fm3m space group.21,22 This soft magnetic phase has high saturation magnetization (μ0MS = 1.7 T) and a relatively low anisotropy constant (0.01 MJ m−3).12 The lattice structure of Fe23B6 crystal along [001], [111], and [112] are shown in Fig. 3g–i, respectively. The lattice map is generated by Vesta software23 based on a crystal CIF file obtained from standard database24 and overlaid on STEM image manually. The supercell (Fe92B24) of the Fe23B6 crystal, including four unit cells (Fe23B6), is presented in atomic resolution STEM images that illustrate the full symmetry elements of the lattice. Namely, as defined by Wyckoff notation, there are four inequivalent iron sites, 4a, 8c, 32f, and 48h, and 24 boron sites at 24e within the Fe92B24 supercell.25 The lattice constant could be represented as a = b = c = 10.6 Å, α = β = γ = 90°. Notably, the metastable Fe23B6 phase is considered as the precursor of RE2Fe14B type magnet during the crystallization sequence,12 and so the interface reaction with the REE-enriched phase is worth investigating in detail to explore the early phase formation mechanism.
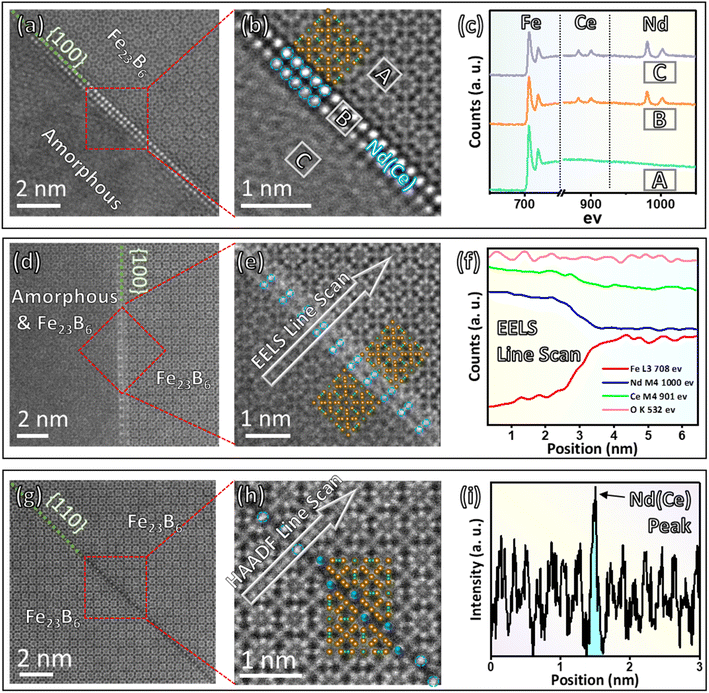
The interface dynamics between the REE-enriched phase and the Fe23B6 metastable phase are analyzed at the sub-nano scale through STEM images and EELS/intensity profiles acquired simultaneously. Fig. 4 exhibits three types of crystal–liquid interface structure and the corresponding elemental distribution analysis that reveals the REE embedded into the Fe23B6 lattice.
(I) The interface structure between Fe23B6 and the liquid phase is illustrated in Fig. 4a, which could be considered as the initial reaction state between rare earth elements and the Fe23B6 phase. The amorphous shown in the lower-left corner is a liquid phase formed after 700 °C annealing, and the atomic ratio of the amorphous area is about Nd (3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce (1)
Ce (1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe (1), verified by EDS. The upper-right corner is Fe23B6 lattice with 〈001〉 zone axis, and the coherent edge is along {100} plane. The high-magnification HAADF image (Fig. 4b) shows the interface structure at an atomic scale. The two-atom-thick interphase layer is marked by a blue circle that separates the Fe23B6 lattice and liquid phase on two sides. The atomic configuration of the interphase layer along the edge is incorporated with the {004} plane of the Fe23B6 lattice, exhibiting good crystallographic coherency at the interface in between. EELS spectrum (Fig. 4c) provides qualitative element analysis at Fe23B6 area ‘A’, the interphase film ‘B’, and the liquid phase ‘C’. Compared with “A” site, the interface “B” site is enriched with Nd and Ce, which is very similar to the liquid phase. By comparison, the intensity of Nd and Ce characteristic peaks is negligible at the ‘A’ site, indicating a minimum Nd and Ce concentration in the Fe23B6 lattice.
