Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Incredible use of plant-derived bioactives as anticancer agents
Kiran Kangraa,
Saloni Kakkara,
Vineet Mittala,
Virender Kumarb,
Navidha Aggarwalc,
Hitesh Choprad,
Tabarak Malik *ef and
Vandana Garg*a
*ef and
Vandana Garg*a
aDepartment of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, 124001, India. E-mail: kirankangra90@gmail.com; drsaloni.pharma@mdurohtak.ac.in; drvineet.pharma@mdurohtak.ac.in; drvandana.pharma@mdurohtak.ac.in
bCollege of Pharmacy, Pandit Bhagwat Dayal Sharma University of Health Sciences, Rohtak, 124001, India. E-mail: sachdeva.virender5@gmail.com
cMM College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana, Ambala 133207, Haryana, India
dDepartment of Biosciences, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Chennai 602105, Tamil Nadu, India
eDepartment of Biomedical Sciences, Jimma University, Jimma, Ethiopia. E-mail: tabarak.malik@ju.edu.et
fDivision of Research & Development, Lovely Professional University, Phagwara, Punjab-144411, India
First published on 20th January 2025
Abstract
Cancer is a major global concern. Despite considerable advancements in cancer therapy and control, there are still large gaps and requirements for development. In recent years, various naturally occurring anticancer drugs have been derived from natural resources, such as alkaloids, glycosides, terpenes, terpenoids, flavones, and polyphenols. Plant-derived substances exhibit their anticancer potential through antiproliferative activity, cytotoxicity, apoptosis, angiogenesis and cell cycle arrest. Natural compounds can affect the molecular activity of cells through various signaling pathways, like the cell cycle pathway, STAT-3 pathway, PI3K/Akt, and Ras/MAP-kinase pathways. Capsaicin, ouabain, and lycopene show their anticancer potential through the STAT-3 pathway in breast, colorectal, pancreatic, lung, cervical, ovarian and colon cancers. Epigallocatechin gallate and emodin target the JNK protein in skin, breast, and lung cancers, while berberine, evodiamine, lycorine, and astragalin exhibit anticancer activity against breast, liver, prostate, pancreatic and skin cancers and leukemia through the PI3K/Akt and Ras/MAP-kinase pathways. In vitro/in vivo investigations revealed that secondary metabolites suppress cancer cells by causing DNA damage and activating apoptosis-inducing enzymes. After a meticulous literature review, the anti-cancer potential, mode of action, and clinical trials of 144 bioactive compounds and their synthetic analogues are included in the present work, which could pave the way for using plant-derived bioactives as anticancer agents.
1 Introduction
Among the non-communicable diseases, cancer is the second most life-threatening disease after cardiovascular diseases.1 It is caused by a combination of genetic factors, environmental stress on cellular activity,2 obesity, poor diet, excessive alcohol intake, smoking, and vitamin B12 deficiency.3 According to Sung et al., transitional cases (64%) would increase significantly more than transitioned cases (32%), reaching 28.4 million cases globally in 2040, with an increase of 47% cases from 2020. Approximately 10 million people would die from cancer in 2024, out of which approximately 19.3 million would be new cases.4 According to the site of occurrence, there are 131 different types of cancers, including skin cancer, lung cancer, oral cancer, and breast cancer.5 It is predicted that there would be 2.3 million more cases of female breast cancer (11.7%), followed by lung (11.4%), colorectal (10.1%), prostate (7.3%), and stomach (5.6%) cancers. Different types of cancers are caused by a variety of variables. Particularly, in the case of skin cancer, ozone depletion, melanin and microbial impact are responsible for its onset.6 Lung cancer is primarily caused by smoking but can also occur in non-smokers owing to other factors, like exposure to radon gas or secondhand smoke. Prostate cancer affects men and is one of the most common cancers in older men. Colorectal cancer affects the colon or rectum and is more common in older adults. It can be developed due to hereditary reasons or develop sporadically. Breast cancer occurs primarily in women but can also affect men. Thus, the pathophysiology of cancer involves a multitude of genetic, molecular, and environmental factors. Cancer arises from mutations in the DNA of cells that disrupt normal control mechanisms governing cell growth and division. These mutations can be inherited or acquired over time due to exposure to carcinogens such as chemicals, radiation, or viruses.1.1 Pathophysiology of cancer
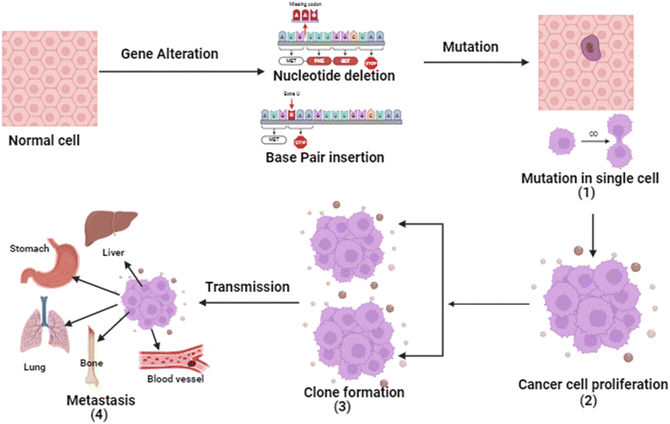
Cancer is a four-step process involving mutation along with cell's proliferative, survival, invasion, and metastatic capacities. In cancer, the cell's genetic system (DNA) and anti-tumor genes are suppressed by environmental factors or unhealthy diet, smoking, drinking obesity etc. Tumor suppressor gene inactivation is a natural physiological reaction of the organism and the cancer develops when this reaction becomes pathologic.7 Except for histological types, almost all cancers share basic pathogenesis. From extensive research, it is evident that the genetic system is involved in the development of malignant tumors. Due to inhibition of angiogenesis and alteration of cells.8The initial stage in the progression of cancer involves the occurrence of a mutation and the subsequent formation of a tumor. This process occurs when a genetic alteration triggers a mutation within a cell, leading to the growth of tumor cells. Following this, the mutation induces cell proliferation and the advancement of the tumor as the mutated cells rapidly multiply and divide, ultimately becoming dominant within the tumor cell population.9 Subsequently, clonal selection occurs among the proliferating cells, resulting in the generation of a new clone of rapidly growing cells with distinct characteristics. This step is repeated throughout the development of the tumor. Finally, metastasis occurs, wherein cancer cells detach from the primary tumor and travel through either the bloodstream or the lymphatic system to distant areas of the body.10 Consequently, these cells continue to multiply in the new locations, ultimately giving rise to new tumors composed of cells that bear resemblance to the original tissue. The propensity of tumors to metastasis is a major factor in the lethality of some malignancies, such as pancreatic and uveal cancers.11 The basic pathophysiology of cancer is described in Fig. 1.
2 Review methodology
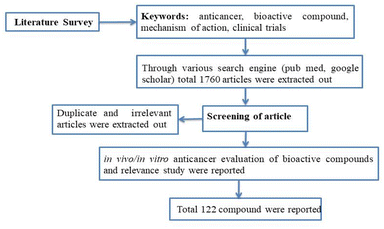
A concise summary of the methodology employed in this review is shown in Fig. 2. A comprehensive search was conducted using various search engines such as PubMed, Google Scholar, ScienceDirect, Scopus, Web of Science, and Chemical Abstracts. The search utilized different keywords including “anti-cancer”, “phytochemical”, “plant bioactive”, “clinical trials”, “mechanism of action”, and more. Irrelevant, duplicate, and incomplete data were excluded, while the literature pertaining to the in vitro or in vivo anticancer potential of plant-based bioactives was included by studying the 1600–1700 review and research article. Additionally, this review focused on articles that described the mechanism of action and clinical trial data for the anti-cancer potential of herbal compounds. The present article primarily reviews data published within the past decade.3 Role of traditional plants and derived bioactives in cancer
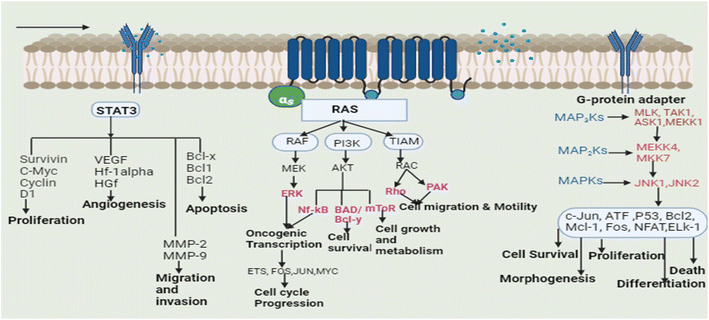
Plants are used to cure many ailments and natural or plant-based medications are preferred by 60–70 percent of the population over synthetic medicines for one reason or the other. These plants may aid the patient resistance to sickness by arbitrating physiological homeostasis and retraining the body tissues.12 Based on the traditional uses of plants and scientific reports, a lot of research has also been dedicated to the study of plants in order to cure cancer, and several plants have been successfully used in the treatment of cancer.13 Isolated phytoconstituents from these plants such as vincristine, vinblastine, chlorogenic acid, gingerol, apigenin, catechin, gallic acid, cinnamic acid, and podophyllotoxin, along with their derivatives and analogues, are used for the treatment of cancer by inhibiting several signaling pathways. Different types of tumors have altered cell signaling pathways (cell death pathways: apoptosis and autophagy, embryonic developmental pathways: Notch, Wnt, Hedgehog, Janus kinase pathway, signal transducer and activator of the transcription factor pathway and RAF/MAPK pathway). Cells integrate the signals received from various growth factors and receptors to control different cellular functions, including cell motility, differentiation, architecture, and polarity. Signalling pathways control cellular growth and induce various alterations in various cell types.14The transcription (STAT3) pathway with signal transducers and activators is a major intrinsic pathway in cancer development (Fig. 3). It transmits intracellular signals that are normally generated at cell surface receptors to the nucleus. STAT3 activation involved a number of human tumors, including haematological and solid tumors. The evidence suggests that oncogenic cell transformation activates STAT-3, providing the survival signal. The dysfunctioning of STAT-3 during mammary gland involution demonstrates that it has proapoptotic functions. Functioning STAT-3 can prevent apoptosis in most cells. These effects are arbitrated by STAT-3-regulated cell survival gene products, i.e. Bcl Bcl1, Bcl-2, Survivin, Mcl-1, and cIAP2. Thus, inhibiting the STAT-3 activation can reduce the activity of these gene products, thereby increasing apoptosis.15 Furthermore, the master protein kinases known as c-Jun N-terminal kinases (JNKs) control a variety of physiological processes, such as inflammatory reactions, morphogenesis, cell proliferation, differentiation, survival, and death. It is becoming clear that persistent JNK activation contributes to cancer development and progression. Further, RAS proteins can interact with other well-known effectors such as phosphatidyl inositol 3-kinases (PI3Ks) via the RAF/MAPK pathway (PI3Ks). The interaction of different RAS proteins with PI3Ks could lead to DNA damage, and finally, to tumor development.
In the present study, different bio-actives from various categories that are reported to possess anti-cancer potential against various cell lines and in experimental animals are summarised.
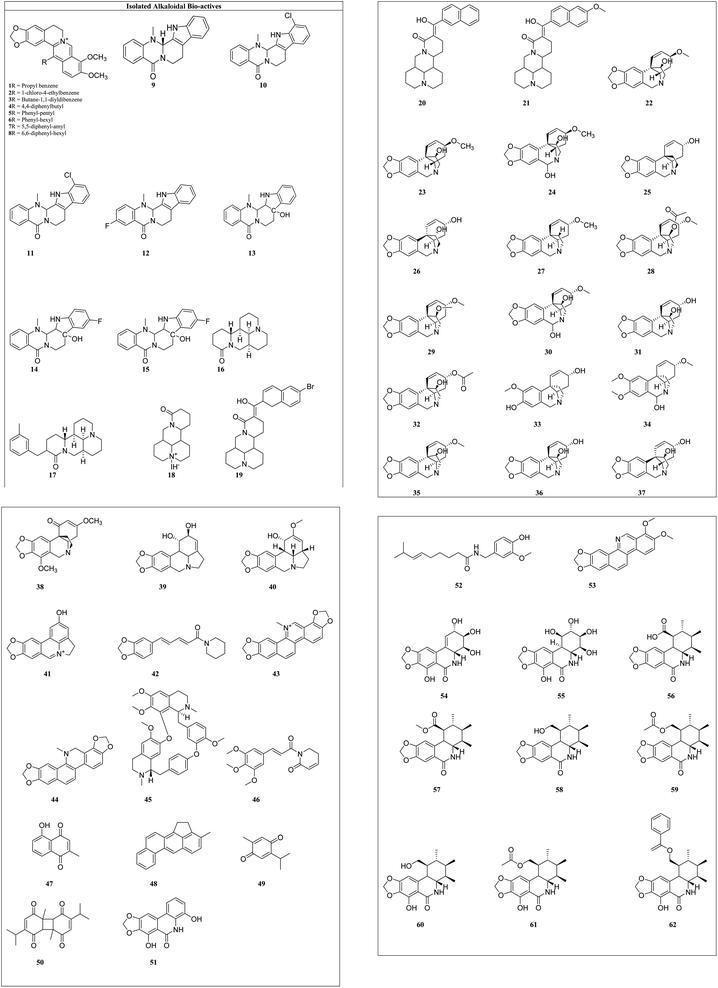
Alkaloids: these are the largest group of phytochemicals with a heterocyclic ring structure and at least one nitrogen atom. To distinguish various alkaloids, a categorization based on biosynthetic pathways is commonly used. Alkaloids can be found in all types of plants, although they are most prevalent in the Ranunculaceae, Leguminosae, Papaveraceae, Menispermaceae, and Loganiaceae families.16 Vinca alkaloids (vincristine, vinblastine, vinorelbine, vindesine, and vinflunine) were the first microtubule-targeting agents (MTAs) and approved for clinical use in hematological and lymphatic neoplasms.17 Various alkaloids from plant sources and their synthetic analogues with cytotoxicity on different cell lines are reported in Table 1 and the structures of isolated alkaloids are shown in Fig. 4.
