Open Access Article
Open Access ArticleOrigin of 13C NMR chemical shifts elucidated based on molecular orbital theory: paramagnetic contributions from orbital-to-orbital transitions for the pre-α, α, β, α-X, β-X and ipso-X effects, along with effects from characteristic bonds and groups†
Waro Nakanishi *,
Satoko Hayashi
*,
Satoko Hayashi * and
Keigo Matsuzaki
* and
Keigo Matsuzaki
Faculty of Systems Engineering, Wakayama University, 930 Sakaedani, Wakayama 640-8510, Japan. E-mail: nakanisi@wakayama-u.ac.jp; hayashi3@wakayama-u.ac.jp
First published on 21st January 2025
Abstract
13C NMR chemical shifts (δ(C)) were analysed via MO theory, together with the origin, using σd(C), σp(C) and σt(C), where C4− was selected as the standard for the analysis since σp(C: C4−) = 0 ppm. An excellent relationship was observed between σd(C) and the charges on C for (C4+, C2+, C0, C2− and C4−) and (C4−, CH22−, CH3− and CH4). However, such a relationship was not observed for the carbon species other than those above. The occupied-to-unoccupied orbital (ψi→ψa) transitions were mainly employed for the analysis. The origin was explained by the pre-α, α, β, α-X, β-X and ipso-X effects. The pre-α effect of an approximately 20 ppm downfield shift is theoretically predicted, and the observed α and β effects of approximately 10–15 ppm downfield shifts are well reproduced by the calculations, as are the variations in the α-X, β-X and ipso-X effects. Large downfield shifts caused by the formation of ethene (∼120 ppm), ethyne (∼60 ppm) and benzene (∼126 ppm) from ethane and carbonyl (∼146 ppm) and carboxyl (∼110 ppm) groups from CH3OH are also reproduced well by the calculations. The analysis and illustration of σp(C) through the ψi→ψa transitions enables us to visualize the effects and to understand the δ(C) values for the C atoms in the specific positions of the species. The occupied-to-occupied orbital (ψi→ψj) transitions are also examined. The theoretical investigations reproduce the observed results of δ(C). The origin for δ(C) and the mechanism are visualized, which allows us to image the process in principle. The role of C in the specific position of a compound in question can be more easily understood, which will aid in the development of highly functional compounds based on NMR.
Introduction
NMR spectroscopy is one of the most important tools in current chemical and biological science research. 1H and 13C NMR spectra are commonly measured and analysed on a daily basis to determine the structures and/or follow up the reactions.1–4 Lots of 1H and 13C NMR data have been reported thus far. The shift values spread over 20 and 300 ppm, respectively. NMR spectra, other than those above, are also measured on a daily basis.5–8 The NMR chemical shifts of the atoms in the 3rd and higher periods, such as 31P, 77Se and 125Te, are predominantly controlled by the paramagnetic terms, whereas those in the 1st period of 1H are controlled by the diamagnetic terms. In the case of the atoms of the 2nd period, such as 15N, 17O and 19F, the chemical shifts are controlled both by the two terms; therefore, the mechanisms are more complex.5–8 The relativistic effect cannot be avoided when considering those in the 5th and higher periods, whereas the effect can be treated as (much) smaller for the atoms in the 4th and lower periods.Our research interest lies in establishablishing the plain rules founded in theory for the origin and mechanism of the NMR chemical shifts of nuclei N [δ(N)]. The mechanism of the origin will help experimental chemists understand the role of N at a specific position in the species over the empirical rules,5,6 which are usually employed in assigning the spectra. The plain rules, which are established based on the theory, must be simple, easily imaged and easily understood for experimental scientists who are not specialists in this field. In our work, this purpose is given more importance than the usual NMR parameter calculations, which accurately predict the shift values of target compounds.
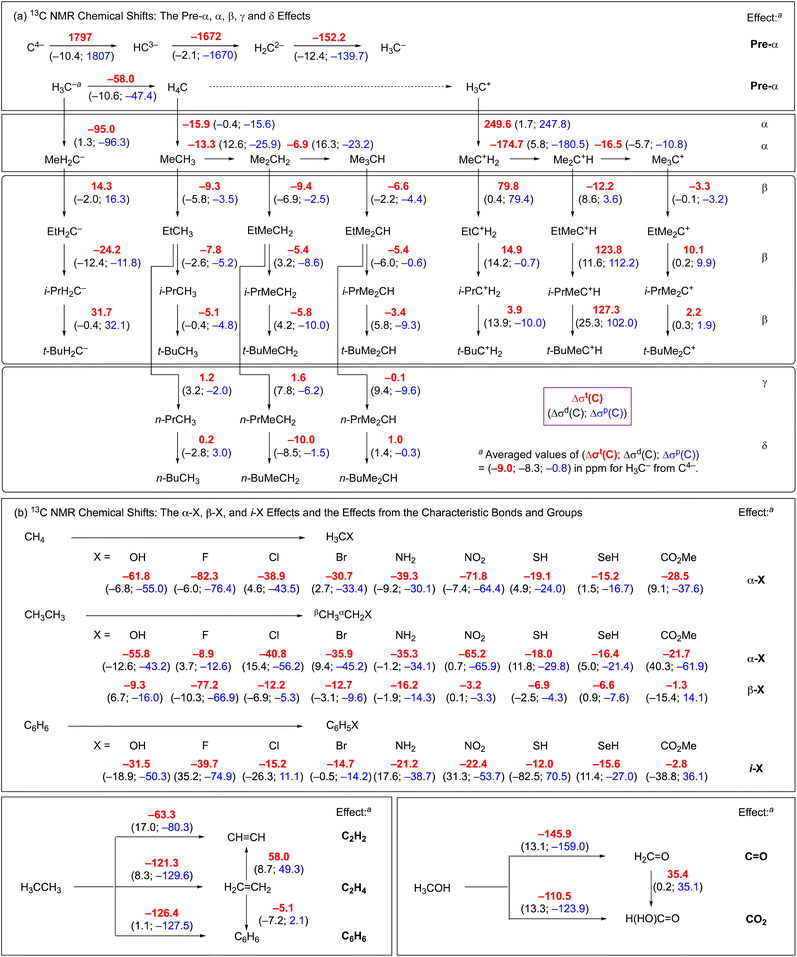
Scheme 1 illustrates the pre-α, α, β, γ and δ effects on δ(C), which are often employed to understand δ(C) uniformly. The typically encountered α, β and γ effects are 12–16 ppm (downfield shifts), 5–9 ppm (downfield shifts) and −2 ppm (upfield shifts), respectively, with the δ effect being negligibly small. The “pre-α effect” has been proposed to establish plain rules and understand the mechanisms for δ(Se) in a unified form.9 Scheme 1 contains the α-X, β-X and ipso-X (i-X) effects, where X stands for atoms or groups other than H and Me. The pre-α, α, and β effects are analysed based on the MO theory. The effects are calculated per unit group (per Me or H) and are discussed using the average values, if suitable. The effects of the characteristic bonds and groups are also examined.
 | ||
| Scheme 1 The pre-α (a), α (b), β (c), γ (d), δ (e), α-X (f), β-X (g) and ipso-X (i-X) (h) effects, which are discussed in this paper. | ||
The chemical shifts of the respective structures can be theoretically calculated with satisfactory accuracy. The total absolute magnetic shielding tensor (σt) is used for the analysis. As shown in eqn (1), σt is decomposed into the contributions from the diamagnetic shielding tensors (σd) and the paramagnetic shielding tensors (σp) under the DFT levels.10–12 The magnetic shielding tensors consist of three components: σmxx, σmyy and σmzz, (m = d, p and t). Eqn (2) shows the relationship. As shown in eqn (3), σd is simply expressed as the sum of the contributions over the occupied orbitals (ψi, so is ψj), where the contribution from each ψi to σd (σdi) is proportional to the average inverse distance of electrons from the nuclei in ψi, 〈ri−1〉 (eqn (4)).13 σp is evaluated by the coupled Hartree–Fock (CPHF) method. σp can be decomposed into contributions from the occupied orbitals or the orbital-to-orbital transitions,14 under the DFT levels. σp is shown in eqn (5), although the contributions from the occupied-to-occupied orbital transitions are neglected.9,13 The process to evaluate σp is highly complex; therefore, σp is discussed based on the approximate image derived from eqn (6),14 where (εa − εi)−1 is the reciprocal orbital energy gap, ψk is the k-th orbital function, and ![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z,N is the orbital angular momentum around the resonance nucleus N, and rN is the distance of electrons from N. The origin for δ(C) can be elucidated based on MO theory.
z,N is the orbital angular momentum around the resonance nucleus N, and rN is the distance of electrons from N. The origin for δ(C) can be elucidated based on MO theory.
| σt = σd + σp | (1) |
| σm = (σmxx + σmyy + σmzz)/3 (m = d, p and t) | (2) |
| σd = Σocci σdi | (3) |
| σdi = (μoe2/12πme)〈ri−1〉 | (4) |
| σp = Σocci σpi = Σocci × Σunocca × σpi→a | (5) |
σpzz = –(μoe2/2me2)ΣocciΣunocca(εa − εi)−1 × {〈ψi|![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z|ψa〉〈ψa| z|ψa〉〈ψa|![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z,NrN−3|ψi〉 + 〈ψi| z,NrN−3|ψi〉 + 〈ψi|![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z,NrN−3|ψa〉〈ψa| z,NrN−3|ψa〉〈ψa|![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z|ψi〉} z|ψi〉}
| (6) |
The interpretation of NMR chemical shifts based on the empirical rules has traditionally been achieved by considering the distribution of electrons of molecules through the inductive and resonance effects, which has been developed in the theory of reactivity, such as the Hammett plots.15 Indeed, this treatment has achieved a lot of success, but it does not eliminate the gap arising from the differences in the mechanisms controlling the energy and electromagnetic terms. The 1H NMR chemical shifts could be essentially explained based on the inductive and resonance effects, if the magnetic anisotropic effect is suitably evaluated. However, the effect on the 13C NMR chemical shifts would be complexly distributed in the σd and σp terms as shown by eqn (3)–(6). Therefore, the effects will not discuss in this paper.
Here, we report the analysis of 13C NMR chemical shifts, based on the molecular orbital (MO) theory. It is intended to establish the plain rules founded in theory. The origin and mechanisms for δ(C) are discussed based on the MO theory for the effects shown in Scheme 1, together with the effects of ethene, ethyne, benzene and carbonyl and carboxyl groups. Similar investigations on δ(O), reported very recently,16 with early investigations on δ(Se),9 will aid in an easier understanding of δ(C).