Fe (1), verified by EDS. The upper-right corner is Fe23B6 lattice with 〈001〉 zone axis, and the coherent edge is along {100} plane. The high-magnification HAADF image (Fig. 4b) shows the interface structure at an atomic scale. The two-atom-thick interphase layer is marked by a blue circle that separates the Fe23B6 lattice and liquid phase on two sides. The atomic configuration of the interphase layer along the edge is incorporated with the {004} plane of the Fe23B6 lattice, exhibiting good crystallographic coherency at the interface in between. EELS spectrum (Fig. 4c) provides qualitative element analysis at Fe23B6 area ‘A’, the interphase film ‘B’, and the liquid phase ‘C’. Compared with “A” site, the interface “B” site is enriched with Nd and Ce, which is very similar to the liquid phase. By comparison, the intensity of Nd and Ce characteristic peaks is negligible at the ‘A’ site, indicating a minimum Nd and Ce concentration in the Fe23B6 lattice.
(II) The Fe23B6 and (liquid + Fe23B6) interface structure is featured in Fig. 4d. At this state, the Fe23B6 lattice grows toward the liquid phase side, and the Nd and Ce atom start to embed into the Fe23B6 lattice. The (liquid + Fe23B6) phase on the lower-left side is the combination of the Fe23B6 phase and rare earth elements enriched liquid phase, as the lattice fringe is nearly visible with relatively poor contrast compared with the pure Fe23B6 phase area on the upper-right side. Fig. 4e exhibits the interface structure at an atomic scale. Still, a rare earth element enriched two-atom-thick film could be identified by brightness difference at the interface, as the intensity in HAADF-STEM images is approximatively proportional to Z2 (Z is the atomic number).26 The intergranular films crystallize coherently with {002} plane of the Fe23B6 lattice marked by a blue circle. Yet, the atomic configuration of the interface area could not be fully distinguished as the crystallization process has not finished, shown as bright stripes and random dots in the region of the intergranular films. The elemental distribution of Fe23B6 and (liquid + Fe23B6) interface structure is analyzed by EELS line scan (Fig. 4f). Similar to the previous result, the rare earth elements (Nd and Ce) are enriched at the liquid phase and interface area. The Fe intensity exhibits a gradient drop from the pure Fe23B6 lattice side to the (liquid + Fe23B6) phase side, indicating the directional diffusion behavior, so the crystal growth direction is predictable on the liquid side.
(III) At the crystallized area, the rare earth element atoms from the liquid phase dissolve inside the Fe23B6 lattice. Fig. 4g demonstrates a substitutional defect structure of Fe23B6 lattice along the {110} plane with the one-atom-thick layer. The 4a site atoms are marked by a blue circle (Fig. 4h), and the intensity line scan profile (Fig. 4i) shows the brightness of marked atoms is significantly higher than other sites Fe atoms inside the Fe23B6 lattice. The exceptional brightness peak observed by the STEM-HAADF image indicates the selective metallic site occupation behavior of Nd and Ce atoms in the Fe23B6 lattice. The similar selective substitutional behavior in Fe23B6 type crystal was predicted via the atomistic simulation method and proved 4a site is the most energy-favorable atomic replacement site preference.27