| Isolated compound | Alkaloid (biological source) | Cancer cell line along with IC50 (nM) value | References |
|---|---|---|---|
| 1 | Berberis aetnensis (Berberidaceae) | MCF-7-230 | 18 and 19 |
| 2 | HepG2-170 | ||
| 3 | LNCaP-190 | ||
| 4 | PC-3165 | ||
| 5 | MHCC97-L-400 | ||
| 6 | MDA-MB21-250 | ||
| 7 | HTB-94-200 | ||
| 8 | SMMC-7721-180 | ||
| 9 | Evodia rutaecarpa (Rutaceae) | MDA-MB-435-49 | 20 |
| 10 | HCT116-90 | ||
| 11 | U20S-26 | ||
| 12 | Panc-1-39 | ||
| 13 | PC-3-65 | ||
| 14 | HL-60-60 | ||
| 15 | Saos-2-95 | ||
| 16 | Sophora flavescens (Fabaceae) | A549-200 | 21 and 22 |
| 17 | HepG2-250 | ||
| 18 | Panc-1-170 | ||
| 19 | CCRFCEM-250 | ||
| 20 | SGC7901-209 | ||
| 21 | PC-3-170, DU145-175 | ||
| 22 | Galanthus nivalis (Amaryllidaceae) | A-431-90 | 23 and 24 |
| 23 | A549-255 | ||
| 24 | BCA-1-200 | ||
| 25 | B16F10-200 | ||
| 26 | CEM-140 | ||
| 27 | HT29-230 | ||
| 28 | HeLa-120 | ||
| 29 | HepG2-156 | ||
| 30 | Hs683-130 | ||
| 31 | HL-60-145 | ||
| 32 | B16F10-250 | ||
| 33 | CEM-180 | ||
| 34 | BCA-1-220 | ||
| 35 | A549-275 | ||
| 36 | HT29-280 | ||
| 37 | Crinum bulbispermum (Amaryllidaceae) | U373-280 | 25 and 26 |
| HL-60-120 | |||
| 38 | Boophone disticha (Amaryllidaceae) | HeLa-150 | 27 |
| G-361-250 | |||
| MCF-7-200 | |||
| K562-280 | |||
| 39 | Hymenocallis littoralis (Amaryllidaceae) | HL-60-150 | 28–30 |
| K562-180 | |||
| PC-3M-200 | |||
| 40 | Amaryllis belladonna L., (Amaryllidaceae) | A549-280 | |
| OE21-220 | |||
| B16F10-290 | |||
| U373-360 | |||
| 41 | Nerine bowdenii (Amaryllidaceae) | HL-60-200 | 30 |
| U937-290 | |||
| K562-360 | |||
| MOLT-4-270 | |||
| LXFL 529L-240 | |||
| 42 | Piper nigrum L. (Piperaceae) | DU145-150 | 31 and 32 |
| HT-29-180, Caco-2-200, SW480-220 | |||
| HRT-18-220 | |||
| A549-140 | |||
| 43 | Sanguinaria canadensis (Papaveraceae) | DU145-210 | 33 |
| BEL-7402-280 | |||
| 44 | Hela-180 | ||
| 45 | Stephania tetrandra (Menispermaceae) | BGC-823-180 | 34 and 35 |
| HCT116-260 | |||
| Hep G2-210 | |||
| A549-160 | |||
| 46 | Piper arborescens (Piperaceae) | KB-140 | 36 and 37 |
| A549-180 | |||
| P388-180 | |||
| HT29-260 | |||
| 47 | Plumbago zeylanica L. (Plumbaginaceae) | MG63-160 | 38 and 39 |
| MCF7-230 | |||
| 48 | Nigella sativa (Iridaceae) | PC3-300 | 40 |
| 49 | LL/2-260 | ||
| 50 | HeLa-280 | ||
| 51 | Cyrtanthus contractus (Amaryllidaceae) | HeLa-200 | 41 |
| MCF7-290 | |||
| A431-260 | |||
| 52 | Capsicum annuum (Solanaceae) | HCT LoVo-250 | 42 |
| MCF7-200, MDA-MB231-240 | |||
| LNCaP-180 | |||
| HL-60-255 | |||
| PANC1-200 | |||
| 53 | Broussonetia papyrifera (L.) (Moraceae) | BEL-7402-185 | 27 |
| Hela-150 | |||
| 54 | Narcissus jonquilla (Amaryllidaceae) | PC3-290 | 43 |
| LoVo-300 | |||
| A549-350 | |||
| MCF-7-380 | |||
| 55 | Hymenocallis littoralis (Amaryllidaceae) | PANC1-250 | |
| 56 | MV4-11-110 | 44 | |
| 57 | U87-160 | ||
| 58 | MCF7-145 | ||
| 59 | OVCAR3-135 | ||
| 60 | Hep G2-230 | ||
| 61 | PANC1-280 | ||
| 62 | U87-260 |
Berberine (1) and its seven synthetic isomers having different substituents, such as [propyl benzene (1), 1-chloro-4-ethylbenzene (2), butane-1,1-diyldibenzene (3), 4,4-diphenylbutyl (4), phenyl-pentyl (5), phenyl-hexyl (6), 5,5-diphenyl-amyl (7) and 6,6-diphenyl-hexyl (8)], were screened for prostate cancer, lung cancer, liver cancer, and chondrosarcoma and these were found to work in a variety of ways to prevent cancer. It inhibited cyclin D1 and E1 in lung cancer and CDK4 expression and modulating cyclin D1 in colorectal cancer and hepatoma cancer. Berberine upregulated the level of p53 and p21 in chondrosarcoma by regulating the PI3K/Akt and p38 signaling pathways.45
Evodiamine (9) and its six derivatives with varied substituents [4-chlorobenzoyl (10), 12-chloroevodiamine (11), 3-fluoroevodiamine (12), 10-hydroxyevodiamine (13), 3-fluoro-10 hydroxyevodiamine (14) and 3-amino-10-hydroxyevodiamine (15)] were screened for the treatment of colon cancer, osteosarcoma, pancreatic carcinoma, prostate cancer, leukemia and breast cancer. By inhibiting the caspase inhibitor evodiamine inhibits cervical cancer. Caspase inhibition causes alteration in Bax and Bcl-2 balance, which decreases apoptosis.46 It suppresses the liver cancer by inducing apoptosis and inhibiting the PI3K/Akt pathway.47
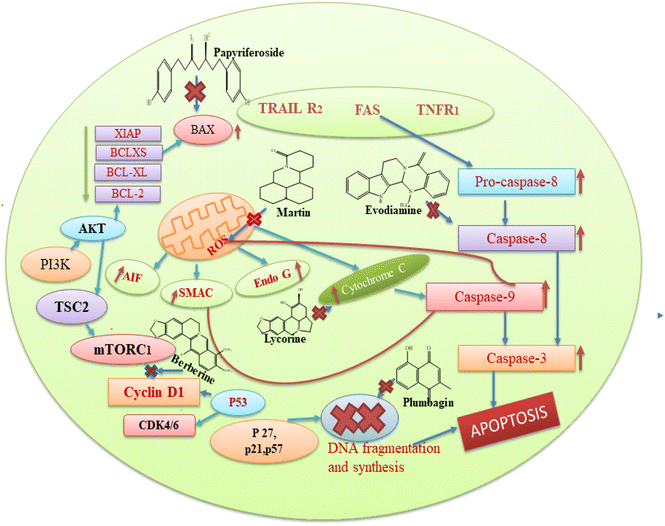
Matrine (16) and its five derivatives with varying substituents, such as [11-(3-methylbenzyl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one (17), 4-methyl-10-oxotetradecahydro-1H,5H-dipyrido[2,1-f.3′,2′-ij][1,6]naphthyridin-4-ium iodide (18), (11(Z)-11-(6-bromonaphthalen-2-yl)(hydroxy)methylene)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one (19), 11-(hydroxy(naphthalen-2-yl)methyl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one (20) and 11-(hydroxy(6-methoxynaph-thalen-2-yl)methyl)dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one (21)], showed anticancer potential against lung cancer, breast cancer, liver cancer, prostate cancer, leukemia and sarcoma. It causes caspase-mediated cell death in lung cancer by impeding the G1/G0 phase of the cell cycle.48 Matrine showed anticancer activity against pancreatic cancer by inducing ROS generation, and induced death.49 Crinine-type (22) alkaloids and their fourteen derivatives [haemanthamine (23), haemanthidine (24), vitattine (25), hydroxyvitattine (26), crinamine (27) 11-O-acetylcrinamine (28) 11-O-methylcrinamine (29), 6-hydroxycrinamine (30), hamayne (31), 3-O-acetylhamayne (32), 8-O-demethylmaritidine (33), papyramine (34), dihydrocrinamine (35) and dihydrohamayne (36)] are also promising therapeutic candidates for the treatment of apoptosis-resistant tumors, particularly glioblastoma. The crinine-type alkaloid inhibits glioblastoma cell proliferation via cytostatic effects resulting from the rigidification of the actin cytoskeleton. Bulbispermine (37) showed anticancer activity against glioblastoma and leukemia by inhibiting apoptosis resistance.25 Distichamine (38) in leukemia alter the cell cycle and induce death by activating the caspase 3 and 7.50 lycorine (39) and amarbellisine (40) reduce Mcl-1 at the translational level, which causes cell death in leukemia cells. Lycorine promotes the intrinsic apoptotic cascade in bladder cancer by decreasing the PI3K–Akt pathway and boosting the expression of the PTEN protein, which acts as a negative regulator of p-Akt.51 ungeremine (41) showed cytotoxic effects against leukemia by inhibiting cell proliferation through caspase activation, matrix metalloproteinases (MMP) modification, and also increasing ROS production.52 Piperine (42) inhibits cell proliferation by activation of apoptotic signalling pathways, modulation of ER stress and induction of detoxification of enzymes.53 Sanguinarine (43) and its one derivative dihydrosanguinarine (44) showed their anticancer potential by suppressing the abnormally active signal transduction pathways, cell apoptosis, and cancer cell proliferation.54 Tetrandrine (45) showed anti-cancer properties against lung, colon, bladder prostate, and many more, as shown in Table 1. Tetrandrine's anticancer properties may be linked to autophagy, cell cycle arrest, alleviate metastasis and suppression of tumor cell proliferation.55 Piplartine (46) caused G2/M cell cycle arrest, followed by mitochondrial-dependent apoptosis, as shown by chromatin condensation and inter-nucleosomal DNA breakage.56 Plumbagin (47) showed its anticancer potential through the NF-k, STAT3, and Akt regulatory signaling pathways. It was also a potent ROS inducer, a suppressor of cellular glutathione, and a novel proteasome inhibitor generating DNA double-strand breaks via oxidative DNA base damage.57
Thymoquinone (48) and its two derivatives [thymoquinone (49) and dithymoquinone (50)] have anti-cancer properties through a variety of mechanisms, including selective antioxidant activity, DNA structural interference, effects on carcinogenic signaling molecules/pathways, and immunomodulation.58 Narciprimine's (51) effects on DNA topoisomerase have also been studied. The findings demonstrated that narciprimine was dose-dependently efficacious in DNA topoisomerase processes. The potential of this alkaloid to interfere with topoisomerase was somewhat associated with anticancer activity measured in HeLa, MCF-7, and A341 cells.41 Multiple mechanisms were involved in capsaicin's (52) anticancer activity, including increased intracellular calcium, inhibition of p53, STAT3 and nuclear factor B.59 Norchelerythrine (53) works as an anticancer agent by various methods, including apoptosis, inhibiting aromatase, disrupting tubulin aggregation, inhibiting topoisomerase, and inhibiting ER.60 In the prostate and breast cancer cells, narciclasine (54) causes inactivation of mitochondrial membrane potential, cytochrome release and caspase activations.27 Pancratistatin (55) and its seven synthetic analogues with varied substituents [JCTH-1 (56), JCTH-2 (57), JCTH-3 (58), JCTH-4 (59), SVTH-5 (60) SVTH-6 (61) and SVTH-7 (62)] inhibit tumor xenograft growth by disrupting mitochondrial activity and by activating the intrinsic apoptotic pathway. SVTH-7 inhibits mitochondrial complex II and III, reducing pro-apoptotic effects on cancer cells and on mitochondria.44
3.1 In vivo anti-cancer studies of alkaloids
In the drug development process, preclinical data give complete information, including preliminary efficacy, toxicity, pharmacokinetics, and safety of potential lead compound. This information can be used to determine whether or not a compound should be pursued further for clinical trials. In this context, various in vivo studies reporting the anti-cancer evaluation of alkaloids have also been summarised as follows:Berberine anticancer activity against colorectal cancer was tested in a xenograft model of BALB/c nude mice. Mice were injected with KM12C cell sublines, shCtrl, and shRXR. After the tumor had grown, the infected mice were given berberine (10 mg kg−1). Berberine reduced the length of the tumor which could be due to the induction of nuclear-catenin degradation, significantly reducing endogenous c-Cbl, Ki67, Cdc2, c-Myc, and CIP1. Berberine also shows its activity by inhibiting the β-catenin signaling pathway.61 A xenograft model was used to test berberine's anticancer activity against endometrial cancer. Mice were injected with HEC-1-A. When the tumor had grown, mice were divided into three groups. Groups were given either 0.5% MC (vehicle control) or berberine (50 mg kg−1, p.o.qd or 100 mg kg−1, p.o.qd), orally. Berberine treatment significantly reduced the invasion of HEC-1-A cells at 50 mg kg−1 and 100 mg kg−1,62 and in lung cancer, it showed its potential at 200 mg kg−1 and 25 mg kg−1 in nude mice.63 The anticancer activity of matrine against lung cancer was tested in a xenograft model of BALB/c nude mice by inserting the LA795 cell. The infected mice were given matrine (80 mg kg−1) and a vehicle. It reduced the length of the tumor by regulating transmembrane protein 16A.64 Matrine anticancer activity in breast cancer was investigated by inserting the C57BL cell subcutaneously. Then 50 mg per kg matrine was injected once a day at an early stage of cancer. Mice were forfeited after 21 days. The tumor was collected and evaluated. The results indicated that matrine reduced breast cancer angiogenesis by inhibiting the Wnt/β-catenin signaling pathway.65 Piperine anticancer activity against breast cancer was investigated in BALB/c mice. Then, 2 × 105 EEMT6/P cells were injected subcutaneously. Following this, 25 mg per kg matrine was injected once a day at an early stage of cancer. Mice were forfeited after 14 days. The tumor was collected and evaluated.66 Lycorine anticancer activity against prostate cancer was tested in a xenograft model of BALB/c nude mice by inserting the RM-1 cells. After the tumor had grown to about 20 mm3 in diameter, the infected mice were given lycorine (10 mg kg−1) and a vehicle. It shows its anticancer potential by inhibiting the p65 and IKK-β phosphorylation, downregulating the Ki-67 expression and increasing caspase 3 in tumor tissue.67 Lycorine anticancer activity against liver cancer was tested in a xenograft model of Kunming mice. Then, 5 × 106 of H22 cells were injected into the axillary region of the right fore limb. The infected mice were given lycorine (10 mg kg−1, 20 mg kg−1 and 40 mg kg−1) and a vehicle. Lycorine reduced the length of the tumor in a dose-dependent manner.68 Evodiamine was tested for anticancer activity against tongue cancer in a xenograft model of BALB/c nude male mice. For 35 days, infected mice were given evodiamine (10 mg kg−1) intraperitoneally. It reduced the tumor length by regulating the NF-B pathway.69 Evodiamine was tested for anticancer activity against lung cancer in a xenograft model of BALB/c nude female mice. For 22 days, the infected mice were given evodiamine (20 mg kg−1) via gavage. Evodiamine reduced the tumor length by increasing CD8 + T cells and decreasing the MUC1-C/PD-L1 axis.70 The anticancer activity of evodiamine against lymphoma was tested in a KM male mouse xenograft model. For 21 days, infected mice were given evodiamine (20 mg kg−1) via gavage three times a day. Evodiamine shortened the tumor length by downregulating Ki-67 expression.71 Evodiamine was tested for anticancer activity against colorectal carcinoma in a xenograft model of BALB/c nude female mice. For 22 days, infected mice were given evodiamine (10 mg kg−1, i.p.). Evodiamine shortened the tumor's length by suppressing hypoxia-inducible factor 1-α-mediated angiogenesis.72 The anticancer activity of evodiamine against lung cancer was tested in a xenograft model SCID nude mice. For 14 days, the infected mice were given evodiamine (20 mg kg−1) via gavage. Evodiamine showed its potential by inhibiting heat shock protein.73
Glycosides: the secondary metabolites, which produce at least one sugar fraction as well as one non-sugar fraction on hydrolysis, are termed glycosides. These include bufalin, antiaroside, papyriferoside, calotropin, ouabain, hyrcanoside, and many more glycosides. The antiproliferative activity of cardiac glycosides has attracted a lot of attention, because the sugar fraction increased solubility and their stereochemistry affected the binding affinity of the receptor protein.74 Various isolated glycosides IC50 values with their cytotoxity on different cell lines are reported in Table 2, and the structures of the isolated glycosides are shown in Fig. 5.