Methodological details in the calculations
The calculations were optimized for various carbon species via the Gaussian 09 program package, including GaussView.17 No specific conditions, such as the long-range interactions, were considered, unless otherwise noted. Optimizations were performed with opt = tight. The threshold (cutoff) values are 0.000015 au, 0.000010 au, 0.000060 au and 0.000040 au for the maximum force, the root mean square (RMS) force, the maximum displacement and the RMS displacement, respectively, where the predicted change in energy seems around 10−10 au. The structural optimizations were performed at the various DFT levels of B3LYP,18–21 CAM-B3LYP,22 PBE,23 PBE0,24 LC-ωPBE25 and ωB97X-D26 (L1) and MP2 (L2),27–29 employing the 6–311++G(3df,3pd) (6D10F) basis set (BSS-A) (L/BSS-A where L = L1 + L2). HOMO and LUMO of the species, together with other MOs, were drawn using GaussView with an isovalue of 0.04 au. The gauge-independent atomic orbital (GIAO) method30–34 was applied to calculate the absolute magnetic shielding tensors of C [σ*(C: * = d, p and t)], employing the same method as that used in the optimizations (La/BSS-A//La/BSS-A, where La stands for each level of L). The solvent effect was not considered in the discussion. The charge on C (Q(C)) was obtained via natural population analysis (NPA).35 A utility program36 was applied to evaluate the contributions from each ψi and/or ψi→ψa transition. The procedure is explained in the Appendix of the ESI.†Results and discussion
Selection of standard and suitable level for the analysis of 13C NMR chemical shifts based on σd(C), σp(C) and σt(C)
The σt(C) values of some carbon species (S) [σt(C: S)] were calculated with the GIAO method under B3LYP/BSS-A, for instance, as were the σd(C: S) and σp(C: S) values. The σt(C) values were similarly calculated with MP2/MSS-A, where σd(C: S) and σp(C: S) are not obtained at the MP2 level. Table 1 lists the calculated σm(C: S) (m = d, p and/or t) values for S = C4+, C2+, C0, C2− and C4−. The σt(C) values for C4+, C2+ and C4−, calculated with the two levels, were very close to each other. Among them, C4− is taken as the standard for the analysis in this work. The reason is as follows: σp(C: C4−) = 0.0 ppm, which is very favourable for our purpose, although σp(C: C2+) and σp(C: C4+) are also 0.0 ppm, as shown in Table 1. The electronic 1So state of C4− with eight valence electrons according to the octet rule and its spherical electron distribution are also favourable for this purpose.| Nuclear | Configoration | σdB3LYP(C: 1s) | σdB3LYP(C: 2s) | σdB3LYP(C: 2p) | σdB3LYP(C) | σpB3LYP(C) | σtB3LYP(C) | σtMP2(C) |
|---|---|---|---|---|---|---|---|---|
| a Calculated by applying the GIAO method under B3LYP/BSS-A and MP2/BSS-A. | ||||||||
| C4+ | (2s)0(2p)0 | 201.71 | 0.00 | 0.00 | 201.71 | 0.00 | 201.71 | 201.77 |
| C2+ | (2s)2(2p)0 | 200.65 | 37.58 | 0.00 | 238.23 | 0.00 | 238.23 | 238.09 |
| C0 | (2s)2(2p)2 | 200.40 | 32.63 | 27.19 (×1) | 260.22 | 3986.91 | 4247.13 | 2980.47 |
| C2− | (2s)2(2p)4 | 200.42 | 31.40 | 18.74 (×2) | 269.23 | 4542.23 | 4811.46 | 3316.42 |
| C4− | (2s)2(2p)6 | 274.39 | 31.72 | 14.08 (×3) | 274.39 | 0.00 | 274.39 | 276.12 |
It is necessary to determine the suitable level in this work, next. The σt(C: S) values were calculated for the 40 neutral carbon species (54 plots) at the DFT levels of B3LYP,18–21 CAM-B3LYP,22 PBE,23 PBE0,24 LC-ωPBE25 and ωB97X-D26 (L1) with BSS-A, together with σd(C: S) and σp(C: S). The MP2 level (L2) is also applied to obtain the σt(C: S) values. The basis set of def2TZVP37,38 was also applied at the B3LYP level (B3LYP/def2TZVP). The results are collected in Tables S1–S7 and S9 of the ESI.†
The calculated σt(C: S) values are very close with each other. The −Δσt(C: S) values calculated at the L (= L1 + L2) levels are plotted versus the corresponding δ(C: S), respectively. The plot for S of the 40 neutral carbon species (54 plots) at B3LYP is drawn in Fig. S1 of the ESI.† The plot is analysed assuming the linear relationship (y = ax + b: Rc2 (the square of the correlation coefficient)), where (a, b, Rc2) = (1.05, 1.90, 0.998). (The plot is very similar to Fig. 3 for the neutral and charged 75 carbon species). Similar treatments were performed at L = L1 + L2. Table 2 collects the results. The correlations were very similar with each other. The Rc2 values at the B3LYP, PBE and ωB97X-D levels (0.998) seem slightly better than others, therefore, the B3LYP level is selected for the calculations. The B3LYP level is most popularly accepted also by the experimental researchers, which is significant for our purposes.
| Entry | Level (L) | a | b | Rc2 | N |
|---|---|---|---|---|---|
| a Calculated with the GIAO method under L/BSS-A.b Observed data are used for the corresponding species in the plot.c Under the solvent effect of CHCl3.d Calculated with B3LYP/def2TZVP. | |||||
| 1 | B3LYP | 1.049 | 1.90 | 0.998 | 54 |
| 2 | CAM-B3LYP | 1.075 | 0.00 | 0.997 | 54 |
| 3 | PBE | 1.037 | 2.18 | 0.997 | 54 |
| 4 | PBE0 | 1.056 | 0.31 | 0.997 | 54 |
| 5 | LC-ωPBE | 1.102 | −1.55 | 0.998 | 54 |
| 6 | ωB97X-D | 1.058 | 0.46 | 0.998 | 54 |
| 7 | MP2 | 1.017 | 1.89 | 0.995 | 54 |
| 8c | B3LYP | 1.053 | 2.44 | 0.997 | 54 |
| 9d | B3LYP | 1.036 | 1.37 | 0.997 | 54 |
Conformational and solvent effects on 13C NMR chemical shifts
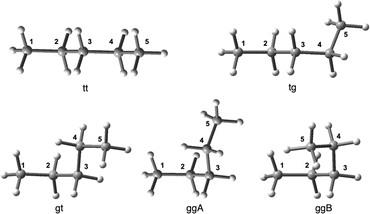
It is an important issue to examine the conformational effect on σt(C) in the calculations. The effect is examined exemplified by n-pentane. Five conformers of the different energies are optimized, which are called tt, tg, gt, ggA and ggB, together with the topological isomers of tg, where t and g stand for the trans and gauche conformations, respectively. Fig. 1 shows the structures of tt, tg, gt, ggA and ggB. The calculated σt(Ci: i = 1–5) values are collected in Table S10 of the ESI,† together with the observed values. The energies of tg, gt, ggA and ggB from tt are also given in the Table, which are 3.7, 7.2, 6.8 and 14.3 kJ mol−1, respectively. The σt(Ci: i = 1–5)calcd values are plotted versus the δ(Ci: i = 1–5)obsd values for the five conformers. Fig. 2 shows the plots, which are also analysed assuming the linear relationship. Table 3 collects the (a, b, Rc2) values, which are also given in Fig. 2.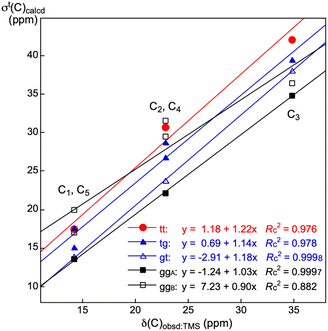 | ||
| Fig. 2 Plots of −Δσt(Ci: i = 1–5)calcd:TMS versus δ(Ci: i = 1–5)obsd:TMS for five conformers in n-pentane. | ||
As shown in Table 3, the correlation seems excellent for gt and ggA, good for tt, and moderate for ggB. The gt and/or ggA conformers seem most suitable for the purpose, at first glance, however, they are less stable than tt by about 7 kJ mol−1. Judging comprehensively, the tt conformer should also be recommended as the suitable one. The extended conformers would be recommended as the nice ones for the long-chained species in the calculations, since it is inferred that they would be less three-dimensionally crowded than other conformers. The selection of the extended conformers would not damage so much on our discussion, judging from the above discussion. However, other conformers must also be considered for the better discussion since other conformers contribute depending on their populations among the conformers.
The solvent effect of chloroform was also examined with the polarizable continuum model (PCM).39 The calculated results are collected in Table S8 of the ESI† and the plot is contained in Fig. S8 of the ESI.† The correlation is shown in Table 2 (entry 8). The correlation seems very similar to (but very slightly poorer than) that without the solvent effect (entry 1). Therefore, the solvent effect is not considered in this work.
Calculated σt(C) and observed δ(C) values
Table 4 lists the σm(C: S), Δσm(C: S) [ =σm(C: S) − σm(C: C4−): Δσp(C) (=σp(C)) since σp(C: C4−) = 0 ppm] and Δσm(C: S)e (m = d, p and t) values, calculated with B3LYP/BSS-A for various carbon species of 1–56, together with the Q(C) values. The Δσm(C: S)e values are explained later. The values are similar calculated for the carbon species of S1–S20 and S21–S70. The calculated values are provided in Tables S11 and S12, respectively, of the ESI.†| Species (nos: sym) | Q(C) | σd(C) | Δσd(C) | σp(C)c | σt(C) | Δσt(C) | Δσd(C)ed | Δσp(C)ed | Δσt(C)ed | Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated with the GIAO-DFT method under B3LYP/BSS-A.b Δσm(C: S) = σm(C: S) − σm(C: C4−) (m = d, p and t).c Δσp(C) = σp(C), since σp(C: C4−) = 0 ppm.d Δσm(C: S)e = (1/n)(Δσm(C: S) − Δσm(C: Se)); see the text for n, S and Se.e The effect not being defined.f From C2H6. | ||||||||||
| C4− (1: Oh) | −4.000 | 274.39 | 0.00 | 0.00 | 274.39 | 0.00 | 0.00 | 0.00 | 0.00 | — |
| HC3− (2: C∞v) | −1.815 | 264.04 | −10.35 | 1807.41 | 2071.45 | 1797.06 | −10.35 | 1807.41 | 1797.06 | Pre-α |
| H2C2− (3: C2v) | −2.156 | 261.96 | −12.43 | 137.46 | 399.42 | 125.03 | −6.22 | 68.73 | 62.51 | Pre-α |
| H3C− (4: C3v) | −1.400 | 249.53 | −24.87 | −2.26 | 247.26 | −27.13 | −8.29 | −0.75 | −9.04 | Pre-α |
| CH4 (5: Td) | −0.805 | 238.98 | −35.42 | −49.66 | 189.32 | −85.08 | −8.85 | −12.42 | −21.27 | Pre-α |
| CH3H2C– (6: Cs) | −1.000 | 250.87 | −23.53 | −98.55 | 152.32 | −122.08 | 1.34 | −96.29 | −94.95 | α |
| EtH2C− (7: Cs) | −0.915 | 248.89 | −25.51 | −82.29 | 166.59 | −107.80 | −1.98 | 16.26 | 14.28 | β |
| i-PrH2C− (8: C1) | −0.923 | 236.54 | −37.85 | −94.11 | 142.43 | −131.96 | −7.16 | 2.22 | −4.94 | β |
| t-BuH2C− (9: Cs) | −0.985 | 236.18 | −38.22 | −62.01 | 174.16 | −100.23 | −4.90 | 12.18 | 7.28 | β |
| n-PrH2C− (10: Cs) | −0.990 | 260.85 | −13.54 | −87.90 | 172.96 | −101.44 | 11.97 | −5.61 | −5.61 | γ |
| n-BuH2C− (11: Cs) | −0.938 | 262.85 | −11.55 | −88.82 | 174.03 | −100.37 | 1.99 | −0.92 | 1.07 | ε |
| CH3CH3 (12: D3d) | −0.571 | 238.60 | −35.80 | −65.21 | 173.39 | −101.00 | −0.38 | −15.55 | −15.92 | α |
| CH3CH2CH3 (13a: C2v) | −0.568 | 232.81 | −41.58 | −68.71 | 164.10 | −110.30 | −5.79 | −3.51 | −9.29 | β |
| CH3CH2CH3 (13b: C2v) | −0.382 | 251.23 | −23.17 | −91.12 | 160.10 | −114.29 | 6.12 | −20.73 | −14.61 | α |