4. Discussion
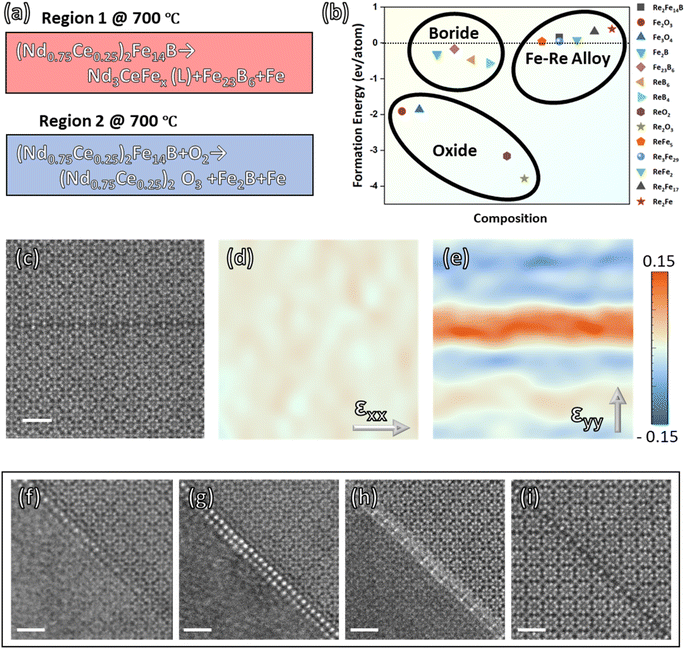
This work describes two types of phase transformation mechanisms of (RE)2Fe14B phase (Fig. 5a). Oxygen concentration is the major factor governing the reaction mechanism at 700 °C of (Nd0.75Ce0.25)2Fe14B material. In situ STEM experiment investigates the thermally induced phase evolution behavior in regions 1 (oxygen-free) and 2 (oxygen-involved) with the same heat condition but different oxygen concentrations. In region 1, the main phase decomposes as Fe23B6, α-Fe, and REE enriched liquid phases. In contrast, region 2 generates Fe2B, α-Fe, and REE oxide phases that are more thermodynamically favorable compared with phases in region 1 (Fig. 5b). Especially, the formation energy of oxide phases is significantly lower compared with boride phases and Fe–Re alloy phases in the system.24 Thus, oxygen tends to deprive the REE elements in the Nd–Ce–Fe–B system and form the REE oxide phase, which hinders the regeneration of the hard magnetic phase. The REE deprivation discussed in this work also explains the thermally induced irreversible degeneration of hard magnetic properties. By comparison, in the oxygen-free system, the REE enriches into the liquid phase and seems to stabilize the metastable phase Fe23B6, which is considered the precursor of the Re2Fe14B type phase. Therefore, the interface evolution between Fe23B6 and the liquid phase is worth exploring to understand the reaction dynamics.The structure of metastable phase Fe23B6 allows partial occupation of metal sites and lattice constant variations that optimize magnetic moment.28–30 Also, the substitution REE atoms introduce a significant lattice distortion into the lattice along the [110] direction inside the matrix lattice (Fig. 5c–e) and enhance the crystal magnetic anisotropy according to Neumann's principle.31–33 Previous studies indicate that dissolving REE atoms is the critical step that transfers the iron boride matrix from ‘soft’ to ‘hard’ magnetic properties.12–14 The dissolving behavior of REE into the Fe23B6 matrix is investigated directly via in situ annealing & quenching STEM experiments for the first time in this work. After annealing and main phase dissociation at 700 °C, the Fe23B6 & liquid phase crystal–liquid interface (Fig. 5f) is fixed during the reaction process via rapid quenching with a 100 °C s−1 cooling rate. As a result, Nd and Ce atoms are found to separate from the liquid phase and crystallize at the surface of the Fe23B6 phase (Fig. 5g). The atomic configuration of the interphase layer is coherent with the Fe23B6 lattice. Likewise, Fe atoms are found to diffuse across the interphase layer and form an iron boride phase at the liquid phase side, and as a result, Nd and Ce atoms embed inside the Fe23B6 lattice (Fig. 5h–i). Although the morphology observed is ‘static’ at room temperature, the unbalanced solidification process creates various interface morphology that provides insights into the early nucleation dynamics of the Nd–Ce–Fe–B phase.
5. Summary
The present work extensively used advanced FIB-STEM and elemental analysis techniques to study the thermally induced phase evolution and crystal–liquid interface structure of the Nd–Ce–Fe–B system with unprecedented detail. The sample preparation and in situ experiment procedure are specifically designed to achieve both oxygen-involved and oxygen-free reaction mechanisms within one FIB-prepared specimen. (Nd0.75Ce0.25)2Fe14B sample is annealed at 700 °C for 7 min and then quenched to 25 °C with a 100 °C s−1 cooling rate that ‘frozen’ the crystal–liquid interface for observation. The angstrom scale atomic structural analysis and sub-nanometer spatial resolution elemental analysis clearly revealed the lattice evolution and element diffusion behavior between metastable Fe23B6 solid phase and REE enriched liquid phase. This combination of advanced FIB and in situ STEM techniques was proved as a powerful method to reveal the phase evolution mechanisms and reaction dynamics of complex composition systems. The following summaries briefly introduce the results presented above.(I) Oxygen concentration is the major factor determining the phase evolution mechanism of (Nd0.75Ce0.25)2Fe14B magnet, depriving REE atoms of matrix forming oxide phase. Therefore, the unbalanced phase transformation equations at 700 °C could be represented as:
• (Nd0.75Ce0.25)2Fe14B → Nd3CeFex (L) + Fe23B6 + α-Fe
• (Nd0.75Ce0.25)2Fe14B + O2 → (Nd0.75Ce0.25)2O3 + Fe2B + α-Fe
(II) The REE atoms from the liquid phase are found to crystallize at the surface of the Fe23B6 lattice, forming an atomic coherent interphase layer. Fe elements diffused toward the liquid phase side that facilitates the Fe23B6 crystal growth across the REE-enriched interface layer. As a result, Nd and Ce atoms become substitutional atoms and are embedded in the Fe23B6 lattice, forming the Nd–Ce–Fe–B phase.
This study demonstrates that oxygen concentration is a dominant factor influencing Nd–Ce–Fe–B alloys' reaction dynamics and phase evolution. Specifically, the substitution of Nd and Ce into the Fe23B6 lattice was directly observed for the first time, providing atomic-scale evidence of the mechanisms underlying hard magnetic phase formation. These findings not only enhance our fundamental understanding of the Nd–Ce–Fe–B system but also offer guidance to the optimization of thermal treatment protocols and oxygen control strategies to enhance the performance and longevity of REE–Fe–B magnets, with potential benefits for automotive, energy, and electronic device applications. Additionally, this research highlights the extraordinary capabilities of combining advanced FIB and in situ STEM technologies to investigate high-temperature reaction dynamics at unprecedented spatial resolution.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.Author contributions
Xiangyu Zhu wrote the manuscript and carried out the FIB and STEM experiment with Qingxiao Wang. Li Shan and Xianming Dai provided finite element analysis. Byung Oh Jung, Myungshin Choi, Sunyong Song, Seok Namkung, Namseok Kang, Hui-Youn Shin, and Minho Joo synthesize the material. M. J. Kim and Xiangyu Zhu supervised and conceived this project.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the Materials & Devices Advanced Research Institute (MDARI), one of the research centers at LG Electronics. The authors are grateful for the assistance of Dr Jeong Soo Lee (Head of MDARI). The UTD research was supported in part by the Louis Beecherl, Jr Endowed funds. Finally, the author acknowledges Yaoqiao Hu for providing suggestions for thermodynamics analysis.References
- M. V. Reimer, H. Y. Schenk-Mathes, M. F. Hoffmann and T. Elwert, Metals, 2018, 8, 867 CrossRef.
- C. Li, J. M. Mogollón, A. Tukker, J. Dong, D. von Terzi, C. Zhang and B. Steubing, Renewable Sustainable Energy Rev., 2022, 164, 112603 CrossRef CAS.
- J. M. D. Coey, Engineering, 2020, 6, 119–131 CrossRef CAS.
- M. Zhang, W. Zhang, F. Chen, Y. Guo, F. Li and W. Liu, J. Supercond. Novel Magn., 2018, 31, 2811–2816 CrossRef CAS.
- T. Miyake, Y. Harashima, T. Fukazawa and H. Akai, Sci. Technol. Adv. Mater., 2021, 22, 543–556 CrossRef PubMed.
- G. Y. Kim, T. H. Kim, H. R. Cha, S. hyub Lee, D. H. Kim, Y. Do Kim and J. G. Lee, J. Mater. Sci. Nanotechnol., 2022, 126, 71–79 CrossRef CAS.
- G. Y. Kim, T. H. Kim, H. R. Cha, S. hyub Lee, D. H. Kim, Y. Do Kim and J. G. Lee, Scr. Mater., 2022, 214, 114676 CrossRef CAS.
- D. C. Nababan, R. Mukhlis, Y. Durandet, M. I. Pownceby, L. Prentice and M. A. Rhamdhani, Corros. Sci., 2021, 182, 109290 CrossRef CAS.
- D. C. Nababan, R. Mukhlis, Y. Durandet, M. I. Pownceby, L. Prentice and M. A. Rhamdhani, Corros. Sci., 2021, 189, 109560 CrossRef CAS.
- T. Abe, M. Morishita, Y. Chen, A. Saengdeejing, K. Hashimoto, Y. Kobayashi, I. Ohnuma, T. Koyama and S. Hirosawa, Sci. Technol. Adv. Mater., 2021, 22, 557–570 CrossRef CAS PubMed.
- S. Kobayashi, T. Abe, A. Martín-Cid, S. Kawaguchi, M. Suzuki, S. Hirosawa and T. Nakamura, J. Alloys Compd., 2022, 892, 162188 CrossRef CAS.
- O. Gutfleisch, J. Phys. D Appl. Phys., 2000, 33(17), R157 CrossRef CAS.
- E. Kneller and R. Hawig, IEEE Trans. Magn., 1991, 27, 3588–3560 CAS.
- Y. C. Jung and K. Nakai, Met. Mater. Int., 2003, 9, 337–344 CrossRef CAS.
- X. Zhu, B. Oh Jung, Q. Wang, Y. Hu, M. Choi, S. Song, S. Namkung, N. Kang, H. Y. Shin, M. Joo and M. J. Kim, Mater. Des., 2022, 216, 110525 CrossRef CAS.
- Z. Liu, J. He and R. V. Ramanujan, Mater. Des., 2021, 209, 110004 CrossRef CAS.
- L. Zhao, J. He, W. Li, X. Liu, J. Zhang, L. Wen, Z. Zhang, J. Hu, J. Zhang, X. Liao, K. Xu, W. Fan, W. Song, H. Yu, X. Zhong, Z. Liu, X. Zhang, L. Zhao, X. Liu, J. Zhang, L. Wen, Z. Zhang, X. Zhang, J. He, J. Hu, X. Liao, K. Xu, W. Fan, W. Song, H. Yu, X. Zhong, Z. Liu and W. Li, Adv. Funct. Mater., 2022, 32, 2109529 CrossRef CAS.
- J. Ni, T. Ma and M. Yan, J. Magn. Magn. Mater., 2011, 323, 2549–2553 CrossRef CAS.
- H. Zheng, W. Wang, J. Yu, Q. Zhai and Z. Luo, J. Mater. Res., 2014, 29, 880–886 CrossRef CAS.
- D. J. Sordelet, X. Y. Yang, E. A. Rozhkova, M. F. Besser and M. J. Kramer, Appl. Phys. Lett., 2003, 83, 69–71 CrossRef CAS.
- V. A. Barinov and V. T. Surikov, Phys. Met. Metallogr., 2008, 105(3), 245–253 Search PubMed.
- V. A. Barinov, V. A. Tsurin, V. I. Voronin, S. I. Novikov and V. T. Surikov, Phys. Met. Metallogr., 2006, 101(5), 456–466 CrossRef.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2008, 41, 653–658 CrossRef CAS.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- M. Souissi, M. H. F. Sluiter, T. Matsunaga, M. Tabuchi, M. J. Mills and R. Sahara, Sci. Rep., 2018, 8(1), 1–9 Search PubMed.
- S. J. Pennycook and D. E. Jesson, Ultramicroscopy, 1991, 37, 14–38 CrossRef.
- J. Xie, N. Chen, L. Teng and S. Seetharaman, Acta Mater., 2005, 53, 5305–5312 CrossRef CAS.
- V. A. Barinov, V. A. Tsurin and V. T. Surikov, Phys. Met. Metall., 2012, 113, 48–61 CrossRef.
- D. G. Quirinale, D. Messina, G. E. Rustan, A. Kreyssig, R. Prozorov and A. I. Goldman, Phys. Rev. Appl., 2017, 8(5), 054046 CrossRef.
- C. M. Fang, M. A. van Huis, M. H. F. Sluiter and H. W. Zandbergen, Acta Mater., 2010, 58, 2968–2977 CrossRef CAS.
- A. R. Biedermann, Geosciences, 2018, 8(8), 302 CrossRef.
- F. Neumann and O. Meyer, Vorlesungen über die Theorie der Elasticität der festen Körper und des Lichtäthers, BG Teubner, 1885 Search PubMed.
- P. J. Dobson, Phys. Bull., 1985, 36, 506 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03708a |
| This journal is © The Royal Society of Chemistry 2025 |