| Isolated compound | Glycoside (biological source) | Cancer cell line along with IC50 (nM) value | References |
|---|---|---|---|
| 63 | Betula papyrifera (Betulaceae) | A-549-50 | 75 |
| 64 | DLD-1-90 | ||
| 65 | WS1-60 | ||
| 66 | Antiaris toxicaria (Moraceae) | KB-150 | 76 |
| 67 | 1A9-190 | ||
| 68 | CAKI-1-130 | ||
| 69 | S-KMEL-2-200 | ||
| 70 | KB-250 | ||
| 71 | S-KMEL-2-320 | ||
| 72 | Asclepias subulata (Apocynaceae) | A549-180 | 77 |
| LS 180, 147 | |||
| 73 | PC-3-90 | ||
| 74 | Salix acmophylla (Salicaceae) | MCF7-184 | 78 |
| 75 | NCI-H460-210 | ||
| 76 | Strophanthus gratus (Apocynaceae) | A549-12.66 | 79 |
| HCT116-10.44 | |||
| PANC1-42.36 | |||
| Hela-22.6 | |||
| 77 | Coronilla varia (Fabaceae) | HCT116-144 | 80 |
| 78 | MCF-7-165 | ||
| 79 | U-2 OS-44 | ||
| 80 | Digitalis purpurea (Plantaginaceae) | Hela-25.44 | 74 |
| 81 | Digitalis purpurea (Plantaginaceae) | GSC-22 | |
| 82 | Digitalis purpurea (Plantaginaceae) | U2OS-18 | |
| SaOS2-15 | |||
| 83 | Digitalis purpurea (Plantaginaceae) | Huh7-22 | |
| Mahlavu-19 | |||
| 84 | Digitalis purpurea (Plantaginaceae) | DAOY-50 | |
| 85 | Digitalis purpurea (Plantaginaceae) | U2OS-95 | |
| SaOS2-90 | |||
| 86 | Bufo melanostictus Schneider (Bufonidae) | MDA-MB231-20 | 81 |
| Hela-16.6 | |||
| SW620 15.6 | |||
| A549-15.57 | |||
| 87 | Rubia philippinensis (Rubiaceae) | MCF7-240 | 82 |
| 88 | SK-MEL5-175 | ||
| 89 | SK-MEL5-235 | ||
| 90 | Rubia philippinensis (Rubiaceae) | B16 F10-80 | |
| 91 | Rubia philippinensis (Rubiaceae) | MCF-7-178 | |
| 92 | Amygdalin, Amygdalus communis (Rosaceae) | TCCSUP-22.8 | 83 |
| HeLa-16.8 | |||
| SNU-C4-34.8 | |||
| 93 | Angelica archangelica (Apiaceae) | HepG2-39.34 | 84 |
| SPC-A1-80 | |||
| SGC-7901-160 | |||
| HeLa-52.86 | |||
| K562-183 | |||
| 94 | Artemisia capillaris (Asteraceae) | HN22-50.34 | 85 |
| HSC4-20.24 | |||
| 95 | Fraxinus rhynchophylla (Oleaceae) | Hep3B-19.34 | 86 |
| 96 | Ferulago campestris (Apiaceae) | A549-29.34 | 87 |
| 97 | Ferulago campestris (Apiaceae) | A549-180.4 | |
| 98 | A549-205.4 | ||
| 99 | Streptomyces chartreusis (Streptomycetaceae) | L1210-20 | 88 |
| P388-70 | |||
| B16-90.34 | |||
| 100 | Vitellaria paradoxa (Sapotaceae) | HL60-30 | 89 |
| 101 | A549-170 | ||
| 103 | AZ521-78 | ||
| 104 | SKBR-3120 | ||
| 105 | AZ521-108 | ||
| 106 | HL60-90 | ||
| 107 | A549-270 | ||
| 108 | Solanum lycopersicum (Solanaceae) | HT-29-70.89 | 90 |
| 109 | Malus pumila (Rosaceae) | HeLa-70.12 | 91 and 92 |
| AGS-40 | |||
| A549-50 | |||
| HepG2-13.16 | |||
| 110 | Brassica napus (Brassicaceae) | PC3-100.9 | 93 |
| HCT116-360 | |||
| NCIH929-100.73 |
Papyriferoside (63) and its two derivatives with different substitutions [(R1 = O, R2 = α-L-araf-[1-6]-β-D-Glcp) (63) (R1 = O, R2 = β-D-Api-[1-2]-β-D-Glcp) (64) (R1 = H2, R2 = β-D-Glcp) (65)] show cytotoxic effects against lung cancer, colorectal cancer, and normal skin cancer by inducing apoptosis, resulting in cell cycle arrest, downregulation of IB phosphorylation and BCL-2, and over expression of cleaved caspase and BAX proteins.94 Antiaroside (66) and its five derivatives with varied substituents [(R1 = CHO, R2 = OH, R3 = α-OH, R4 = β-O-α-L-rhamnose) (67), (R1 = CHO, R2 = H, R3 = β-OH, R4 = β-O-6-deoxy-β-D-glucose) (68), (R1 = CHO, R2 = H, R3 = β-OH, R4 = β-O-6-deoxy-β-D-allose) (69), (R1 = CHO, R2 = H, R3 = β-OH, R4 = β-O-α-L-rhamnose) (70) and (R1 = CH2OH, R2 = H, R3 = β-OH, R4 = β-O-6-deoxy-β-D-glucose) (71)] suppress lung cancer cell proliferation by inhibiting the cell migration and the epithelial–mesenchymal transition (EMT) processes.95 Calotropin (72) and its derivative 12,16-dihydroxycalotropin (73) induce cell death through an apoptotic process that is caspase-dependent and ideally driven by an extrinsic pathway. These A. subulata cardenolide glycosides could be used as anticancer drugs. Acmophyllin A (74) and Acmophyllin B (75) both promote apoptosis, damage DNA, and/or denature proteins, which trap free radicals and protect cellular macromolecules from oxidative mutilation.96 Ouabain's (76) administration causes an increase in programmed cell death, intracellular ROS production, and breakage of DNA strands. Ouabain also inhibited STAT3-mediated transcription and downstream target proteins, as well as suppressing STAT3 levels and phosphorylation.79 Hyrcanoside (77) and its two derivatives [deglucohyrcanoside (78) and cymarin (79)] showed anticancer potential against leukemia, lung adenocarcinoma, colorectal carcinoma, adenocarcinoma, breast carcinoma, and osteosarcoma by inducing cell cycle arrest in the G2/M phase.80 Phyto-compounds digoxin (80), digitoxin (81), digitoxigenin (82), lanatoside (83), oleandrin (84) and neritaloside (85), reported in Table 2 are cardiac glycosides. The cardiac glycoside binding site has been investigated, in what manner the multifunctional groups of sodium pump is blocked. The first extracellular subunit channel is the most critical component of the binding site. The −1 subunit is overexpressed in several cancers including lung cancer, renal carcinoma, glioma, and melanoma.97 Anti-proliferation, Na+/K+-ATPase activity targeting, and steroid receptor coactivator inhibitions were the key anti-cancer molecular mechanisms of bufalin (86).81 2-Methyl-1,3,6-trihydroxy-9,10-anthraquinone 3-O-(6′-O-acetyl)-α-rhamnosyl(1 → 2)-β-glucoside (87), 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone (88), alizarin (89), purpurin (90) and lucidin-ω-methyl ether (91) can cause cell death in CNE cells by arresting CNE cells at the G1 stage.98 Amygdalin (92) has also been demonstrated to prevent various cancer cells by reducing integrin expression and catenin levels, and inhibiting the Akt-mTOR pathway, which may contribute to cancer cell metastasis suppression.99 Imperatorin (93) decreases the viability of HeLa cells and laryngeal carcinoma (Hep-2) cells by inducing apoptosis and elevating the activity of apoptosis mediator's caspase-3 and caspase-8 in both cell lines.84 Esculetin (94) treats HN22 and HSC4 cells resulted in a substantial reduction of cancer cells, as well as the regulation of Sp1 regulatory protein.85 Fraxini's (95) anti-proliferative effect in Hep3 cells was related to apoptosis and alterations in the mitochondrial structure.86 Coumarin glycosides grandivittin (96), agasyllin (97) and aegelinol benzoate (98) have anticancer properties and showed their anticancer potential by lowering the mitochondrial depolarization potential, modulating the mitochondrial protein pathway, enhancing Bid, Bad, and Box protein expression, and lowering Bcl-xl and Mcl-1 expression.100 Chartreusin's (99) anticancer actions are due to DNA binding and inhibition of topoisomerase II.101 Paradoxoside (100) and its seven derivatives with disparate substituents [(R = GlcA R1 = OH R2 = Xyl-(1-4)-Rha-(1-2)-Ara) (101), (R = GlcA R1 = OH R2 = Rha-(1-3)-Xyl-(1-2)-Rha-(1-2)-Ara) (102), (R = GlcA R1 = OH R2 = Api-(1-3)-Xyl-(1-2)-Rha-(1-2)-Ara) (103), (R = GlcA R1 = OH R2 = Api-(1-3)-Xyl-(1-2)-Rha-(1-2)-Ara) (104), (R = MeGlcA R1 = H R2 = H) (105), (R = Glc-(1-3)-Glc R1 = H R2 = H) (106) and (R = GlcR1 = H R2 = H) (107)] showed anticancer activity on human leukemia, lung cancer, stomach cancer and breast cancer by regulating the microphthalmia-associated transcription factor (MITF), TRP-1 and TRP-2 expression.89 Lycopene90 (108) showed its potential against colon cancer by significantly elevated cleaved caspase 3, BAX, cleaved PARP, and 8-oxo-dG levels in cancer cells. Quercetin91,92 (109) diminished the viability of cervical cancer cells through the induction of G2/M phase cell cycle arrest and apoptosis, alongside the suppression of cell migration and invasion. In the context of gastric cancer, quercetin was observed to inhibit miR-143, while in HepG2 cells, p53 and miR-34a were found to be inhibited. Apigenin [110] showed its anticancer potential by inhibiting the STAT1/COX-2/iNOS signaling pathway.93
3.2 In vivo anti-cancer studies of glycosides
Recent pre-clinical studies of various glycosides from the natural sources with anti-cancer potential are summarised below:Lycopene anticancer activity against ovarian cancer was evaluated in egg-laying hens. First, 200 mg kg−1 and 400 mg kg−1 of lycopene were given to the hens daily for 12 months. At the end of 12 months, hens were sacrificed and ovarian tissues and blood were collected and evaluated. By reducing the expression of NF-κB and STAT3 and increasing the expression of heme oxygenase 1, lycopene shows its anticancer potential.102 Lycopene consumption significantly reduced the metastatic load in an ovarian carcinoma-bearing rat model. Its consumption reduces the expression of CA125. The anti-proliferative and anti-metastatic effects were augmented by the down regulation of ITGB1, MMP9, ITGA5, FAK, ILK, and EMT markers, which reduced the MAPK activity and inhibited integrin 5 protein expression. Lycopene activity against tobacco-induced carcinogens was evaluated in male ferrets. For one month, six groups of ferrets were given 50 mg kg−1 of NNK to induce lung and liver lesions. Following the induction of the lesions, each group was given dietary lycopene for 26 weeks at doses of 2.2 and 6.6 mg kg−1 BW per day, respectively. Lycopene supplementation inhibited NNK-induced pulmonary α7 nAChR and hepatic CYP2E1, which were linked to lower mortality and occurrences of both pulmonary and hepatic lesions.103 The anticancer potential of quercetin against colon cancer was tested in 4 week-old Balb/C mice. The control group received no treatment, whereas the treatment group received 10 mg kg−1 of quercetin per day. The tumor volume was significantly reduced in the treatment group. According to the findings, quercetin has anticancer properties by inhibiting the expression of Notch-1, Jagged 1, Hes-1, and Presenilin-1.104 Then 2 × 105 MCF-7 cells were inserted into mice. Two groups were divided simultaneously; one group was the untreated group which receive only vehicle while the second group received quercetin (50 mg kg−1 i.p.) twice a day for a month. Quercetin inhibits tumor by downregulation of VEGF, PKM2, beclin-1, and p-Akt/Akt.105 Apigenin's anticancer activity against chondrosarcoma was investigated in athymic nude mouse xenografts. Then, 2 × 105 Sw1353 cells were inserted into mouse. The untreated group received no treatment, while the treatment group received 5 mg kg−1 apigenin daily. In the treatment group, the tumor volume was significantly reduced. Apigenin has anti-cancer properties because it inhibits Ki67 expression. Apigenin-induced cell cycle arrest and apoptosis by regulating the expression of Bcl-2.106 Additionally, apigenin (3 mg kg−1) inhibited NSCLC xenograft growth and metastasis by targeting the dipeptidyl peptidase IV (DPPIV) enzyme.107 Digoxin anticancer activity against human lung cancer was investigated in BALB/c nude mouse xenograft model. Following this, 1 × 107 A549 cells were implanted in mouse. After the tumor had grown to about 100 mm3 in diameter, the infected mice were daily given digoxin (1.0 mg kg−1). After 14 days, mice were forfeited, and the tumor volume was collected and evaluated. The results indicated that digoxin inhibits lung cancer by inhibiting both DNA DSB and SSB repairs.108 Digitoxin anticancer activity against cervical cancer was investigated in a BALB/c nude mouse xenograft model. Then, 5 × 106 HeLa cells were implanted into mouse. After the tumor had grown to about 300 mm3 in diameter, the infected mice were given digitoxin (1.0 to 2.0 mg kg−1) daily. After 19 days, mice were forfeited, and the tumor volume was collected and evaluated. Digitoxin shows its potential by arresting the cell.109 Bufalin anticancer activity against human lung cancer was investigated in a BALB/c nude mouse xenograft model. Then, 8 × 106 A549 cells were implanted into mouse. After the tumor had grown to about 300 mm3 in diameter, the infected mice were given bufalin (1 mg to 6 mg kg−1) daily. After 19 days, mice were forfeited, and the tumor volume was collected and evaluated. Bufalin shows its potential by activation of caspase-3 and the cleavage of PARP in A549 cells.110 Bufalin anticancer activity against breast cancer was investigated in athymic nude mice. Then, 5 × 106 MB-231 cells were injected subcutaneously into both dorsal regions of mice and 10 μl of bufalin was injected once a day at an early stage of cancer. Mice were forfeited after 21 days. The tumor was collected and evaluated. The results indicated that bufalin reduced breast cancer angiogenesis by inhibiting the MAPK and NF-kB pathways.111 Alizarin anticancer activity against pancreatic cancer was investigated in the mouse xenograft model. Then 5 × 106 MIA PaCa-2-luc cells were implanted into the mouse. After the tumor had grown to about 300 mm3 in diameter, the infected mice were given alizarin (10 to 30 mg kg−1) daily. After 19 days, mice were sacrificed, and the tumor volume was collected and evaluated. Digitoxin showed its potential by abrogating NF-κB activation.112
| Isolated compounds | Biological source | Cancer cell line along with IC50 (nM) value | References |
|---|---|---|---|
| 111 | Pothomorphe umbellata (Piperaceae) | SK-MEL2-95 | 113 |
| SK-MEL103-100 | |||
| SK-MEL147-90 | |||
| 112 | Cucumis sativus (Cucurbitaceae) | SK MEL28-45 | 114 |
| A-375-30 | |||
| 113 | Betula pendula (Betulaceae) | SK-MEL 28-200 | 115 |
| MSK-MEL2-198.4 | |||
| G361-190 | |||
| 114 | Cinnamomum camphora (Lauraceae) | A549-18.7 | 116 |
| 115 | Cannabis sativa (Cannabaceae) | A549-120 | 117 |
| 116 | Cannabis sativa (Cannabaceae) | C6-22 | |
| 117 | Colchicum autumnale (Colchicaceae) | HA22T/VGH-90 | 118 |
| OVCAR3-129 | |||
| T24-180 | |||
| MDA-MB-231-30 | |||
| 118 | Robinia pseudoacacia (Fabaceae) | LOVO-100 | 119 |