| Me2CHCH3 (14a: C3v) | −0.565 | 230.20 | −44.20 | −73.87 | 156.33 | −118.06 | −4.20 | −4.33 | −8.53 | β |
| Me3CH (14b: C3v) | −0.232 | 267.54 | −6.85 | −114.35 | 153.19 | −121.21 | 9.52 | −21.56 | −12.04 | α |
| t-BuCH3 (15: Td) | −0.563 | 229.82 | −44.58 | −78.63 | 151.19 | −123.20 | −2.93 | −4.47 | −7.40 | β |
| CH3CH2CH2CH3 (16a: C2h) | −0.565 | 236.01 | −38.38 | −70.67 | 165.34 | −109.05 | 3.20 | −1.96 | 1.24 | γ |
| CH3CH2CH2CH3 (16b: C2h) | −0.377 | 244.34 | −30.06 | −93.60 | 150.74 | −123.66 | −6.89 | −2.48 | −9.37 | β |
| n-BuCH3 (17a: C2v) | −0.375 | 233.25 | −41.15 | −67.69 | 165.55 | −108.84 | −2.77 | 2.98 | 0.21 | δ |
| n-PrMeCH2 (17b: C2v) | −0.564 | 252.13 | −22.27 | −99.81 | 152.32 | −122.08 | 7.79 | −6.21 | −6.21 | γ |
| i-PrMeCH2 (18a: C1) | −0.372 | 247.55 | −26.84 | −102.18 | 145.37 | −129.02 | −1.84 | −5.53 | −7.37 | β |
| EtMe2CH (18b: C1) | −0.225 | 265.39 | −9.01 | −118.76 | 146.63 | −127.77 | −2.15 | −4.41 | −6.56 | β |
| t-BuMeCH2 (19: Cs) | −0.371 | 251.79 | −22.60 | −112.21 | 139.58 | −134.81 | 0.19 | −7.03 | −6.84 | β |
| n-BuMeCH2(20: C2h) | −0.374 | 243.63 | −30.77 | −101.29 | 142.33 | −132.06 | −8.50 | −1.48 | −9.98 | δ |
| i-PrMe2CH (21: C2h) | −0.219 | 259.35 | −15.04 | −118.14 | 141.21 | −133.18 | −4.10 | −1.89 | −5.99 | β |
| t-BuMe2CH (22: Cs) | −0.215 | 265.19 | −9.20 | −127.39 | 137.80 | −136.60 | −0.78 | −4.35 | −5.13 | β |
| n-PrMe2CH (23: C1) | −0.224 | 274.83 | 0.43 | −128.33 | 146.50 | −127.90 | 9.44 | −9.57 | −9.57 | γ |
| n-BuMe2CH (24: C1) | −0.223 | 276.20 | 1.80 | −128.65 | 147.55 | −126.84 | 1.37 | −0.31 | 1.06 | ε |
| H3C+ (25: C3v) | 0.362 | 240.45 | −33.94 | −467.68 | −227.23 | −501.62 | e | e | e | e |
| MeH2C+ (26: Cs) | −0.215 | 242.18 | −32.21 | −219.86 | 22.32 | −252.08 | 1.73 | 247.82 | 249.55 | α |
| EtH2C+ (27: Cs) | −0.256 | 242.53 | −31.87 | −140.46 | 102.07 | −172.33 | 0.35 | 79.40 | 79.75 | β |
| i-PrH2C+ (28: C1) | −0.472 | 256.69 | −17.70 | −139.73 | 116.96 | −157.44 | 7.26 | 40.07 | 47.32 | β |
| t-BuH2C+ (29: C1) | −0.489 | 270.61 | −3.79 | −149.75 | 120.85 | −153.54 | 9.48 | 23.37 | 32.84 | β |
| Me2HC+ (30: C2) | 0.396 | 247.98 | −26.42 | −400.37 | −152.39 | −426.79 | 3.76 | 33.66 | 37.42 | α |
| EtMeHC+ (31: C1) | 0.366 | 256.56 | −17.84 | −396.76 | −140.20 | −414.59 | 256.56 | −396.76 | −140.20 | β |
| i-PrMeHC+ (32: C1) | 0.174 | 268.19 | −6.21 | −284.57 | −16.39 | −290.78 | 134.09 | −142.29 | −8.19 | β |
| t-BuMeHC+ (33: C1) | −0.327 | 293.49 | 19.10 | −182.60 | 110.89 | −163.50 | 97.83 | −60.87 | 36.96 | β |
| Me3C+ (34: C1) | 0.536 | 242.28 | −32.12 | −411.12 | −168.84 | −443.23 | 0.61 | 18.86 | 19.46 | α |
| EtMe2C+ (35: C1) | 0.540 | 242.21 | −32.19 | −414.36 | −172.15 | −446.55 | −0.07 | −3.24 | −3.31 | β |
| i-PrMe2C+ (36: C1) | 0.544 | 242.44 | −31.95 | −404.51 | −162.07 | −436.46 | 0.08 | 3.30 | 3.39 | β |
| t-BuMe2C+ (37: C1) | 0.537 | 242.76 | −31.63 | −402.64 | −159.88 | −434.27 | 0.16 | 2.83 | 2.99 | β |
| CH3OH (38: Cs) | −0.191 | 232.16 | −42.24 | −104.67 | 127.49 | −146.90 | −6.82 | −55.01 | −61.82 | α-X |
| CH3SH (39: Cs) | −0.692 | 243.86 | −30.54 | −73.63 | 170.23 | −104.16 | 4.88 | −23.97 | −19.09 | α-X |
| CH3SeH (40: Cs) | −0.737 | 240.42 | −33.97 | −66.34 | 174.08 | −100.31 | 1.45 | −16.68 | −15.23 | α-X |
| CH3SSMe (41: C2) | −0.709 | 241.38 | −33.02 | −85.75 | 155.63 | −118.77 | 2.40 | −36.09 | −33.69 | α-X |
| CH3F (42: C3v) | −0.066 | 232.99 | −41.40 | −126.01 | 106.99 | −167.41 | −5.98 | −76.35 | −82.33 | α-X |
| CH3Cl (43: C3v) | −0.529 | 243.61 | −30.79 | −93.19 | 150.42 | −123.98 | 4.63 | −43.53 | −38.90 | α-X |
| CH3Br (44: C3v) | −0.601 | 241.70 | −32.69 | −83.06 | 158.65 | −115.75 | 2.73 | −33.40 | −30.67 | α-X |
| CH3I (45: C3v) | −0.719 | 236.32 | −38.08 | −56.17 | 180.15 | −94.24 | −2.66 | −6.51 | −9.17 | α-X |
| CH3CO2Me (46: Cs) | −0.666 | 248.04 | −26.36 | −87.22 | 160.82 | −113.58 | 9.06 | −37.56 | −28.50 | α-X |
| CH3CN (47: C3v) | −0.677 | 220.25 | −54.14 | −38.56 | 181.70 | −92.70 | −18.72 | 11.10 | −7.62 | α-X |
| CH3NH2 (48: CS) | −0.360 | 229.80 | −44.60 | −79.79 | 150.01 | −124.39 | −9.18 | −30.13 | −39.31 | α-X |
| CH3NO2 (49: Cs) | −0.410 | 231.58 | −42.82 | −114.08 | 117.50 | −156.90 | −7.40 | −64.42 | −71.82 | α-X |
| CH3CH2OH (50a: Cs) | −0.016 | 225.96 | −48.44 | −108.36 | 117.60 | −156.80 | −12.64 | −43.15 | −55.79 | α-X |
| CH3CH2OH (50b: Cs) | −0.587 | 245.33 | −29.07 | −81.19 | 164.14 | −110.26 | 6.73 | −15.98 | −9.25 | β-X |
H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (51: D2h) CH2 (51: D2h) |
−0.365 | 246.91 | −27.49 | −194.83 | 52.08 | −222.31 | 8.31 | −129.62 | −121.31 | C2H4f |
HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (52: D∞h) CH (52: D∞h) |
−0.225 | 255.62 | −18.77 | −145.56 | 110.06 | −164.34 | 17.03 | −80.35 | −63.33 | C2H2f |
| C6H6 (53: D6h) | −0.106 | 239.66 | −34.73 | −192.70 | 46.96 | −227.43 | 1.07 | −127.49 | −126.43 | C6H6f |
| C6H5OH (54: Cs) | 0.449 | 258.53 | −15.87 | −243.01 | 15.52 | −258.88 | 18.86 | −50.31 | −31.45 | i-X |
H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (55: C2v) O (55: C2v) |
0.307 | 245.20 | −29.19 | −263.64 | −18.44 | −292.83 | 13.05 | −158.97 | −145.93 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (56: Cs) O (56: Cs) |
0.675 | 245.49 | −28.90 | −228.53 | 16.97 | −257.43 | 13.34 | −123.86 | −110.53 | OC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
The Δσm(C: S)e values are defined by Δσm(C: S)e = (1/n)[Δσm(C: S) − Δσm(C: Se)], for the pre-α, α, β, γ and δ effects, together with the α-X, β-X and i-X effects, where Se are the starting species for the effects and n is the factor used to make Δσm(C: S)e per unit group. Scheme 2 explains the method used to calculate the effects, exemplified by the pre-α, α and β effects. The effects are calculated according to the definition. In the case of the β effect from CH3CH2CH3 to (CH3)2CHCH2CH3, (CH3)2CHCH2CH3, CH3CH2CH3 and 2 correspond to S, Se and n, respectively. The difference in Δσt(C: S) between Se = CH3CH2CH3 (σt(C) = 160 ppm) and S = (CH3)2CHCH2CH3 (145 ppm) is −15 ppm, which corresponds to the 2β effect. The Δσt(C: S) values are abbreviated as Δ in Scheme 2. Therefore, the β effect in this process is evaluated as −8 ppm (=Δ/2), for example. The Δσd(C: S)e and Δσp(C: S)e values for the effect are calculated similarly. Scheme 3 summarizes the pre-α, α, β, γ and δ effects, together with the α-X, β-X and i-X effects and the effects of the characteristic bonds and groups. The Δσd(C)e, Δσp(C)e and Δσt(C)e values are also shown in Scheme 3.
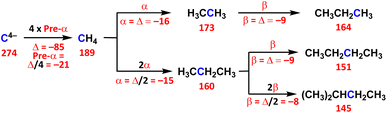 | ||
| Scheme 2 Evaluation of the pre-α, α and β effects. The σt(C: S) values in ppm are given in red bold, and the differences between the two values are Δ. | ||
Fig. 3 shows the plots of δ(C: S)obsd:TMS versus –Δσt(C: S)calcd:TMS for the various species of 1–56 shown in Table 4 and S21–S70 in Table S12 of the ESI,† although the species are limited for those of the available δ(C: S)obsd:TMS values. The −Δσt(C: S)calcd:TMS values are used in Fig. 3 for the convenience of the direct comparison between the observed and calculated values. The plot shows an excellent correlation (y = −1.52 + 0.942x: Rc2 = 0.998), with some systematic deviations. The excellent correlation confirms the high reliability of the calculations. While the data for (CH3F, CH2F2, CHF3 and CF4) are on the correlation line, those for (CH3Cl, CH2Cl2, CHCl3 and CCl4) and (CH3Br, CH2Br2, CHBr3 and CBr4) deviate systematically from the line.
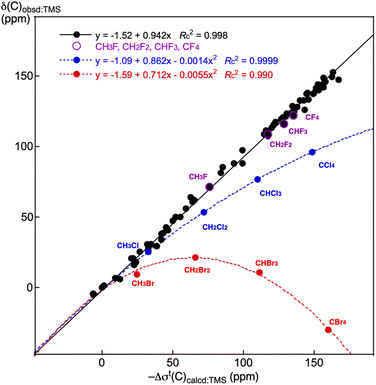 | ||
| Fig. 3 Plots of δ(C: S)obsd:TMS versus −Δσt(C: S)calcd:TMS for 1–56 and S21–S70, CH3X, CH2X2, CHX3 and CX4 (X = F, Cl and Br). | ||
While the data point for CH3Cl is on the line, that from CH3Br seems to deviate slightly downside from the correlation. Data for the chlorine and bromine species are analysed using the quadratic functions. The correlations are described by y = −1.09 + 0.862x − 0.0014x2: Rc2 = 0.9999 and y = −1.59 + 0.712x − 0.0055x2: Rc2 = 0.990, respectively. The relativistic effect must be responsible for the deviations, which will not be discussed here.10,11,40–42
Behaviour of σd(C)
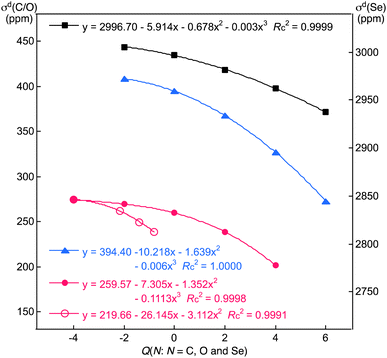
Fig. 4 shows the plot of σd(C) versus Q(C) for (C4+, C2+, C0, C2− and C4−). The correlation is excellent if analysed using the cubic function (y = 0.1113x3 − 1.352x2 − 7.305x + 259.57: Rc2 = 0.9998). The behaviour of the σd(C) values can essentially be understood by considering the two factors shown by eqn (3) and (4). If the number of occupied MOs (ψi) on C increases, the σd(C) values increase, although the magnitude of each σdi(C) will decrease, especially for the outer ψi (as shown by σdB3LYP(C: AO)). The average distance of the electrons from C(ri) in question in ψi becomes longer because of the increase in the electron–electron repulsion if the number of occupied MOs increases. In this case, each 〈ri−1〉 and σdi in eqn (4) decreases. The σdi values collected in Table 4 are well understood as the total effect of the two. Fig. 2 also shows the plot of σd(C) versus Q(C) for (C4−, CH22−, CH3−, and CH4). The correlation is also excellent if analysed using the quadratic function (y = −3.112x2 − 26.145x + 219.66: Rc2 = 0.9998).Fig. 4 contains similar plots for (O6+, O4+, O2+, O0 and O2−)16 and (Se6+, Se4+, Se2+, Se0 and Se2−),9 whose correlations are excellent (y = −0.006x3 − 1.639x2 − 10.218x + 394.40: Rc2 = 1.0000 for O and y = 0.003x3 − 0.678x2 − 5.914x + 2996.70: Rc2 = 0.9999 for Se). The correlations become less sharp in the order of N = O > C > Se. The Δσd(N2−:2+) (=(σd(N2−) − σd(N2+)) value was divided by the overall chemical shift width for each N. The values were compared with each other to estimate roughly the contributions of σd(N) to σt(N). The ratio is 0.103 (=31.0/300) for C, 0.016 (=41.1/2500) for O and 0.003 (=23.9/8000) for Se. The results show that the contributions of σd(N) to the overall chemical shifts decreased in the order of N = C ≫ O >> Se. As a result, the σd(C) term should be considered carefully for N = C relative to the cases of O and Se.