| 119 | Malus domestica (Rosaceae) | Mel 928-25 | 120 |
| 120 | Silybum marianum (Asteraceae) | DU145-24 | 121 and 122 |
| MDAMB-468-46 | |||
| MMP2-63.5 | |||
| CD34-56.4 | |||
| 121 | Anaphalis neelgerriana (Asteraceae) | HaCaT-28 | 123 |
| HL-60-40 | |||
| A549-68 | |||
| H1299-64 | |||
| 122 | Ginkgo biloba (Ginkgoaceae) | MCF7-48, T47D-52 | 124 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Polyphenols | |||
| 123 | Camellia sinensis (Theaceae) | A375 12.8 | 125 |
| Hs294T-8.78 | |||
| 124 | Combretum caffrum (Combretaceae) | P388-28 | 36 |
| 125 | Cajanus cajan (Fabaceae) | HepG2-50.99 | 126 |
| MCF-7-20.56 | |||
| A549-60.18 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Lignan | |||
| 126 | Justicia hyssopifolia L. (Acanthaceae) | MALME-3M-16 | 127 |
| SK-MEL-5.32 | |||
| UACC257-48 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isothiocyanate | |||
| 127 | Sulforaphene | MCF-7-41.1 | 128 |
| HepG2-40.0 | |||
| HT-29-42 | |||
| 128 | Zingiber officinale (Roscoe) | HeLa-250.68 | 129 |
| SiHa-370.52 | |||
| 129 | Allium sativum (Amaryllidaceae) | HepG2-19.26 | 130 |
| MCF7-28.51 | |||
| A549-36 | |||
| PC3-77.92 | |||
| 130 | Derris eriocarpa (Leguminosae) | KB-40.13 | 131 |
| P-388-34.31 | |||
| H2108-56.5 | |||
| 131 | Andrographis paniculata (Acanthaceae) | HL 60-20.4 | 132 |
| HepG2-40.2 | |||
| Lovo-8.6 | |||
| 132 | Scutellaria baicalensis (Lamiaceae) | MDA-MB-231-34.77 | 133 |
| MCF7-41.78 | |||
| 133 | Angelica gigas (Apiaceae) | SNU-216-50 | 134 |
| HT29-293.064 | |||
| A549-200 | |||
| B16F10-80 | |||
| 134 | Angelica gigas (Apiaceae) | PC3-36.3 | 135 |
| 135 | Melilotus officinalis (Fabaceae) | MCF7-40 | 136 |
| 136 | Glycine max (legumes) | MCF7-15 | 137 |
| HepG2-25 | |||
| NCI-H1299-55 | |||
| 137 | Zingiber officinale (Roscoe) | S-180-19.18 | 138 |
| HL-60-111.4 | |||
| 138 | Glycyrrhiza glabra (Fabaceae) | MDA-MB-231-84.22 | 139 |
| 139 | Salvia involucrata (Lamiaceae) | MCF-7-25.44 | 140 |
| HCC38-65.42 | |||
| 140 | Glycyrrhiza glabra (Fabaceae) | MCF-7-17.63 | 141 |
| A549-11.55 | |||
| DU-145-9.45 | |||
| 141 | Azadirachta indica (Meliaceae) | EJ-30 | 142 |
| MDA-MB-231-10.97 | |||
| HT29-40 | |||
| HCT116-75 | |||
| 142 | Physalis pubescens L. (Solanaceae) | SKOV3-60.63 | 143 |
| 143 | Polygonum cuspidatum (Polygonaceae) | HeLa-30 | 144 |
| 144 | Withaferin A | Panc1-10.24 | 145 |
| BxPc-320.78 | |||
| Withania somnifera (Solanaceae) | U87MG-10.4 | ||
| GBM2-19 | |||
In melanoma cell lines, 4-nerolidylcatechol (111) is reported as an inhibitor of cell invasiveness, owing to the G1 cell cycle arrest and inhibition of MMP-2 activity.113 Melanoma has a high prevalence of B-RAF mutations. Cucurbitacin B (112) could be a possibility for inhibiting the signaling kinase pathway. Cucurbitacin B is a kinase inhibitor for B-RAF and MEK1.114 Betulin's (113) anticancer action is based on the stimulation of apoptotic cell death. Betulin treatment caused cytomorphological changes that are typical of apoptotic cells, including cell rounding and the production of apoptotic bodies.115 Camphor (114) white oil caused transcriptional alterations in immune-related genes identified by RNA-sequencing in vivo, leading to tumor regression mediated by cytotoxic T cells.116 The cytotoxicity of myrcene (115) against leukemia cells was shown to be substantial. At 0.01 g ml−1, myrcene decreased t-butyl hydroperoxide-induced DNA damage in human B lymphoid NC–NC cells by 50%.146 Linalool (116) inhibited mitochondrial complexes I and II, increased reactive oxygen species, and lowered ATP and GSH levels in HepG2 cells. Linalool also upregulated p53 and cyclin-dependent kinase inhibitors, which induced strong apoptosis in a variety of leukemia cells.147 By decreasing the mRNA and protein expression of human telomerase reverse transcriptase, costunolide (117) inhibited proliferation in human B cell leukemia cells148 acacetin (118) inhibited epidermal growth factor (EGF)-induced cell transformation and phosphorylation of p70S6K. Acacetin binds to the p110 subunit of PI3-K, interacting with Val828, Glu826 and Tyr813 residues.119 Mitf, a transcription factor related to microphthalmia and found downstream of the Wnt/-catenin pathway, has emerged as a key melanoma prognostic factor. Fisetin (119) (3,7,3′,4′-tetrahydroxyflavone) treatment of melanoma cells resulted in decreased cell survival, G1-phase arrest, and inhibition of Wnt/-catenin signaling. Fisetin-treated cells have higher intracellular levels of Axin and TrCP, as well as reduced glycogen synthase kinase 3 phosphorylation and catenin stabilization.120 Silymarin (120) blocks cyclin-dependent kinase (CDK) activity and increases the levels of the CDK inhibitors p21CIP1 and p27KIP1 such that they are more tightly bound to CDKs, which suppresses EGFR signaling. Silymarin inhibits development at the G1 and G2 checkpoints.121 The bioactive flavonoid, astragalin (121) heptaacetate (AHA) promotes apoptosis in HL-60 cells by releasing cytochrome c into the cytoplasm. Activation of Bax, caspase-3/-7, and p38MAPK, as well as intracellular ROS production and suppression of cell signaling pathways JNK/SAPK and ERK 1/2 also promote apoptosis in HL-60. TNF-induced NF-B activation is significantly inhibited by astragalin in A549 and H1299 cells. Furthermore, astragalin-induced cell death is associated with a time- and dose-dependent increase in the Bax/Bcl-2 ratio, as well as increased cleavage of caspase-3/-9 and PARP123 Ginkgetin (122) decreased cell viability in breast cancer and blocked estrogen receptor (ER) expression at mRNA and protein levels. Ginkgetin therapy also reduced the expression of survivin, and cyclin D1, which are also ER targets.124 Epigallocatechin gallate (123) inhibited cell proliferation by reducing the PCNA protein level and promoted apoptosis in melanoma by assessed cleavage of PARP, TUNEL assay. Treatment of melanoma cells with epigallocatechin gallate leads to a reduction in cyclin D1 and cdk2 protein levels, as well as stimulation of the cyclin kinase inhibitors (ckis) and p27KIP1.125 Combretastatin (124) is the new molecule of vascular disrupting medicines that target tumor blood channels and prevent angiogenesis. Combretastatin affects DNA structure and function by interfering with nucleic acid production and transcription and inhibiting cell proliferation.149 Resveratrol (stilbenoid) [125] was observed to halt the cell cycle at the G2/M phase, also elevating intracellular reactive oxygen species (ROS) and caspase 3 activity, and increasing the Bax/Bcl-2 protein ratio, all of which are indicative of apoptosis in hepatic cancer.126 Elenoside (126) was screened for its anticancer potential on skin cancer cell lines but its mechanism of action is not known.150 Sulforaphane128 (127) induced mitochondrion-mediated apoptosis in cancer cells through the activation of caspase-9, followed by the cleavage and subsequent activation of caspase-3 and caspase-7. 6-Shogaol129 (128) has demonstrated the capability to inhibit the proliferation and migration of cervical cells through the suppression of the PI3K/Akt/mTOR signaling pathway. Allicin130 (129) has been shown to exert its cytotoxic effects by targeting cancer cells during the S and G2/M phases of the cell cycle. Alpinumisoflavone131 (130) modulates several signaling pathways, including PI3K/Akt, MAPK, and those regulating endoplasmic reticulum (ER) stress, ultimately leading to cell death and showcasing its therapeutic potential. Andrographolide132 (131) acts against leukemia by inducing cell cycle arrest in the G0/G1 phase, while also affecting the G2/M, G1, and S phases in hepatoma and colon cancer. The anti-tumor effects of baicalein133 (132) in breast cancer may be attributed to a novel mechanism involving tumor-associated macrophages. Decursin134 (133) reveals its potential by disrupting multiple signaling pathways; for instance, in gastric cancer, it alters the STAT3/c-My pathway and the MAPK/ERK1/2 pathways associated with colon and melanoma cancers. Additionally, decursin affects the PERK/ATF4 pathway, which plays a role in lung cancer. Decursinol135 (134) exerts its cytotoxic properties through the regulation of the G0/G1 phase in prostate cancer cells. The anticancer properties of dicumarol136 (135) have been linked to the inhibition of NQO1. Genistein137 (136) directly inhibits the PLK1 signaling pathway, demonstrating its anticancer efficacy. Gingerol138 (137) has the capacity to induce the generation of reactive oxygen species (ROS) in chronic (K562) and acute myeloid leukemia (U937) tumor cell lines, resulting in the disruption of the G2/M cell cycle, a reduction in cell cycle protein expression (including cyclin B1, Cdk1, Cdc25B, and Cdc25C), and alterations in cellular oxidant status that promote mitochondrial ROS production. Glycyrrhizin139 (138) offers protective and detoxifying effects by reducing the generation of reactive oxygen species, preserving glutathione (GSH), and differentially modulating apoptosis, as well as the Akt, ERK, and JNK pathways within the MAPK signaling cascade. Hispidulin140 (139) has been shown to inhibit TGF-β1-induced Smad2/3 signaling and cell migration across breast cancer. Licochalcone A141 (140) modulates the expression of various signaling pathways, including the EGFR/ERK, PI3K/Akt/mTOR, p38/JNK, MKK4/JNK, mitochondrial apoptosis pathway and the death receptor pathway. It inhibits the expression of proteins involved in the cell cycle and angiogenesis, and regulates both autophagy and apoptosis in cancer cells. Nimbolide142 (141) blocks the attainment of cancer hallmarks such as sustained proliferation, evasion of apoptosis, invasion, angiogenesis, metastasis, and inflammation by influencing kinase-driven oncogenic signaling pathways and shows its potential. Furthermore, physapubescin B143 (142) inhibited the transcriptional activity of STAT3, an oncogenic transcription factor implicated in numerous human malignancies, including ovarian cancer. Pterostilbene144 (143) was linked to the induction of apoptosis in tumor cells, as well as the downregulation of the oncogene E6 and the upregulation of activated caspase-3 levels. Withaferin A145 (144) was found to induce apoptosis and inhibit growth in pancreatic cancer cells through mitochondrial dysfunction and inactivation via the PI3K/Akt pathway.
3.3 In vivo anti-cancer studies of miscellaneous bio-actives
The pre-clinical data of various miscellaneous bio-actives including different terpenoids, flavones, polyphenols, etc., are included as follows:Epigallocatechin anticancer activity against lung cancer was evaluated in A/J female mice. Mice were injected with cisplatin for the induction of cancer. Epigallocatechin (1 mg ml−1, orally) was given to the mice. Male db/db mice were given tap water containing 40 ppm DEN for two weeks, followed by 34 weeks of drinking water containing 0.1% epigallocatechin gallate. The fortified drinking water containing epigallocatechin gallate significantly reduced the development of liver cell adenomas compared to the EGCG-untreated control group. In the livers of experimental mice, epigallocatechin gallate inhibited the phosphorylation of the ERK (extracellular signal-regulated kinase), Akt, Stat3, and JNK proteins. Chitosan-based nano-formulation of epigallocatechin gallate (10 mg ml−1) was also developed for the treatment of prostate cancer and the same was evaluated in a xenograft athymic nude mouse model. The formulation decreased the expression of Ki-67 and VEGF (markers of angiogenesis) in tissues of treated mice.151 Emodin's anticancer activity against human lung epithelial cancer was investigated in BALB/c nude mice with 50 mg per kg emodin daily, which inhibits cell growth (A549) by inducing ER-dependent apoptosis. In hepatocellular cancer, emodin shows its potential by inhibiting the p-JNK expression and increasing ERK and p38 phosphorylation. Emodin's anticancer activity against breast cancer was investigated in C57BL/6 and BALB/c mice. Then, 2 × 105 EO771 or 4T1 cells were inserted into mice and 40 mg per kg emodin was injected once a day at an early stage of cancer. Mice were sacrificed at different time intervals and the tumor was collected and evaluated. The results indicated that emodin reduced breast cancer angiogenesis by inhibiting M2 polarization and macrophage infiltration and increasing T-cell activation.152 Baicalein's anticancer activity against colon cancer was investigated in mouse xenograft. Then 50 mg kg−1 of baicalein was given to the infected (HCT116 cell) mouse. Baicalein shows its activity by downregulating the mitogen-activated protein kinase (MAPK) and p38 signaling pathways.153 A nude mouse model was used to test withaferin A's anticancer activity against colorectal cancer. CRC cells were inserted into the mouse. The mouse was given 5 mg per kg withaferin A orally after the onset of cancer. According to the findings, withaferin A has the potential to inhibit Akt overexpression and micro-vessel formation. Withaferin A's anticancer activity against hepatocellular carcinoma was investigated in athymic nude mouse xenografts. Then, 5 × 106 HepG2 cells were injected subcutaneously into the mice. After 15 days of implantation, mice were divided into the untreated and treatment groups. The untreated group received no treatment, while the treatment group received 4 mg per kg withaferin A orally daily. After 5 weeks of treatment, mice were sacrificed and the tumor was collected and evaluated. Withaferin A showed its anticancer potential by inhibiting Ki67 expression while increasing the ERK, RSK, ELK1, and DR5 levels.154 Some recent pre-clinical data related to the anti-cancer potential of plant bio-actives are also listed in Table 4.