Fig. 5 shows the plot of σd(C: S) versus Q(C) for 1–56 in Table 4, except for those plotted in Fig. 4 (1–5). The σd(C: S) values are analysed separately by the types of S: RH2C− (6–11), RCH3 (12–16), RMeCH2 (17–20), RMe2CH (21–25), RH2C+ (26–30), RMeHC+ (31–34), RMe2C+ (35 and 36) and CH3X (37–50), together with C2H4 (51), C2H2 (52), C6H6 (53), H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (54) and H(HO)C
O (54) and H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (55). The range of σd(C) in each plot is less than approximately 15 ppm, except for that of RMeCH2 (17–20), which ranges from 30 ppm. The σd(C: S) values increase in the order of R = Me < Et < i-Pr < t-Bu for RH2C− and RCH3. However, the behaviour seems complex for others.
O (55). The range of σd(C) in each plot is less than approximately 15 ppm, except for that of RMeCH2 (17–20), which ranges from 30 ppm. The σd(C: S) values increase in the order of R = Me < Et < i-Pr < t-Bu for RH2C− and RCH3. However, the behaviour seems complex for others.
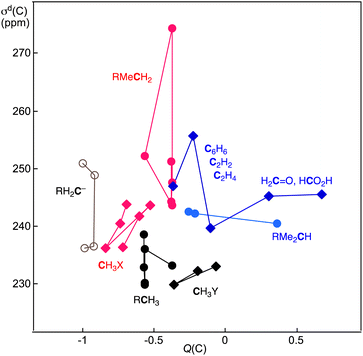 | ||
| Fig. 5 Plots of σd(C) versus Q(C) for various species, 1–56, except for HkC(4−k)− (1–4), collected in Table 4. | ||
The origin and mechanisms of the effects are discussed next, using an approximated image derived from eqn (6).
Origin of the pre-α effect
The analysis of the 13C NMR chemical shifts starts from the pre-α effect,9 this is the important starting point of our NMR analysis. The pre-α effect is evaluated by the (Δσd(C)e, Δσp(C)e, Δσt(C)e) values. The average values from C4− to H4C are (−8.9, −12.4, −21.3 ppm) (see Table 4), which are calculated per unit group (per H in this case). The pre-α effect is theoretically predicted to be a downfield shift of 21 ppm based on the average value of Δσt(C)e from C4− to H4C. The solvent effect is not considered for the pre-α effect on each process from C4− to CH4, although the negative charge would also be of importance (see Scheme 3).Table 5 lists the σd(C), σp(C) and σt(C) values of C4−, H2C2−, H3C− and H4C, separately by each MO. The inner MO of ψ1 is formed by the 1s(C) AO; therefore, it greatly contributes to σd(C) but not σp(C). The second inner ψ2 is constructed mainly by the 2s(C) AO; therefore, it contributes substantially to σd(C) but slightly to σp(C). The outer MOs of ψ3, ψ4 and ψ5 are constructed mainly by the 2px(C), 2py(C) and 2pz(C) AOs with 1s(H) AO(s), if any. Therefore, they contribute greatly to σp(C) but slightly to σd(C), although σp(C) = 0 ppm for all the MOs in C4−. The MOs of ψ3, ψ4 and ψ5 in C4− are equivalent; therefore, the total values of the three are given in the Table, as are ψ3 and ψ4 in H3C−.
| MO (i in ψi) | σdi(C) | σpi(C) | σti(C) |
|---|---|---|---|
| a Calculated with the GIAO-DFT method under B3LYP/BSS-A.b MOs of ψ1, ψ2, ψ3, ψ4 and ψ5 of C4− are constructed mainly by 1s(C), 2s(C), 2px(C), 2py(C) and 2pz(C) AOs, respectively. | |||
| C4– (Oh)b | |||
| 1 (A1g) | 200.45 | 0.00 | 200.45 |
| 2 (A1g) | 31.72 | 0.00 | 31.72 |
| 3 (T1u)–5 (T1u) | 42.04 | 0.00 | 42.04 |
| Total | 274.39 | 0.00 | 274.39 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| H2C2− (C2v) | |||
| 1 (A1) | 200.37 | 0.00 | 200.37 |
| 2 (A1) | 23.73 | −8.06 | 15.68 |
| 3 (B2) | 1.27 | −93.29 | −92.02 |
| 4 (A1) | 20.18 | −16.17 | 4.01 |
| 5 (B1) | 16.41 | 197.59 | 214.00 |
| ψocc to ψocc | 57.39 | ||
| Total | 261.96 | 137.46 | 399.42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| H3C− (C3v) | |||
| 1 (A1) | 200.32 | 0.00 | 200.32 |
| 2 (A1) | 24.93 | −3.19 | 21.74 |
| 3 (E)–4 (E) | 3.28 | −148.47 | −145.19 |
| 5 (A1) | 21.00 | 24.22 | 45.22 |
| ψocc to ψocc | 125.17 | ||
| Total | 249.52 | −2.26 | 247.26 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| H4C (Td) | |||
| 1 (A1) | 200.28 | 0.00 | 200.28 |
| 2 (A1) | 26.97 | 0.33 | 27.30 |
| 3 (T2)–5 (T2) | 11.73 | −175.52 | −163.83 |
| ψocc to ψocc | 125.55 | ||
| Total | 238.98 | −49.66 | 189.32 |
The protonation of C4− introduces σ(C–H) and σ*(C–H) orbitals in the resulting species, resulting in the asymmetrical distribution of electrons. The symmetrical and unsymmetrical components of the electron distribution produce σd(C) and σp(C), respectively, as mentioned above. The σp(C) terms are caused through orbital-to-orbital transitions, such as the ψi→ψa transition, where σ(C–H) and σ*(C–H) are expected to operate as the typical ψi and ψa, respectively, in the transitions. Table 6 lists the ψi→ψa transitions that contributed mainly to σpi→a:kk(C: k = x, y and/or z) for H2C2− and H4C. The magnitudes of σpi→a(C), which are greater than 10 ppm in magnitude, are provided in Table 6. In the case of H2C2−, the ψ3→ψ9 (σp3→9:zz(C) = −113.8 ppm) and ψ4→ψ9 (σp4→9:yy(C) = −87.6 ppm) transitions largely contribute to σpi→a(C), with large positive contributions from the ψ4→ψ7, ψ5→ψ6, ψ5→ψ7 and ψ5→ψ8 transitions. The ψ3→ψ15, ψ3→ψ16, ψ4→ψ14, ψ4→ψ16, ψ5→ψ14 and ψ5→ψ15 transitions contribute greatly to σp(C) (σpi→a:kk(C) = −33.3 ppm) in H4C.
| i→ab | σpi→a:xx(C) | σpi→a:yy(C) | σpi→a:zz(C) | σpi→a(C) |
|---|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A.b The magnitudes of σpi→a(C) larger than 10 ppm are shown. In ψi→ψa. | ||||
| H2C2− (C2v) | ||||
| 3→9 | 0.00 | 0.00 | −113.76 | −37.92 |
| 4→7 | 72.46 | 0.00 | 0.00 | 24.15 |
| 4→9 | 0.00 | −87.63 | 0.00 | −29.21 |
| 5→6 | 0.00 | 216.63 | 0.00 | 72.21 |
| 5→7 | 0.00 | 0.00 | 225.75 | 75.25 |
| 5→8 | 0.00 | 143.42 | 0.00 | 47.81 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| CH4 (Td) | ||||
| 3→15 | −33.27 | 0.00 | 0.00 | −11.09 |
| 3→16 | 0.00 | −33.27 | 0.00 | −11.09 |
| 4→14 | −33.27 | 0.00 | 0.00 | −11.09 |
| 4→16 | 0.00 | 0.00 | −33.27 | −11.09 |
| 5→14 | 0.00 | −33.27 | 0.00 | −11.09 |
| 5→15 | 0.00 | 0.00 | −33.27 | −11.09 |
Fig. 6a and b illustrate the selected ψi→ψa transitions for H2C2− and H4C, respectively, along with the characteristics of ψi and ψa and the orbital energies. Fig. 6a shows the ψ3→ψ9, ψ4→ψ7, ψ4→ψ9, ψ5→ψ6, ψ5→ψ7 and ψ5→ψ8 transitions in H2C2−, whereas the ψ3→ψ15, ψ3→ψ16, ψ4→ψ14, ψ4→ψ16, ψ5→ψ14 and ψ5→ψ15 transitions in H4C are shown in Fig. 6b.
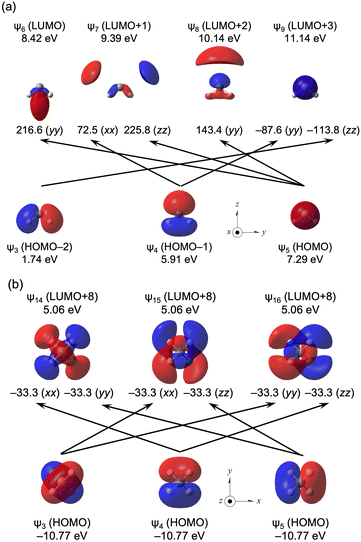 | ||
| Fig. 6 Contributions from each ψi→ψa transition to the components of σp(C) in H2C2− (a) and H4C (b) with an isovalue of 0.04 au. | ||
Origin of the α effect
A downfield shift of approximately 10–15 ppm is usually observed for the α effect, which is well supported by the calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values of (−0.4, −15.6, −15.9 ppm) for CH3CH3 from CH4 (see Table 4 and Scheme 3).Table 7 lists the σd(C), σp(C) and σt(C) values of C2H6, separately for each MO. Similar to the case of H4C, the first and second inner MOs of ψ1 and ψ2 are constructed by the 1s(C) AOs; therefore, they greatly contribute to σd(C) but not σp(C). The third and fourth inner MOs of ψ3 and ψ4 are formed mainly by the 2s(C) AOs; therefore, they contribute to σd(C) and σp(C), more or less. The outer MOs of ψ5–ψ9 are constructed mainly by the 2p(C) AOs with the 1s (H) AOs; therefore, they contribute largely to σpi(C) (−36.6 to −42.3 ppm), with very small contributions to σd(C) (−4.1 to 8.0 ppm). Table 8 lists the ψi→ψa transitions contributing to σpi→a:kk(C: k = x, y and/or z), where a larger than ca. 20 ppm in magnitude. The ψi→ψa transitions of ψ5→ψ29 (σp5→29:yy(C) = −21.0 ppm), ψ6→ψ29 (σp6→29:xx(C) = −20.5 ppm), ψ7→ψ26 (σp7→26:xx(C) = −25.5 ppm), ψ7→ψ27 σp7→27:yy(C) = −25.5 ppm), ψ8→ψ27 (σp8→27:zz(C) = −21.1 ppm) and ψ9→ψ26 (σp9→26:zz(C) = −21.2 ppm) contribute a lot to σpi→a:kk(C: k = x, y and/or z).
| MO (i in ψi) | σdi(C) | σpi(C) | σti(C) |
|---|---|---|---|
| a Calculated with the GIAO-DFT method under B3LYP/BSS-A. | |||
| 1 (A1g); 2 (A2u) | 200.29 | 0.00 | 200.29 |
| 3 (A1g) | 15.73 | −3.49 | 12.24 |
| 4 (A2u) | 17.28 | 5.92 | 23.20 |
| 5 (Eu) | 2.78 | −36.60 | −33.81 |
| 6 (Eu) | 2.78 | −36.74 | −33.95 |
| 7 (A1g) | 7.97 | −42.31 | −34.34 |
| 8 (Eg) | −4.12 | −36.94 | −41.06 |
| 9 (Eg) | −4.12 | −36.94 | −41.06 |
| ψocc to ψocc | 121.88 | ||
| Total | 238.60 | −65.21 | 173.39 |
| i→ab | σpi→a:xx(C) | σpi→a:yy(C) | σpi→a:zz(C) | σpi→a(C) |
|---|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A. Magnitudes of σpi→a(C) larger than 6 ppm are shown.b In ψi→ψa. | ||||
| 5→29 | 0.00 | −20.97 | 0.00 | −6.99 |
| 6→29 | −20.53 | 0.00 | 0.00 | −6.84 |
| 7→26 | −25.48 | 0.00 | 0.00 | −8.49 |
| 7→27 | 0.00 | −25.47 | 0.00 | −8.49 |
| 8→27 | 0.00 | −0.54 | −21.14 | −7.23 |
| 9→26 | 0.00 | −0.54 | −21.16 | −7.23 |
Fig. 7 shows the selected transitions of CH3CH3 (D3d), as shown in Table 8. The occupied-to-unoccupied MO transitions play an important role in the α effect in CH3CH3. While the HOMO of ψ9 acts as a good donor, the LUMOs of ψ10 and ψ11–ψ25 do not seem to contribute substantially to the transitions.
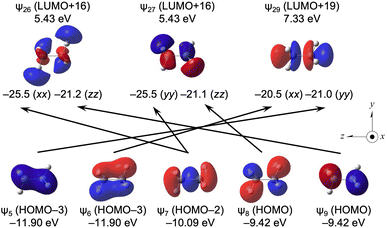 | ||
| Fig. 7 Contributions from each ψi→ψa transition to the σpi→a:kk(C: k = x, y and/or z) in CH3CH3 with an isovalue of 0.04 au. | ||
Origin of the β effect
A downfield shift of approximately 10 ppm is often observed for the β effect. The β effect is evaluated for CH3CH2CH3 (C2v), (CH3)2CHCH3 (C3v) and (CH3)3CCH3 from the corresponding species. The calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values for the species are (−5.8, −3.5, −9.3 ppm), (−4.2, −4.3, −8.5 ppm) and (−2.9, −4.5, −7.4 ppm), respectively, which reproduce the observed β effect well (see Table 4 and Scheme 3).Table 9 lists the σd(C), σp(C) and σt(C) values for CH3CH2CH3 (C2v) separately by ψi. The contributions from ψ7, ψ8, ψ11, ψ12 and ψ13 to σpi(C) are large (−21.6 to −48.7 ppm), although the contributions seem to be widely distributed to ψi. Table 10 shows the main ψi→ψa transitions contributing to σpi→a:kk(C: k = x, y and/or z). The contributions are relatively large for ψ8→ψ41 (σp8→41:xx(C) = −15.2 ppm) and ψ11→ψ36 (σp11→36:zz(C) = −20.1 ppm).
| MO (i in ψi) | σd(C) | σp(C) | σt(C) |
|---|---|---|---|
| a Calculated with the GIAO-DFT method under B3LYP/BSS-A. | |||
| 1 (A1)–3 (A1) | 200.29 | 0.00 | 200.29 |
| 4 (A1) | 16.20 | −2.72 | 13.48 |
| 5 (B2) | 6.36 | −1.23 | 5.13 |
| 6 (A1) | 16.89 | 4.51 | 21.39 |
| 7 (B1) | 4.00 | −33.20 | −29.20 |
| 8 (A1) | 7.27 | −21.55 | −14.28 |
| 9 (B2) | 11.57 | 2.10 | 13.68 |
| 10 (A2) | −0.60 | −2.62 | −3.22 |
| 11 (B2) | 0.28 | −29.63 | −29.35 |
| 12 (A1) | −5.18 | −48.71 | −53.89 |
| 13 (B1) | −5.87 | −41.71 | −47.58 |
| ψocc to ψocc | 83.64 | ||
| Total | 251.23 | −91.12 | 160.10 |
Fig. 8 shows the ψ8→ψ41 and ψ11→ψ36 transitions, together with the orbital energies and the axes of CH3CH2CH3. The transitions are well visualized. However, it is curious that ψ14 (LUMO) and those near it seem not to operate effectively as acceptors in the transitions. Similar results are obtained for EtMeCH2, i-PrMeCH2 and t-BuMeCH2 from the corresponding species. The calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values are (−6.9, −2.5, −9.4 ppm), (−1.8, −5.5, −7.4 ppm) and (0.2, −7.0, −6.8 ppm), respectively, for the species. For ionic EtH2C−, t-BuH2C− and EtH2C+, the calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values are predicted to be (−2.0, 16.3, 14.3 ppm), (−4.9, 12.2, 7.3 ppm) and (0.4, 79.4, 79.8 ppm) respectively, from the corresponding species. The (large) upfield shifts are again predicted for the β effect for the ionic species. A large positive Δσt(C)e value of 79.8 ppm is predicted for EtH2C+ from the corresponding species. The vacant 2p(C) orbital in EtH2C+ is responsible for these results.
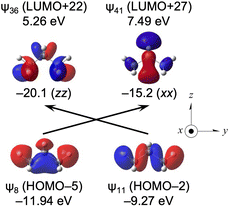 | ||
| Fig. 8 Contributions from each ψi→ψa transition to the σpi→a:kk(C: k = x, y and/or z) of CH3CH2CH3 with an isovalue of 0.04 au. | ||
Origin of the γ and δ effects
An upfield shift of ∼2 ppm is often encountered for the γ effect. The conformational effect cannot be avoided in the γ and δ effects,9 contrary to the α and β effects in the cases of CH4, CH3CH3 and CH3CH2CH3, as discussed above. The γ and δ effects are not discussed further here since they are negligibly small.Origin of the α-X and β-X effects
The α-X effect is examined for CH3–X from CH4 and CH3CH2–X from CH3CH3 (X = OH, SH, SeH, SSCH3, F, Cl, Br, I, CO2Me, CN, NH2 and/or NO2) (see Scheme 3, Table 4 and Table S11 of the ESI†). The (Δσd(C)e, Δσp(C)e, Δσt(C)e) values of (−18.7–40.3, −76.4–−11.1, −82.3–−4.4 ppm) are predicted for the species. The β-X effect is similarly examined for CH3CH2–X from CH3CH3 (X = OH, SH, SeH, SSCH3, F, Cl, Br, I, CO2Me, CN, NH2 and NO2) (see Scheme 3, Table 4 and Table S11 of the ESI†). The (Δσd(C)e, Δσp(C)e, Δσt(C)e) values of (−16.8–0.9, −66.9–14.1, −77.2–−1.3 ppm) are calculated for the species. It seems difficult to find a good relation between the α-X and β-X effects in the same species, as a whole. However, such relation could be found is the species are divided to some groups, as shown in Fig. S10 of the ESI.† The β-X effect of −77.2 ppm for X = F is notable.Table 11 lists the σd(C), σp(C) and σt(C) values for CH3CH2OH and CH3CH2OH, separately for each MO. The outer MOs of HOMO (ψ13), HOMO–1 (ψ12), HOMO-4 (ψ9) and HOMO–5 (ψ8) contribute greatly to σp(C) in CH3CH2OH. On the other hand, the outer MOs of HOMO-2 (ψ11) and HOMO–3 (ψ10) contribute greatly to σp(C) in CH3CH2OH. Table 12 lists the ψi→ψa transitions contributing to σpi→a:kk(C: k = x, y and/or z), which are greater than 5 ppm in magnitude. The contributions from the ψi→ψa transitions seem greater for ψ8→ψ38 (σp8→33:xx(C) = −13.1 ppm and σp8→33:yy(C) = −3.2 ppm) in CH3CH2OH. The contributions are greater for ψ10→ψ31 (σp10→31:yy(C) = −28.7 ppm), ψ10→ψ35 (σp10→35:zz(C) = −17.1 ppm) and ψ10→ψ59 (σp10→59:zz(C) = −15.36 ppm) in CH3CH2OH.
| MO (i in ψi) | σdi(C) | σpi(C) | σti(C) |
|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A. | |||
| CH3CH2OH (Cs) | |||
| 1 (A′)–3 (A′) | 200.33 | 0.00 | 200.32 |
| 4 (A′)–6 (A′) | 30.44 | −6.12 | 24.33 |
| 7 (A′) | 4.08 | −28.13 | −24.05 |
| 8 (A′′) | 4.62 | −50.64 | −46.02 |
| 9 (A′) | −4.90 | −48.07 | −52.97 |
| 10 (A′) | −2.22 | −9.91 | −12.13 |
| 11 (A′′) | −1.61 | −16.19 | −17.80 |
| 12 (A′) | 1.21 | −47.21 | −46.00 |
| 13 (A′′) | −6.00 | −27.71 | −33.70 |
| ψocc to ψocc | 125.61 | ||
| Total | 225.95 | −108.36 | 117.60 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CH3CH2OH (Cs) | |||
| 1 (A′)–3 (A′) | 200.27 | −0.03 | 200.24 |
| 4 (A′)–6 (A′) | 30.09 | 0.84 | 31.14 |
| 7 (A′) | 2.12 | −5.52 | −3.41 |
| 8 (A′′) | 3.12 | −18.92 | −15.80 |
| 9 (A′) | 15.42 | −6.30 | 9.12 |
| 10 (A′) | −3.19 | −72.62 | −75.82 |
| 11 (A′′) | −2.25 | −50.07 | −52.31 |
| 12 (A′) | 0.94 | −20.25 | −19.32 |
| 13 (A′′) | −1.18 | −2.31 | −3.50 |
| ψocc to ψocc | 93.77 | ||
| Total | 245.33 | −81.19 | 164.14 |
| i→ac | σpi→a:xx(C) | σpi→a:yy(C) | σpi→a:zz(C) | σpi→a(C) |
|---|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A.b The magnitudes of σpi→a(C) larger than 5 ppm are shown.c In ψi→ψa. | ||||
| CH3CH2OH (Cs) | ||||
| 8→33 | −13.09 | −3.24 | 0.00 | −5.44 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| CH3CH2OH (Cs) | ||||
| 10→31 | −3.47 | −28.67 | 0.00 | −10.71 |
| 10→35 | 0.00 | 0.00 | −17.07 | −5.69 |
| 10→59 | 0.00 | 0.00 | −15.29 | −5.10 |
Fig. 9a shows the ψ8→ψ33 transition in CH3CH2OH and the ψ10→ψ31, ψ10→ψ35 and ψ10→ψ59 transitions in CH3CH2OH are illustrated in Fig. 9b. The α-X and β-X effects are well visualized, employing the ψi→ψa transitions.
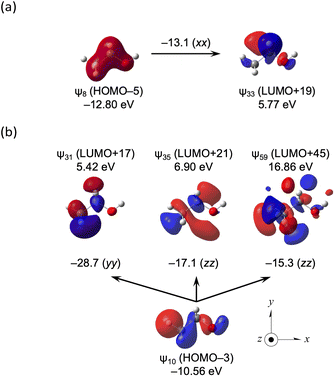 | ||
| Fig. 9 Contributions from each ψi→ψa transition to the components of σp(C) for CH3CH2OH (a) and CH3CH2OH (b), together with the axes with an isovalue of 0.04 au. | ||
The α-X effect is also typical detected in CH3NH2 and CH3NO2. The σd(C), σp(C) and σt(C) values for CH3NH2 and CH3NO2 are listed in Table S13 of the ESI,† separately for each MO. In the case of CH3NH2, which has a very strong electron donating X of NH2, the outer MOs of the HOMO–1 (ψ8) ∼ HOMO–3 (ψ6) contribute greatly to σp(C). In the case of CH3NO2, with the very strong electron accepting group X of NO2, the somewhat more inner HOMO–3 (ψ13) and HOMO–4 (ψ12) also strongly affect σp(C). The ψi→ψa transitions contributing to σpi→a:kk(C: k = x, y and/or z) from the species are collected in Table S14 of the ESI.† The contributions to CH3NH2 are large for the ψi→ψa transitions of ψ7→ψ26 (σp7→26:zz(C) = −19.9 ppm), ψ7→ψ41 (σp7→41:zz(C) = −18.8 ppm), ψ8→ψ23 (σp8→23:yy(C) = −31.4 ppm) and ψ8→ψ26 (σp8→26:xx(C) = −22.9 ppm). Those in CH3NO2 are large for ψ12→ψ28 (σp12→28:xx(C) = −28.7 ppm) and ψ12→ψ29 (σp12→29:yy(C) = −29.2 ppm) and ψ13→ψ28 (σp13→28:zz(C) = −31.6 ppm). The ψi→ψa transitions of ψ7→ψ26, ψ7→ψ41, ψ8→ψ23 and ψ8→ψ26 for CH3NH2 and those of ψ12→ψ28, ψ12→ψ29, ψ13→ψ28 and ψ13→ψ30 for CH3NO2 are shown in Fig. S11a and b of ESI,† respectively. The α-X effect is well visualized both for X of a very good donor and a very good acceptor, employing ψi→ψa transitions.
Effect from ethene and ethyne and the origin
Large downfield shifts in δ(C) are reported for ethene (∼120 ppm) and ethyne (∼60 ppm) from ethane.1–4 The calculated (Δσd(C)e, Δσp(C)e and Δσt(C)e) values for H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and HC
CH2 and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH from CH3CH3 are (8.3, −129.6, −121.3 ppm) and (17.0, −80.4, −63.3 ppm), respectively, which reproduce the observed results well.