| Isolated compound | Plant | Type of cancer | Model/dose | Reference |
|---|---|---|---|---|
| 6-Shogaol | Zingiber officinale (Roscoe) | Non-small cell lung cancer | Nude mice model (10 mg kg−1) | 155 |
| Allicin | Allium sativum (Amaryllidaceae) | Liver bile duct carcinoma | BALB/c nude mice model (10 mg kg−1) | 156 |
| Alpinum | Derris eriocarpa (Leguminosae) | Renal cell carcinoma | In BALB/c nude mice xenograft (40 mg kg−1) | 157 |
| Andrographolide | Andrographis paniculata (Acanthaceae) | Breast cancer | Nude (BALB/c females, 6–8 weeks old) mice (25, 50, and 100 mg kg−1) | 158 |
| Baicalin | Scutellaria baicalensis (Lamiaceae) | Colon cancer | Nude mice (50 mg kg−1) | 159 |
| Curcumin | Curcuma longa (Zingiberaceae) | Melanoma cancer | Six-week-old female BALB/c nude mice (25 mg kg−1) | 160 |
| Decursin | Angelica gigas (Apiaceae) | Prostate cancer | SCID-NSG mice xenograft (4.5 mg kg−1) | 161 |
| Dicumarol | Melilotus officinalis (Fabaceae) | Ovarian carcinoma | BALB/c nude mouse xenograft model, DIC (30 mg kg−1) | 162 |
| Genistein | Glycine max (legumes) | Leukemia | Male athymic BALB/c nu/nu mice 6–8 week (0.2 or 0.4 mg kg−1) | 163 |
| Gingerol | Zingiber officinale (Roscoe) | Breast cancer | Mice model (5 mg kg−1) | 164 |
| Glycyrrhizin | Glycyrrhiza glabra (Fabaceae) | Non-small cell lung cancer | Athymic BALB/c nude mice xenograft (100 mg kg−1) | 165 |
| Hispidulin | Salvia involucrata (Lamiaceae) | Hepatocellular carcinoma | Nude mice (20 mg kg−1) | 166 |
| Stilbenoid | Polygonum cuspidatum (Polygonaceae) | Breast cancer | Nude mouse mode (5 mg kg−1) | 167 |
| Licochalcone A | Glycyrrhiza glabra (Fabaceae) | Glioma cell | Athymic nude mice (10 mg kg−1) | 168 |
| Nimbolide | Azadirachta indica (Meliaceae) | Pancreatic cancer | Athymic nu/nu mouse model, (5 mg kg−1) | 169 |
| Physapubescin B | Physalis pubescens L. (Solanaceae) | Renal cell carcinoma | Xenograft mouse model (30 mg kg−1) | 170 |
| Pterostilbene | Polygonum cuspidatum (Polygonaceae) | Endometrial cancer | Xenograft mouse model (30 mg kg−1) | 171 |
3.4 Clinical trial data for plant-derived bioactives in cancer management
Despite the fact that an enormous number of anti-cancer molecules are currently being developed, clinical trials using phyto-chemicals to manage different cancers are still in the early stages.172 The trials on anticancer moieties are focused on three important components: first, increasing cancer cells response to standard chemo- and radio-therapy; second, minimising the severe side effects of traditional cancer therapy; and third, identifying undesirable interactions with standard therapy. Preclinical studies of various phytoconstituents have revealed a high potential for treating various types of cancers. Due to a lack of research and knowledge regarding their mechanism of action, the specific site of action, and dose, they failed to enter clinical trials. Currently, only seven phytoconstituents are under clinical trial as reported in Table 5.173–175| Isolated compound | Biological source | Type of cancer | Stage of trial | Identifier code |
|---|---|---|---|---|
| Berberine hydrochloride (alkaloid) | Berberis sp. (Berberidaceae) | Colorectal cancer | Placebo-controlled phase 2/3 trial berberine hydrochloride (1000 patients) (300 mg twice per day) | NCT03281096 |
| Curcumin (polyphenol) | Curcuma longa (Zingiberaceae) | Advanced and metastatic breast cancer | Placebo-controlled phase 2/3 trial curcumin (300 mg i.v. per day) along with paclitaxel (80 mg per m2 BS; i.v.) once a week for 12 weeks | NCT03072992 |
| Epigallocatechin (flavonoids) | Camellia sinensis (Theaceae) | Colorectal cancer | Early phase 1 trial Teavigo™ (highly purified and refined green tea extract providing 94% EGCG) (450 mg PO per day) | NCT02891538 |
| Lycopene (carotenoids) | Solanum lycopersicum (Solanaceae) | Metastatic colorectal cancer | Placebo-controlled phase 2 trial lycopene (20 mg PO per day) to reduce skin toxicity | NCT03167268 |
| Quercetin (carotenoids) | Glycyrrhiza glabra (Leguminosae) | Prostate cancer | Phase 1 trial, placebo-controlled, two arm study of quercetin and green tea to enhance the bioavailability of green tea polyphenols in men scheduled for prostatectomy | NCT01912820 |
| Resveratrol (stilbenoid) (polyphenol) | Polygonum cuspidatum (Polygonaceae) | Low-grade GI neuroendocrine tumors | Placebo-controlled phase 1 trail, resveratrol (2.5 g p.o. twice per day) on Notch-1 signaling in low-grade gastrointestinal neuroendocrine tumors | NCT01476592 |
| Sulforaphane (isothiocyanate) | Brassica oleracea (Brassicaceae) | Former smokers with a high risk of developing lung cancer | Placebo-controlled phase 2 trial Avmacol (sulforaphane) tablets (120 μM p.o. twice per day) | NCT03232138 |
3.5 Structure–activity relationship (SAR) analysis of compounds with similar structures
Berberine and its derivatives have efficient cytotoxic potential against breast cancer, liver cancer and pancreatic cancer, and its structural analysis shows that its antitumor activity is mainly concentrated on C-9 and C-13, the derivatives being more potent than the parent compound. Propyl benzene and 4,4-diphenylbutyl on C-9 showed better anti-breast cancer cell toxicity, while 1-chloro-4-ethylbenzene and phenylpentyl on C-13 are strong electron-withdrawing groups and strengthen cytotoxicity potential against hepatic cancer cell toxicity compared to the parent compound.18SAR analysis shows that analogs of pancratistatin depend largely on the hydroxyl group at C-7 and the functional group substitution at C-1. Three new synthetic analogs, SVTH-5, SVTH-6, and SVTH-7, were examined, which possesses the complete anticancer pharmacophore of pancratistatin, including the hydroxyl group at C-7. As a result, SVTH-6 and SVTH-5 were more effective against cancer cells than related compounds JCTH-4 and JCTH 3, respectively, which lack this functional group. In addition, the functional group at C-1 significantly determines the effectiveness of the analogs. For example, JCTH-1 and JCTH-2 differ from JCTH-4 only in the functional group at C-1 and have hardly any anticancer activity. Similarly, SVTH-7 differs from SVTH-6 and SVTH-5 only in the C-1 group and is more effective against most cancer cell lines tested.43
Cymarin, hyrcanoside and deglucohyrcanoside contain a carbonyl group at C-19 and a β-hydroxyl group at C-5. While ouabain contains a β-hydroxyl group at C-1, α-OH groups at C-10 and C-19, but lacks a β-OH group at C-5. The β-hydroxyl group at C-5 may contribute to general cytotoxicity. However, as reported in the literature, cytotoxicity is significantly affected by the carbonyl group at C-19; when the hydroxyl group at C-19 is replaced by a carbonyl group, the cytotoxicity of the resulting derivative increases.80
4 Discussion
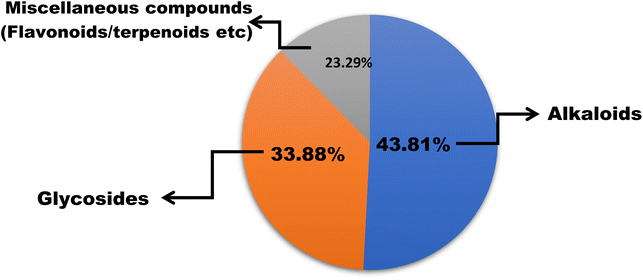
In this work, we reviewed a total of 144 isolated compounds which are having anticancer potential against different cell lines. Out of 144, there are 62 alkaloids, 47 glycosides, and 35 other isolated compounds, which include flavones, terpenoids, terpene, and polyphenols (Fig. 7). Out of 62 alkaloids, only one alkaloid berberine is currently under clinical trials in placebo-controlled phase 2/3 on colorectal cancer.The IC50 value of berberine is 250 nM for breast cancer cell lines, 400 nM for colorectal cancer, and 200 nM for chondrosarcoma and lung cancer. Berberine's structural alteration for anticancer action had primarily focused on C9 and C13. To increase the efficacy and bioavailability, some cycloberberine derivatives were also developed. By enhancing the moderate DNA-binding affinities of protoberberine alkaloids, five derivatives substantially inhibit human HepG2 and human colon cancer cell lines. With an IC50 of 200 nM, evodiamine has anticancer potential in human liver cancer cell lines (HepG2 and PLHC-1). The best derivative was 4-chlorobenzene, which had IC50 values of 8.6, 4.9, and 260 nM against A549 (lung cancer), MDA-MB435 (breast cancer), and HCT116 (colon cancer) cell lines with N-substitution series of evodiamine derivatives. Bulbispermine shows cytotoxic effects against glioblastoma (T98G and U373) and human leukemia (HL-60) with IC50 values of 90 nM, 380 nM, and 80 nM. Distichamine shows anticancer activity against HeLa, CEM, K562, MCF-7, and G-361 with IC50 values of 22–147 nM. Lycorine had IC50 ranging from 50 nM to 100 nM for different cell lines such as PC-3M, DU145, LNCaP, and 22RV1 and in vivo lycorine (5 mg per kg per day or 10 mg per kg per day) reduces prostate cancer.
We have also reviewed 47 glycosides that have anticancer potential against various cancer cell lines and digoxin was found to have the greatest potential to treat various cancers. Digoxin has undergone 27 clinical trials in which 11 trails are completed, 7 trails are under recruiting, 1 trail is not yet recruiting, 2 trails are active, 2 trails are terminated, 3 studies have unknown status, and 1 study is withdrawn. All trials involved digoxin alone or a combination of digoxin with other drugs such as enzalutamide, rosuvastatin, capecitabine, lapatinib, metformin, and simvastatin. These trials are conducted on various cancer cell lines such as prostate, head and neck, pancreatic lung, and breast cancer on neoplasm and solid tumours. Ouabain had IC50 values for H460 and PANC1 of 10.44 nM and 42.36 nM respectively. The IC50 value of bufalin is 20.0 nM for breast cancer, 16.6 nM for cervical cancer, 28.23 nM for gallbladder cancer and 15.57 nM for lung cancer. Imperatorin inhibits colon cancer with an IC50 value of 78 nM. In combination with quercetin, imperatorin showed the synergistic effect by reducing the cell viability of HeLa cells to 52.86% and for Hep-2 cells to 39.34%. Esculetin inhibits the HCC cell with an IC50 value of 2.24 nM and reduces the tumor growth by 20.33, 40.37, and 55.42% in Hepa1-6 cell-containing mice. Cardiac glycosides such as digitoxin, digitoxigenin, lanatoside, oleandrin and neritaloside showed anticancer potential with significant IC50 values of 22 nM, 15 nM, 19 nM, 50 nM, and 90 nM on lung cancer (A549 and H1975), and of 59 nM, 43 nM, 45 nM, 1104 nM, and 165 nM on osteosarcoma (U2OS and SaOS-2) respectively.
Amongst miscellaneous isolated compounds epigallocatechin curcumin, lycopene, and resveratrol are under clinical trials. Totally 32 clinical trials were studied on epigallocatechin, of which 16 studies are completed, 6 trials are under recruiting, 1 study is enrolled by invitation, 1 study is active but this study is not recruiting, 6 studies are terminated and 2 trials are withdrawn. Camphor has one completed clinical trial related to the feasibility of recruiting pediatric patients receiving chemotherapy for cancer towards homeopathy. Fisetin has one clinical trial and this trial is not yet recruiting, which is related to the efficacy of the combination of dasatinib with quercetin and fisetin to reduce senescence and to improve frailty in adult survivors of childhood cancer. Silymarin has 6 clinical trials and all 6 are completed, and these trials were for colorectal, breast cancer, prostate cancer, upper GI cancer, colon, and leukemia. A total of 17 clinical trials have been done on combretastatin, in which 11 studies are completed, 4 studies are terminated, 1 trail has unknown status and 1 trail is withdrawn. These trails include head and neck cancer, sarcoma, neuroendocrine tumor, and solid tumors. Linalool shows anticancer potential against prostate cancer with IC50 values of 28.3 and 10.5 nM at 24 h and 48 h respectively.
Based on the aforementioned findings from the literature, we can state that each plant bioactive has a different method of action that targets different cancer cells. Alkaloids typically prevent cancer by blocking the replication of DNA and causing protein denaturation, and this leads to apoptosis. Moreover, alkaloids inhibit the caspase inhibitor and the G2/M phase, while glycosides decrease the proliferation of cells by altering the expression of IB phosphorylation, BCL-2, caspase 3, and BAX proteins. Cardiac glycosides block the transport of sodium ions across the membrane, and this causes an increase in the concentration of calcium ions in the plasma membrane, which is involved in the regulation of multiple signal pathways, including apoptosis. The basic mechanism of action of these secondary metabolites is also represented in Fig. 8. Among the seven compounds currently undergoing clinical trials, curcumin, resveratrol and berberine stand out as the most significant. Clinical investigations of curcumin are assessing its efficacy against various cancers, such as colorectal, pancreatic, and breast cancers. However, curcumin's low solubility in water restricts its absorption within the gastrointestinal tract, leading to a low concentration of the compound in the bloodstream and posing challenge in achieving therapeutic levels.177 Researchers are actively seeking methods to improve its bioavailability, given that the natural form of curcumin is poorly absorbed by the body. Additionally, there is some evidence regarding the ideal dosage and administration frequency for curcumin, especially in the context of cancer treatment. This ambiguity complicates the design of clinical trials and hinders the ability to compare findings across different studies. In the case of resveratrol, clinical trials are investigating its potential in the prevention or treatment of cancers, including breast, prostate, and colon cancer. However, similar to curcumin, the low bioavailability of resveratrol presents a challenge for therapeutic use, with resveratrol being rapidly metabolized and excreted from the body, resulting in low plasma concentrations. It is extensively metabolized in the liver and intestines, limiting the amount that enters the bloodstream and target tissues.178 While resveratrol is generally considered safe at lower doses, higher doses may cause side effects, such as gastrointestinal upset and, in some cases, kidney damage. Clinical investigations of berberine are currently underway to test the effectiveness of berberine in various types of cancer, including lung and colon cancer, as well as to determine its potential synergistic effects with conventional chemotherapy. Berberine can inhibit enzymes involved in drug metabolism, such as cytochrome P450 enzymes, which may interact with other medications and reduce their effectiveness or increase the risk of side effects.179
5 Conclusion
Medicinal plants contain a significant number of secondary metabolites belonging to various categories such as alkaloids, glycosides, flavonoids, terpenes, and terpenoids. These compounds have shown promising anticancer properties against multiple types of cancers. Extensive literature reviews have indicated that each active compound from plants exhibits a distinct mechanism of action in the treatment of different cancers. Additionally, certain phytoconstituents such as vinca alkaloids, taxane diterpenoids, camptothecin derivatives, and epipodophyllotoxin are currently utilized in cancer therapy. Meanwhile, berberine, curcumin, lycopene, quercetin, resveratrol, and sulforaphane are currently under clinical trials. In this work, the authors reviewed a large number of secondary metabolites, which play an important role in preventing and treating various types of cancers and are under different stages of clinical trials. To conclude, we can say that, plant-derived bioactives hold tremendous anticancer potential, which could lead to the establishment of novel therapeutic agents. However, persistent study is required to discover the uncovered moieties of new plants with anticancer potential, which may offer breakthrough for improving anticancer therapy.Data availability
No primary research results have been included and no new data were generated or analysed as part of this review.Conflicts of interest
All the authors declared no conflict for the submitted manuscript.Acknowledgements
The authors acknowledge the Vivekanand Library, Maharshi Dayanand University Rohtak, for providing necessary literature facilities for the present study.References
- D. T. Debela, S. G. Muzazu, K. D. Heraro, M. T. Ndalama, B. W. Mesele, D. C. Haile, S. K. Kitui and T. Manyazewal, New approaches and procedures for cancer treatment: current perspectives, SAGE Open Med., 2021, 9, 20503121211034370 CrossRef.
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Cancer statistics, Ca-Cancer J. Clin., 2024, 74, 12–49 Search PubMed.
- F. Artosi, G. Costanza, M. Di Prete, V. Garofalo, F. Lozzi, E. Dika, T. Cosio, L. Diluvio, R. G. Shumak, S. Lambiase, C. Di Raimondo, S. Campa, P. Piscitelli, A. Miani, L. Bianchi and E. Campione, Epidemiological and clinical analysis of exposure-related factors in non-melanoma skin cancer: a retrospective cohort study, Environ. Res., 2024, 247, 118117 Search PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, Ca-Cancer J. Clin., 2021, 71, 209–249 Search PubMed.
- M. Kapałczyńska, T. Kolenda, W. Przybyła, M. Zajączkowska, A. Teresiak, V. Filas, M. Ibbs, R. Bliźniak, Ł. Łuczewski and K. Lamperska, 2D and 3D cell cultures–a comparison of different types of cancer cell cultures, Arch. Med. Sci., 2018, 14, 910–919 Search PubMed.
- U. Leiter, U. Keim and C. Garbe, Epidemiology of Skin Cancer: Update 2019, in: Sunlight, Vitamin D and Skin Cancer, Adv. Exp. Med. Bio., ed. J. Reichrath, 2020, p. 1268 Search PubMed.
- I. G. Lempesis, V. E. Georgakopoulou, P. Papalexis, G. P. Chrousos and D. A. Spandidos, Role of stress in the pathogenesis of cancer, Int. J. Oncol., 2023, 63, 1–4 Search PubMed.
- O. V. Bukhtoyarov and D. M. Samarin, Pathogenesis of Cancer: Cancer Reparative Trap, J. Cancer Ther., 2015, 6, 399–412 Search PubMed.
- Z. Wang and X. Wu, Abnormal function of telomere protein TRF2 induces cell mutation and the effects of environmental tumor-promoting factors, Oncol. Rep., 2021, 46, 1–20 Search PubMed.
- S. P. Leong and M. H. Witte, Cancer metastasis through the lymphatic versus blood vessels, Clin. Exp. Metastasis, 2024, 41, 387–402 CrossRef PubMed.
- E. N. Kontomanolis, A. Koutras, A. Syllaios, D. Schizas, S. Kalagasidou, A. Pagkalos, D. Alatzidou, P. Kantari, T. Ntounis and Z. Fasoulakis, Basic principles of molecular biology of cancer cell-Molecular cancer indicators, J. BUON, 2021, 26, 1723–1734 Search PubMed.
- G. Gaobotse, S. Venkataraman, P. D. Brown, K. Masisi, T. E. Kwape, D. O. Nkwe, G. Rantong and A. Makhzoum, The use of African medicinal plants in cancer management, Front. Pharmacol., 2023, 14, 1122388 CrossRef CAS PubMed.
- P. Bajpai, S. Usmani, R. Kumar and O. Prakash, Recent advances in anticancer approach of traditional medicinal plants: a novel strategy for cancer chemotherapy, Intell. Pharm., 2024, 2, 291–304 Search PubMed.
- H. Y. Yip and A. Papa, Signaling pathways in cancer: therapeutic targets, combinatorial treatments, and new developments, Cells, 2021, 10, 659 CrossRef CAS PubMed.
- J. Yi, F. Peng, J. Zhao and X. Gong, METTL3/IGF2BP2 axis affects the progression of colorectal cancer by regulating m6A modification of STAG3, Sci. Rep., 2023, 13, 17292 Search PubMed.
- M. Ghanbari-Movahed, T. Kaceli, A. Mondal, M. H. Farzaei and A. Bishayee, Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review, Biomedicines, 2021, 9, 480 CrossRef CAS PubMed.
- E. Matrineo, G. Casamassima, S. Castiglione, E. Cellupica, S. Pantalone, F. Papagni, M. Rui, A. M. Siciliano and S. Collina, Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead, Bioorg. Med. Chem. Lett., 2018, 28(17), 2816–2826 CrossRef.
- C. Zhang, J. Sheng, G. Li, L. Zhao, Y. Wang, W. Yang, X. Yao, L. Sun, Z. Zhang and R. Cui, Effects of Berberine and Its Derivatives on Cancer: A Systems Pharmacology Review, Front. Pharmacol., 2020, 10, 1461 CrossRef PubMed.
- C. Wen, L. Wu, L. Fu, X. Zhang and H. Zhou, Berberine enhances the anti-tumor activity of tamoxifen in drug-sensitive MCF-7 and drug-resistant MCF-7/TAM cells, Mol. Med. Rep., 2016, 14, 2250–2256 CrossRef CAS.
- C. Sheng, Z. Miao and W. Zhang, Topoisomerase I Inhibitors Derived from Natural Products: Structure–Activity Relationships and Antitumor Potency, Atta-ur-Rahman editors, Stud. Nat. Prod. Chem., 2016, 47, 1–28 CAS.
- H. Zhang, L. Chen, X. Sun, Q. Yang, L. Wan and C. Guo, Matrine: A Promising Natural Product With Various Pharmacological Activities, Front. Pharmacol., 2020, 11, 588 CrossRef CAS PubMed.
- L. Jiang, L. Wu, F. Yang, N. Almosnid, X. Liu, J. Jiang, E. Altman, L. Wang and Y. Gao, Synthesis, biological evaluation and mechanism studies of matrine derivatives as anticancer agents, Oncol. Lett., 2017, 14, 3057–3064 Search PubMed.
- J. J. Nair, J. Bastida, F. Viladomat and J. van Staden, Cytotoxic agents of the crinane series of amaryllidaceae alkaloids, Nat. Prod. Commun., 2012, 7, 1677–1688 Search PubMed.
- B. Abebe, S. Tadesse, A. Hymete and D. Bisrat, Antiproliferative Effects of Alkaloids from the Bulbs of Crinum abyscinicum Hochst. ExA. Rich, Evid.-Based Complementary Altern. Med., 2020, 2020, 2529730 CrossRef.
- G. Luchetti, R. Johnston, V. Mathieu, F. Lefranc, K. Hayden, A. Andolfi, D. Lamoral-Theys, M. R. Reisenauer, C. Champion, S. C. Pelly, W. A. Van Otterlo, I. V. Magedov, R. Kiss, A. Evidente, S. Rogelj and A. Kornienko, Bulbispermine: a crinine-type Amaryllidaceae alkaloid exhibiting cytostatic activity toward apoptosis-resistant glioma cells, ChemMedChem, 2012, 7, 815–822 CrossRef CAS PubMed.
- M. M. He, C. R. Qu, O. D. Gao, X. M. Hu and X. C. Hong, Biological and pharmacological activities of amaryllidaceae alkaloids, RSC Adv., 2015, 5, 16562–16574 RSC.
- K. Habartová, L. Cahlíková, M. Řezáčová and R. Havelek, The Biological Activity of Alkaloids from the Amaryllidaceae: From Cholinesterases Inhibition to Anticancer Activity, Nat. Prod. Commun., 2016, 11, 1587–1594 CrossRef.
- M. Roy, L. Liang, X. Xiao, P. Feng, M. Ye and J. Liu, Lycorine: a prospective natural lead for anticancer drug discovery, Biomed. Pharmacother., 2018, 107, 615–624 CrossRef CAS.
- G. Van Goietsenoven, A. Andolfi, B. Lallemand, A. Cimmino, D. Lamoral-Theys, T. Gras, A. Abou-Donia, J. Dubois, F. Lefranc, V. Mathieu, A. Kornienko, R. Kiss and A. Evidente, Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells, J. Nat. Prod., 2010, 73, 1223–1227 CrossRef CAS PubMed.
- J. J. Nair and J. van Staden, Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae, Nat. Prod. Commun., 2014, 9(8), 1193–1210 CrossRef CAS.
- A. Manayi, S. M. Nabavi, W. N. Setzer and S. Jafari, Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies, Curr. Med. Chem., 2018, 25, 4918–4928 CrossRef CAS PubMed.
- E. Turrini, P. Sestili and C. Fimognari, Overview of the anticancer potential of the “King of Spices” Piper nigrum and its main constituent piperine, Toxins, 2020, 12, 747 Search PubMed.
- M. Sun, W. Lou, J. Y. Chun, D. S. Cho, N. Nadiminty, C. P. Evans, J. Chen, J. Yue, Q. Zhou and A. C. Gao, Sanguinarine suppresses prostate tumor growth and inhibits survivin expression, Genes Cancer, 2010, 1, 283–292 Search PubMed.
- S. Q. Pang, G. Q. Wang, J. S. Lin, Y. Diao and R. A. Xu, Cytotoxic activity of the alkaloids from Broussonetiapapyrifera fruits, Pharm. Biol., 2014, 52, 1315–1319 CrossRef CAS.
- J. Lu, J. Bao, X. Chen, M. C. Huang and Y. Wang, Alkaloids Isolated from Natural Herbs as the Anticancer Agents, Evid.-Based Complementary Altern. Med., 2012, 1–12 Search PubMed.
- K. Piska, A. Gunia-Krzyżak, P. Koczurkiewicz, K. Wójcik-Pszczoła and E. Pękala, Piperlongumine (piplartine) as a lead compound for anticancer agents - synthesis and properties of analogues: a mini-review, Eur. J. Med. Chem., 2018, 156, 13–20 CrossRef CAS PubMed.
- K. I. Mohd, A. Barman and N. Qais, Anti-Cancer constituents from plants: a brief review, Dhaka Univ. J. Pharm. Sci., 2020, 19, 83–96 Search PubMed.
- C. H. Yan, F. Li and Y. C. Ma, Plumbagin shows anticancer activity in human osteosarcoma (MG-63) cells via the inhibition of S-phase checkpoints and down-regulation of c-myc, Int. J. Clin. Exp. Med., 2015, 8, 14432–14439 CAS.
- X. Q. Zhang, C. Y. Yang, X. F. Rao and J. P. Xiong, Plumbagin shows anti-cancer activity in human breast cancer cells by the upregulation of p53 and p21 and suppression of G1 cell cycle regulators, Eur. J. Gynaecol. Oncol., 2016, 37, 30–35 CAS.
- M. A. Khan, H. C. Chen, M. Tania and D. Z. Zhang, Anticancer activities of Nigella sativa (black cumin), Afr. J. Tradit., Complementary Altern. Med., 2011, 8, 226–232 Search PubMed.
- J. J. Nair, J. van Staden and J. Bastida, Apoptosis-Inducing Effects of Amaryllidaceae Alkaloids, Curr. Med. Chem., 2016, 23, 161–185 CrossRef CAS PubMed.
- A. M. Chapa-Oliver and L. Mejía-Teniente, Capsaicin: From Plants to a Cancer-Suppressing Agent, Molecules, 2016, 21, 931 CrossRef PubMed.
- D. Ma, C. Pignanelli, D. Tarade, T. Gilbert, M. Noel, F. Mansour, S. Adams, A. Dowhayko, K. Stokes, S. Vshyvenko, T. Hudlicky, J. McNulty and S. Pandey, Cancer cell mitochondria targeting by pancratistatin analogs is dependent on functional complex ii and iii, Sci. Rep., 2017, 7, 42957 Search PubMed.
- C. Griffin, C. Hamm, J. McNulty and S. Pandey, Pancratistatin induces apoptosis in clinical leukemia samples with minimal effect on non-cancerous peripheral blood mononuclear cells, Cancer Cell Int., 2010, 10, 6 CrossRef PubMed.
- Y. Wang, Y. Liu, X. Du, H. Ma and J. Yao, The Anti-Cancer Mechanisms of Berberine: A Review, Cancer Manage. Res., 2020, 12, 695–702 CrossRef CAS.
- T. Koltai, Re-Purposing Evodiamine as an Anti-Cancer Drug: Effects on Migration and Apoptosis, Open Access J. Oncol. Med., 2018, 1(3), 37–51 Search PubMed.
- J. Jia, X. Kang, Y. Liu and J. Zhang, Inhibition of human liver cancer cell growth by evodiamine involves apoptosis and deactivation of PI3K/AKT pathway, Appl. Biol. Chem., 2020, 63, 67 Search PubMed.
- Q. An, C. Han, Y. Zhou, F. Li, D. Li, X. Zhang, Z. Yu, Z. Duan and Q. Kan, Matrine induces cell cycle arrest and apoptosis with recovery of the expression of miR-126 in the A549 non-small cell lung cancer cell line, Mol. Med. Rep., 2016, 14, 4042–4048 Search PubMed.
- H. Huang, Q. Wang, T. Du, C. Lin, Y. Lai, D. Zhu, W. Wu, X. Ma, S. Bai, Z. Li, L. Liu and Q. Li, Matrine inhibits the progression of prostate cancer by promoting expression of GADD45B, Prostate, 2018, 78, 327–335 CrossRef CAS PubMed.
- J. J. Nair, L. Rárová, M. Strnad, J. Bastida and J. van Staden, Apoptosis-inducing effects of distichamine and narciprimine, rare alkaloids of the plant family Amaryllidaceae, Bioorg. Med. Chem. Lett., 2012, 22, 6195–6199 CrossRef CAS PubMed.
- G. Xin, M. Yu, Y. Hu, S. Gao, Z. Qi, Y. Sun, W. Yu and J. He, Yubin, Effect of lycorine on the structure and function of hepatoma cell membrane in vitro and in vivo, Biotechnol. Biotechnol. Equip., 2020, 34, 104–114 Search PubMed.
- A. T. Mbaveng, G. Bitchagno, V. Kuete, P. Tane and T. Efferth, Cytotoxicity of ungeremine towards multi-factorial drug resistant cancer cells and induction of apoptosis, ferroptosis, necroptosis and autophagy, Phytomedicine, 2019, 60, 152832 CrossRef CAS PubMed.
- R. A. Rather and M. Bhagat, Cancer chemoprevention and piperine: molecular mechanisms and therapeutic opportunities, Front. Cell Dev. Biol., 2018, 6, 10 CrossRef PubMed.
- I. W. Achkar, F. Mraiche, R. M. Mohammad and S. Uddin, Anticancer potential of sanguinarine for various human malignancies, Future Med. Chem., 2017, 9, 933–950 CrossRef CAS PubMed.
- F. Luan, X. He and N. Zeng, Tetrandrine: a review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems, J. Pharm. Pharmacol., 2020, 72, 1491–1512 CrossRef CAS PubMed.
- D. P. Bezerra, C. Pessoa, M. O. de Moraes, N. Saker-Neto, E. R. Silveira and L. V. Costa-Lotufo, Overview of the therapeutic potential of piplartine (piperlongumine), Eur. J. Pharm. Sci., 2013, 48, 453–463 CrossRef CAS PubMed.
- Z. Yin, J. Zhang, L. Chen, Q. Guo, B. Yang, W. Zhang and W. Kang, Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances, BioMed Res. Int., 2020, 2020, 6940953 CrossRef PubMed.
- M. A. Khan, M. Tania, S. Fu and J. Fu, Thymoquinone, as an anticancer molecule: from basic research to clinical investigation, Oncotarget, 2017, 8, 51907–51919 CrossRef PubMed.
- J. R. Friedman, N. A. Nolan, K. C. Brown, S. L. Miles, A. T. Akers, K. W. Colclough, J. M. Seidler, J. M. Rimoldi, M. A. Valentovic and P. Dasgupta, Anticancer Activity of Natural and Synthetic Capsaicin Analogs, J. Pharmacol. Exp. Ther., 2018, 364, 462–473 Search PubMed.
- G. C. Arya, K. Kaur and V. Jaitak, Isoxazole derivatives as anticancer agent: a review on synthetic strategies, mechanism of action and SAR studies, Eur. J. Med. Chem., 2021, 221, 113511 CrossRef CAS.
- H. Ruan, Y. Y. Zhan, J. Hou, B. Xu, B. Chen, Y. Tian, D. Wu, Y. Zhao, Y. Zhang, X. Chen, P. Mi, L. Zhang, S. Zhang, X. Wang, H. Cao, W. Zhang, H. Wang, H. Li, Y. Su, X. K. Hu and T. Zhang, Berberine binds RXR alpha to suppress beta-catenin signaling in colon cancer cells, Oncogene, 2017, 36, 6906–6918 Search PubMed.
- Y. Wang and S. L. Zhang, Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2, Biomed. Pharmacother., 2018, 103, 1287–1293 Search PubMed.
- R. G. Xiong, S. Y. Huang, S. X. Wu, D. D. Zhou, Z. J. Yang, A. Saimaiti, C. N. Zhao, A. Shang, Y. J. Zhang, R. Y. Gan and H. B. Li, Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review, Molecules, 2022, 27, 4523 CrossRef CAS PubMed.
- S. Guo, Y. Chen, C. Pang, X. Wang, S. Shi, H. Zhang, H. An and Y. Zhan, Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects, J. Cell. Physiol., 2019, 234(6), 8698–8708 CrossRef CAS PubMed.
- X. Xiao, M. Ao, F. Xu, X. Li, J. Hu, Y. Wang, D. Li, X. Zhu, C. Xin and W. Shi, Effect of matrine against breast cancer by downregulating the vascular endothelial growth factor via the Wnt/β-catenin pathway, Oncol. Lett., 2018, 15(2), 1691–1697 Search PubMed.
- W. H. Talib, Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone, Sci. Pharm., 2017, 85, 27 CrossRef.
- J. Liu, S. Sun, C. Zhou, Z. Sun, Q. Wang and C. Sun, In vitro and in vivo anticancer activity of Lycorine in prostate cancer by inhibiting NF-κB signaling pathway, J. Cancer, 2022, 13, 3151–3159 CrossRef CAS PubMed.