CH from CH3CH3 are (8.3, −129.6, −121.3 ppm) and (17.0, −80.4, −63.3 ppm), respectively, which reproduce the observed results well.
Table 13 lists the σd(C), σp(C) and σt(C) values of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h) and HC
CH2 (D2h) and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h), separately for each MO, whereas HOMO–1 (ψ7) ∼ HOMO–3 (ψ5) contribute a lot to σp(C) in H2C
CH (D∞h), separately for each MO, whereas HOMO–1 (ψ7) ∼ HOMO–3 (ψ5) contribute a lot to σp(C) in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h), HOMO–2 (ψ5) and HOMO–3 (ψ4) strongly affect HC
CH2 (D2h), HOMO–2 (ψ5) and HOMO–3 (ψ4) strongly affect HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h). Table 14 shows that the ψi→ψa transitions largely contribute to σp(C) in H2C
CH (D∞h). Table 14 shows that the ψi→ψa transitions largely contribute to σp(C) in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h) and HC
CH2 (D2h) and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h). The ψi→ψa transitions of ψ6→ψ9 (σp6→9:yy(C) = −175.0 ppm), ψ6→ψ17 (σp6→17:yy(C) = −47.7 ppm), ψ6→ψ37 (σp6→37:yy(C) = −31.9 ppm), ψ7→ψ9 (σp7→9:zz(C) = −126.0 ppm) and ψ7→ψ17 (σp7→17:zz(C) = −32.1 ppm) provide great contributions to H2C
CH (D∞h). The ψi→ψa transitions of ψ6→ψ9 (σp6→9:yy(C) = −175.0 ppm), ψ6→ψ17 (σp6→17:yy(C) = −47.7 ppm), ψ6→ψ37 (σp6→37:yy(C) = −31.9 ppm), ψ7→ψ9 (σp7→9:zz(C) = −126.0 ppm) and ψ7→ψ17 (σp7→17:zz(C) = −32.1 ppm) provide great contributions to H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h). Similarly, the transitions of ψ5→ψ10 (σp5→10:yy(C) = −84.2 ppm), ψ5→ψ11 (σp5→11:xx(C) = −84.2 ppm), ψ5→ψ17 (σp5→17:xx(C) = −48.7 ppm) and ψ5→ψ18 (σp5→18:yy(C) = −48.7 ppm) greatly contribute to HC
CH2 (D2h). Similarly, the transitions of ψ5→ψ10 (σp5→10:yy(C) = −84.2 ppm), ψ5→ψ11 (σp5→11:xx(C) = −84.2 ppm), ψ5→ψ17 (σp5→17:xx(C) = −48.7 ppm) and ψ5→ψ18 (σp5→18:yy(C) = −48.7 ppm) greatly contribute to HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h).
CH (D∞h).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h) and HC
CH2 (D2h) and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h), given separately by each ψia
CH (D∞h), given separately by each ψia
| MO (i in ψi) | σdi(C) | σpi(C) | σti(C) |
|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A. | |||
| H2C=CH2 (D2h) | |||
| 1 (Ag); 2 (B1u) | 200.30 | 0.02 | 200.32 |
| 3 (Ag) | 16.54 | −10.56 | 5.98 |
| 4 (B1u) | 13.95 | −0.39 | 13.56 |
| 5 (B2u) | 1.14 | −33.89 | −32.75 |
| 6 (Ag) | 5.18 | −112.14 | −106.96 |
| 7 (B3g) | −7.49 | −87.51 | −94.99 |
| 8 (B3u) | 17.28 | −19.65 | −2.37 |
| ψocc to ψocc | 69.30 | ||
| Total | 246.91 | −194.83 | 52.08 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CH (D∞h) CH (D∞h) |
|||
| 1 (Σg); 2 (Σu) | 200.21 | −0.46 | 199.74 |
| 3 (Σg) | 17.82 | −22.76 | −4.95 |
| 4 (Σu) | 2.99 | −34.63 | −31.64 |
| 5 (Σg) | −2.03 | −131.94 | −133.97 |
| 6 (Πu) | 18.32 | −10.20 | 8.12 |
| 7 (Πu) | 18.32 | 0.04 | 18.36 |
| ψocc to ψocc | 54.39 | ||
| Total | 255.62 | −145.56 | 110.06 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h) and HC
CH2 (D2h) and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h)a,b
CH (D∞h)a,b
| i→ac | σpi→a:xx(C) | σpi→a:yy(C) | σpi→a:zz(C) | σpi→a(C) |
|---|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A.b The magnitudes of σpi→a(C) larger than 5 ppm are shown.c In ψi→ψa. | ||||
| H2C=CH2 (D2h) | ||||
| 6→9 | 0.00 | −174.98 | 0.00 | −58.33 |
| 6→17 | 0.00 | −47.66 | 0.00 | −15.89 |
| 6→37 | 0.00 | −31.93 | 0.00 | −10.64 |
| 7→9 | 0.00 | 0.00 | −126.04 | −42.01 |
| 7→17 | 0.00 | 0.00 | −32.05 | −10.68 |
| 7→19 | −21.82 | 0.00 | 0.00 | −7.27 |
| 7→37 | 0.00 | 0.00 | −16.43 | −5.48 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e002.gif) CH (D∞h) CH (D∞h) |
||||
| 5→10 | 0.00 | −84.16 | 0.00 | −28.05 |
| 5→11 | −84.16 | 0.00 | 0.00 | −28.05 |
| 5→17 | −48.66 | 0.00 | 0.00 | −16.22 |
| 5→18 | 0.00 | −48.66 | 0.00 | −16.22 |
| 5→30 | −22.78 | 0.00 | 0.00 | −7.59 |
| 5→31 | 0.00 | −22.78 | 0.00 | −7.59 |
| 5→37 | −23.40 | 0.00 | 0.00 | −7.80 |
| 5→38 | 0.00 | −23.40 | 0.00 | −7.80 |
Fig. 10a shows the ψ6→ψ9, ψ6→ψ17, ψ6→ψ37, ψ7→ψ9 and ψ7→ψ37 transitions in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h), and Fig. 10b shows the ψ5→ψ10, ψ5→ψ11, ψ5→ψ17 and ψ5→ψ18 transitions in HC
CH2 (D2h), and Fig. 10b shows the ψ5→ψ10, ψ5→ψ11, ψ5→ψ17 and ψ5→ψ18 transitions in HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h). The MO energies and axes are also shown in the figure. The main characteristics of ψ6, ψ7, ψ9, ψ17 and ψ37 with H2C
CH (D∞h). The MO energies and axes are also shown in the figure. The main characteristics of ψ6, ψ7, ψ9, ψ17 and ψ37 with H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 are occupied σ(C
CH2 are occupied σ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), σ(C–H), vacant π*(C
C), σ(C–H), vacant π*(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), σ*(C–C) and σ*(C–H), respectively. The LUMO of ψ9 acts as a great acceptor in the transition, whereas the HOMO of ψ8 does not seem to do so. The main characteristic of ψ5 in HC
C), σ*(C–C) and σ*(C–H), respectively. The LUMO of ψ9 acts as a great acceptor in the transition, whereas the HOMO of ψ8 does not seem to do so. The main characteristic of ψ5 in HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH is the occupied σ(C–C), whereas those of ψ10 and ψ11 are the vacant π*(C
CH is the occupied σ(C–C), whereas those of ψ10 and ψ11 are the vacant π*(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), and those of ψ17 and ψ18 seem to be higher vacant π*(C
C), and those of ψ17 and ψ18 seem to be higher vacant π*(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The HOMO and LUMO in HC
C). The HOMO and LUMO in HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH seem not to act as good donors and acceptors, respectively.
CH seem not to act as good donors and acceptors, respectively.
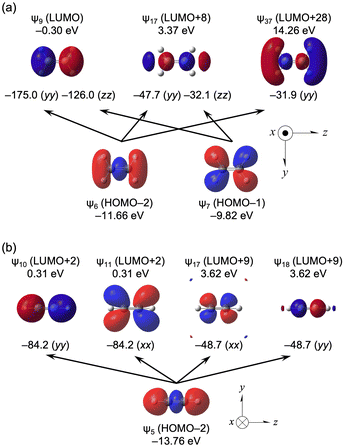 | ||
Fig. 10 Contributions from each ψi→ψa transition to the components of σp(C) in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (D2h) (a) and HC CH2 (D2h) (a) and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (D∞h) (b), together with the axes with an isovalue of 0.04 au. CH (D∞h) (b), together with the axes with an isovalue of 0.04 au. | ||
Effect of benzene and its origin
Large downfield shifts in δ(C) (∼127 ppm) are reported for benzene.1–4 The calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values from CH3CH3 are (1.1, −127.5, −126.4 ppm). The calculations reproduced the observed results well.Table 15 lists the σd(C), σp(C) and σt(C) values of C6H6, separately, by ψi. The contributions from HOMO–2 (ψ19), HOMO–5 (ψ16) and HOMO–7 (ψ14) are very large, together with HOMO–3 (ψ18). Table 16 lists the ψi→ψa transitions that greatly contribute to σp(C). The transitions of ψ14→ψ22 (σp14→22:yy(C) = −95.9 ppm), ψ16→ψ22 (σp16→22:xx(C) = −95.3 ppm), ψ16→ψ78 (σp16→78:xx(C) = −40.1 ppm) and ψ19→ψ40 (σp19→40:xx(C) = −38.3 ppm) contribute greatly, together with ψ18→ψ40 (σp18→40:yy(C) = −43.9 ppm) and ψ18→ψ50 (σp18→50:yy(C) = −31.2 ppm).
| MO (i in ψi) | σdi(C) | σpi(C) | σti(C) |
|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A. | |||
| 1 (A1g) | 33.37 | −0.01 | 33.37 |
| 2 (E1u)–6 (B1u) | 167.08 | 0.17 | 167.23 |
| 7 (A1g) | 5.82 | −5.41 | 0.42 |
| 8 (E1u) | 2.95 | −4.84 | −1.89 |
| 9 (E1u) | 11.07 | −3.02 | 8.05 |
| 10 (E2g) | 0.88 | −15.67 | −14.78 |
| 11 (E2g) | 7.07 | 2.34 | 9.41 |
| 12 (A1g) | 1.95 | −11.67 | −9.72 |
| 13 (B2u) | 3.33 | −10.46 | −7.13 |
| 14 (B1u) | 2.84 | −48.38 | −45.54 |
| 15 (E1u) | 6.87 | −0.43 | 6.44 |
| 16 (E1u) | −19.97 | −107.31 | −127.28 |
| 17 (A2u) | 8.37 | −9.28 | −0.91 |
| 18 (E2g) | 1.65 | −30.56 | −28.91 |
| 19 (E2g) | −5.76 | −48.14 | −53.90 |
| 20 (E1g) | 11.06 | −11.58 | −0.51 |
| 21 (E1g) | 2.08 | −1.36 | 0.72 |
| ψocc to ψocc | 111.93 | ||
| Total | 240.68 | −193.67 | 47.00 |
| i→ac | σpi→a:xx(C) | σpi→a:yy(C) | σpi→a:zz(C) | σpi→a(C) |
|---|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A.b The magnitudes of σpi→a(C) larger than 8 ppm are shown.c In ψi→ψa. | ||||
| 3→22 | −36.14 | 0.00 | 0.00 | −12.05 |
| 14→22 | 0.00 | −95.93 | 0.00 | −31.98 |
| 15→22 | 0.00 | −27.30 | 0.00 | −9.10 |
| 16→22 | −95.30 | 0.00 | 0.00 | −31.77 |
| 16→68 | 0.00 | 0.00 | −26.05 | −8.68 |
| 16→78 | −40.08 | 0.00 | 0.00 | −13.36 |
| 16→93 | 0.00 | 0.00 | −25.58 | −8.53 |
| 16→117 | 0.00 | 0.00 | −28.12 | −9.37 |
| 18→40 | 0.00 | −43.94 | 0.00 | −14.65 |
| 18→50 | 0.00 | −31.15 | 0.00 | −10.38 |
| 19→40 | −38.27 | 0.00 | 0.00 | −12.76 |
| 19→50 | −26.96 | 0.00 | 0.00 | −8.99 |
| 19→59 | 0.00 | 0.00 | −26.04 | −8.68 |
Fig. 11 shows the ψi→ψa transitions in C6H6, shown in Table 16, although they are limited to those discussed above, together with the MO energies and axes. The main characteristics of ψ14, ψ16 and ψ19 are the occupied σ(C–C), σ(C–C) with σ(C–H) and σ(C–C) with σ(C–H), respectively, whereas those of ψ22, ψ40 and ψ78 are vacant π*(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), π*(C6H6), and much higher π*(C6H6), respectively. The LUMO of ψ22 acts as a great acceptor in the transition, whereas the HOMO does not seem to do so.
C), π*(C6H6), and much higher π*(C6H6), respectively. The LUMO of ψ22 acts as a great acceptor in the transition, whereas the HOMO does not seem to do so.
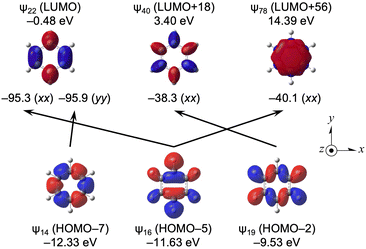 | ||
| Fig. 11 Contributions from each ψi→ψa transition to the components of σp(C) in C6H6 (D6h), together with their axes by the isovalue of 0.04 au. | ||
Origin of the i-X effect
The i-X effect is examined for C6H5–X from C6H6 (X = OH, SH, SeH, F, Cl, Br, I, NH2, CO2Me, CN and NO2) (see Scheme 3, Table 4 and Table S11 of the ESI†). The calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values are (−82.5–35.2, −74.9–70.5, −39.7–14.0 ppm) for the species.The σd(C), σp(C) and σt(C) values of C6H5OH, are listed in Table S15 of the ESI,† separately for each MO. The outer MOs of HOMO–3 (ψ22), HOMO–8 (ψ17) and HOMO–9 (ψ16) contribute greatly to σp(C). The ψi→ψa transitions contributing to σpi→a:kk(C: k = x, y and/or z), greater than 18 ppm, are listed in Table S16 of the ESI.† The ψi→ψa transitions of ψ16→ψ28 (σp16→28:yy(C) = −53.5 ppm), ψ17→ψ28 (σp17→28:xx(C) = −25.5 ppm), ψ17→ψ87 (σp17→87:xx(C) = −24.9 ppm) and ψ22→ψ44 (σp22→44:yy(C) = −28.1 ppm) contribute a lot to σpi→a:kk(C: k = x, y and/or z). The ψi→ψa transitions of ψ16→ψ28, ψ17→ψ28, ψ17→ψ87 and ψ22→ψ44 are drawn in Fig. S15 of the ESI,† where the transitions of large contributions are omitted if the contributions from ψi are small in C6H5OH. The i-X effect is well visualized through the occupied-to-unoccupied orbital transitions.
Effect of the carbonyl group and its origin
Very large downfield shifts of δ(13C) (∼200 ppm) are usually observed for the carbonyl group.1–4 The calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values for H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from MeOH are (13.1, −159.0, −145.9 ppm), which explains the observed results well.
O from MeOH are (13.1, −159.0, −145.9 ppm), which explains the observed results well.
Table 17 lists the σd(C), σp(C) and σt(C) values of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, separately, by ψi. The outer MOs of HOMO (ψ8), HOMO–2 (ψ6) and HOMO–3 (ψ5) contribute a lot to σp(C), whose values amount to −95.7, −118.8 and −64.5 ppm, respectively. The ψi→ψa transitions of ψ5→ψ9 (σp5→9:zz(C) = −62.6 ppm), ψ6→ψ9 (σp6→9:yy(C) = −195.3 ppm), ψ6→ψ13 (σp6→13:yy(C) = −30,3 ppm) and ψ8→ψ9 (σp8→9:zz(C) = −185.7 ppm) contribute predominantly to σi→ap, as shown in Table 18.
O, separately, by ψi. The outer MOs of HOMO (ψ8), HOMO–2 (ψ6) and HOMO–3 (ψ5) contribute a lot to σp(C), whose values amount to −95.7, −118.8 and −64.5 ppm, respectively. The ψi→ψa transitions of ψ5→ψ9 (σp5→9:zz(C) = −62.6 ppm), ψ6→ψ9 (σp6→9:yy(C) = −195.3 ppm), ψ6→ψ13 (σp6→13:yy(C) = −30,3 ppm) and ψ8→ψ9 (σp8→9:zz(C) = −185.7 ppm) contribute predominantly to σi→ap, as shown in Table 18.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (C2v), given separately by each ψia
O (C2v), given separately by each ψia
| MO (i in ψi) | σd(C) | σp(C) | σt(C) |
|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A. | |||
| 1 (A1); 2 (A1) | 200.29 | −0.02 | 200.27 |
| 3 (A1) | 7.42 | −21.88 | −14.46 |
| 4 (A1) | 22.01 | −2.20 | 19.82 |
| 5 (B2) | 6.81 | −64.47 | −57.66 |
| 6 (A1) | 1.98 | −118.84 | −116.86 |
| 7 (B1) | 14.00 | −13.21 | 0.79 |
| 8 (B2) | −7.32 | −95.73 | −103.05 |
| ψocc to ψocc | 52.70 | ||
| Total | 245.20 | −263.64 | −18.44 |
Fig. 12 shows the ψ6→ψ9 and ψ8→ψ9 transitions of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, along with the MO energies and the molecular axes. The main characteristics of ψ6, ψ8 and ψ9 are the occupied σ(C
O, along with the MO energies and the molecular axes. The main characteristics of ψ6, ψ8 and ψ9 are the occupied σ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), np(C) and vacant π*(C
O), np(C) and vacant π*(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. HOMO (ψ8) and LUMO (ψ9) act as excellent donors and acceptors, respectively, to produce very large σp(C) values in H2C
O), respectively. HOMO (ψ8) and LUMO (ψ9) act as excellent donors and acceptors, respectively, to produce very large σp(C) values in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. HOMO–1 (ψ7) (π(C
O. HOMO–1 (ψ7) (π(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)) in H2C
O)) in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O does not seem to act as a good donor, contrary to the excellent donors of HOMO (ψ8) (np(C)) and HOMO–2 (ψ6) (σ(C
O does not seem to act as a good donor, contrary to the excellent donors of HOMO (ψ8) (np(C)) and HOMO–2 (ψ6) (σ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)).
O)).
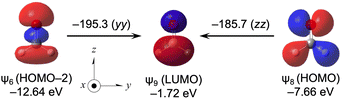 | ||
Fig. 12 Contributions from each ψi→ψa transition to the components of σp(C) in H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (C2v), together with the axes with an isovalue of 0.04 au. O (C2v), together with the axes with an isovalue of 0.04 au. | ||
Effect of the carboxyl group and its origin
The carboxyl effect is closely related to the carbonyl effect. Very large downfield shifts of δ(13C) (∼180 ppm) are usually recorded for the carboxyl group.1–4 The calculated (Δσd(C)e, Δσp(C)e, Δσt(C)e) values are (13.3, −123.9, −111.5 ppm) for H(OH)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from MeOH, which explains the observed results well.
O from MeOH, which explains the observed results well.
Table 19 lists the σd(C), σp(C) and σt(C) values of H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, separately, by ψi. The contributions from HOMO–2 (ψ10) and HOMO–5 (ψ7) to σip(C) are very large, with values of −86.2 and −68.8 ppm, respectively. Table 20 lists the ψi→ψa transition in H(HO)C
O, separately, by ψi. The contributions from HOMO–2 (ψ10) and HOMO–5 (ψ7) to σip(C) are very large, with values of −86.2 and −68.8 ppm, respectively. Table 20 lists the ψi→ψa transition in H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which mainly contributes to σpi→a:kk(C: k = x, y and/or z), larger than approximately 10 ppm. The ψi→ψa transitions of ψ7→ψ13 (σp7→13:xx(C) = −83.7 ppm with σp7→13:yy(C) = −12.7 ppm), ψ8→ψ13 (σp8→13:yy(C) = −38.1 ppm) and ψ10→ψ13 (σp10→13:yy(C) = −108.6 ppm with σp10→13:xx(C) = −29.2 ppm) contribute predominantly to σpi→a(C), together with ψ5→ψ13 (σp5→13:yy(C) = −30.6 ppm) and ψ12→ψ13 (σp12→13:xx(C) = −45.6 ppm).
O, which mainly contributes to σpi→a:kk(C: k = x, y and/or z), larger than approximately 10 ppm. The ψi→ψa transitions of ψ7→ψ13 (σp7→13:xx(C) = −83.7 ppm with σp7→13:yy(C) = −12.7 ppm), ψ8→ψ13 (σp8→13:yy(C) = −38.1 ppm) and ψ10→ψ13 (σp10→13:yy(C) = −108.6 ppm with σp10→13:xx(C) = −29.2 ppm) contribute predominantly to σpi→a(C), together with ψ5→ψ13 (σp5→13:yy(C) = −30.6 ppm) and ψ12→ψ13 (σp12→13:xx(C) = −45.6 ppm).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Cs), given separately by each ψia
O (Cs), given separately by each ψia
| MO (i in ψi) | σd(C) | σp(C) | σt(C) |
|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A. | |||
| 1 (A′)–3 (A′) | 200.31 | −0.03 | 200.28 |
| 4 (A′) | 8.56 | −8.27 | 0.29 |
| 5 (A′) | 3.00 | −23.65 | −20.65 |
| 6 (A′) | 13.84 | −11.59 | 2.25 |
| 7 (A′) | 6.02 | −68.76 | −62.74 |
| 8 (A′) | 5.29 | −30.62 | −25.34 |
| 9 (A′′) | 14.46 | −20.58 | −6.13 |
| 10 (A′) | −4.35 | −86.16 | −90.51 |
| 11 (A′′) | 1.96 | −0.95 | 1.01 |
| 12 (A′) | −3.59 | −24.60 | −28.19 |
| ψocc to ψocc | 46.69 | ||
| Total | 245.49 | −228.53 | 16.97 |
| i→ac | σpi→a:xx(C) | σpi→a:yy(C) | σpi→a:zz(C) | σpi→a(C) |
|---|---|---|---|---|
| a Calculated with the GIAO method under B3LYP/BSS-A.b The magnitudes of σpi→a(C) larger than 10 ppm are shown.c In ψi→ψa. | ||||
| 5→13 | −0.10 | −30.55 | 0.00 | −10.22 |
| 7→13 | −83.72 | −12.66 | 0.00 | −32.13 |
| 8→13 | −0.90 | −38.10 | 0.00 | −13.00 |
| 10→13 | −29.23 | −108.63 | 0.00 | −45.95 |
| 12→13 | −45.55 | 2.78 | 0.00 | −14.25 |
Fig. 13 shows the ψ7→ψ13 and ψ10→ψ13 transitions in H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, along with the MO energies and the molecular axes. The characters of ψ7 and ψ10 are the occupied σ(O–C
O, along with the MO energies and the molecular axes. The characters of ψ7 and ψ10 are the occupied σ(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of A′ spread over the whole molecule, whereas that of ψ13 is the vacant π*(O–C
O) of A′ spread over the whole molecule, whereas that of ψ13 is the vacant π*(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). While ψ7 and ψ10 operate as excellent donors, ψ13 (LUMO) is an excellent acceptor in H(HO)C
O). While ψ7 and ψ10 operate as excellent donors, ψ13 (LUMO) is an excellent acceptor in H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
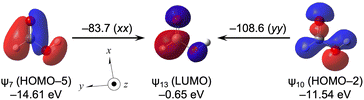 | ||
Fig. 13 Contributions from each ψi→ψa transition to the components of σp(C) in H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Cs), together with the axes with an isovalue of 0.04 au. O (Cs), together with the axes with an isovalue of 0.04 au. | ||
The (Δσd(C)e, Δσp(C)e, Δσt(C)e) values are (13.3, −123.9, −111.5 ppm) for H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from CH3OH, as mentioned above. However, the values for the conversion of H(HO)C
O from CH3OH, as mentioned above. However, the values for the conversion of H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from H2C
O from H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are also of interest. The values are (0.3, 35.1, 35.4 ppm). Specifically, the NMR signal of H(HO)C
O are also of interest. The values are (0.3, 35.1, 35.4 ppm). Specifically, the NMR signal of H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is predicted to appear at a higher field of approximately 35 ppm than that of H2C
O is predicted to appear at a higher field of approximately 35 ppm than that of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The specific π-allyl type O–C
O. The specific π-allyl type O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interaction is responsible for these results. The charge on C of H(HO)C
O interaction is responsible for these results. The charge on C of H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is less positive than that of H2C
O is less positive than that of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O because of the donation from HO to C
O because of the donation from HO to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in H(HO)C
O in H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which should lead to an upfield shift.
O, which should lead to an upfield shift.
It is necessary to consider the effect of the wider extension of the MOs over the entire molecule in H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. One explanation is as follows: It does not directly increase the σp(C) value when the occupancy of electrons becomes higher at the carbon atom in question in an important orbital of H(HO)C
O. One explanation is as follows: It does not directly increase the σp(C) value when the occupancy of electrons becomes higher at the carbon atom in question in an important orbital of H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O relative to the case of H2C
O relative to the case of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The energy differences between the two orbitals that make up the transitions also affect σp(C). Nevertheless, a more upfield shift in σp(C) is expected to be predicted when the charge on C becomes less positive. However, more complex mechanisms control the real shift values.
O. The energy differences between the two orbitals that make up the transitions also affect σp(C). Nevertheless, a more upfield shift in σp(C) is expected to be predicted when the charge on C becomes less positive. However, more complex mechanisms control the real shift values.
The analysis of H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and H(HO)C
O and H(HO)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O will help to understand the δ(C) values of similar structures. The upfield shifts in RC(=O)NHR' and ROC(=O)OR' relative to R2C
O will help to understand the δ(C) values of similar structures. The upfield shifts in RC(=O)NHR' and ROC(=O)OR' relative to R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can also be understood based on the structural similarities to R(R’O)C
O can also be understood based on the structural similarities to R(R’O)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. However, further investigations are necessary to understand the much greater downfield shifts of the species containing low-lying vacant orbitals, such as some cationic species, carbenes and radicals.
O. However, further investigations are necessary to understand the much greater downfield shifts of the species containing low-lying vacant orbitals, such as some cationic species, carbenes and radicals.
Illustration of the terms used to control Δσt(C)
The Δσd(C), Δσp(C), Δσt(C) (=Δσd(C) + Δσp(C)) values and the components are plotted for CH3CH3, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, CH
CH2, CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, C6H6 and H2C
CH, C6H6 and H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Fig. 14 shows the plot. The Δσd(C) and Δσp(C) values are all negative for the species, where C4– is taken as the standard. However, the contributions from the occupied-to-occupied (occ-to-occ) orbital (ψi→ψj) transitions are all positive for the species shown in Fig. 14. The magnitudes of Δσd(C) seem almost constant for the species, whereas those of Δσp(C) change widely depending on the structure of the species, which increases the variety of the Δσt(C) values. The magnitudes of Δσt(C) (=Δσd(C) + Δσp(C)) increase in the order of S
O. Fig. 14 shows the plot. The Δσd(C) and Δσp(C) values are all negative for the species, where C4– is taken as the standard. However, the contributions from the occupied-to-occupied (occ-to-occ) orbital (ψi→ψj) transitions are all positive for the species shown in Fig. 14. The magnitudes of Δσd(C) seem almost constant for the species, whereas those of Δσp(C) change widely depending on the structure of the species, which increases the variety of the Δσt(C) values. The magnitudes of Δσt(C) (=Δσd(C) + Δσp(C)) increase in the order of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH3CH3 < CH
CH3CH3 < CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH < CH2
CH < CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 < C6H6 < H2C
CH2 < C6H6 < H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, as expected. The σp(C) values originate from the unsymmetric component of the electron distribution derived from the unsymmetric MOs on the unsymmetric structure of the species.
O, as expected. The σp(C) values originate from the unsymmetric component of the electron distribution derived from the unsymmetric MOs on the unsymmetric structure of the species.
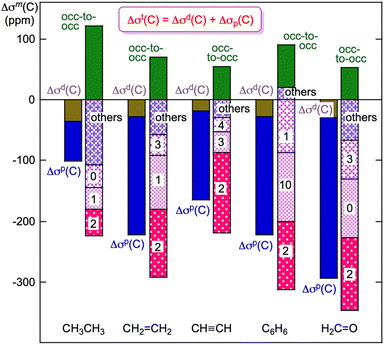 | ||
Fig. 14 Plots of Δσd(C), Δσp(C), Δσt(C) and the components for CH3CH3, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, CH CH2, CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, C6H6 and H2C CH, C6H6 and H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Each MO contributing to σp(C) is shown by n in HOMO–n. O. Each MO contributing to σp(C) is shown by n in HOMO–n. | ||
The unsymmetric MOs are constructed mainly by the 2px(C), 2py(C) and 2pz(C) AOs. These MOs are expected to exist near the HOMO; however, they sometimes reside in (much) deeper areas. The magnitudes of the terms, such as Δσd(C), Δσp(C), Δσt(C) and the components, are well visualized in Fig. 14, which helps us to understand the plain rules founded in theory, supported by the origin and mechanism, for 13C NMR chemical shifts.
The occupied-to-occupied orbital (ψi→ψj) transitions arise through the redistribution of electrons in a species under an applied magnetic field. They are usually not considered to be important; therefore, they are often neglected in analyses and discussions. However, they contribute to σp(C) more than those expected in some cases, as shown in Fig. 14. Specifically, the occupied-to-occupied orbital transitions may play an important role in the (observed) 13C NMR chemical shifts, which are hidden in the unconscious realm of the measurers.
Conclusions
The plain rules associated with the origin and mechanism for δ(C) are intended to be established, without compromising theoretical requirements, in this work. The rules should be simple, easily imaged and easily understood for experimental scientists, who are not specialists. The rules should help experimental scientists image the role of C at a specific position in a species over the empirical rules usually employed in assigning the spectra. The δ(C) values are analysed by employing the calculated σd, σp and σt terms. The contributions from σd(C) to σt(C) are approximately one tenth of those from σp(C), although the ratio changes depending on the species. The plot of σd(C) versus Q(C) for C4+, C2+, C0, C2− and C4− gave excellent correlations if analysed via a cubic regression curve, as did the plots for C4−, CH22−, CH3− and CH4 with a quadratic curve. The σd(C) values can be understood based on Q(C) for the species; however, σd(C) of those other than those above do not correlate with Q(C). The relationship between σp(C) and Q(C) was not examined further, which would be hidden in the complex combinations of the ψi→ψa transitions, as shown in eqn (6).The pre-α effect of an approximately 20 ppm downfield shift is theoretically predicted based on the average value of Δσt(C)e from C4– to H4C. The α and β effects of approximately 10 ppm downfield shifts are reproduced well by the calculated Δσt(C)e values. The variety of the α-X, β-X and i-X effects are clarified by the calculations. Large downfield shifts by ethene (∼120 ppm), benzene (∼127 ppm) and ethyne (∼60 ppm) and very large downfield shifts by the carbonyl group (∼200 ppm) and carboxyl group (∼180 ppm) are also well reproduced by the calculations.
The orbital-to-orbital transitions in σp(C) are widely employed to clarify the origin and the mechanism for δ(C) in this work. The occupied-to-unoccupied orbital (ψi→ψa) transitions in σp(C) enable us to visualize the origin and the mechanism. The occupied-to-occupied orbital (ψi→ψj) transitions, which are usually neglected, are also examined: The contributions from the ψi→ψj transitions are greater than those expected in some cases. The examination provided useful information for σp(C). As a result, the plain rules with the origin and the mechanism are formulated for δ(C). The origin and the mechanism for δ(C) can be imaged and understood more easily based on the treatments also by experimental scientists. The results will help in understanding the role of C in question in the specific position of a compound. By expanding the image of the orbital-to-orbital transitions, it would be possible to envision the molecular orbitals around the carbon in question in the compound and their interactions. The expansion will hopefully lead to the development of the highly functionality based on the inherent properties of the compound. Namely, this work also has the potential to provide an understanding δ(C) of unknown species and facilitate new concepts for strategies to create highly functional materials on the basis of the δ(C) values.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
W. N. and S. H. formulated the project and organized the data; contributed to supervision, data curation and resources; and wrote, reviewed and edited the paper. K. M. contributed to the investigation. All of the authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors are very grateful to Prof. Masahiko Hada and Dr Daisuke Yamaki of Tokyo Metropolitan University for the utility programs.Notes and references
- J. Pople, W. G. Schneider and H. J. Bernstein, High-resolution Magnetic Resonance, McGraw-Hill, New York, 1959 Search PubMed.
- J. B. Stothers, Carbon-13 NMR Spectroscopy, Academic Press, New York and London, 1972 Search PubMed.
- H.-O. Kalinowski, S. Berger and S. Braun, Carbon-13 NMR Spectroscopy, John Wiley & Sons: New York, 1988 Search PubMed.
- Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press, vol. 83, 2014 Search PubMed.
- Encyclopedia of Nuclear Magnetic Resonance, ed. D. M. Grant and R. K. Harris, John Wiley & Sons, New York, 1996 Search PubMed.
- Encyclopedia of Nuclear Magnetic Resonance, ed. R. K. Harris and R. E. Wasylishen, John Wiley & Sons, New York, 2012 Search PubMed.
- Nuclear Magnetic Shieldings and Molecular Structure, ed. J. A. Tossell, Kluwer Academic Publishers, Dordrecht, Boston, London, 1993 Search PubMed.
- Calculation of NMR and EPR Parameters; Theory and Applications, ed. M. Kaupp, M. Bühl and V. G. Malkin, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004 Search PubMed.
- W. Nakanishi, S. Hayashi and M. Hada, Chem. E. J., 2007, 13, 5282–5293 CAS.
- K. Kanda, H. Nakatsuji and T. Yonezawa, J. Am. Chem. Soc., 1984, 106, 5888–5892 CrossRef CAS.
- Molecular Quantum Mechanics, ed. P. W. Atkins and R. S. Friedman), Oxford: New York, 1997, Chapter 13 Search PubMed.
- Indeed, this decomposition includes small arbitrariness due to the coordinate origin dependence, but it does not damage our insights into the 13C NMR spectroscopy.
- The occupied-to-unoccupied orbital (ψi→ψa) transitions mainly contribute to σp, whereas the occupied-to-occupied orbital (ψi→ψj) transitions sometimes play an important role in σp.
- Based on the second-order perturbation theory at the level of the HF and single-excitation CI approximation, σpi→a on a resonance nucleus N is shown to be proportional to reciprocal orbital energy gap (εa − εi)−1 as expressed in eqn (5), where ψk is the k-th orbital function,
![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z,N is orbital angular momentum around the resonance nucleus, and rN is the distance from the nucleus N.
z,N is orbital angular momentum around the resonance nucleus, and rN is the distance from the nucleus N. - L. P. Hammett, Chem. Rev., 1935, 17, 125–136 CrossRef CAS.
- K. Matsuzaki, S. Hayashi and W. Nakanishi, RSC Adv., 2024, 14, 14340–14356 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. D. Becke, Phys. Rev., 1988, A38, 3098–3100 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- B. Miehlich, A. Savin, H. Stall and H. Preuss, Chem. Phys. Lett., 1989, 157, 200–206 CrossRef CAS.
- T. Yanai, D. P. Tew and N. C. A. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6169 CrossRef CAS.
- T. M. Henderson, A. F. Izmaylov, G. Scalmani and G. E. Scuseria, J. Chem. Phys., 2009, 131, 044108 CrossRef PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618–622 CrossRef.
- J. Gauss, J. Chem. Phys., 1993, 99, 3629–3643 CrossRef CAS.
- J. Gauss and B. Bunsenges, Phys. Chem., 1995, 99, 1001–1008 CAS.
- K. Wolinski, J. F. Hinton and P. Pulay, J. Am. Chem. Soc., 1990, 112, 8251–8260 CrossRef CAS.
- K. Wolinski and A. Sadlej, Mol. Phys., 1980, 41, 1419–1430 CrossRef CAS.
- R. Ditchfield, Mol. Phys., 1974, 27, 789–807 CrossRef CAS.
- R. McWeeny, Phys. Rev., 1962, 126, 1028–1034 CrossRef.
- F. London, J. Phys. Radium, 1937, 8, 397–409 CrossRef CAS.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, C. R. Landis and F. Weinhold, NBO Version 6.0. 2013 Search PubMed.
- The utility program was provided by Prof. Hada and Dr Yamaki of Tokyo Metropolitan University. It is derived from the NMR program in the Gaussian program, which made some processes, necessary for our discussion, possible to be printed out, such as the contributions from the occupied orbitals and/or the orbital-to-orbital transitions.43.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS.
- W. Nakanishi, S. Hayashi, D. Shimizu and M. Hada, Chem.–Eur. J., 2006, 12, 3829–3846 CrossRef CAS PubMed.
- W. Nakanishi, S. Hayashi, Y. Katsura and M. Hada, J. Phys. Chem. A, 2011, 115, 8721–8730 CrossRef CAS.
- S. Hayashi, K. Matsuiwa and W. Nakanishi, RSC Adv., 2014, 4, 44795–44810 RSC.
- Essentially the same analysis can be achieved by using the ADF program.
Footnote |
| † Electronic supplementary information (ESI) available: Additional tables and the fully optimized structures given by Cartesian coordinates, together with total energies. See DOI: https://doi.org/10.1039/d4ra05980h |
| This journal is © The Royal Society of Chemistry 2025 |