- G. Xin, M. Yu, Y. Hu, S. Gao, Z. Qi, Y. Sun, W. Yu, J. He and Y. Ji, Effect of lycorine on the structure and function of hepatoma cell membrane in vitro and in vivo, Biotechnol. Biotechnol. Equip., 2020, 34, 104–114 CrossRef CAS.
- Q. Guo, Y. Liu, J. Zhao, J. Wang, Y. Li, Y. Pang, J. Chen and J. Wang, Evodiamine inactivates NF-κB and potentiates the antitumor effects of gemcitabine on tongue cancer both in vitro and in vivo, OncoTargets Ther., 2018, 12, 257–267 CrossRef PubMed.
- Z. B. Jiang, J. M. Huang, Y. J. Xie, Y. Z. Zhang, C. Chang, H. L. Lai, W. Wang, X. J. Yao, X. X. Fan, Q. B. Wu, C. Xie, M. F. Wang and E. L. Leung, Evodiamine suppresses non-small cell lung cancer by elevating CD8+ T cells and downregulating the MUC1-C/PD-L1 axis, J. Exp. Clin. Cancer Res., 2020, 39, 249 CrossRef CAS.
- Y. H. Deng, Y. C. Dai and B. Tang, Effect of Evodiamine on the Ki-67 Expression in Tumor Tissue of Lymphoma Transplanted Mice Model, Clin. Basic Bridging Res., 2020, 36, 655–657 Search PubMed.
- D. Zeng, P. Zhou, R. Jiang, X. P. Li, S. Y. Huang, D. Y. Li, G. L. Li, L. S. Li, S. Zhao, L. Hu, J. H. Ran, D. L. Chen, Y. P. Wang and J. Li, Evodiamine inhibits vasculogenic mimicry in HCT116 cells by suppressing hypoxia-inducible factor 1-alpha-mediated angiogenesis, Anticancer Drugs, 2021, 32, 314–322 CrossRef CAS PubMed.
- S. Y. Hyun, H. T. Le, H. Y. Min, H. Pei, Y. Lim, I. Song, Y. T. K. Nguyen, S. Hong, B. W. Han and H. Y. Lee, Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70, Theranostics, 2021, 11, 2932–2952 CrossRef CAS.
- N. F. Z. Schneider, C. Cerella, C. M. O. Simões and M. Diederich, Anticancer and Immunogenic Properties of Cardiac Glycosides, Molecules, 2017, 22, 1932 CrossRef CAS.
- V. L. Muilenburg, P. L. Phelan, P. Bonello and D. A. Herms, Inter- and intra-specific variation in stem phloem phenolics of paper birch (Betula papyrifera) and European white birch (Betula pendula), J. Chem. Ecol., 2011, 37, 1193–1202 CrossRef CAS.
- H. Khan, M. Saeedi, S. M. Nabavi, M. S. Mubarak and A. Bishayee, Glycosides from medicinal plants as potential anticancer agents: emerging trends towards future drugs, Curr. Med. Chem., 2019, 26, 2389–2406 CrossRef CAS.
- L. A. Rascón-Valenzuela, C. Velázquez, A. Garibay-Escobar, W. Vilegas, L. A. Medina-Juárez, N. Gámez-Meza and R. E. Robles-Zepeda, Apoptotic activities of cardenolide glycosides from Asclepias subulata, J. Ethnopharmacol., 2016, 193, 303–311 CrossRef.
- Z. A. Shah, A. Hameed, A. Ahmed, S. U. Simjee, A. Jabeen, A. Ullah and F. Shaheen, Cytotoxic and anti-inflammatory salicin glycosides from leaves of Salix acmophylla, Phytochem. Lett., 2016, 17, 107–113 CrossRef CAS.
- J. Du, L. Jiang, F. Chen, H. Hu and M. Zhou, Cardiac glycoside ouabain exerts anticancer activity via downregulation of STAT3, Front. Oncol., 2021, 11, 684316 CrossRef CAS PubMed.
- S. Rimpelová, T. Zimmermann, P. B. Drašar, B. Dolenský, J. Bejček, E. Kmoníčková, P. Cihlářová, S. Gurská, L. Kuklíková, M. Hajdůch, T. Ruml, L. Opletal, P. Džubák and M. Jurášek, Steroid glycosides hyrcanoside and deglucohyrcanoside: on isolation, structural identification, and anticancer activity, Foods, 2021, 10, 136 CrossRef.
- C. S. Cheng, J. Wang, J. Chen, K. T. Kuo, J. Tang, H. Gao, L. Chen, Z. Chen and Z. Meng, New therapeutic aspects of steroidal cardiac glycosides: the anticancer properties of Huachansu and its main active constituent Bufalin, Cancer Cell Int., 2019, 19, 92 CrossRef PubMed.
- V. K. Bajpai, M. B. Alam, K. T. Quan, H. J. Choi, H. An, M. K. Ju, S. H. Lee, Y. S. Huh, Y. K. Han and M. Na, Cytotoxic properties of the anthraquinone derivatives isolated from the roots of Rubia philippinensis, BMC Complementary Altern. Med., 2018, 18, 200 CrossRef PubMed.
- E. Jaszczak-Wilke, Ż. Polkowska, M. Koprowski, K. Owsianik, A. E. Mitchell and P. Bałczewski, Amygdalin: toxicity, anticancer activity and analytical procedures for its determination in plant seeds, Molecules, 2021, 26, 2253 CrossRef PubMed.
- E. Kozioł and K. Skalicka-Woźniak, Imperatorin-pharmacological meaning and analytical clues: profound investigation, Phytochem. Rev., 2016, 15, 627–649 CrossRef.
- J. H. Cho, J. C. Shin, J. J. Cho, Y. H. Choi, J. H. Shim and J. I. Chae, Esculetin (6,7-dihydroxycoumarin): a potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells, Int. J. Oncol., 2015, 46, 265–271 CrossRef CAS.
- P. Yang, Y. Jiang, Y. Pan, X. Ding, P. Rhea, J. Ding, D. H. Hawke, D. Felsher, G. Narla, Z. Lu and R. T. Lee, Mistletoe extract Fraxini inhibits the proliferation of liver cancer by down-regulating c-Myc expression, Sci. Rep., 2019, 9, 6428 CrossRef PubMed.
- S. Rosselli, A. M. Maggio, N. Faraone, V. Spadaro, S. L. Morris-Natschke, K. F. Bastow, K. H. Lee and M. Bruno, The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol, Nat. Prod. Commun., 2009, 4, 1701–1706 CrossRef CAS.
- J. Portugal, Chartreusin, elsamicin A and related anti-cancer antibiotics, Curr. Med. Chem.:Anti-Cancer Agents, 2003, 3, 411–420 CrossRef CAS PubMed.
- J. Zhang, M. Kurita, T. Shinozaki, M. Ukiya, K. Yasukawa, N. Shimizu, H. Tokuda, E. T. Masters, M. Akihisa and T. Akihisa, Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities, Phytochemicals, 2014, 108, 157–170 CrossRef CAS.
- D. Ataseven, A. Öztürk, M. Özkaraca and Z. Joha, Anticancer activity of lycopene in HT-29 colon cancer cell line, Med. Oncol., 2023, 24(40), 127 CrossRef.
- X. Chen, P. Xu, H. Zhang, X. Su, L. Guo, X. Zhou, J. Wang, P. Huang, Q. Zhang and R. Sun, EGFR and ERK activation resists flavonoid quercetin-induced anticancer activities in human cervical cancer cells in vitro, Oncol. Lett., 2021, 22, 1–3 Search PubMed.
- K. Andrijauskaite, J. Morris and M. J. Wargovich, Natural Anticancer Agents, Academic Press, 2019, pp. 49–73 Search PubMed.
- A. N. Adham, S. Abdelfatah, A. M. Naqishbandi, N. Mahmoud and T. Efferth, Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells, Phytomedicine, 2021, 80, 153371 CrossRef CAS.
- J. E. Lee, N. T. T. Thuy, J. Lee, N. Cho and H. M. Yoo, Platyphylloside isolated from betula platyphylla is antiproliferative and induces apoptosis in colon cancer and leukemic cells, Molecules, 2019, 24, 2960 CrossRef CAS.
- W. P. Zheng, F. Y. Huang, S. Z. Dai, J. Y. Wang, Y. Y. Lin, Y. Sun, G. H. Tan and Y. H. Huang, Toxicarioside O Inhibits Cell Proliferation and Epithelial-Mesenchymal Transition by Downregulation of Trop2 in Lung Cancer Cells, Front. Oncol., 2021, 10, 609275 CrossRef.
- N. Tawfeek, M. F. Mahmoud, D. I. Hamdan, M. Sobeh, N. Farrag, M. Wink and A. M. El-Shazly, Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review, Front. Pharmacol., 2021, 12, 593856 CrossRef CAS PubMed.
- H. A. Elbaz, T. A. Stueckle, W. Tse, Y. Rojanasakul and C. Z. Dinu, Digitoxin and its analogs as novel cancer therapeutics, Exp. Hematol. Oncol., 2012, 1, 4 CrossRef CAS PubMed.
- G. Y. Yao, W. Dai, M. Ye, R. Huang, Y. M. Pan, Z. Liao and H. S. Wang, Synthesis and antitumor properties of novel alizarin analogs, Med. Chem. Res., 2014, 23, 5031–5042 CrossRef CAS.
- P. Liczbiński and B. Bukowska, Molecular mechanism of amygdalin action in- vitro: review of the latest research, Immunopharmacol. Immunotoxicol., 2018, 40, 212–218 Search PubMed.
- R. K. Singh, T. S. Lange, K. K. Kim and L. Brard, A coumarin derivative (RKS262) inhibits cell-cycle progression, causes pro-apoptotic signaling and cytotoxicity in ovarian cancer cells, Invest. New Drugs, 2011, 29, 63–72 Search PubMed.
- J. Rohr and C. Hertweck, Type II PKS, in Comprehensive Natural Products II, Elsevier, 2010, pp. 227–303 Search PubMed.
- K. Sahin, E. Yenice, M. Tuzcu, C. Orhan, C. Mizrak, I. H. Ozercan, N. Sahin, B. Yilmaz, B. Bilir, B. Ozpolat and O. Kucuk, Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens, J. Cancer Prev., 2018, 23, 25–36 CrossRef PubMed.
- K. Aizawa, C. Liu, S. Tang, S. Veeramachaneni, K. Q. Hu, D. E. Smith and X. D. Wang, Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation, Int. J. Cancer, 2016, 139, 1171–1181 Search PubMed.
- Y. Li, Z. Wang, J. Jin, S. X. Zhu, G. Q. He, S. H. Li, J. Wang and Y. Cai, Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway, Biochem. Biophys. Res. Commun., 2020, 523, 947–953 CrossRef CAS PubMed.
- L. Jia, S. Huang, X. Yin, Y. Zan, Y. Guo and L. Han, Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction, Life Sci., 2018, 208, 123–130 Search PubMed.
- X. B. Yan, T. Xie, S. D. Wang, Z. Wang, H. Y. Li and Z. M. Ye, Apigenin inhibits proliferation of human chondrosarcoma cells via cell cycle arrest and mitochondrial apoptosis induced by ROS generation-an in vitro and in vivo study, Int. J. Clin. Exp. Med., 2018, 11, 1615–1631 Search PubMed.
- J. H. Chang, C. W. Cheng, Y. C. Yang, W. S. Chen, W. Y. Hung, J. M. Chow, P. S. Chen, M. Hsiao, W. J. Lee and M. H. Chien, Downregulating CD26/DPPIV by apigenin modulates the interplay between Akt and Snail/Slug signaling to restrain metastasis of lung cancer with multiple EGFR statuses, J. Exp. Clin. Cancer Res., 2018, 37, 199 CrossRef PubMed.
- Y. Wang, Q. Ma, S. Zhang, H. Liu, B. Zhao, B. Du, W. Wang, P. Lin, Z. Zhang, Y. Zhong and D. Kong, Digoxin enhances the anticancer effect on non-small cell lung cancer while reducing the cardiotoxicity of adriamycin, Front. Pharmacol., 2020, 11, 186 CrossRef CAS PubMed.
- H. Gan, M. Qi, C. Chan, P. Leung, G. Ye, Y. Lei, A. Liu, F. Xue, D. Liu, W. Ye, D. Zhang, L. Deng and J. Chen, Digitoxin inhibits HeLa cell growth through the induction of G2/M cell cycle arrest and apoptosis in vitro and in vivo, Int. J. Oncol., 2020, 57, 562–573 CrossRef CAS PubMed.
- M. Liu, L. X. Feng, P. Sun, W. Liu, W. Y. Wu, B. H. Jiang, M. Yang, L. H. Hu, D. A. Guo and X. Liu, A novel bufalin derivative exhibited stronger apoptosis-inducing effect than bufalin in a549 lung cancer cells and lower acute toxicity in mice, PLoS One, 2016, 11, e0159789 CrossRef PubMed.
- H. T. Chen, D. Sun, Y. C. Peng, P. H. Kao and Y. L. Wu, Novel augmentation by bufalin of protein kinase C-induced cyclooxygenase-2 and IL-8 production in human breast cancer cells, Innate Immun., 2017, 23, 54–66 CrossRef CAS.
- Z. Xu, Y. Hou, C. Zou, H. Liang, J. Mu, X. Jiao, Y. Zhu, L. Su, M. Liu, X. Chen, C. Qian, X. Zhu, W. Gong, Q. Dong and F. Zhang, Alizarin, a nature compound, inhibits the growth of pancreatic cancer cells by abrogating NF-κB activation, Int. J. Biol. Sci., 2022, 18, 2759–2774 CrossRef CAS PubMed.
- C. A. Brohem, T. C. Sawada, R. R. Massaro, R. L. Almeida, D. P. Rivelli, C. D. Ropke, V. V. da Silva, T. M. de Lima, R. Curi, S. B. Barros and S. S. Maria-Engler, Apoptosis induction by 4-nerolidylcatechol in melanoma cell lines, Toxicol. In Vitro, 2008, 23, 111–119 CrossRef PubMed.
- M. S. Ahmed and F. T. Halaweish, Cucurbitacins: potential candidates targeting mitogen-activated protein kinase pathway for treatment of melanoma, J. Enzyme Inhib. Med. Chem., 2014, 29, 162–167 CrossRef CAS.
- S. K. Król, M. Kiełbus, A. Rivero-Müller and A. Stepulak, Comprehensive review on betulin as a potent anticancer agent, BioMed Res. Int., 2015, 2015, 584189 Search PubMed.
- M. Schröder, M. Petrova, Z. Vlahova, G. M. Dobrikov, I. Slavchev, E. Pasheva and I. Ugrinova, In vitro anticancer activity of two ferrocene-containing camphor sulfonamides as promising agents against lung cancer cells, Biomedicines, 2022, 10, 1353 CrossRef PubMed.
- I. Begum, R. Sharma and H. K. Sharma, A review on plants having anti-cancer activity, Curr. Trends Pharm. Res., 2017, 4, 39–62 Search PubMed.
- X. Lin, Z. Peng and C. Su, Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone, Int. J. Mol. Sci., 2015, 16, 10888–10906 CrossRef CAS PubMed.
- S. Singh, P. Gupta, A. Meena and S. Luqman, Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders, Food Chem. Toxicol., 2020, 145, 111708 CrossRef CAS PubMed.
- D. N. Syed, F. Afaq, N. Maddodi, J. J. Johnson, S. Sarfaraz, A. Ahmad, V. Setaluri and H. Mukhtar, Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased Mitf levels, J. Invest. Dermatol., 2011, 131, 1291–1299 CrossRef CAS.
- K. Ramasamy and R. Agarwal, Multitargeted therapy of cancer by silymarin, Cancer Lett., 2008, 269, 352–362 CrossRef CAS PubMed.
- D. Delmas, J. Xiao, A. Vejux and V. Aires, Silymarin and cancer: a dual strategy in both in chemoprevention and chemosensitivity, Molecules, 2020, 25, 2009 CrossRef CAS PubMed.
- A. Riaz, A. Rasul, G. Hussain, M. K. Zahoor, F. Jabeen, Z. Subhani, T. Younis, M. Ali, I. Sarfraz and Z. Selamoglu, Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities, Adv. Pharmacol. Sci., 2018, 2018, 9794625 Search PubMed.
- Y. Park, S. H. Woo, S. K. Seo, H. Kim, W. C. Noh, J. K. Lee, B. M. Kwon, K. N. Min, T. B. Choe and I. C. Park, Ginkgetin induces cell death in breast cancer cells via downregulation of the estrogen receptor, Oncol. Lett., 2017, 14, 5027–5033 CrossRef PubMed.
- M. Nihal, N. Ahmad, H. Mukhtar and G. S. Wood, Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma, Int. J. Cancer, 2005, 114, 513–521 CrossRef CAS.
- N. Zhang, X. Shen, X. Jiang, J. Cai, X. Shen, Y. Hu and S. X. Qiu, Two new cytotoxic stilbenoid dimers isolated from Cajanus cajan, J. Nat. Med., 2018, 201(72), 304–309 CrossRef PubMed.
- E. Navarro, S. J. Alonso, P. J. Alonso, J. Trujillo, E. Jorge and C. Perez, Pharmacological effects of elenoside, an arylnaphthalene lignan, Biol. Pharm. Bull., 2001, 24, 254–258 CrossRef CAS.
- S. B. Kntayya, M. D. Ibrahim, N. M. Ain, R. Iori, C. Ioannides and A. F. A. Razis, Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells, Nutrients, 2018, 10, 718 CrossRef PubMed.
- X. D. Pei, Z. L. He, H. L. Yao, J. S. Xiao, L. Li, J. Z. Gu, P. Z. Shi, J. H. Wang and L. H. Jiang, 6-Shogaol from ginger shows anti-tumor effect in cervical carcinoma via PI3K/Akt/mTOR pathway, Eur. J. Nutr., 2021, 1, 1–3 Search PubMed.
- M. Ossama, R. M. Hathout, D. A. Attia and N. D. Mortada, Enhanced allicin cytotoxicity on HEPG-2 cells using glycyrrhetinic acid surface-decorated gelatin nanoparticles, ACS Omega, 2019, 4, 11293–11300 CrossRef CAS PubMed.
- S. B. Ateba, M. A. Mvondo, S. Djiogue, S. Zingué, L. Krenn and D. Njamen, A pharmacological overview of alpinumisoflavone, a natural prenylated isoflavonoid, Front. Pharmacol., 2019, 10, 952 CrossRef CAS PubMed.
- R. Raghavan, S. Cheriyamundath and J. Madassery, Exploring the mechanisms of cytotoxic and anti-inflammatory property of andrographolide and its derivatives, Pharmacogn. Rev., 2018, 12, 56–65 CrossRef CAS.
- X. Zhao, J. Qu, X. Liu, J. Wang, X. Ma, X. Zhao, Q. Yang, W. Yan, Z. Zhao, Y. Hui and H. Bai, Baicalein suppress EMT of breast cancer by mediating tumor-associated macrophages polarization, Am. J. Cancer Res., 2018, 8, 1528–1540 CAS.
- Y. Chu, Q. Yuan, H. Jiang, L. Wu, Y. Xie, X. Zhang and L. Li, A comprehensive review of the anticancer effects of decursin, Front. Pharmacol., 2024, 15, 1303412 CrossRef CAS.
- Z. A. Rahman, F. Hidayatullah, P. K. Pratama, D. P. Andhika and L. Hakim, Effects of decursinol angelate on viability and apoptosis in PC-3 prostate cancer cells: in vitro study, Narra J, 2024, 4, e948 CrossRef.
- D. Aras, O. Cinar, Z. Cakar, S. Ozkavukcu and A. Can, Can dicoumarol be used as a gonad-safe anticancer agent: an in vitro and in vivo experimental study, Mol. Hum. Reprod., 2016, 22, 57–67 CrossRef CAS.
- H. S. Chae, R. Xu, J. Y. Won, Y. W. Chin and H. Yim, Molecular targets of genistein and its related flavonoids to exert anticancer effects, Int. J. Mol. Sci., 2019, 20, 2420 CrossRef PubMed.
- S. Nafees, M. Zafaryab, S. H. Mehdi, B. Zia, M. A. Rizvi and M. A. Khan, Anti-cancer effect of gingerol in cancer prevention and treatment, Anti-Cancer Agents Med. Chem., 2021, 21, 428–432 CrossRef CAS.
- Y. Cai, B. Zhao, Q. Liang, Y. Zhang, J. Cai and G. Li, The selective effect of glycyrrhizin and glycyrrhetinic acid on topoisomerase IIα and apoptosis in combination with etoposide on triple negative breast cancer MDA-MB-231 cells, Eur. J. Pharmacol., 2017, 809, 87–97 CrossRef CAS.
- H. A. Kim and J. Lee, Hispidulin modulates epithelial-mesenchymal transition in breast cancer cells, Oncol. Lett., 2021, 21, 155 CrossRef CAS PubMed.
- N. Deng, M. Qiao, Y. Li, F. Liang, J. Li and Y. Liu, Anticancer effects of licochalcones: a review of the mechanisms, Front. Pharmacol., 2023, 14, 1074506 CrossRef CAS PubMed.
- S. Nagini, R. Nivetha, M. Palrasu and R. Mishra, Nimbolide, a neem limonoid, is a promising candidate for the anticancer drug arsenal, J. Med. Chem., 2021, 64, 3560–3577 CrossRef CAS.
- X. Zhao, L. Huang, W. Xu, X. Chen, Y. Shen, W. Zeng and X. Chen, Physapubescin B inhibits tumorgenesis and circumvents taxol resistance of ovarian cancer cells through STAT3 signaling, Oncotarget, 2017, 8, 70130 CrossRef PubMed.
- K. Chatterjee, S. Mukherjee, J. Vanmanen, P. Banerjee and J. E. Fata, Dietary polyphenols, resveratrol and pterostilbene exhibit antitumor activity on an HPV E6-positive cervical cancer model: an in vitro and in vivo analysis, Front. Oncol., 2019, 9, 352 CrossRef PubMed.
- V. S. Sivasankarapillai, R. M. K. Nair, A. Rahdar, S. Bungau, D. C. Zaha, L. Aleya and D. M. Tit, Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera, Environ. Sci. Pollut. Res., 2020, 27, 26025–26035 CrossRef CAS.
- A. M. Tomko, E. G. Whynot, L. D. Ellis and D. J. Dupré, Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis, Cancers, 2020, 12, 1985 CrossRef CAS PubMed.
- M. V. Sobral, A. L. Xavier, T. C. Lima and D. P. de Sousa, Antitumor activity of monoterpenes found in essential oils, Sci. World J., 2014, 2014, 953451 Search PubMed.
- D. Y. Kim and B. Y. Choi, Costunolide-A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential, Int. J. Mol. Sci., 2019, 20, 2926 CrossRef CAS PubMed.
- G. Ş. Karatoprak, E. K. Akkol, Y. Genç, H. Bardakci, Ç. Yücel and E. Sobarzo-Sánchez, Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications, Molecules, 2020, 25, 2560 CrossRef CAS.
- S. Ijaz, N. Akhtar, M. S. Khan, A. Hameed, M. Irfan, M. A. Arshad, S. Ali and M. Asrar, Plant derived anticancer agents: a green approach towards skin cancers, Biomed. Pharmacother., 2018, 103, 1643–1651 CrossRef CAS.
- K. J. Min and T. K. Kwon, Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate, Integr. Med. Res., 2014, 3, 16–24 CrossRef PubMed.
- A. S. Choudhari, P. C. Mandave, M. Deshpande, P. Ranjekar and O. Prakash, Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice, Front. Pharmacol., 2020, 10, 1614 CrossRef.
- J. Dou, Z. Wang, L. Ma, B. Peng, K. Mao, C. Li, M. Su, C. Zhou and G. Peng, Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence, Oncotarget, 2018, 9, 20089–20102 CrossRef.
- P. Kuppusamy, A. Nagalingam, N. Muniraj, N. K. Saxena and D. Sharma, Concomitant activation of ETS-like transcription factor-1 and death receptor-5 via extracellular signal-regulated kinase in withaferin a-mediated inhibition of hepatocarcinogenesis in mice, Sci. Rep., 2017, 7, 17943 CrossRef.
- M. O. Kim, M. H. Lee, N. Oi, S. H. Kim, K. B. Bae, Z. Huang, D. J. Kim, K. Reddy, S. Y. Lee, S. J. Park, J. Y. Kim, H. Xie, J. K. Kundu, Z. Y. Ryoo, A. M. Bode, Y. J. Surh and Z. Dong, [6]-Shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2, Carcinogenesis, 2014, 35, 683–691 CrossRef CAS PubMed.
- H. Chen, B. Zhu, L. Zhao, Y. Liu, F. Zhao, J. Feng, Y. Jin, J. Sun, R. Geng and Y. Wei, Allicin inhibits proliferation and invasion in vitro and in vivo via shp-1-mediated stat3 signaling in cholangiocarcinoma, Cell. Physiol. Biochem., 2018, 47, 641–653 CrossRef CAS PubMed.
- T. Wang, Y. Jiang, L. Chu, T. Wu and J. You, Alpinumisoflavone suppresses tumour growth and metastasis of clear-cell renal cell carcinoma, Am. J. Cancer Res., 2017, 7, 999–1015 CAS.
- J. Li, C. Zhang, H. Jiang and J. Cheng, Andrographolide inhibits hypoxia-inducible factor-1 through phosphatidylinositol 3-kinase/AKT pathway and suppresses breast cancer growth, OncoTargets Ther., 2015, 8, 427–435 CrossRef.
- Y. Tao, S. Zhan, Y. Wang, G. Zhou, H. Liang, X. Chen and H. Shen, Baicalin, the major component of traditional Chinese medicine Scutellariabaicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs, Sci. Rep., 2018, 8, 14477 CrossRef.
- G. Zhao, X. Han, S. Zheng, Z. Li, Y. Sha, J. Ni, Z. Sun, S. Qiao and Z. Song, Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells, Oncol. Rep., 2016, 35, 1065–1074 CrossRef CAS PubMed.
- W. Wu, S. N. Tang, Y. Zhang, M. Puppala, T. K. Cooper, C. Xing, C. Jiang and J. Lü, Prostate cancer xenograft inhibitory activity and pharmacokinetics of decursinol, a metabolite of angelica gigas pyranocoumarins, in mouse models, Am. J. Chin. Med., 2017, 45, 1773–1792 CrossRef CAS PubMed.
- W. Zhang, J. Su, H. Xu, S. Yu, Y. Liu, Y. Zhang, L. Sun, Y. Yue and X. Zhou, Dicumarol inhibits PDK1 and targets multiple malignant behaviors of ovarian cancer cells, PLoS One, 2017, 12, e0179672 CrossRef PubMed.
- Y. C. Hsiao, S. F. Peng, K. C. Lai, C. L. Liao, Y. P. Huang, C. C. Lin, M. L. Lin, K. C. Liu, C. C. Tsai, Y. S. Ma and J. G. Chung, Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo, Environ. Toxicol., 2019, 34, 443–456 CrossRef CAS.
- A. C. B. M. Matrine, A. M. Fuzer, A. B. Becceneri, J. A. da Silva, R. Tomasin, D. Denoyer, S. H. Kim, K. A. McIntyre, H. B. Pearson, B. Yeo, A. Nagpal, X. Ling, H. S. Selistre-de-Araújo, P. C. Vieira, M. R. Cominetti and N. Pouliot, [10]-Gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo, Oncotarget, 2017, 8, 72260–72271 CrossRef PubMed.
- X. Wu, W. Wang, Y. Chen, X. Liu, J. Wang, X. Qin, D. Yuan, T. Yu, G. Chen, Y. Mi, J. Mou, J. Cui, A. Hu, Y. E and D. Pei, Glycyrrhizin Suppresses the Growth of Human NSCLC Cell Line HCC827 by Downregulating HMGB1 Level, BioMed Res. Int., 2018, 2018, 6916797 Search PubMed.
- M. Han, H. Gao, J. Xie, Y. P. Yuan, Q. Yuan, M. Q. Gao, K. L. Liu, X. H. Chen, Y. T. Han and Z. W. Han, Hispidulin induces ER stress-mediated apoptosis in human hepatocellular carcinoma cells in vitro and in vivo by activating AMPK signaling pathway, Acta Pharmacol. Sin., 2019, 40, 666–676 CrossRef CAS PubMed.
- A. S. Choudhari, P. C. Mandave, M. Deshpande, P. Ranjekar and O. Prakash, Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice, Front. Pharmacol., 2020, 10, 1614 CrossRef.
- W. J. Lu, G. J. Wu, R. J. Chen, C. C. Chang, L. M. Lien, C. C. Chiu, M. F. Tseng, L. T. Huang and K. H. Lin, Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest, Food Funct., 2018, 9, 4500–4507 RSC.
- R. Subramani, E. Gonzalez, A. Arumugam, S. Nandy, V. Gonzalez, J. Medel, F. Camacho, A. Ortega, S. Bonkoungou, M. Narayan, A. K. Dwivedi and R. Lakshmanaswamy, Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition, Sci. Rep., 2018, 6, 19819 CrossRef.
- L. Chen, G. Xia, F. Qiu, C. Wu, A. P. Denmon and X. Zi, Physapubescin selectively induces apoptosis in VHL-null renal cell carcinoma cells through down-regulation of HIF-2α and inhibits tumor growth, Sci. Rep., 2016, 6, 32582 CrossRef CAS.
- W. Wen, G. Lowe, C. M. Roberts, J. Finlay, E. S. Han, C. A. Glackin and T. H. Dellinger, Pterostilbene, a natural phenolic compound, synergizes the antineoplastic effects of megestrol acetate in endometrial cancer, Sci. Rep., 2017, 7, 12754 CrossRef PubMed.
- A. Bishayee and G. Sethi, Bioactive natural products in cancer prevention and therapy: progress and promise, in Seminars in Cancer Biology, Academic Press, 2016, pp. 1–3 Search PubMed.
- F. Majolo, L. K. Delwing, D. J. Marmitt, I. C. Bustamante-Filho and M. I. Goettert, Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery, Phytochem. Lett., 2019, 1, 196–207 CrossRef.
- S. Kumar, D. Kumar, A. Bhat and A. Kumar, Phytochemicals in Clinical Studies: Current Perspective, in Functional Food and Human Health, ed. V. Rani and U. Yadav, Springer Nature Singapore Pte Ltd, 2018, pp. 471–511 Search PubMed.
- J. Fleminger and B. Goldacre, Prevalence of clinical trial status discrepancies: a cross-sectional study of 10,492 trials registered on both clinicaltrials.gov and the european union clinical trials register, PLoS One, 2018, 13, e0193088 CrossRef.
- H. ur Rashid, S. Rasool, Y. Ali, K. Khan and M. A. U. Martines, Martines Anti-cancer potential of sophoridine and its derivatives: recent progress and future perspectives, Bioorg. Chem., 2020, 99, 103863 CrossRef CAS PubMed.
- S. Sabet, A. Rashidinejad, L. D. Melton and D. J. McGillivray, Recent advances to improve curcumin oral bioavailability, Trends Food Sci. Technol., 2021, 110, 253–266 CrossRef CAS.
- L. X. Zhang, C. X. Li, M. U. Kakar, M. S. Khan, P. F. Wu, R. M. Amir, D. F. Dai, M. Naveed, Q. Y. Li, M. Saeed and J. Q. Shen, Resveratrol (RV): a pharmacological review and call for further research, Biomed. Pharmacother., 2021, 43, 112164 CrossRef PubMed.
- P. Bathaei, M. Imenshahidi and H. Hosseinzadeh, Effects of Berberis vulgaris, and its active constituent berberine on cytochrome P450: a review. Naunyn Schmiedebergs, Arch. Pharmacol., 2024, 14, 1–24 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |