Open Access Article
Open Access ArticleRecent advances in microwave-assisted synthesis of triazoles and their derivatives: a green approach toward sustainable development methods
Suman Swami *a,
Neelam Sharmab,
Garvit Sharmac and
Rahul Shrivastava
*a,
Neelam Sharmab,
Garvit Sharmac and
Rahul Shrivastava *b
*b
aDepartment of Chemistry, Chandigarh University, NH-05, Ludhiana-Chandigarh State Hwy, Mohali, Punjab 140413, India. E-mail: sumanswami1994@gmail.com
bDepartment of Chemistry, Manipal University Jaipur, VPO-Dehmi-Kalan, Off Jaipur Ajmer Express Way, Jaipur, Rajasthan 303007, India. E-mail: chem.rahul@gmail.com
cDepartment of Computational Science, Central University of Punjab, Bathinda, Punjab 151401, India
First published on 24th January 2025
Abstract
Triazole, a nitrogen-containing five-membered heterocycle with two isomeric forms, 1,2,3-triazole and 1,2,4-triazole, has proven to be a valuable component in the pharmaceutical domain. Owing to its widespread utility in drug development, pharmaceutical and medicinal chemistry, several synthetic methods have been explored, such as different catalytic systems, solvents, and heating methodologies in recent years. However, some methods were associated with several limitations, such as harsh reaction conditions, high temperatures, low atom economy, and long reaction times. Conversely, the ongoing demand from the chemical industry has led to increased attention on overcoming these limitations and developing sustainable laboratory methods. In recent years, the microwave heating method in organic synthesis has evolved as a new, environmentally friendly approach with benefits such as atom economy, reduced use of hazardous chemicals, safer chemical design, few derivatives and enhanced energy efficiency. This review summarizes recent progress in microwave-assisted synthesis of triazoles (1,2,3-triazole and 1,2,4-triazole), with a comparative analysis between conventional methods and microwave-assisted methods in terms of reaction time, yield, green synthesis, sustainability and other relevant factors.
Introduction
Heterocycles are important components in most commercially available drugs because of their ability to manipulate lipophilicity, hydrogen bonding, polarity, pharmacokinetic, pharmacological, physicochemical and toxicological properties.1 A potent method for designing novel bioactive molecules is directly and indirectly associated with the synthesis of various heterocyclic derivatives.2 Therefore, significant interest has been generated in the synthesis of various heterocycles, multi-heterocycles and hybrid heterocyclic molecules in recent years.3,4 Among the diverse range of heterocyclic entities, nitrogen-containing heterocyclic molecules have received massive attention because of their role as core structures in numerous natural products and bioactive molecules, such as vitamins, antibiotics, hormones, glycosides, and alkaloids, which are important for human and animal health. Thus, nitrogen-containing heterocycles are specifically regarded as “privileged” structures for synthesis and development of novel drug molecules.5,6 Triazoles, with two isomeric forms, 1,2,3-triazole and 1,2,4-triazole derivatives, are an important class of molecules due to their biological importance and potential as drug development agents.7 Due to the diverse pharmacological spectrum of both isomeric forms of triazoles, they have emerged as viable candidates in the drug development process over the past few decades. Generally, triazoles and their derivatives are uncommon in nature; hence, synthetic methodologies for molecules containing triazole have been widely explored for the formation of pharmaceutical molecules.8 Strong chemical stability (usually inert to reducing and oxidizing agents), dipole moment, rigidity and hydrogen bonding capabilities are some favorable characteristics of the triazole ring.9 It was found that the production of triazole-containing molecules using conventional methods is associated with longer reaction times, poor yields and harsh reaction conditions. In view of all these limitations, efforts to discover novel methodologies and techniques for the synthesis of complex triazole-containing heterocyclic molecules have been continuously pursued over the past few decades.10–17In recent decades, the concept of sustainability has received significant attention both in industry and society, with the notion of “meeting the needs of the present generation without compromising the future generation's needs”.18,19 In the progress of sustainable development, the principle of green chemistry addresses the fundamental scientific challenges of protecting both the environment and human health. Therefore, modern green chemistry techniques have gained increasing popularity as non-conventional methods for rapid organic transformations. It was found that non-classical techniques adopting green chemistry principles could reduce/eliminate the formation of hazardous substances and the use of traditional hazardous volatile organic solvents. Among the various non-classical methodologies, microwave-assisted synthesis is one of the promising green methodologies for the rapid synthesis of different organic entities.20
Chemical synthesis has been traditionally carried out by conductive heating with the aid of an external heat source, such as a hot plate, heating mantel, water bath, sand bath and oil bath.21 In traditional methods, generally heat must first pass through the vessel/container walls before it can reach the solvent and reactants (Fig. 1a). This methodology depends on the thermal conductivities of several components that must be stabilized; thus, this method of transferring energy into the system is cumbersome and ineffective. Additionally, the temperature of the reactor/reaction vessel was higher than the reaction mixture until adequate time had elapsed to allow the reaction vessel and contents inside the vessel to establish thermal equilibrium, which took more time. Additionally, conductive heating impairs the chemist's capacity to control the process. Subsequently, labor-intensive extra efforts are required to eliminate the heat source after the completion of the reaction, and cooling must be applied to reduce the bulk internal temperature.22 Moreover, microwave heating is a completely different approach. As shown in Fig. 1b, the direct connection between the microwaves and molecules in the reaction mixture causes a sharp temperature rise. The process operates independently of the thermal conductivity of the vessel materials. Consequently, it achieves immediate, concentrated heating of substances responsive to either ionic conduction or dipole rotation.23–25
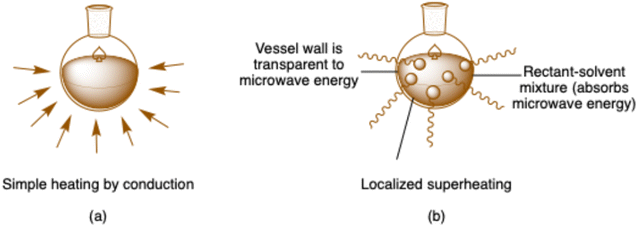 | ||
| Fig. 1 (a) Illustration of reaction mixture heating using the traditional heating method and (b) reaction mixture heating via microwave. | ||
In general, microwave heating has several benefits that are closely related to green chemistry concepts.
• Reaction times can be significantly shortened (a few minutes or seconds) compared to traditional heating.
• Remote energy introduction is possible without physical contact between the substrates and the heating source.
• The energy inputs received by the sample can be stopped as soon as the power is turned off.
• A slower rate of thermal inertia compared with traditional conductive heating is observed.
• Energy is distributed throughout the product's mass rather than at the surface.
• If microwaves can strongly couple with some components, heating rates can drastically increase.
• This method is easily applied to parallel or sequential synthesis.
• It provides a clean, mild, easier workup procedure; greater selectivity; convenient, spontaneous reaction procedure; catalyst-free condition; regio- and stereo-selectivity and utilization of ecofriendly solvents.
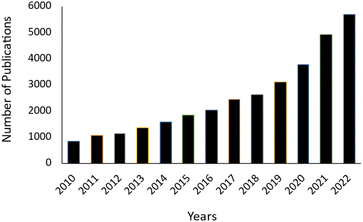
These benefits are important for green and sustainable chemistry because they could boost productivity, improve safety in various ways and eliminate or reduce waste formation through more efficient processing. Therefore, interest in the microwave-assisted synthesis of heterocyclic moieties has incessantly increased because of its efficacy in all types of chemical reactions, including cycloadditions, additions, eliminations, fragmentations, substitutions and multicomponent reactions, which was witnessed as extraordinary growth in the form of studies conducted in the last few years (Fig. 2). As shown in Fig. 2, ample numbers of research articles have been published since 2010 on the synthesis of tetrazole derivatives based on a microwave-assisted approach as a green protocol. In this review, we summarize the recent progress (from 2010 to 2023) in microwave-assisted triazole (1,2,3-triazole and 1,2,4-triazole) synthesis.
Microwave-assisted synthesis of 1,2,4-triazole and derivatives
The 1,2,4-triazole is one of the isomers of triazole that has been widely explored in drug developments owing to its characteristic properties, such as antibacterial, antitubercular, antifungal, anti-inflammatory, analgesic, anti-tumor, antiviral, anticonvulsant, antidepressant, insecticidal and influence the central nervous system.26–30 In addition, the ambident nucleophilicity of these moieties makes them excellent starting materials for the synthesis of several intriguing N- and S-bridged heterocycles.31 For example, Zaheer et al. synthesized a series of 4-(benzylideneamino)-3-(1-(2-fluoro-[1,1′-biphenyl]-4-yl)ethyl)-1H-1,2,4-triazole-5(4H)-thione derivatives (1a–m) via microwave-assisted (MW) condensation reaction. In the condensation process, the use of MW was more effective in the reaction time minimum compared to the traditional heating method with the advantage of green reaction conditions. It was observed that the reaction was completed within 10–25 minutes with 97% yield under MW radiation, while in the conventional method, it takes 290 minutes to complete with 78% yield7 (Scheme 1). It was also reported that compound 1e exhibited significant analgesic activities using different processes, such as tail flick and writhing methods.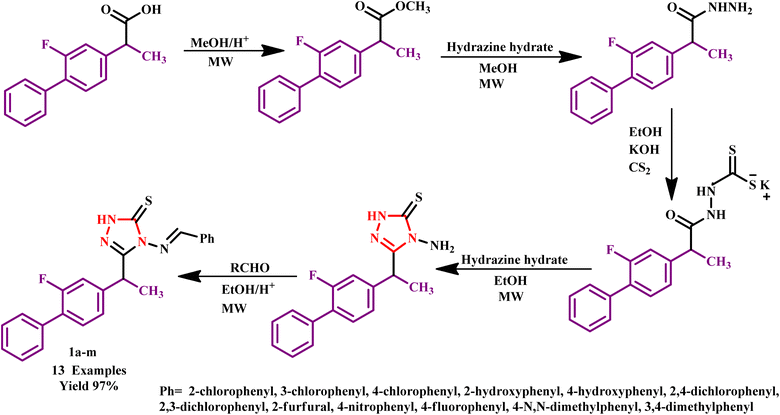 | ||
| Scheme 1 Synthetic route of 4-(benzylideneamino)-3-(1-(2-fluoro-[1,1′-biphenyl]-4-yl)ethyl)-1H-1,2,4-triazole-5(4H)-thione derivatives (1a–m).7 | ||
Similarly, Shiradkar and his co-workers reported the synthesis of N-{4-[(4-amino-5-sulfanyl-4H-1,2,4-triazol-3-yl)methyl]-1,3-thiazol-2-yl}-2-substituted amide derivatives using the microwave organic reaction enhancement method. The synthesized compounds were further screened for antitubercular activity against the Mycobacterium TB H37 Rv strain using the Microplate Alamar Blue Assay MABA assay method. Some compounds, such as 3b, 3c, 5a, 5b and 5c, exhibited better anti-bacterial activities against Escherichia coli, Staphylococcus aureus, Salmonella typhosa, and Pseudomonas aeruginosa, while compounds 5a, 5b and 5c exhibited better antitubercular activities against M. tuberculosis strain H37 Rv (Scheme 2).32
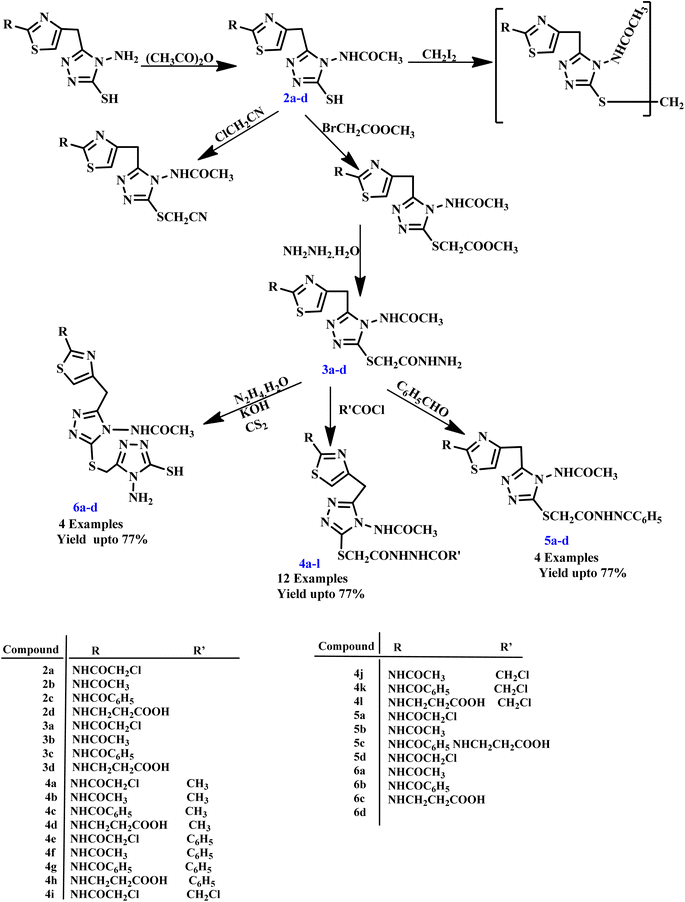 | ||
| Scheme 2 Synthesis of N-{4-[(4-amino-5-sulfanyl-4H-1,2,4-triazol-3-yl)methyl]-1,3-thiazol-2-yl}-2-substituted amide derivatives.32 | ||
Virk et al. investigated the synthesis of N-substituted-2-[(5-{1-[(4-methoxyphenyl)sulfonyl]-4-piperidinyl}-4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]propenamide derivative (7a–r) in which 1,2,4-triazole and piperidine rings were incorporated through microwave-assisted and conventional methods (Scheme 3). They reported that microwave-assisted synthesis was carried out in just 33–90 seconds with a remarkable 82% yield, while the conventional method required several hours to complete. The research group also evaluated the biological potential of these compounds against α-glucosidase and acetylcholinesterase (AChE) enzymes. The methodology provides valuable insights into applications of microwave technology in organic synthesis for rapid and efficient compound production.33
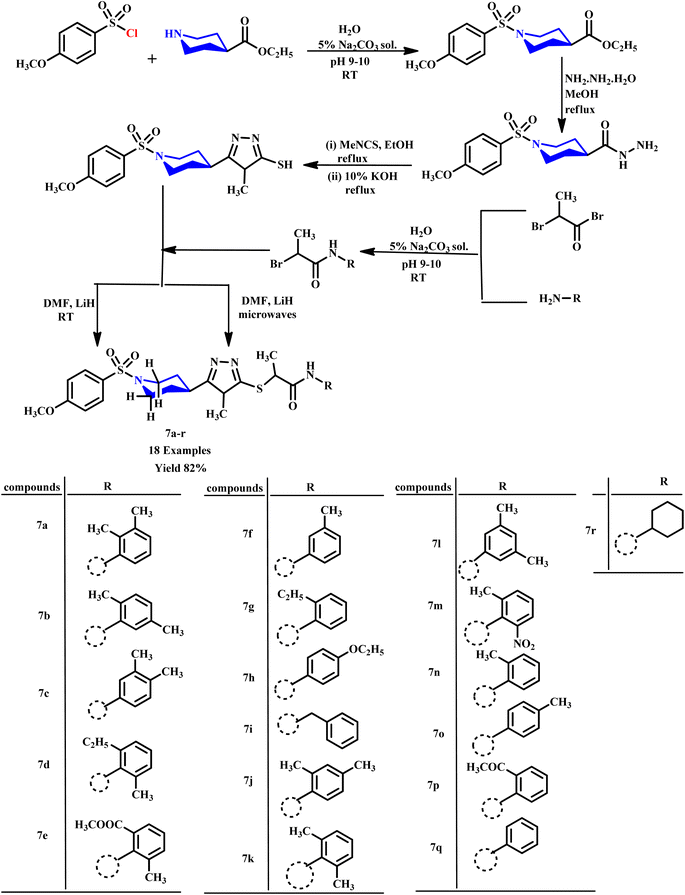 | ||
| Scheme 3 Synthetic route of N-substituted-2-[(5-{1-[(4-methoxyphenyl)sulfonyl]-4-piperidinyl}-4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]propenamide derivatives (7a–r).33 | ||
Further, Shaikh et al. developed triazole-derived Schiff bases featuring pyrazole and triazole nuclei (8a–e) through microwave (MW) irradiation at temperatures of 70–75 °C using polyethylene glycol-400 as a solvent. This approach provides desired products in a short time frame of just 15–20 minutes with an excellent yield (Scheme 4). The resulting Schiff bases can act as pivotal precursors for the synthesis of various biologically active ligands and heterocyclic compounds, highlighting their significance in the development of valuable coordination compounds. This method enhances reaction efficiency and opens new avenues for research in medicinal chemistry and material science.34
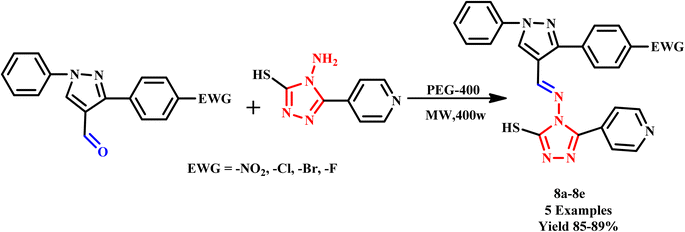 | ||
| Scheme 4 Synthetic method for the synthesis of triazole-based Schiff bases (8a–e).34 | ||
In the progress of green protocol development for triazoles, Shcherbyna utilized a Milestone Flexi Wave Microwave system for the synthesis of novel series of 4-((5-((cyclohexylmethyl)thio)-4-R1-4H-1,2,4-triazol-3-yl)methyl)morpholines (9a–e) and 4-((4-R1-5-(pyridin-2-ylthio)-4H-1,2,4-triazol-3-yl)methyl)morpholines (10a–e) as depicted in Scheme 5. The Milestone Flexi Wave Microwave system facilitates reaction completion in a minimum time (10.0 minutes) compared to the traditional method of heating.35
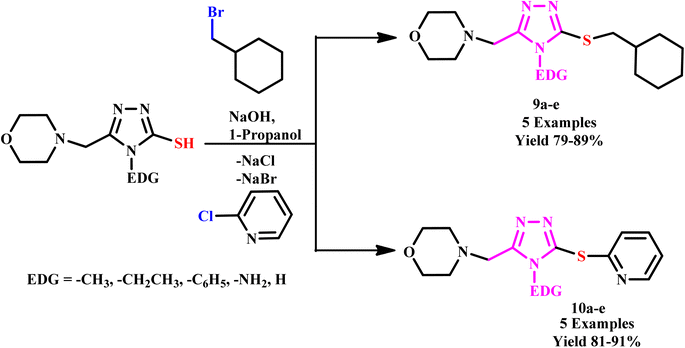 | ||
| Scheme 5 Synthesis of 4-((5-((cyclohexylmethyl)thio)-4-R1-4H-1,2,4-triazol-3-yl)methyl)morpholines and 4-((4-R1-5-(pyridin-2-ylthio)-4H-1,2,4-triazol-3-yl)methyl) morpholines (9a–e & 10a–e).35 | ||
Similarly, Virk et al. synthesized N-(substituted)-5-((1-(4-methoxy phenylsulfonyl)piperidin-4-yl)-4H-1,2,4-triazol-3-ylthio) acetamide moieties (11a–k) using microwave-assisted methods. In this methodology, the reaction was completed within 31–68 seconds with extraordinary product yield, as shown in Scheme 6. The synthesized compounds were further examined owing to their inhibition potential against acetylcholinesterase (AChE), bovine carbonic anhydrase (bCA-II) and butyrylcholinesterase (BChE) enzymes. Notably, microwave-assisted synthesis significantly outperformed conventional methods, which typically required 9–19 hours for product formation, while reported methodologies drastically reduced reaction times from 19 hours to just 31–68 seconds.36
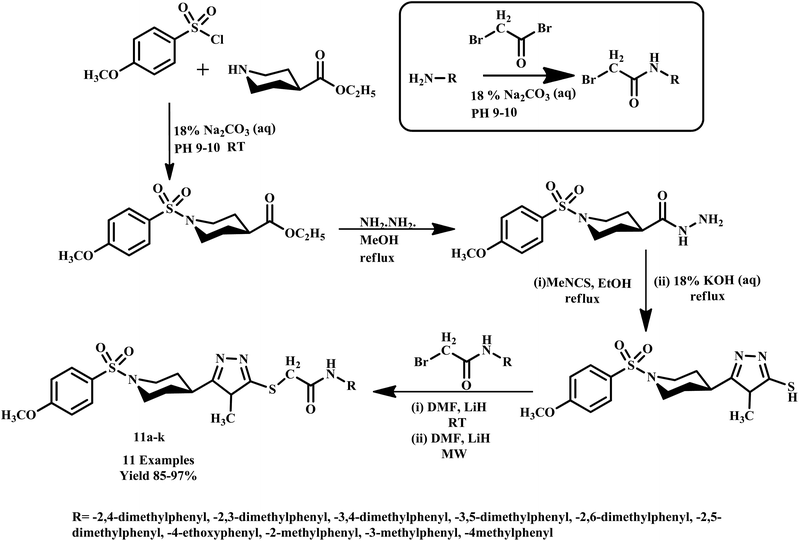 | ||
| Scheme 6 Synthesis method of N-(substituted)-2-[(5-{1-[(4-methoxyphenyl)sulfonyl]-4-piperidinyl}-4-methyl-4H-1,2,4-triazol-3-yl)sulfanyl]acetamide (11a–k).36 | ||
Piperazine-azole-fluoroquinolone-based 1,2,4-triazole derivatives were synthesized by Ozdemir et al. using an ethanol solvent under microwave irradiation with an impressive yield of 96%. This method significantly reduced the reaction time from 27 hours (under conventional heating) to just 30 minutes, highlighting the efficiency of microwave-assisted synthesis. The synthesized compounds were subsequently tested for antioxidant and antibacterial activities, which demonstrated good to moderate efficacy in both assays. These results demonstrate the potential of these derivatives as promising candidates for further pharmaceutical development.37 In another study, Al-Soud and co-workers employed microwave irradiation for the synthesis of acyclic C-nucleosides, specifically 6-alkyl/aryl-3-(1,2-O-isopropylidene-D-ribo-tetritol-1-yl)[1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles and their 6-arylthiomethyl analogues. This method reduced reaction time to 15–20 minutes at 120 °C, significantly accelerating the synthesis process. Among the synthesized compounds, 6-(3,4-dichlorophenyl)-3-(1,2-O-isopropylidene-D-ribo-tetritol-1-yl)-7H-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole demonstrated notable antiviral activity against HIV-2.38 Similarly, Cebeci and co-workers reported the synthesis of a novel hybrid combining 1,2,4-triazole with conazole and fluoroquinolone moieties under microwave irradiation within a minimal time. They investigated the activity of these derivatives against various common infection strains. It was found that most of the reported compounds exhibited potent antibacterial activity against both Gram-positive and Gram-negative bacteria. Furthermore, certain derivatives have shown promising antiproliferative effects against HeLa cervical cancer cells and cytotoxicity towards normal cells.39 In another report, ethyl 2-chloroacetate and 4-chlorophenol were utilized as starting materials for the synthesis of a novel series of thioether derivatives containing 1,2,4-triazole moieties (12a–k), as depicted in Scheme 7. The synthetic protocol developed by Min et al. employed microwave irradiation, resulting in an efficient reaction with an 81% yield within just 15 minutes. Additionally, several compounds from this series demonstrated notable antifungal activities, showcasing their potential as effective antifungal agents. This microwave-assisted synthesis of thioether derivatives containing 1,2,4-triazole moieties is an efficient and high-yielding method. Additionally, significant antifungal activities highlight their potential for further development in pharmaceutical applications.40
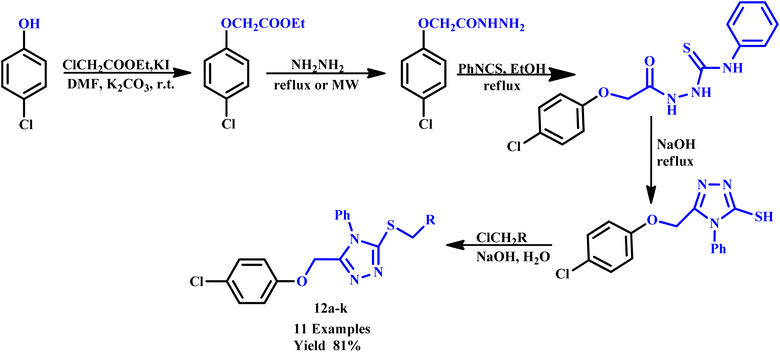 | ||
| Scheme 7 Synthetic route of thioether derivatives containing 1,2,4-triazole moieties (12a–k).40 | ||
In a subsequent report, Banerjee and co-workers developed Schiff base complexes of bis(cyclopentadienyl) titanium(IV) from 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles using both conventional and microwave-assisted methods (Fig. 3). Under microwave irradiation, the reactions were completed within 10–15 minutes with good yields, while the conventional heating method required 6–7 hours. Remarkably, the microwave method saved time and enhanced the overall efficiency of the synthesis. Additionally, Schiff base complexes demonstrated moderate to good antibacterial activity against various bacterial strains, highlighting their potential for medical applications.41
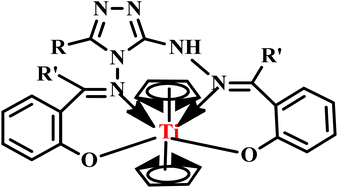 | ||
| Fig. 3 Structure of Schiff base complex bis(cyclopentadienyl) titanium(IV).41 | ||
Franziska A. Brede synthesized transition metal complexes [ZnCl2(TzH)2] and [MCl2(TzH)4] (where M = Mn, Fe; TzH = 1,2,4-H-triazole) as precursors for the formation of coordination polymer ethers under microwave-assisted reactions. Remarkably, reported stiff chain-like coordination polymers and their precursor complexes exhibit significant differences in permittivity. In the synthesis, the utilization of microwave irradiation expedited the reaction process and resulted in a higher degree of polymerization. These findings highlight the potential of microwave-assisted synthesis in creating advanced materials with unique electrical properties.42 Similarly, in another report, biologically active nalidixic acid-containing hybrid compounds were synthesized by Ceylan et al. through a microwave-assisted synthetic route. The reported compounds were produced with yields ranging from 43% to 99% within 5.0 minutes at 50 °C. This rapid and efficient synthesis highlights the significant potential of microwave-assisted techniques in streamlining the production of complex pharmaceutical compounds. Further, Dandia et al. described a one-pot synthesis of a series of trifluoromethyl-substituted spiro[3H-indole-3,3′-[3H-1,2,4]triazole]-2(1H)-ones (13a–g) via the condensation of thiosemicarbazide and 3-arylimino-2H-indole-2-ones under microwave irradiation with yields of 85–90%, as depicted in Scheme 8. This methodology not only offers greater selectivity and high purity but also significantly enhances the yield compared to conventional methods.43
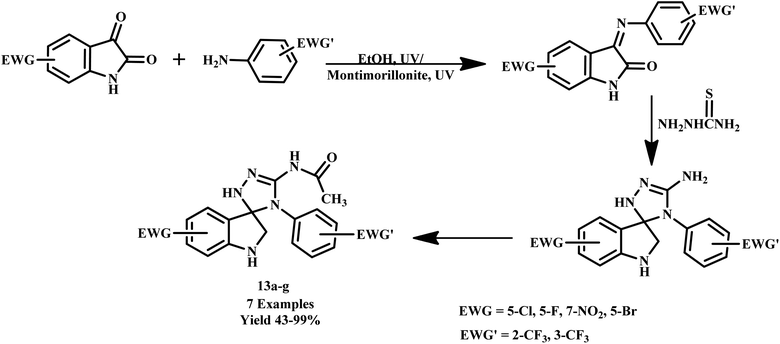 | ||
| Scheme 8 Synthesis of tri fluoromethyl-substituted spiro[3H-indole-3,3′-[3H-1,2,4]triazole]-2(1H)-ones (13a–g).43 | ||
Similarly, Dengale and his coworker reported the synthesis of 5-[2-(3-fluorophenyl)-4-methylthiazol-5-yl]-N-phenyl-1,3,4-thiadiazol-2-amine}(14a–e) and {5-[2-(3-fluorophenyl)-4-methylthiazol-5-yl]-4-phenyl-4H-1,2,4-triazole-3-thiol (15a–e) from a 2-(3-fluorophenyl)-4-methylthiazole-5-carbohydrazide via cyclization under microwave irradiation with 77% yield, as depicted in Scheme 9. In this series of compounds, N-(3,4-dichlorophenyl)-2-{[2-(3-fluorophenyl)-4-methyl-1,3-thiazol-5-yl]carbonyl}hydrazine carbothioamide and compound 15a showed better antibacterial activities against the E. coli strain. The reported compound 2-{[2-(3-fluorophenyl)-4-methyl-1,3-thiazol-5-yl]-carbonyl}-N-(3-methylphenyl)hydrazine carbothioamide (62.5 μg mL−1) exhibited high activities against the P. aeruginosa strain. Compound 14c (250 μg mL−1) and compound 15c (200 μg mL−1) showed the highest activities against the C. albicans strain, and compound 14b (250 μg mL−1) was examined to be highly active against A. niger.44
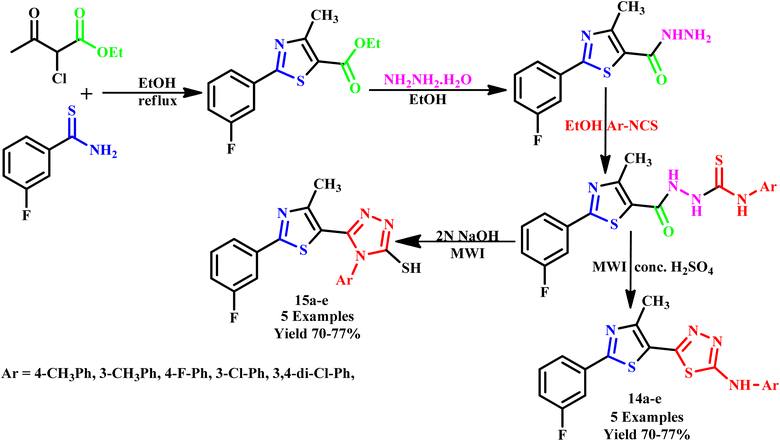 | ||
| Scheme 9 . Synthetic route of {5-[2-(3-fluorophenyl)-4-methylthiazol-5-yl]-4-phenyl-4H-1,2,4-triazole-3-thiol or 5-[2-(3-fluorophenyl)-4-methylthiazol-5-yl]-N-phenyl-1,3,4-thiadiazol-2-amine} [14a–e and 15a–e].44 | ||
Later, in the progress of sustainable method development for triazoles, El Ashry et al. synthesized 4-amino-3-(D-gluco- and D-galacto-pentitol-1-yl)-5-mercapto-1,2,4-triazole (16a and 16b) via the condensation of thiocarbohydrazide and D-glucono- and D-galactono-1,5-lactone under a microwave-assisted protocol, as depicted in Scheme 10. The reaction yielded the product in an impressive 88% yield within just 5–6 minutes.45
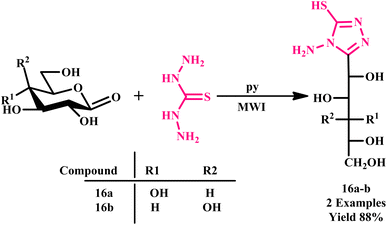 | ||
| Scheme 10 Microwave-assisted synthetic protocol for 4-amino 3-(D-gluco- and D-galacto-pentitol-1-yl)-5-mercapto-1,2,4-triazole (16a and 16b).45 | ||
Further in a report, Jaisankar and his co-workers described easy and efficient microwave-assisted one-pot synthesis of 1,2,4-triazole-3-carboxamides derivatives (17a–h) using ester and amine in toluene under neutral conditions. The outcomes of this protocol were compared with conventional methods, revealing that the microwave-assisted reaction proceeded without the need for any catalyst and achieved high yields within just 30 minutes. In contrast, the same product formation takes approximately 12–16 hours using conventional methods (Scheme 11). This remarkable reduction in reaction time, along with the elimination of catalysts, underscores the significant advantages of microwave-assisted sustainable synthesis.46
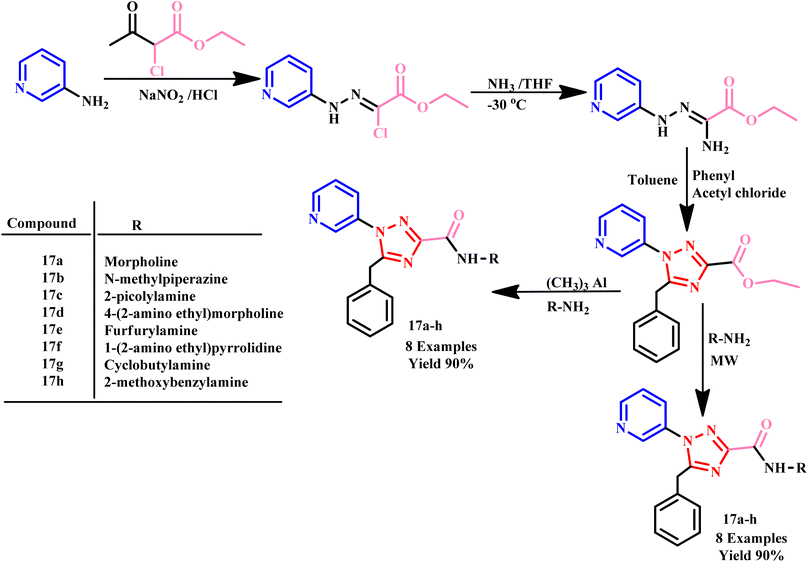 | ||
| Scheme 11 Microwave-assisted synthesis of 1,2,4-triazole-3-carboxamide derivatives (17a–h).46 | ||
A microwave-assisted synthesis of 3,5-dibenzyl-4-amino-1,2,4-triazole (18) was efficiently conducted in just 8–9 minutes at 250 W utilizing coupling of 2,4-pentanedione and diazotization reported by Jha and her co-workers (Scheme 12). In contrast, the traditional method required a lengthy reflux time of 10 hours with equal ratios of reactants, highlighting the significant time-saving advantage of the microwave approach. Moreover, the study involved the formation of metal complexes screened for anticancer activity. Spectral analyses revealed that these metal complexes exhibited octahedral geometry. Notably, owing to the steric hindrance posed by large benzyl groups, only mononuclear complexes were successfully formed. The reported innovative methodology enhances synthesis efficiency and contributes to the development of potential therapeutic agents against cancer.47
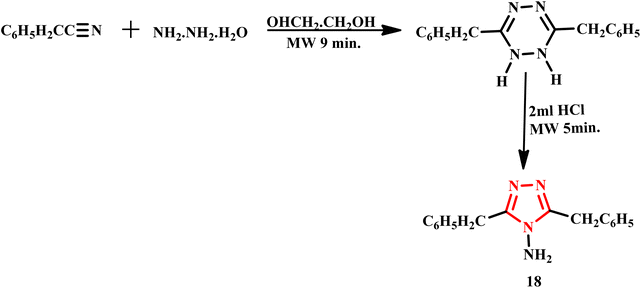 | ||
| Scheme 12 Microwave-assisted synthetic protocol for 3,5-dibenzyl-4-amino-1,2,4-triazole (18).47 | ||
Similarly, Kahveci et al. developed a microwave-assisted green synthetic protocol for the rapid synthesis of 3-aryl-4-arylmethylideneamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives (19a–p). It was observed that utilization of microwave irradiation, reported compounds were formed in just 5.0 minutes with an impressive yield of 96%, as illustrated in Scheme 13. The synthesized derivatives demonstrated significant mycostatic activity against various Candida species, highlighting their potential application in antifungal therapy.48
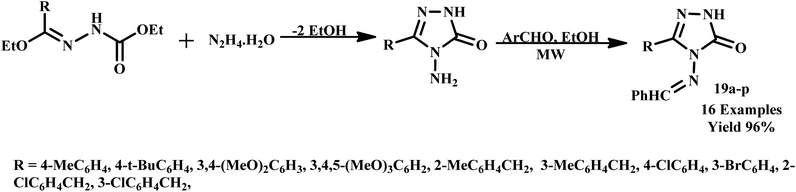 | ||
| Scheme 13 Synthetic route of 3-aryl-4-arylmethylideneamino-4,5-dihydro-1H-1,2,4-triazol-5-ones (19a–p).48 | ||
The novel zinc(II) phthalocyanines and phthalonitrile containing pharmacologically active molecules, such as triazole and morpholine, were synthesized through microwave irradiation and the classical method by Kantar and his co-workers. The reported microwave approach was a viable alternative process and an appealing practical method for the synthesis of phthalocyanines, including morpholino-substituted triazole derivatives, due to outstanding product yields (92%), simple workup procedure and quick reaction time, as depicted in Scheme 14. It was found that under the microwave, the reaction was carried out within 10–13 minutes, while the same product formation took approximately 48.0 hours with 64–80% yield in the conventional method. The compound 4-[4-(3-morpholinopropyl)-5-phenyl-2H-1,2,4-triazol-3-one-1-yl] phthalonitrile exhibited promising anti-xanthine oxidase (anti-XO) inhibition with an IC50 value of 0.082 ± 0.004 mM. The authors also reported that the low solubility and high aggregation tendency of phthalocyanine compounds did not exhibit xanthine oxidase (XO) inhibition.49
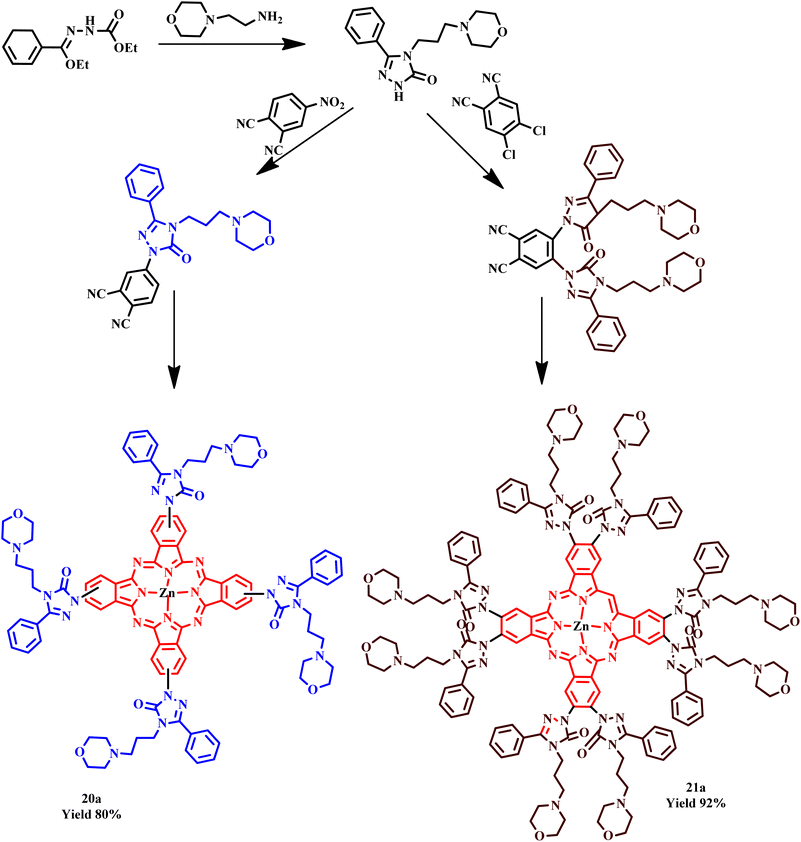 | ||
| Scheme 14 Synthetic route of 4-(3-morpholinopropyl)-5-phenyl-2H-1,2,4-triazol-3-one, 4-[4-(3-morpholinopropyl)-5-phenyl-2H-1,2,4-triazol-3-one-1-yl] phthalonitrile and 4,5-bis[4-(3-morpholinopropyl)-5-phenyl-2H-1,2,4-triazol-3-one1-yl] phthalonitrile and zinc phthalocyanines (20a and 21a).49 | ||
Furthermore, Kidwai et al. synthesized 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazepine derivatives (22a–e and 23a–e) using microwave irradiation. The reaction involved 1-amino-2-mercapto-5-substituted triazoles and substituted chalcones and was completed within an impressive 60–120 seconds, as shown in Scheme 15. In contrast, the conventional heating method required a lengthy duration of 10–18 hours. This significant reduction in reaction time demonstrates the efficiency of microwave-assisted synthesis and offers a valuable approach for the rapid development of complex organic compounds.50
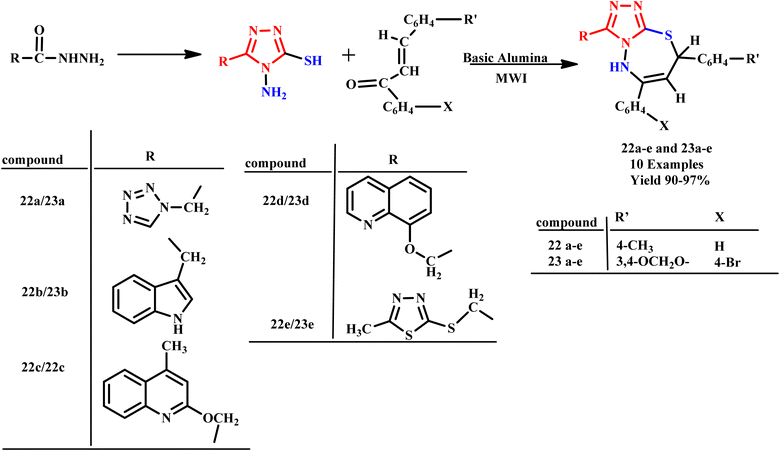 | ||
| Scheme 15 Synthesis of 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazepine compounds (22a–e and 23a–e).50 | ||
Similarly, in the progress of green microwave-assisted protocol developments, Kumar et al. developed one pot protocol for the synthesis of 3-phenyl-2-[4-{(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy}phenyl] thiazolidin-4-ones derivative from substituted phenyl azide, propargyl oxy-benzaldehyde, thioglycolic acid and substituted aniline using of Cu(I)-catalyzed microwave-assisted synthesis.51 Further, an efficient, easy and mild microwave-assisted method was developed by Shelkea and his coworker for the synthesis of substituted 1,2,4-triazoles derivative (24a–q) using formamide and hydrazines (Scheme 16). The MW-assisted protocol offered certain benefits, such as excellent yields, catalyst free conditions and short reaction time (in minutes) compared to the conventional method.52
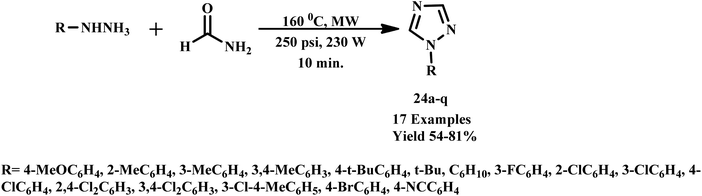 | ||
| Scheme 16 Microwave-assisted 1,2,4-trizole synthesis using formamide and hydrazine (24a–q).52 | ||
Lee et al. developed a microwave-assisted facile and efficient one pot synthetic method for 1,3,5-trisubstituted-1,2,4-triazoles (25a–f) synthesis via cyclization with hydrazines, followed by an N-acylation reaction of amide derivatives. The reaction was completed within one minute with an 85% yield (Scheme 17) compared to the conventional method, which takes more than 4.0 hours.53
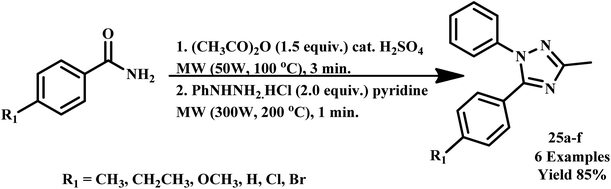 | ||
| Scheme 17 Microwave-assisted synthetic protocol for 1,3,5-trisubstituted-1,2,4-triazole synthesis (25a–f).53 | ||
Further, microwave-assisted efficient and highly selective one pot synthetic method for 3,3′(5,5′)-polymethylene-bis(1H-1,2,4-triazol-5(3))-amines from the reaction of aminoguanidine and dicarboxylic acids in an aqueous medium was developed by Lim et al. The reaction was completed within 5.0 minutes at 200 °C under the MW method. This microwave-enabled one-pot approach yielded good yields and high purity of the desired products, demonstrating its scalability. The method's scope was effectively investigated by creating a small library of polymethylene-bis(1H-1,2,4-triazol-5(3)-amines) with various alkyl chain linkers.54 Similarly, Liu et al. developed some new fluorinated 1,2,4-triazole derivatives using the microwave irradiation method. Additionally, initial bioassays revealed that some of the compounds showed effective herbicidal properties. It was observed that when the reaction took place at room temp, it required more than 24.0 hours and obtained a low amount of yield <5%, but under MW conditions, the reaction time interval from hours to few minutes (10.0 minutes) with a good amount of yield (56%). It was found that most of the reported compounds inhibited the growth of B. campestris (efficacy 66–89%), while all compounds showed low activities against E. crusgalli.55 A robust regioselective microwave-assisted synthetic protocol for the synthesis of N-substituted 1,2,4-triazoles derivative (26a–j) was developed by Meng and Kung. This innovative approach involves the formation of several intermediates, evading target compounds through a cyclization reaction, as illustrated in Scheme 18. The advantages of this green protocol include high yields and minimal reaction times, making it an efficient choice for synthetic chemistry. The synthesized triazoles serve as valuable starting materials for subsequent alkylation or acylation reactions, enabling the production of trisubstituted triazoles with well-defined regioselective substitution patterns. This methodology not only streamlines the synthesis process but also enhances the potential for further functionalization in drug development and material science.56
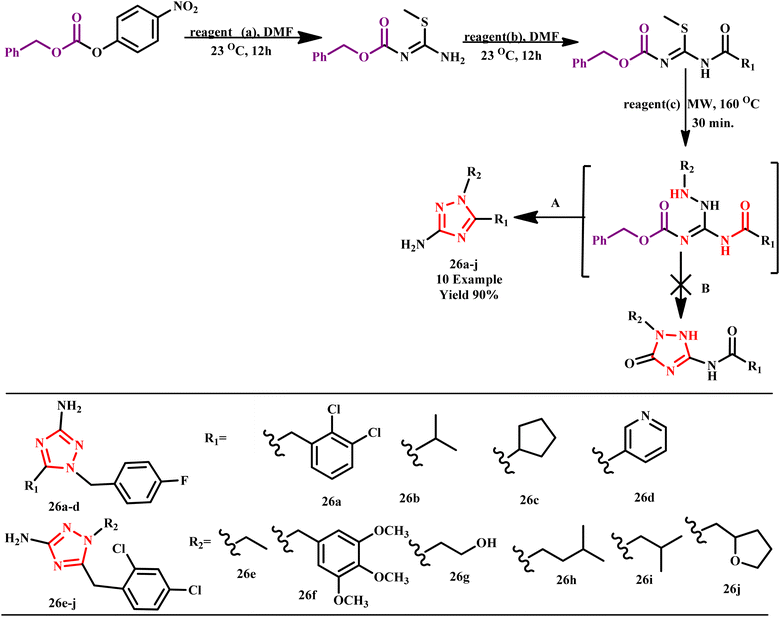 | ||
| Scheme 18 Microwave-assisted synthesis of N1-substituted 1,2,4-triazoles (26a–j). Condition: (a) 2-methyl-2-thiopseudourea sulfate; (b) carboxylic acid, 4-methylmorpholine, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride, 1-hydroxybenzotrizole; (c) substituted hydrazine, triethylamine.56 | ||
Similarly, Meng et al. synthesized 1,2,4-triazole thioether derivatives in few minutes using the microwave-assisted methodology. The synthesized triazole derivatives exhibited moderate to good inhibition activity against R. solani and C. orbiculare at 50 μg mL−1.57 Further, Sun and his co-worker synthesized a series of 1,2,3-thiadiazole rings containing 1,2,4-triazole derivatives (27a–q) under a multistep reaction through MW-assisted conditions (Scheme 19). According to the preliminary bioassays, some of the compounds displayed good fungicidal activities. It was observed that the best reaction conditions were 90 °C for 15 minutes under microwave irradiation.58
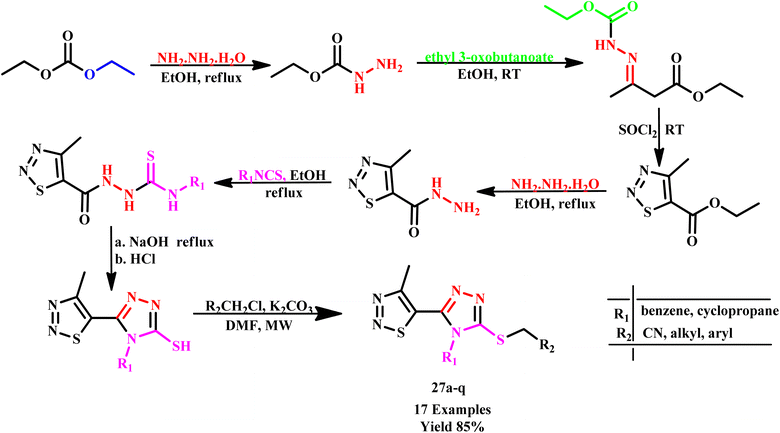 | ||
| Scheme 19 Microwave-assisted synthesis of 1,2,3-thiadiazole rings containing 1,2,4-triazole derivatives (27a–q).58 | ||
Further, Nas et al. synthesized metal-free 4-(2-(4-(4-methoxybenzylamino)-5-oxo-3-p-tolyl-4,5-dihydro-1H-1,2,4-triazol-1-yl)ethoxy) phthalonitrile and its complex with Ni(II), Zn(II), Co(II), Pb(II) and Cu(II) derivatives (28a–f) (Scheme 20).59
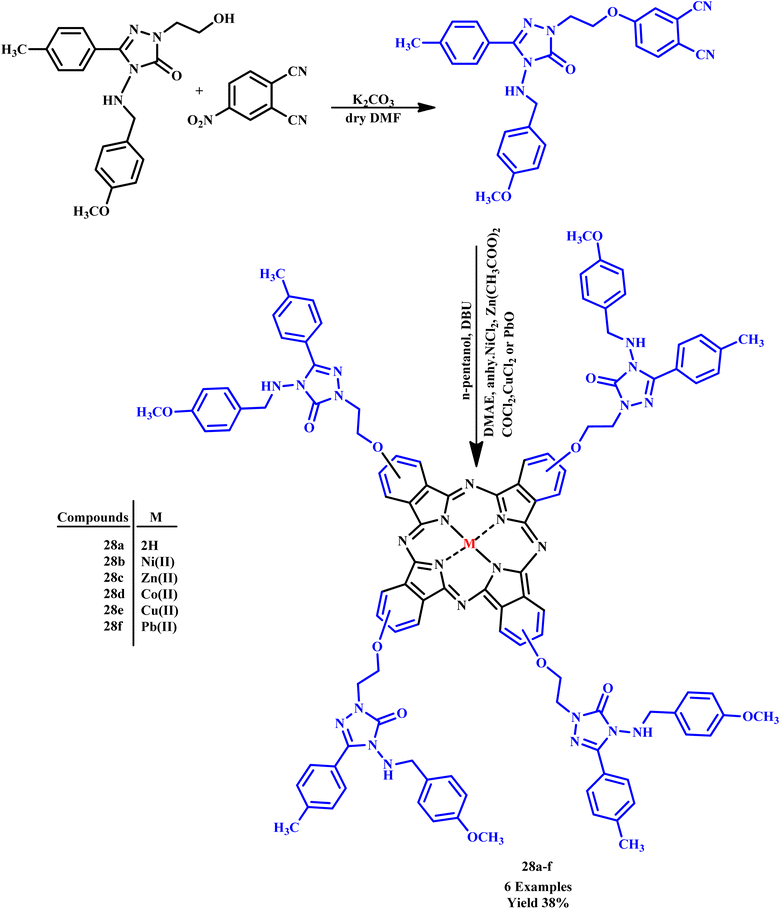 | ||
| Scheme 20 Synthetic method for the synthesis of metallophthalocyanine and metal-free phthalocyanine (28a–f).59 | ||
Nesaragi et al. developed a regioselective microwave-assisted synthesis of quinolin-3-yl-methyl-1,2,3-triazolyl-1,2,4-triazol-3(4H)-one derivatives (29a–m). This methodology was achieved through the [3 + 2] cycloaddition reaction of azides with terminal alkynes, resulting in impressive yields of 88–92%, as shown in Scheme 21. The reported efficient method highlights the versatility of microwave-assisted techniques and demonstrates its potential for producing complex triazole derivatives with high regioselectivity.60
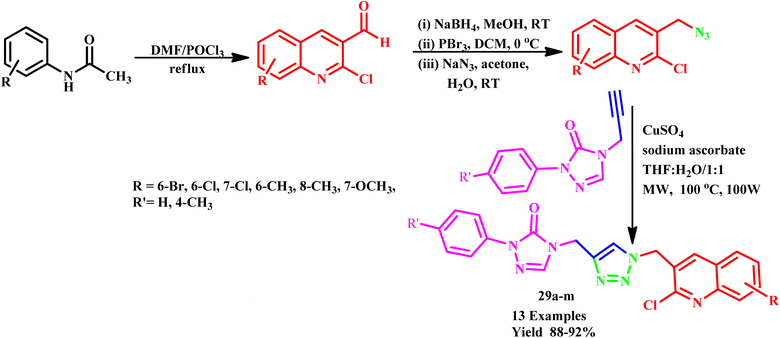 | ||
| Scheme 21 Synthetic route of quinolinyl-1,2,3-triazolyl-1,2,4-triazol-3(4H)-one derivatives (29a–m).60 | ||
An efficient, fast and straightforward method was developed by Rahimizadeh et al. for one-pot synthesis of 6-aryl-3-substituted 5H-1,2,4-triazolo[4,3-b][1,2,4]triazoles. This synthesis involved the heterocyclization of the N-(3-methylthio-5-substituted-4H-1,2,4-triazol-4-yl)benzene carboximidamide derivative (30a–e) using microwave irradiation under solvent-free conditions. Compared to conventional heating methods, microwave irradiation was 36–72 times faster and resulted in significantly higher yields, as illustrated in Scheme 22. It was found that the conventional heating methodology required 130 minutes to achieve a modest 25% yield, while the microwave method completed the reaction in just 10.0 minutes with an impressive yield of 77%. This advancement highlights the effectiveness of microwave-assisted synthesis in enhancing both efficiency and productivity in chemical reactions.61
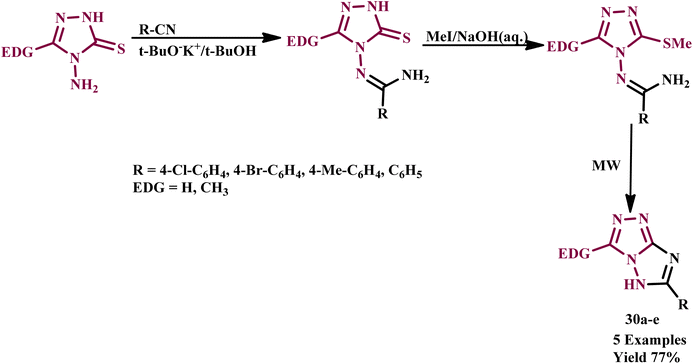 | ||
| Scheme 22 Synthesis of N-(3-methylthio-5-substituted-4H-1,2,4-triazol-4yl)benzene carboximidamides (30a–e).61 | ||
Similarly, Aouad et al. reported a simultaneous inhibition study of CA II, VEGFR-2 and MMP-2 using 1,2,3- and 1,2,4-triazole hybrids that were carefully crafted using a pharmacophore hybridization technique and bearing diverse sulfonamide appendages. In the synthesis, a microwave-assisted click 1,3-dipolar cycloaddition process involving 4-azido benzene sulfonamides and different alkynes was used for the production of 1,2,4-triazole derivatives in a quantitative yield.62 In another report, Wei et al. described a new and convenient in situ metal/ligand reaction for the synthesis of a series of 3,5-disubstituted-1,2,4-triazole derivative (31a–f) via the cycloaddition reaction of ammonia and organo-nitriles under the microwave-assisted condition with 85% yield within 1.5 hours. It was reported that a similar reaction was completed within 72 hours using a hydrothermal process (Scheme 23).63 A comparative analysis of microwave-assisted 1,2,4-triazole synthesis with conventional methods is presented in Table 1.
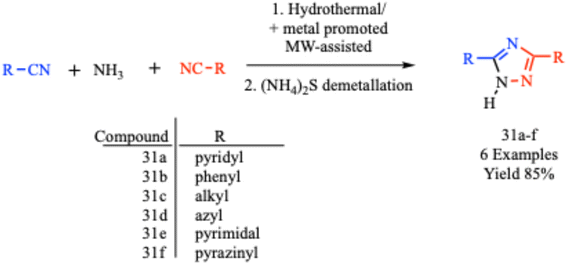 | ||
| Scheme 23 Synthetic route for 3,5-disubstituted-1,2,4-triazole derivatives (31a–f).63 | ||
| Entry | Structure | Name | Conventional method | Microwave method | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Time (hours) | Temp. (°C) | Yield (%) | Time (min) | Temp. (°C) | Yield (%) | ||||
| 1 | 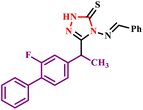 |
4(Benzylideneamino)-3-(1-(2-fluoro-[1,1′-biphenyl]-4-yl)ethyl)-1H-1,2,4-triazole-5(4H)-thione derivatives | 3–5 | 170–213 | 63–78 | 9–20 | 280–317 | 89–97 | 7 |
| 2 |  |
N-{4-[(4-amino-5-sulfanyl-4H-1,2,4-triazol-3-yl)methyl]-1,3-thiazol-2-yl}-2-substituted amide derivatives | 0.5–0.75 | — | 77 | 5–6 | — | 77 | 32 |
| 3 | 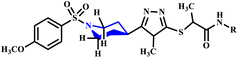 |
N-substituted-2-[(5-{1-[(4-methoxyphenyl) sulfonyl]-4-piperidinyl}-4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl] propanamide based on 1,2,4-trizole ring derivatives | 6–17 | RT | 45–83 | 37–90 seconds | — | 82–97 | 33 |
| 4 |  |
4-((1,3-Diphenyl-1H-pyrazol-4-yl)methyleneamino)-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiols | — | — | 15–20 | 70–75 | 85–89 | 34 | |
| 5 | 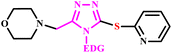 |
4-((5-((cyclohexylmethyl)thio)-4-R1-4H-1,2,4-triazol-3-yl)methyl)morpholines and 4-((4-R1-5-(pyridin-2-ylthio)-4H-1,2,4-triazol-3-yl)methyl)morpholines | — | — | 10 | 160 | 79–81 | 35 | |
| 6 | 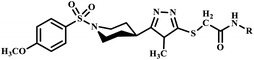 |
N-(substituted)-5-(1-(4-methoxyphenylsulfonyl)piperidin-4-yl)-4H-1,2,4-triazol-3-ylthio) acetamide derivatives | 9–19 | — | 44–88 | 31–68 seconds | — | 85–97 | 36 |
| 7 | 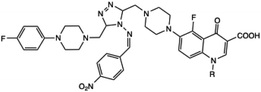 |
Piperazine-azole-fluoroquinolone based 1,2,4-triazole derivatives | 27 | — | 91 | 30 | 125 | 96 | 37 |
| 8 | 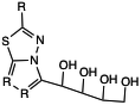 |
Acyclic (nucleosides of 3-(D-ribo-tetritol-1-yl)-5-mercapto-1,2,4-triazoles | 12 | 80 | 73 | 15 | 120 | 87 | 38 |
| 9 | 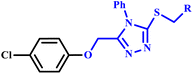 |
Thioethers containing 1,2,4-triazole derivatives | 24 | RT − 90 | 78–42 | 15 | 90 | 81–87 | 40 |
| 10 | 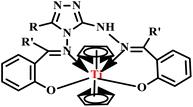 |
Schiff base complexes of bis(cyclopentadienyl)titanium(IV) | 6–7 | 55–62 | 10–15 | 65–75 | 41 | ||
| 11 | — | Transition metal-based 1,2,4 trizole | — | — | — | 15 | 85–120 | — | 42 |
| 12 | 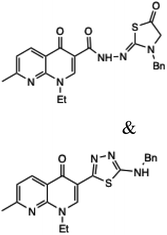 |
3-(4-Benzyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one or 1-ethyl-3-(4-ethyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-7-methyl-1,8-naphthyridin-4(1H)-one | 14–16 | Ethanol/water reflux | 63–67 | 4 | 150 | 86–88 | 31 |
| 13 | 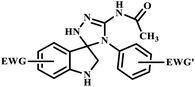 |
Trifluoromethyl substituted spiro[3H-indole-3,3′-[3H-1,2,4]triazole]-2(1H)-ones | — | — | — | 5–7 | 78 | 90–95 | 43 |
| 14 | 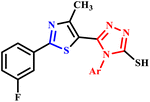 |
5-[2-(3-Fluorophenyl)-4-methylthiazol-5-yl]-4-phenyl-4H-1,2,4-triazole-3-thiol | 2–3 | — | 60–69 | 5–8 | 350 W | 70–77 | 44 |
| 15 | 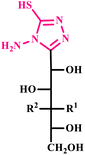 |
3-(D-Alditol-1-yl)-4-amino-5-mercapto-1,2,4-triazole derivatives | 1.5–2 | — | 70–80 | 5–6 | 201–217 | 80–88 | 45 |
| 16 | 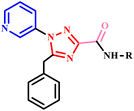 |
1,2,4-Triazole-3-carboxamide derivatives | 12–18 | 100 | 60–80 | 30 | 130 | 65–90 | 46 |
| 17 | 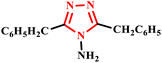 |
3,5-Dibenzyl-4-amino-1,2,4-triazole | 10–14 | Reflux | 8–9 | 250–300 W | — | 47 | |
| 18 | 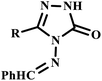 |
3-Aryl-4-arylmethylideneamino-4,5-dihydro-1H-1,2,4-triazol-5-onederivatives | 5 | 350 W | 96 | 48 | |||
| 19 | 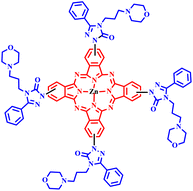 |
Morpholino-substituted 1,2,4-triazole-3-one derivatives | 5 | 150–155 | 30–64 | 13 | 125 | 70–92 | 49 |
| 20 | 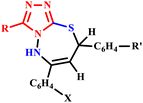 |
1,2,4-Trizolo[3,4-b]-1,3,4-thiadiazepine derivatives | 10–18 | 50–72 | 6–7 | 90–97 | 50 | ||
| 21 | 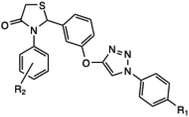 |
3-Phenyl-2-[4-{(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy}phenyl] thiazolidin-4-one derivative | — | — | — | 40–50 | 70 | 62–87 | 51 |
| 22 | 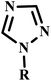 |
Substituted 1,2,4-triazoles derivative | — | — | — | 10 | 160 | 54–81 | 52 |
| 23 | 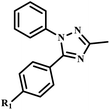 |
1,3,5-Trisubstituted-1,2,4-triazoles derivative | 4 | 45–76 | 1.0 | 200 | 68–85 | 53 | |
| 24 | 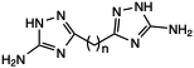 |
Polymethylene-bis(1H1,2,4-triazol-5(3)-amine) derivative | — | — | 5 | 200 | 52–88 | 54 | |
| 25 | 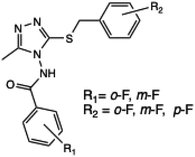 |
Fluorinated 1,2,4-triazole derivatives | — | — | — | 10 | 90 | 32–56 | 55 |
| 26 | 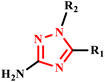 |
N1-substituted 3-amino-1,2,4-triazole derivative | — | — | — | 30 | 160 | 34–70 | 56 |
| 27 | 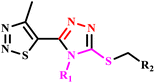 |
1,2,3-Thiadiazole rings containing 1,2,4-triazole derivatives | 24 | rt | 78 | 15 | 90 | 85 | 58 |
| 28 |  |
Metallophthalocyanine derivatives | 24 | 160 | 18 | — | 175 | 38 | 59 |
| 29 |  |
Quinolin-3-yl-methyl-1,2,3-triazolyl-1,2,4-triazol-3(4H)-ones | 4–6 | 74–80 | 3–5 | 88–92 | 60 | ||
| 30 |  |
6-Aryl-3-substituted-5H-[1,2,4]-triazolo[4,3-b][1,2,4]triazole derivative | 6 | 110–130 | 15–25 | 5–10 | 700–1000 W | 55–77 | 61 |
| 31 |  |
3,5-Disubstituted-1,2,4-triazole derivative | 72 | 120–170 | 13–25 | 1.5 | 120–170 | 72–85 | 63 |
Microwave-assisted synthesis of 1,2,3-triazole and derivatives
Another member of the triazole family, the 1,2,3-triazole ring, is another important heterocyclic scaffold that exhibits a wide range of applications in various domains. Owing to the wide range of applications, 1,2,3-triazoles with different substituents have drawn enormous attention for utilization in the fields of chemistry, medicine, and biology. For example, this scaffold is extensively explored in the development of anticonvulsant, antifungal, and notably well-liked anti-cancer medicines.64 In this line, a convenient and simple method was reported by Subhashini et al. for the synthesis of a new series of imidazole linked-1,2,3-triazole compounds (32a–h) through the MW method from 4-hydroxy benzaldehyde using click reaction within 3–5 minutes, as depicted in Scheme 24. It was observed by a research group that most of the synthesized hybrid compounds showed in vitro anti-microbial and antioxidant activity. In contrast, in conventional methods, the desired product was formed in 5.0 h at 80 °C. The antibacterial, antioxidant and molecular docking properties of these compounds were investigated using the Schrodinger suite. Preliminary results showed that several target compounds showed potential antibacterial potency owing to their in vitro antimicrobial activity against Gram-positive and Gram-negative pathogens. Additionally, these compounds were examined using four distinct techniques for the in vitro antioxidant activity. A small number of them showed excellent antioxidant activity. Furthermore, in silico molecular docking experiments provided additional support for the activity relationship between these compounds.65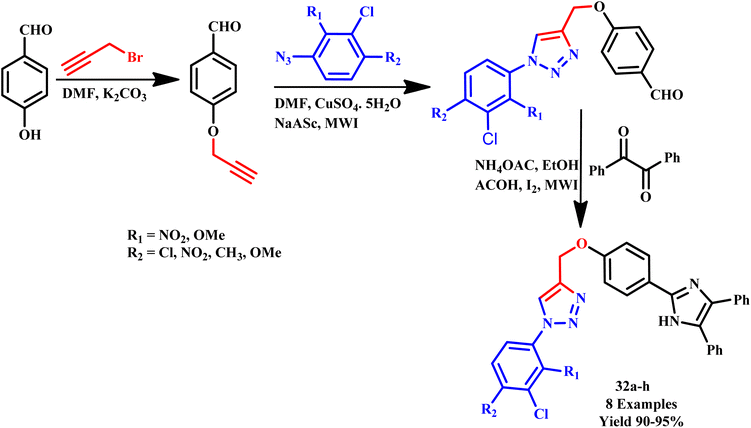 | ||
| Scheme 24 Microwave-assisted synthetic protocol for 1,2,3 triazole derivatives (32a–h).65 | ||
Similarly, Roshandel et al. reported solvent and catalyst-free microwave-assisted synthetic protocol for 1,2,3-triazole derivatives (33a–p) via cycloaddition reaction of acetylenes and trimethylsilylazide, as depicted in Scheme 25. In their synthetic method, diphenylacetylene was utilized as a model compound. By employing a thermally stable azide source and eliminating the need for solvents, metal catalysts, or other additives, the researchers were able to achieve the synthesis of 1,2,3-triazoles on a practical scale. This approach resulted in good to excellent yields (55–99%) and showcased a more environmentally friendly methodology that adheres to green chemistry principles while maintaining efficiency in triazole production.66
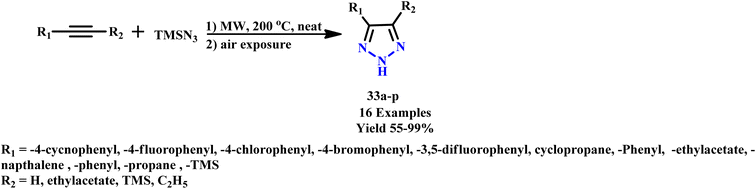 | ||
| Scheme 25 Synthesis of 1,2,3-triazole derivatives (33a–p) from acetylenes and trimethylsilylazide under microwave.66 | ||
Further, in another report, an efficient microwave-assisted synthetic method was developed by Krim and his co-workers for the synthesis of 1,2,3-triazole and bis-1,2,3-triazole acyclonucleoside analogues of acyclovir. Remarkably, the reaction was completed in only 1.0 minutes with a yield of 93%, while conventional methodologies required three hours for completion. A series of novel nucleobase-linked 1,2,3-triazole acyclonucleosides were synthesized using copper(I) as a catalyst to facilitate the 1,3-dipolar cycloaddition of N-1-propargylpyrimidines, N-1-propargylindazoles or N-9-propargylpurine with azido-pseudo sugars under microwave conditions.67 Further, in a report, Singh synthesized a series of triazole organo-silanes and triazole functionalized alkynes (34a–e) through microwave protocol. Surprisingly, microwave protocol offers a great alternative with 86–98% yield within 21.0 minutes, which is almost sixteen times lower than conventional techniques (Scheme 26). Another research group reported that synthesized tris-triazole compounds exhibited high effectiveness for anti-parasitic and antioxidant treatment. Tris-triazole silane compound Giardia lamblia (Z)-N-(2,4-bis((1-(3-(triethoxysilyl) propyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-4H-1,2,4-triazol-4-amine was determined to be the most effective treatment against Trichomonas vaginalis. This low-cost, multipurpose medication is hopefully useful in the eradication of parasitic diseases from the human population.68
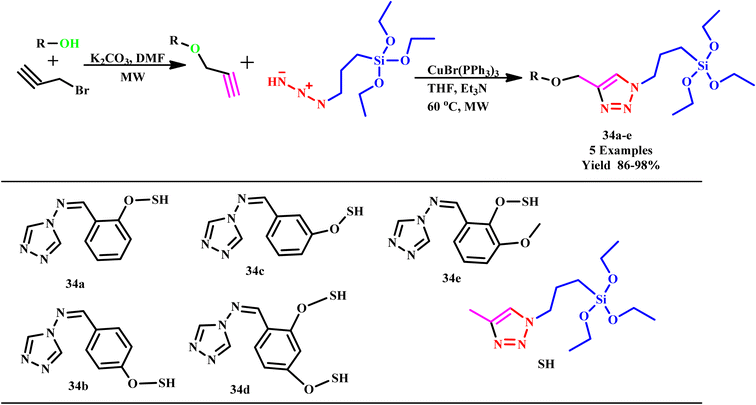 | ||
| Scheme 26 Microwave-assisted synthetic protocol for silicon-based multidentate triazole scaffolds (34a–e).68 | ||
Ashok and his co-workers reported a microwave-assisted synthetic protocol for a series of 3-(5-methyl-1-aryl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehydes and their benzimidazole derivatives (35a–g) with an 89% yield (Scheme 27). Among the synthesized compounds, 3-(5-methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4 carbaldehyde was proven to be a more effective antiproliferative agent for C6 cell, while 2-(3-(1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1Hbenzo [d]imidazole compound was proven to be a more effective antiproliferative agent for MCF-7 cell line. In conventional heating methodology, the reported reaction time required more than 6 hours to complete at room temperature with 66% yield, while it required 6–10 min at 300 W under MW irradiation conditions.69
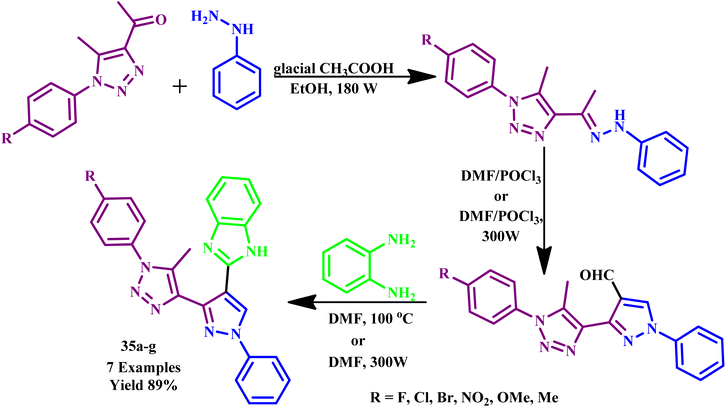 | ||
| Scheme 27 Microwave-assisted synthetic route for 3-(5-methyl-1-aryl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehydes and benzimidazole derivatives (35a–g).69 | ||
Narsimha et al. reported a one-pot, copper-catalyzed synthesis of fused benzothiazino[1,2,3]triazolo[4,5-c]quinolinone derivatives (36a–f) and (37a–i) from 1-iodoalkynes and various aryl azides via intramolecular C–H arylation of the in situ generated 5-iodotriazole intermediate, as illustrated in Scheme 28. This innovative method showcases the efficiency of copper catalysis in complex organic syntheses. Notably, some of the synthesized compounds demonstrated significant anticancer activities against the A-549 and MCF-7 cancer cell lines. In particular, compounds 37b and 37c exhibited high activity against both the A-549 and MCF-7 cell lines. These findings suggest that such compounds could be promising candidates for further development as anticancer agents.70
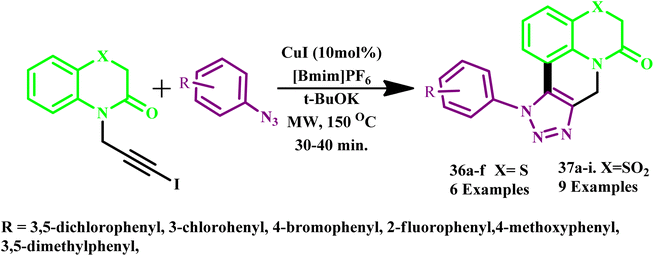 | ||
| Scheme 28 Synthetic protocol for the synthesis of fused 1,2,3-triazole derivatives (36a–f and 37a–i).70 | ||
Further, a novel series of acridone-based 1,2,3-triazole hybrid derivatives (38a–h) were synthesized via copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) by Aarjane and his coworker within 10.0 minutes through the MW-assisted method with 69–86% yields (Scheme 29). The traditional methodology required at least 10.0 hours to complete the reaction, and overall yield was found to be in a range of 60–75%, while under microwave-assisted reaction, product yield was improved, and reaction time was also reduced to few minutes. The structures of the acridon-1,2,3-triazole hybrid derivatives were identified using FTIR, NMR, and mass spectrometry. The synthesized derivatives exhibit antibacterial activity against S. aureus, Klebsiella pneumonia, Escherichia coli, Pseudomonas putida, and Serratia marcescens, indicating that the substitution of the acridone ring by the 1,2,3-triazole nucleus enhances the antibacterial potential of the compounds. Compound 38f shows an excellent antibacterial effect against S. aureus.71
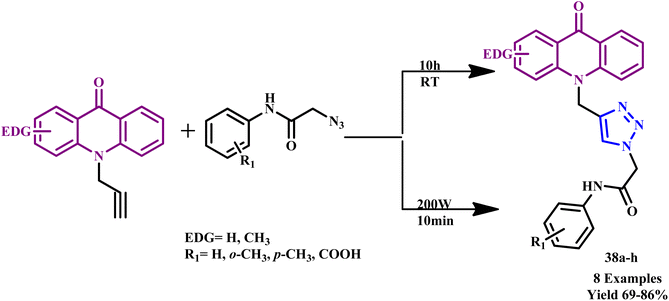 | ||
| Scheme 29 Synthetic route for a novel series of acridone-based 1,2,3-triazole hybrid derivatives (38a–h).71 | ||
Similarly, a series of novel 1-(5-methyl1-aryl-1H-1,2,3-triazol-4-yl)ethanone O-((1-aryl-1H1,2,3-triazol-4-yl)methyl) oxime derivatives (39a–p) was prepared through Cu(I)-catalyzed 1,3-dipolar cycloaddition of organic azides and alkynes under microwave irradiation (Scheme 30) by Ashok and his coworker. The O-alkoxy oxime linkage was used in this methodology to connect the two triazole moieties. Furthermore, some of these compounds showed good anti-fungal and antibacterial activities against the tested microorganisms. To obtain the desired product via the conventional method, the reaction was heated at 80 °C for 8 hours and produced 52% yield, while the MW method required 180 W for 12 minutes with 92% yield. Furthermore, synthesized compounds 39g and 39i showed excellent anti-fungal activities and compounds 39g, 39i, 39l and 39h showed excellent anti-bacterial activities against the tested microorganism.9
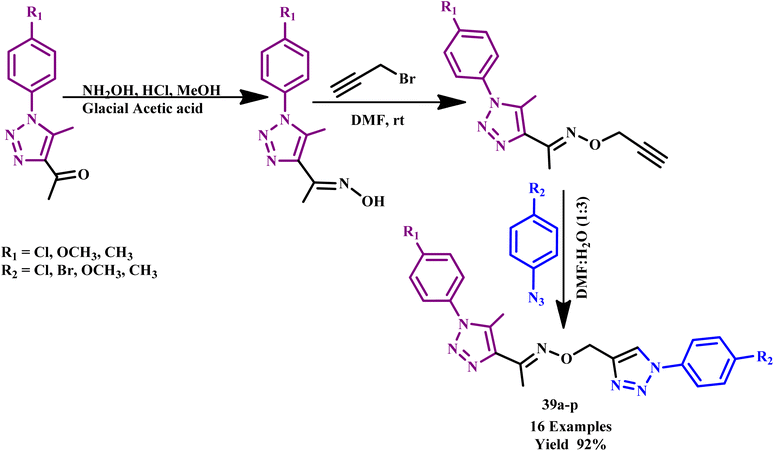 | ||
| Scheme 30 Synthesis of 1,2,3-triazole derivatives (39a–p).9 | ||
Du and team developed a highly efficient, rapid and facile microwave-assisted one-pot synthesis of 1-monosubstituted 1,2,3-triazoles (40a–n). This method utilized commercially available arylboronic acids, 3-butyn-2-ol and sodium azide, and achieved excellent yields within just 15.0 minutes at 80 °C (Scheme 31). In contrast, conventional methods require significantly longer reaction times to produce the target products. This innovative approach speeds up the synthesis process and enhances overall efficiency, which makes it a valuable advancement in the field of organic chemistry.72
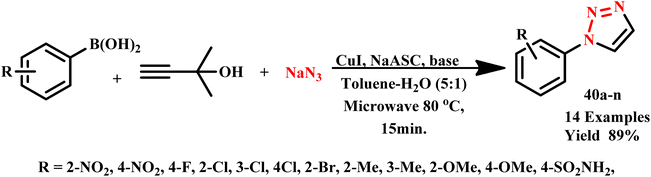 | ||
| Scheme 31 Synthesis of 1-monosubstituted 1,2,3-triazole (40a–n).72 | ||
A new series of p-tolyloxyquinoline-triazole hybrid derivatives (41a, 42a, 43a–41d, 42d, and 43d) were prepared by Vishnuvardhan and his coworker under microwave irradiation from hydroxylacetophenes and 2-(ptolyloxy)quinoline-3-carbaldehyde, as depicted in Scheme 32. It was observed that in the microwave method, the reaction was completed within 5–6 min with 93% yield, and in the conventional method, the reaction required more than 8–10 hours to complete with 75% yield. The desired 1,2,3-triazole compounds were produced through the 1,3-dipolar cycloaddition of alkynes with azides using CuI as a catalyst under a microwave-assisted process. All the reported compounds were examined with antimicrobial activities in which most of the compounds revealed the highest powerful zone of inhibition against all tested organisms. For example, some derivatives (41d, 42d and 43c) exhibited anti-fungal activities against Candida metapsilosis and Aspergillus Niger organisms using Griseofulvin as standard drug whereas some derivatives (41d, 42d and 43a) exhibited excellent anti-bacterial activities against S. aeureus, B. faecalis, B. faecalis, E. coli, and B. faecalis using Ampicillin as standard drug.4
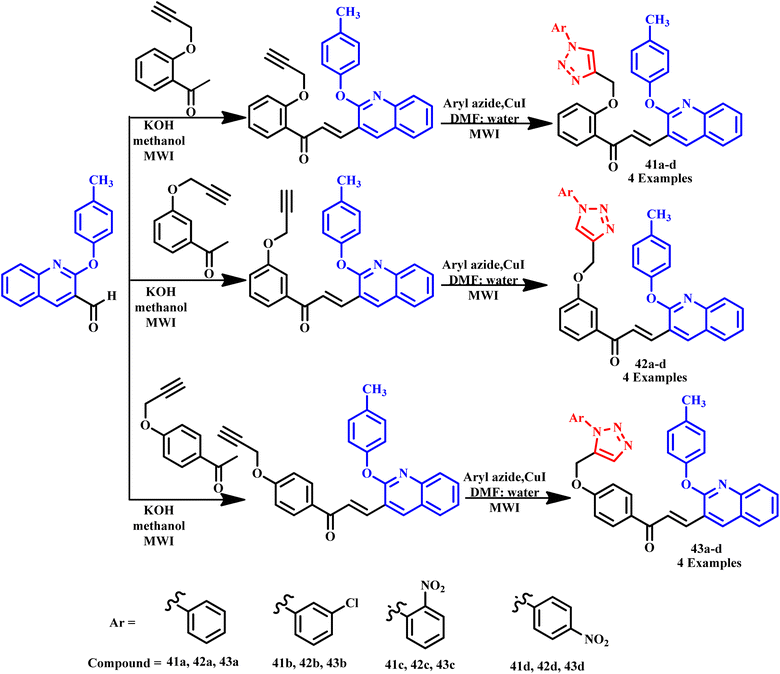 | ||
| Scheme 32 Synthesis of p-tolyloxyquinoline-triazole hybrid compounds (41a–43d).4 | ||
Similarly, Jaafar and his co-workers developed a regioselective synthesis of N-bis-1,2,3-triazolo-linked-1,5-benzodiazepin-2-ones (BZD) using various aliphatic/aromatic moieties through the microwave irradiation method for 6–12 minutes at 400 W with 67–92% yield as depicted in Scheme 33. All the synthesized compounds were evaluated for their antioxidant and antimicrobial activities. Compounds 44h and 44k showed anti-fungal activities. Furthermore, compounds 44g and 44h exhibited anti-oxidant activities rather than other bis-triazole benzodiazepines.73
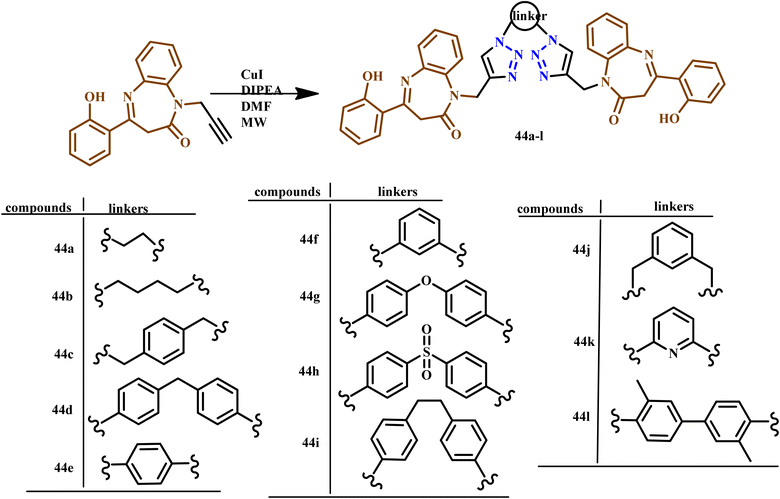 | ||
| Scheme 33 Microwave-assisted synthesis of 1,1′-((1,1′-(hexane-1,6-diyl)bis(1H-1,2,3-triazole-4,1-diyl)) bis(methylene))bis(1,5-benzodiazepin-2-one) and 1-((1-(6-azidohexyl)-1H-1,2,3-triazol-4-yl)methyl)-(1,5-benzodiazepin-2-one) (44a–i).73 | ||
In another report, Dürüst and Karakuş reported simple microwave-assisted one pot synthesis of 1,2,4-oxadiazol-5-ylmethyl-1,2,3-triazoles derivatives (45a–k) through 1,3-dipolar cycloaddition of phenyl propiolic acid and azidomethyl 1,2,4-oxadiazolesas, as depicted in Scheme 34. In this methodology, sodium-ascorbate and CuSO4 are used as catalysts in ethanol/water solvent. The reaction mixture was then irradiated on the Cem Discover microwave reactor for 2.5 minutes at 0–80 W at 65 °C, with 75% yield, while the traditional method required 16.0 h to complete at 65 °C with 73% product yield. The benefits of the microwave-assisted method include slower reaction time, easy separation, simple work-up and reasonable yields.64
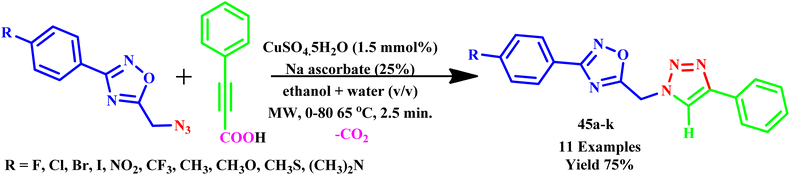 | ||
| Scheme 34 Synthesis of 1,2,4-oxadiazol-5-ylmethyl-1,2,3-triazoles (45a–k) via 1,3-dipolar cycloadditions of azide and phenyl propiolic acid.64 | ||
J. I. Sarmiento-Sánchez and co-workers reported the synthesis of 1,4-disubstituted 1,2,3-triazole derivatives (46a–u) via an in situ reaction of alkyl azides with various alkynes. They established that microwave irradiation significantly reduces reaction times from hours to minutes, which is an important advantage for the viability of novel synthetic techniques (Scheme 35). Furthermore, the yields of products obtained using microwave irradiation were higher than those achieved under standard thermal conditions. This advancement underscores the efficiency and practicality of microwave-assisted synthesis in organic chemistry.74
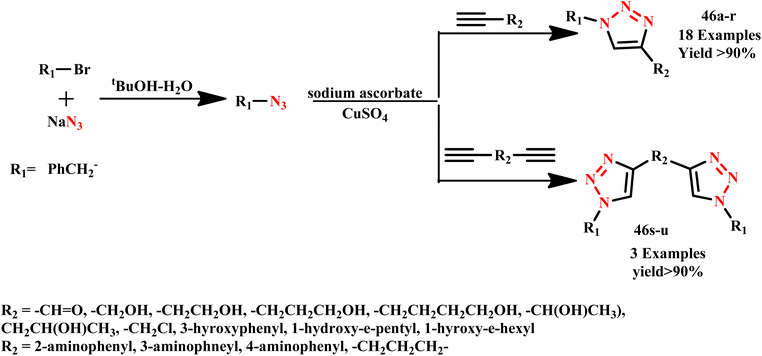 | ||
| Scheme 35 Microwave-assisted synthetic route for 1,4-disubstituted 1,2,3-triazole derivatives (46a–u).74 | ||
Similarly, a straightforward microwave-assisted methodology for the synthesis of novel 1,2,3-triazole compounds (47a–h) from acridone was developed by Aarjane et al. The typical synthesis includes regiospecific 1,3-dipolar cycloaddition between aromatic azides and 10-(prop-2-yn-1-yl)acridone derivatives using a Cu(I) catalyst (Scheme 36). In comparison to the traditional approach, microwave-assisted synthesis significantly shortened the reaction times and increased the yields of all reported compounds. It was observed that the microwave-assisted reaction was completed within 10.0 minutes at 200 W with 94% yield, while the conventional reaction required 4–8 h at 80 °C to complete with 62% yield. The FTIR, NMR, and mass spectrometry were used to determine the structures of the novel compounds.75
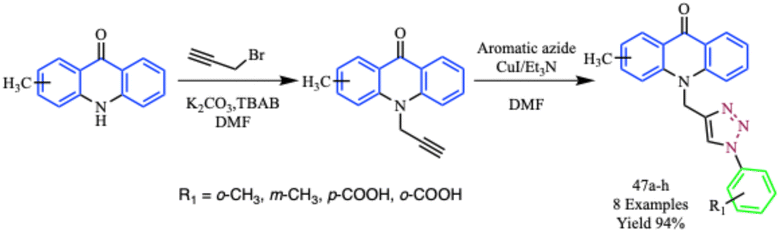 | ||
| Scheme 36 Microwave-assisted synthesis of 1,2,3-triazole derivatives (47a–h) from acridone.75 | ||
Further, a three-component reaction under microwave irradiation using allyl or benzyl halides, terminal alkynes, and sodium azide in the presence of neutral alumina-supported copper iodide was reported by Agalave et al. for 1,4-disubstituted 1,2,3-triazole derivative (48a–s) synthesis, as shown in Scheme 37. In this methodology, simple filtering was used to separate the products and catalyst and achieved a 98% yield of products in nearly pure form. It was found that the reaction was completed within 12.0 hours at room temperature, but in the microwave irradiation process (100 W, 78 °C), the reaction was completed within 6.0 minutes with 98% yield. This straightforward heterogeneous catalyst was reused for more than eight cycles while maintaining its high catalytic activities using water as a green solvent in a microwave process. Another environmentally friendly feature of this reaction was the ability to isolate desired products in high yields through straightforward filtration without the need for column chromatography.76
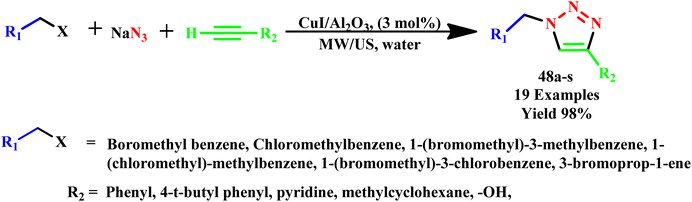 | ||
| Scheme 37 Microwave-assisted synthesis of 1,4-disubstituted 1,2,3-triazoles (48a–s) via CuI on Al2O3 catalyzed.76 | ||
In another study, Appukkuttan and his co-workers developed a microwave-assisted, regioselective, and efficient three-component protocol for the rapid synthesis of 1,4-disubstituted 1,2,3-triazole derivatives (49a–m) (Scheme 38). This approach enhances the effectiveness of the click chemistry process by making it more user friendly and safer by eliminating the need for organic azides by producing them in situ. In this methodology, the reaction time was drastically reduced from hours to just 10–15 minutes along with an improvement of yields (up to 90%).77
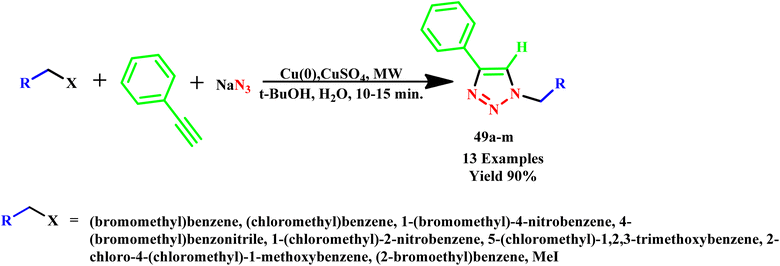 | ||
| Scheme 38 Microwave-assisted synthetic route for 1,4-disubstituted 1,2,3-triazoles (49a–m).77 | ||
Similarly, an effective microwave-assisted synthesis of 1-[5-(1-benzyl-1H-[1,2,3]triazol-4-ylmethoxy)-2,2-dimethylchroman-6-yl]-3-aryl-propen-1-one and 1-[7-(1-benzyl-1H-[1,2,3]triazol-4-ylmethoxy)-2,2-dimethyl-chroman-6-yl]-3-aryl-2-propen-1-ones was developed by Ashok and his coworker. They reported that CuI-catalyzed reaction required 24.0 h for completion and produced 72% yield, while microwave-assisted reaction required 8–10 h with 92% product yield. Most compounds were synthesized with ease of reaction conditions, such as smooth reaction conditions, purity and lower reaction times. Further, the in vitro antibacterial activity of all reported compounds was examined, and it was observed that phenyl ring containing compounds exhibited significantly higher antibacterial activity compared to other derivatives. Compound 1-[7-(1-benzyl-1H-[1,2,3])triazol-4-ylmethoxy-2,2-dimethyl-chroman-6-yl]-3-phenyl-propenone, 1-[7-(1-benzyl-1H-[1,2,3])triazol-4-ylmethoxy-2,2-dimethyl-chroman-6-yl]-3-(3,4-dimethoxy-phenyl)-propenone, 1-[7-(1-benzyl-1H-[1,2,3]) triazol-4-ylmethoxy-2,2-dimethyl-chroman-6-yl]-3-(3,4,5-trimethoxy-phenyl)-propenone displayed maximum zone inhibition. All prepared compounds were screened in vitro for their antifungal activities against Candida albicans (ATCC-2091), Aspergillus foetidus (NCIM-505) and Aspergillus niger (ATCC9029).78 Similarly, Ashok et al. synthesized bis-1,2,3-triazole derivatives based on 2-indolinone under microwave irradiation through copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition (CuAAC) reaction of O-,N-propargylated indolinone derivatives (50a–t) with in situ-generated organic azides (Scheme 39). The conventional method gave a 70% yield at 80 °C for 7.0 hours, while the microwave-assisted synthetic protocol offered a 92% yield at 180 W within 3–5 minutes. Some derivatives, such as 50e, 50f and 50o exhibited outstanding radical scavenging activities compared with standard drugs BHT and ascorbic acid and showed promising antioxidant activities toward H2O2, NO radical and DPPH radical. Compounds 50g, 50h, 50n, 50q and 50r displayed excellent antimicrobial activities against fungal and bacterial strain.79
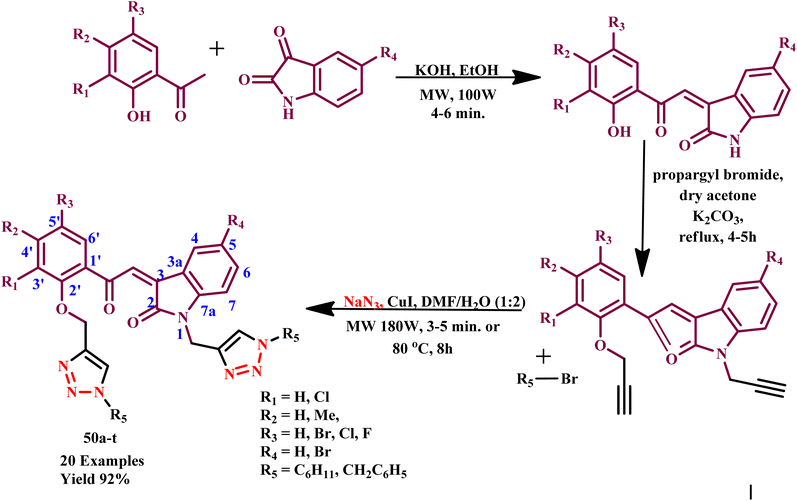 | ||
| Scheme 39 Microwave-assisted synthetic route for 2-indolinone-based bis-1,2,3-triazole (50a–t) synthesis.79 | ||
Ashok and his co-workers synthesized a series (51a–j) of biologically active dimers containing 1,2,3-triazole and coumarin pharmacophores, as depicted in Scheme 40. They examined the antimicrobial and antimycobacterial properties of these compounds. Using traditional methods, the reaction mixture was stirred for 24 hours at 80 °C giving 52% product yield. However, when irradiated under microwave radiation at 180 W for 10.0 minutes, a 79% product yield was obtained. Notably, two derivatives exhibited outstanding antitubercular activities, with a minimum inhibitory concentration (MIC) of 1.56 μg mL−1, while other derivatives showed potential antibacterial activity against various bacterial and fungal strains. Structure–activity relationship studies revealed that a long lipophilic alkyl spacer with a carbon chain length of 5–8 and the presence of an electronegative chlorine atom at the C-6 position of the coumarin nucleus played essential roles in the broad range of biological activities observed in these compounds.80
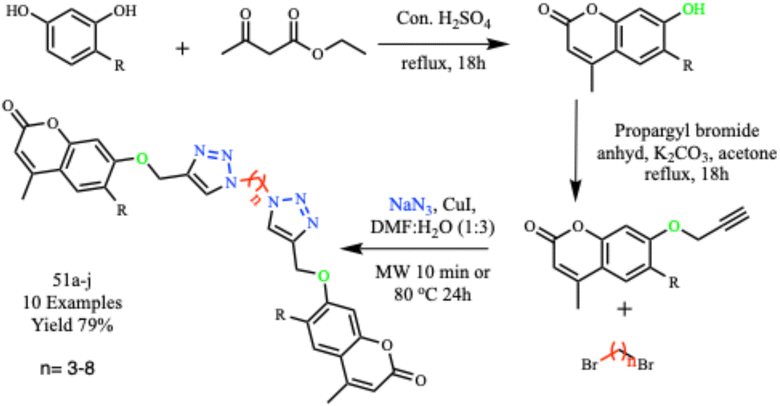 | ||
| Scheme 40 Synthesis of dimers of coumarin-tethered 1,2,3-triazole hybrids (51a–j).80 | ||
Similarly, Mohammed et al. reported perfluoro-butylethylazide and 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl azides along with various terminal alkynes to create 1,2,3-triazoles using the microwave-assisted method.81 Further, a small library of enantiopure a-[4-(1-substituted)-1,2,3-triazol-4-yl]benzylacetamides derivatives (52a–h) (R) and (52a–h) (S) from racemic propargyl amines were synthesized by Castagnolo et al. using microwave-assisted Cu(I)-catalyzed click chemistry method (Scheme 41). These triazole derivatives are important and easily accessible intermediates for the synthesis of enantiopure azole analogues because they were synthesized in a short time with good yields and substantial enantiomeric excess.82
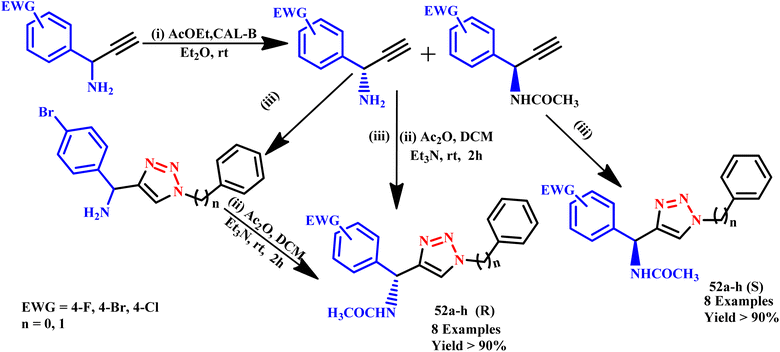 | ||
| Scheme 41 Microwave-assisted synthesis of a-[4-(1-substituted)-1,2,3-triazol-4-yl]benzylacetamides. Conditions: (iii) (for n = 1) NaN3, benzyl chloride, sodium ascorbate/Cu(SO)4·H2O/tBuOH, MW, 125 °C, 10 min; (for n = 0) phN3, sodium ascorbate/CuSO4·H2O/tBuOH, MW, 120 °C, 10 min.82 | ||
Librando developed a reproducible and straightforward synthetic procedure for recyclable Cu(I)–DAPTA complexes on carbon materials (such as carbon nanotubes (CNT) and activated carbon (AC)) to create a heterogeneous catalyst. These immobilized Cu(I)–DAPTA complexes were employed for catalytic synthesis of 1,2,3-triazole derivatives (53a–h) via cycloaddition reaction of azides and alkynes under microwave irradiation (Scheme 42). Microwave heating significantly reduced reaction times compared to traditional methods and increased yield purity of product by minimizing unfavorable side reactions.83
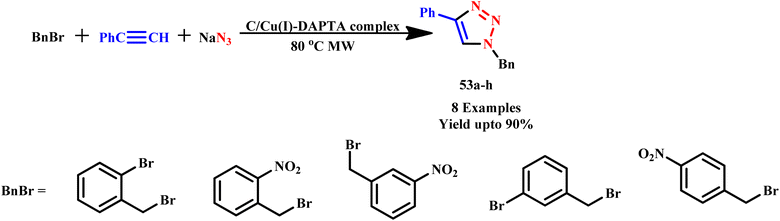 | ||
| Scheme 42 Microwave-assisted synthetic protocol for the synthesis of 1,2,3-triazoles (53a–h) via 1,3 dipolar cycloadditions of azide–alkyne.83 | ||
Dharavath et al. utilized microwave irradiation to develop a highly effective and environmentally friendly method for creating 1,4-disubstituted 1,2,3-triazole derivatives (54a–i) based on coumarin. This method employs a Cu(I)-catalyzed click reaction involving terminal alkynes and various substituted aryl azides (Scheme 43). The author reported that compared to the conventional approach (which required 6.0 hours and 72% yield), this innovative approach was simpler and more efficient and offered the advantages of high yields (90%) and a shorter reaction time (7 minutes). Additionally, some of the synthesized compounds exhibited good hydrogen-bonding interactions with Val-191 and His-88 amino acids as well as with water molecules, indicating their potential for biological activity.84
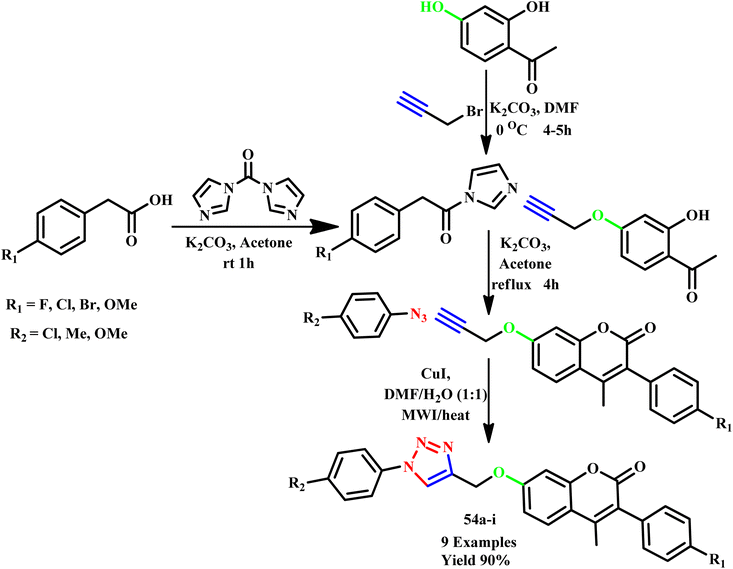 | ||
| Scheme 43 Microwave-assisted synthesis of coumarin-based 1,2,3-triazole compounds (54a–i).84 | ||
Alves et al. developed a metal-free, one-pot method for the synthesis of multiple 4-aryl-1,4-dihydrochromene-triazoles derivatives (55a–o) using PEG 400 as a solvent under microwave irradiation conditions, as illustrated in Scheme 44. This innovative approach leverages microwave irradiation to produce triazole compounds from readily available building blocks, achieving good yields and significantly reducing reaction times. The method was not only efficient but also environmentally friendly due to the use of PEG400 as a green solvent. Additionally, microwave-assisted synthesis ensures better control over reaction parameters, minimizing side reactions and enhancing product purity. This technique showcases the potential for the rapid and sustainable synthesis of biologically active compounds.85
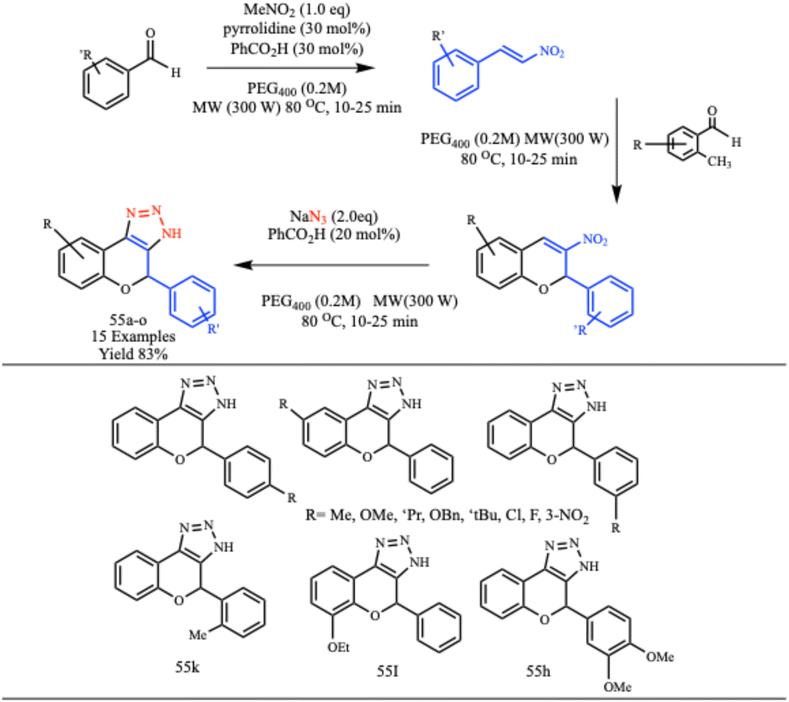 | ||
| Scheme 44 Microwave-assisted protocol for the synthesis of 4-aryl-1,4-dihydrochromene-triazoles (55a–o).85 | ||
Dutta et al. established a green method for synthesizing various 5-amino-1,2,3-triazoles from phenyl azide derivatives and benzyl cyanide. This innovative approach utilizes a bifunctional ionic liquid, [DHIM][OH], in combination with microwave irradiation conditions. The use of microwave heating not only accelerates the reaction but also enhances the overall efficiency and yield of the desired triazole products. This method exemplifies sustainable chemistry by minimizing waste and utilizing environmentally friendly reagents, making it a valuable contribution to the field of organic synthesis. Furthermore, the versatility of ionic liquid as a solvent allows for easy recovery and reuse, promoting the principles of green chemistry.86 Costa produced 4-substituted-1,2,3-triazolyl 1′-homo-3′-isoazanucleosides derivatives (56a–k/(±) −3 (±) −14) through MW assist cycloaddition reaction through different alkyne with 3,5-disubstituted proline derivative, as shown in Scheme 45. The compounds were tested in vitro for their cytotoxic effects on human ovary carcinoma cell lines (A2780), human lung cancer cell lines (NCIeH460) and human breast carcinoma cell lines (MCF-7). Compounds 61f (±) −8 and 61g (±) −9 exhibited inhibitory activity, with IC50 values of 56 and 942 mM against the A2780 cell lines.87
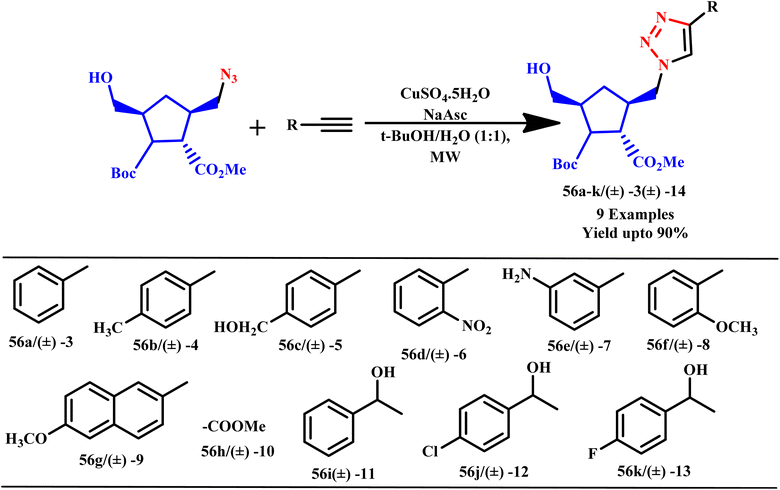 | ||
| Scheme 45 Microwave-assisted synthesis of triazoles.87 | ||
Further, Garg and his coworker established a microwave-induced multicomponent reaction from nitroalkane, aldehyde and sodium azide in polyethylene glycol 400 to produce biologically significant 4-aryl-NH-1,2,3-triazole derivatives (57a–q) using anthranilic acid as a catalyst. The protocol was optimized using inexpensive and easily accessible reagents. The reported protocol gave excellent product yields, metal-free conditions, use of MW irradiation as an unconventional energy source, gram scale synthesis and easy solvent recycling (Scheme 46).88
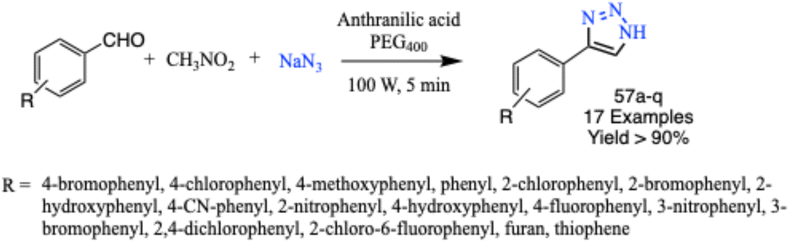 | ||
| Scheme 46 Microwave-assisted synthesis route for 4-aryl-NH-1,2,3-triazoles (57a–q).88 | ||
Further, He et al. developed a straightforward procedure for the effective and rapid synthesis of difunctional glycosyl triazolyl acids using a click reaction facilitated by microwave irradiation. This innovative approach allows for the efficient production of glycosyl mono-, di- and tyrosine derivatives, which were identified as novel inhibitors of PTP1B and demonstrated selectivity over other homologous protein tyrosine phosphatases several fold. The significant selectivity and effectiveness of these synthesized compounds highlighted their potential for therapeutic applications, particularly in targeting diseases related to dysregulated PTP activity.89 Iwasaki et al. developed an efficient method for the direct arylation of 1,4-disubstituted 1,2,3-triazole derivative (58a–q) using aryl chloride in the presence of palladium, employing microwave irradiation at 250 °C for 15 minutes, as illustrated in Scheme 47. This method successfully synthesizes several 1,4-disubstituted 1,2,3-triazoles through copper-catalyzed [3 + 2] cycloaddition of terminal alkynes and organic azides. The present procedure not only provides a clear and rapid pathway for synthesizing 1,4,5-trisubstituted 1,2,3-triazoles but also enhances overall yield and purity. This advancement underscores the potential of microwave-assisted techniques in streamlining synthetic processes and expanding the utility of triazole derivatives in pharmaceutical applications.90
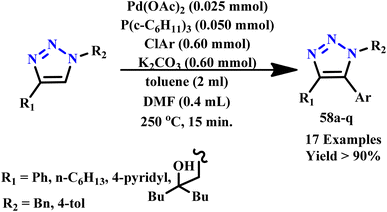 | ||
| Scheme 47 Microwave-assisted Pd catalyzed arylation of 1,4-disubstituted 1,2,3-triazole derivatives (58a–q).90 | ||
Joosten et al. reported microwave-assisted regioselective synthesis of multivalent 1,4-disubstituted 1,2,3-triazole-linked glycodendrimers (59a–s) through a copper-catalyzed [3 + 2] cycloaddition process, achieving excellent yields. By utilizing microwave irradiation, azido carbohydrates were effectively linked to dendritic acetylenes via a CuI-catalyzed reaction, as shown in Scheme 48. The method streamlined synthesis and enhanced the efficiency of the process, resulting in yields of up to 98% when conducted at 80 °C for 20 minutes. Additionally, two glycoconjugates incorporating fluorescent labels were developed, which are essential for the biological evaluation of these molecules.91
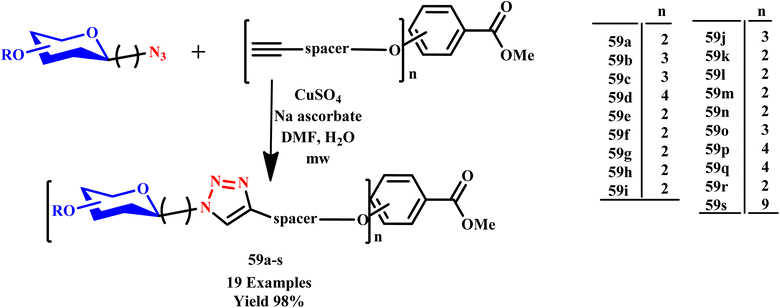 | ||
| Scheme 48 Synthetic route of copper-mediated triazole glycodendrimers (59a–s).91 | ||
Sravanthi et al. synthesized (E)-NO-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)-3-(4-phenyl1H-1,2,3-triazol-1-yl)benzohydrazide derivatives (60a–l) under microwave irradiation and conventional conditions (Scheme 49). The microwave irradiation approach offered simpler, straightforward and environmentally friendly synthesis with shorter reaction times and higher yields. Most heteroaryl benzohydrazide derivatives were synthesized using this approach, which offers diverse environmental flexibility. However, the traditional method reaction was completed within 1–2 h with an 80% yield, while the MW-assisted reaction took only 4–6 minutes with a 98% yield. In addition, compound 60e and 60g derivatives showed a significant broad spectrum of activities against both Gram-positive and Gram-negative bacterial strains.92
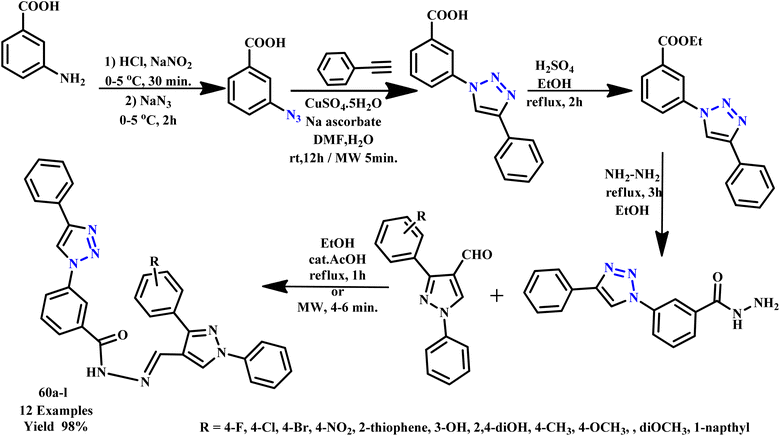 | ||
| Scheme 49 Synthetic route for 3-(4-phenyl-1H-1,2,3-triazol-1-yl)benzohydrazide derivatives (60a–l).92 | ||
J. Li et al. synthesized 5-{[(1-benzyl-1H-1,2,3-triazol-4-yl)methoxy]methyl}-3-(4-substituted-phenyl)isoxazole derivatives (61a–i) and substituted 2-[(4-{[(3-phenylisoxazol-5-yl)methoxy]-methyl}-1H-1,2,3-triazol-1-yl)methyl]-2-[(5-{[(3-phenylisoxazol5-yl)methoxy]methyl}-1H-1,2,3-triazol-1-yl)methyl]propane1,3-diol compounds via click reaction under MW irradiation using copper(I) ion as catalyst, which was prepared in situ from sodium ascorbate and copper acetate, as depicted in Scheme 50. Compared to the conventional method, the microwave irradiation method offers a shorter reaction time and a higher yield. The conventional method required 9–10 hours to complete with a 69% yield, while the MW-assisted reaction was completed within 10.0 minutes with a 93% yield.93
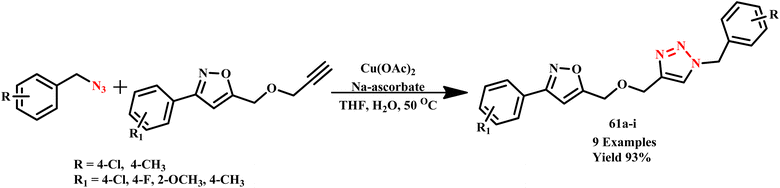 | ||
| Scheme 50 Microwave-assisted synthetic route for single 1,2,3-triazole derivatives (61a–i).93 | ||
Zabrodski developed a quick and effective way to incorporate 2-(1H-1,2,3-triazol-4-yl)pyridine and 2-(1H-1,2,3-triazol-1-ylmethyl)-pyridine into N-substituted glycine peptoidoligomers via an alkyne–azide click reaction on a solid support under microwave irradiation. The novel peptoid chelator was used to create intermolecular and intramolecular chiral helical peptoid–copper(II) complexes.94 Similarly, a novel, highly effective, solvent and metal free three-component green synthesis of 5-(1,3-thiazolan-2-yl)-1H-1,2,3-triazoles and element-substituted NH-1,2,3-triazoloimines derivatives (62a–d) was developed by Medvedeva et al. using 3-silicon(germanium)-substituted 2-propyn-1-als functionalized primary amine and trimethylsilyl azide through MW irradiation (Scheme 51). The reported MW-assisted process required 10.0 minutes at 100 °C to obtain the target product with 92% yield, and at room temperature with stirring, the same reaction required 10.0 days for completion. The advantages of this approach include the accessibility of a straightforward experimental process, great product yields and fast reaction time.95
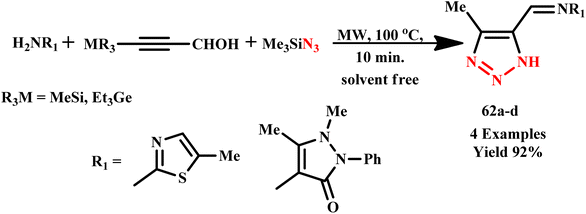 | ||
| Scheme 51 Microwave-assisted synthesis of NH-1,2,3-triazoloimines (62a–d).95 | ||
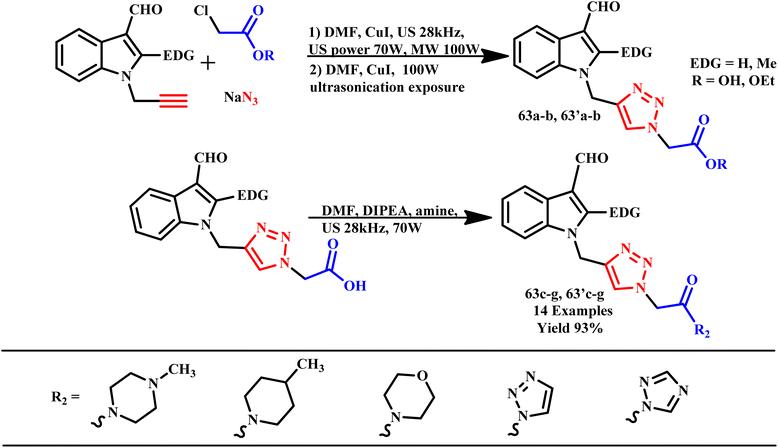
The 1,2,3-triazole and isoxazole derivatives-based flavonoids were developed by Ashok and his coworker using microwave irradiation and traditional heating techniques. The research group reported that using streptomycin as a reference medication, these compounds exhibit excellent action against Gram positive pathogens, such as Staphylococcus aureus, Bacillus subtilis, and Gram-negative organisms, such as Pseudomonas aeruginosa and Escherichia coli. The reported product showed the ability to scavenge free radicals examined using ascorbic acid as a reference medication. HeLa cell lines and MCF-7 were used for testing anticancer activities.96 Further, a novel class of tridentate ligands was prepared using microwave irradiation to synthesize diversely functionalized pyridyl-1,2,3-triazole derivatives without the need for metals or azides by Gulledge and coworkers. The typical work uses N-tosylhydrazones and anilines as feasible synthetic substitutes and avoids the use of thermally sensitive and poisonous organoazides.97 Mnasri and his coworkers synthesized Cu-doped rod-shaped mesoporous silica nanoparticles (Cu-SN) and used them as an effective heterogeneous catalyst for the synthesis of 1,4-dibustituted-1,2,3-triazole derivatives via a 1,3-dipolar cycloaddition reaction in aqueous solution through a microwave irradiation procedure at 45–80 °C and obtained up to 98% yield. The synthesis of 1,4-dibustituted-1,2,3-triazole derivatives in a single pot was simple and produced good yields because of the high copper dispersion at the pore surface that increased active site accessibility.98 Similarly, a regioselective three-component, reliable and sustainable synthetic process for 3-formylindole clubbed 1,4-disubstituted-1,2,3-triazole derivatives (63a–g) was developed by Mokariya and coworker using the MW-assisted method (Scheme 52). The reported protocol avoids time-consuming isolation and the use of organic azides and produces high yields of products with extraordinary purity and a low reaction time. The research group found that compared to standard drugs chloramphenicol and ampicillin, some of the derivatives exhibited excellent inhibitory activities against P. aeruginosa and greater effectiveness against the albicans fungus strain. A molecular docking study was also conducted to comprehend the binding interaction with a protein. Compound (63d) displayed outstanding inhibitory activities against P. aeruginosa compared to reference/standard drugs chloramphenicol and ampicillin. Compound 63b exhibited better potency against fungi strain C. albicans compared to the reference drugs fluconazole and griseofulvin. Further, it was observed that the reaction at room temperature takes 120.0 minutes at 100 °C for completion, while ultrasonicate-MW 100 W took 15.0 minutes and microwave irradiation took 15–30 minutes at 100 W to obtain the desired product.99
 | ||
| Scheme 52 Microwave-assisted synthesis of 3-formyl-indole clubbed 1,2,3-triazole derivatives (63a–g).99 | ||
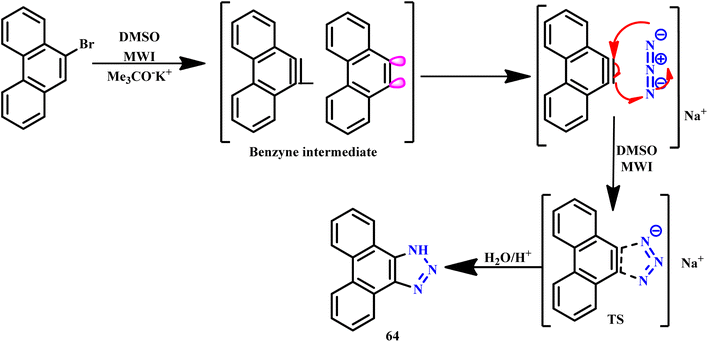
Similarly, an efficient microwave-assisted 1,3-dipolar cycloaddition reaction between 9-bromophenanthrene and sodium azide (NaN3) in the presence of potassium tert-butoxide facilitated the one-pot synthesis of 1H-phenanthro[9,10-d][1,2,3]triazole (64), as illustrated in Scheme 53. Notably, this protocol demonstrated significant efficiency, shorter reaction time (45 seconds), and high product yield (62%), while traditional methods required much longer reaction times. This rapid synthesis highlights the advantages of microwave irradiation in organic transformations. It was suggested that under these reaction conditions, 9-bromophenanthrene was converted to benzyne intermediate, which subsequently participated in the cycloaddition with azide, underscoring the innovative mechanistic insights gained from this approach.100
 | ||
| Scheme 53 Microwave-assisted synthetic route for 1H-phenanthro[9,10-d][1,2,3]triazole (64).100 | ||
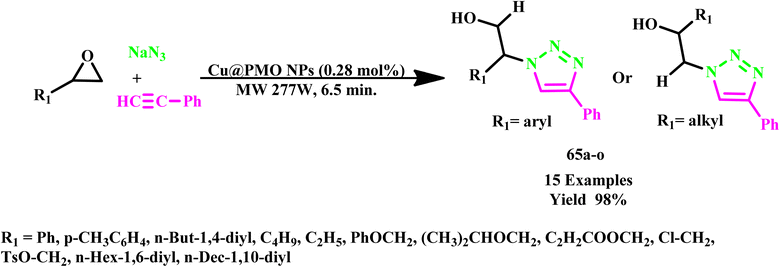
In a recent report, H. Naeimi et al. described the copper@PMO-catalyzed synthesis of β-hydroxy-1,2,3-triazolesderivaties (65a–o) via multicomponent reaction under microwave irradiation at 277 W for just 7.0 minutes, achieving an impressive yield of 98% (Scheme 54). This protocol utilized solid-supported catalyst and microwave energy and offered numerous advantages, such as cost-effectiveness, environmentally friendly reaction conditions, non-toxic reagents, high conversion rates and significantly reduced reaction times. Furthermore, the catalyst was easily recyclable and demonstrated no leaching, contributing to the high product yields. This approach enhances the efficiency of synthesis and aligns with the principles of green chemistry, making it a valuable advancement in organic synthesis.101
 | ||
| Scheme 54 Microwave-assisted synthetic route for beta-hydroxy-1,2,3-triazoles (65a–o).101 | ||
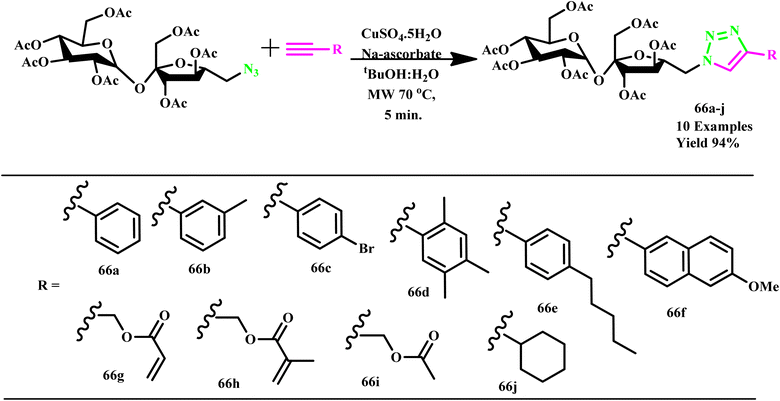
Similarly, Potewar et al. developed a microwave-assisted protocol for synthesizing novel 1-(1′,2,3,3′,4,4′,6-hepta-O-acetyl-6′-deoxy-sucros-6′-yl)-4-substituted-1,2,3-triazoles derivative (66a–j) through Cu-catalyzed 1,3-dipolar cycloaddition of sucrose-derived azides and terminal alkynes and achieved higher yields and reduced reaction times (Scheme 55). This innovative approach resulted in the creation of a diverse library of 1,2,3-triazole-sucrose conjugates (66a–j) by integrating structural motifs from carbohydrates and triazoles. The compound 1′,2,3,3′,4,4′,6-hepta-O-acetyl-6′-azido-6′-deoxy-sucrose was regioselectively synthesized from sucrose using an improved procedure and was employed in cycloaddition reactions. Remarkably, the microwave method yielded 94% of the target product within just 5.0 minutes at 400 W, while traditional methods required much longer reaction times. This advancement enhances the efficiency of synthesis and underscores the potential of microwave-assisted techniques in the development of complex glycosylated compounds.102
 | ||
| Scheme 55 Microwave-assisted synthetic route for 1,2,3-triazole-sucrose derivatives (66a–j).102 | ||
Sucharitha described an effective one-pot three-component Cu-catalyzed synthesis of benzo[1,3]thiazine-derived fused 1,2,3-triazole derivatives (67a–m) under MW conditions in ionic liquid (Scheme 56). It was found that the conventional method required a longer reaction time and high temperature and produced a poor yield (140 °C, 20 h and 45% yield) compared to the MW method (140 °C, 30 min. and 81% yield). The advantage of this method is a simple workup process, high yields and quick reaction times. It was observed that when compared to standard medicine, these reported compounds exhibited potent efficacy and showed considerable effect on biofilm inhibition. Some of the reported derivatives also exhibited a two-fold increase in inhibitory activity (MIC value of 3.12 μg mL−1) against S. epidermidis and B. subtilis compared to standard ciprofloxacin (6.25 μg mL−1) against tested bacteria and showed equipotent activities against S. aureus (MIC value of 6.25 μg mL−1) in comparison to the positive control. Promising results were obtained using conjugates of series against S. aureus and E. coli, with an MIC value of 6.25 μg mL−1, which is equivalent to ciprofloxacin. The findings indicate that the two derivatives are effective inhibitors of B. subtilis and S. aureus biofilm growth and strong antibacterial agents.103
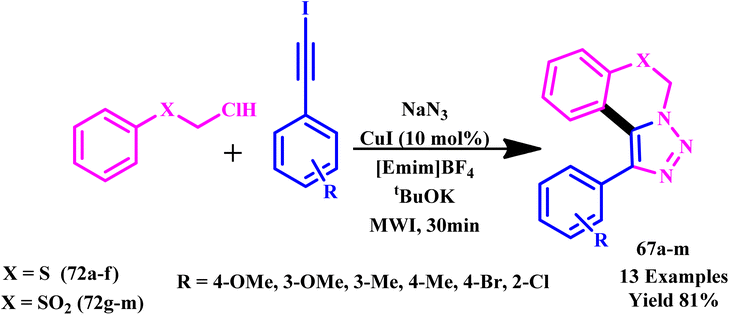 | ||
| Scheme 56 Microwave-assisted synthetic route for fused benzo[e]1,2,3-triazoles (67a–m).103 | ||
Reddy and coworkers reported unique hybrids of benzofuran-based triazole analogues (68a–f) using both traditional methods and microwave irradiation methodology (Scheme 57). They reported that the MW irradiation methodology was more environmentally friendly, quick, easy and high yielding. In the conventional method, the reaction was completed in 23–24 hours at room temperature and produced a lower amount of yield (65–67%), while under microwave irradiation conditions, it produced the desired product within 6–8 minutes at 180 W with 86–89% yield. The prepared compounds were tested for their in vitro antibacterial activity against the Gram positive bacterial strains of Bacillus subtilis, Staphylococcus aureus, and Gram negative bacterial strains of Klebsiella pneumoniae and Escherichia coli at two separate concentrations of 20 and 40 μg mL−1. In this case, compounds 68a, 68b, and 68f showed maximum zone inhibition against the tested bacterial strains.104
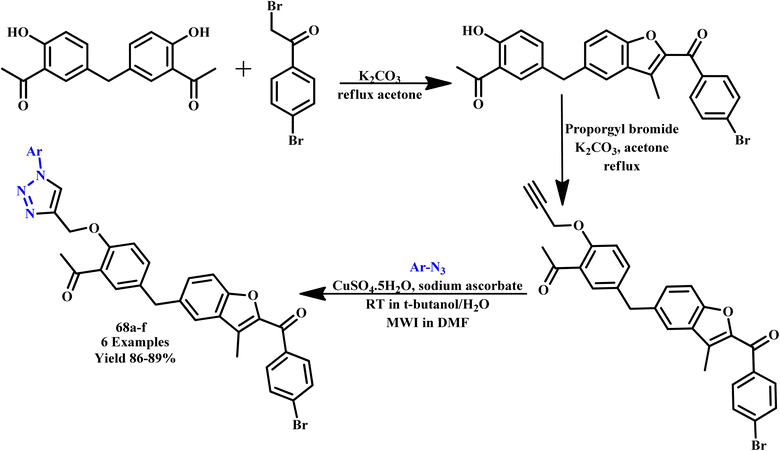 | ||
| Scheme 57 Microwave-assisted synthesis of benzofuran-based 1,2,3-triazoles (68a–f).104 | ||
Znati et al. designed and synthesized a new series of 1,4-disubstitued triazole-linked flavonol derivatives (69a–x) via a combination of the library of variably substituted aryl azides and three cytotoxic flavonols under microwave irradiation conditions with 78–93% yield within 5 minutes (Scheme 58). The newly synthesized compounds were tested for their cytotoxic activities against three different human cancer cell lines, including breast (MCF-7), ovarian (OVCAR-3), and colon (HCT-116). It was found that reported compounds 69c and 69p demonstrated excellent activity against OVCAR-3 and compounds 69f, 69g and 69q showed high activity against HCT-116 with IC50 values < 3.0 μM.105
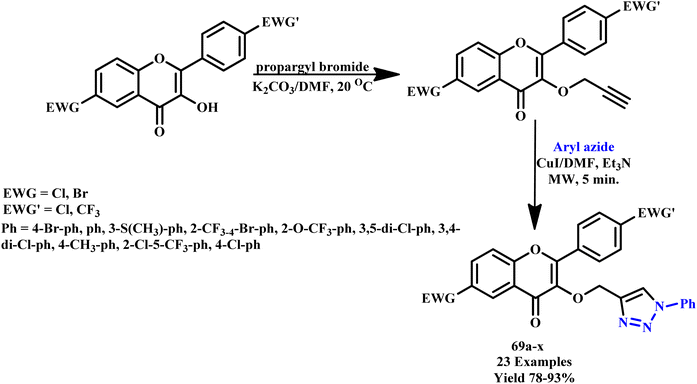 | ||
| Scheme 58 Microwave-assisted synthesis of 1,2,3-triazole derivatives (69a–x).105 | ||
In another report, Saikia et al. reported the microwave-assisted regioselective synthesis of 1,4-disubstituted 1,2,3-triazole derivatives (70a–m) in methanol using a Cu(II) catalytic and ionic liquid under MW irradiation (Scheme 59). The synthesis of 1,2,3-triazole moieties in a one-pot multicomponent pathway was followed by in situ reduction of an ionic liquid-assisted Cu(II) catalyst to Cu(I). Additionally, it was shown that using microwave irradiation in the reported synthetic sequence resulted in a remarkable reduction in reaction times with outstanding yields and high selectivity.106
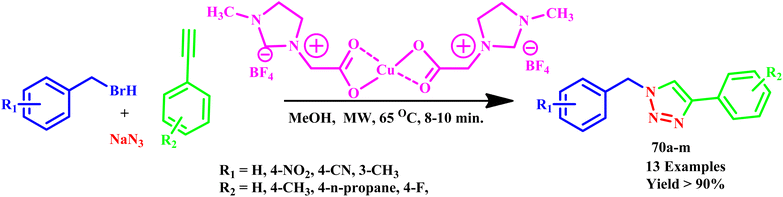 | ||
| Scheme 59 Microwave-assisted synthetic route of 1,4-disubstituted 1,2,3-triazoles (70a–m).106 | ||
A series of highly symmetrical phthalocyanines (71) with eight peptide residues were developed by Sokolova and coworkers using a Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction under microwave irradiation (Scheme 60). The phthalocyanine conjugates were non-toxic in nature over the whole concentration range, making them potential therapeutic photosensitizers.107
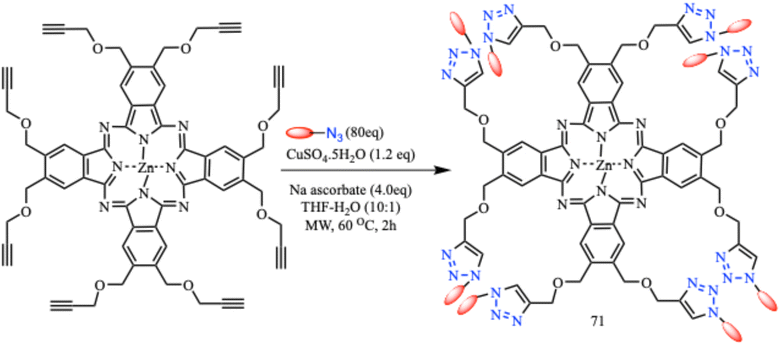 | ||
| Scheme 60 Microwave-assisted synthesis and deprotection of ZnPc-peptide conjugates (71).107 | ||
Similarly, Sood et al. reported a quick and high-yielding method for 1,2,3-triazole functionalized polysulfone derivatives (72a–e) using microwave-assisted synthesis (Scheme 61). The ultimate characteristics (mechanical, thermal, proton conduction) of the functionalized polymer are determined by the degree of 1,2,3-triazole functionalization of the polymer and the chemical structure of the ring substituent. However, the proton conductivities of phosphoric acid-doped polysulfone carrying 4-heptyl-1,2,3-triazole were found to be greater than those of carrying 4-phenyl-1,2,3-triazole because the former possessed higher chain mobility and lower Tg.108
 | ||
| Scheme 61 Microwave-assisted synthesis of 1,2,3-triazole functionalized polysulfones (72a–e).108 | ||
Further, in another report, a mild and flexible one-pot three-component process for [3 + 2]-cycloaddition for new imine 1,4-disubstituted 1,2,3-triazolesderivaties (73a–k) was developed by Souza and co-worker using propargylamine, α-thio aldehyde, and organoazides under the MW-assisted technique (Scheme 62). This protocol offers several advantages compared to conventional methods, such as less reaction time, easy product recovery and mild reaction conditions.109
 | ||
| Scheme 62 Microwave-assisted synthesis of 1,2,3-triazoles (73a–k).109 | ||
Similarly, Steenackers et al. developed a microwave-assisted one-pot approach for the generation of the 2-AIT framework (74a–z) (74aa–74ab) in which triazole moieties were attached to the 2N-position of the 2-AI ring through alkyl linkage (Scheme 63). The procedure combines a Dimroth-type rearrangement with an azide–alkyne cycloaddition reaction catalyzed by copper nanoparticles. Remarkably, the addition of a triazole moiety to their side chain of 2-aminoimidazoles with a lengthy 2N-alkyl chain (C8–C13) significantly increased their activity. The synthesized 2-amino-1H-imidazole/triazole conjugates exhibited high inhibitory action against the biofilms of E. coli, S. typhimurium, S. aureus, and P. aeruginosa, offering a lead structure for the further design and development of novel biofilm inhibitors.110
 | ||
| Scheme 63 Microwave-assisted synthesis of the 2-AIT framework (74a–z) (74aa–74ab).110 | ||
Using “click chemistry”, five mandipropamid analogues (75a–d) were synthesized and designed by Su and coworkers. A 1,2,3-triazole functional group was replaced with a phenyl ring in mandipropamid (Scheme 64). According to the bioassay results, some of the reported compounds demonstrated a considerable amount of fungicidal activity. For example, N-(3,4-dimethoxyphenethyl)-2-ethoxy-2-(1-(2,2,2-trifluoroethyl)-1H-1,2,3-triazol4-yl)acetamide and N-(3,4-dimethoxyphenethyl)-2-allyloxy-2-(1-(2,2,2-trifluoroethyl)-1H-1,2,3-triazol4-yl)acetamide showed moderate activity (31.0%) at 100 lg mL−1 and moderate activity (38.5%) at 50 lg mL−1 against Brassica and B. berengeriana.111
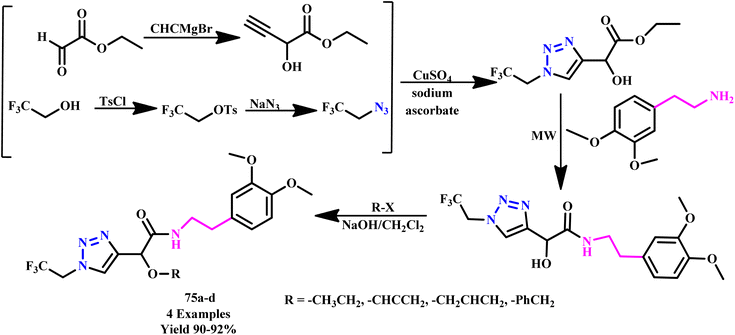 | ||
| Scheme 64 Microwave-assisted synthesis of mandipropamid analogues (75a–d).111 | ||
Similarly, under microwave-irradiation, N. Subhashini and his coworker synthesized novel pyrazole-based 1,2,3-triazole derivatives (76a–o) via a Huisgen click reaction from organic azides and 1-phenyl-3-[3-(prop-2-yn-1-yloxy)phenyl]-1H-pyrazole-4-carbaldehyde using the redox system CuSO4·5H2O–sodium ascorbate (Scheme 65). It was found that compared to the conventional heating method (required 12–16 h at rt), the reported methodology required less time (4–6 minutes) to complete the reaction at 180 W and provide a better yield (up to 80–90%). Additionally, antimicrobial activity was tested on all obtained compounds, and it was found that 3-(3-{[1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl]-methoxy}phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde and 3-(3-{[1-(2-chlorophenyl)-1H-1,2,3-triazol-4-yl]-methoxy}phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde exhibited stronger activities than reference medication against pathogenic bacteria.112
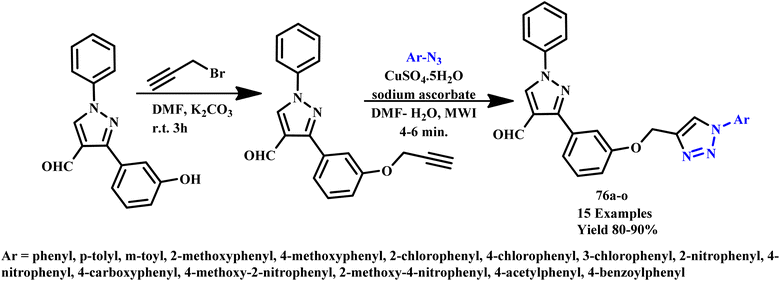 | ||
| Scheme 65 Microwave-assisted synthesis of pyrazole-based 1,2,3-trizoles (76a–o).112 | ||
BasappaVagish reported microwave irradiation-based synthesis of α,β-unsaturated carbonyl-linked coumarin–triazole hybrid compounds (77a–e) (Scheme 66). The intermolecular interactions are quantified by Hirshfeld surface analysis, and crystal structure analysis demonstrates that the C–O⋯π, C–H⋯π and π–π stabilize the crystal structure. Additionally, coumarin–triazole hybrid target compounds were tested in an in vitro study for their anticancer efficacy against the PC-3 and DU-145 cell lines. The outcome demonstrates that 3-(3-(1-(4-bromophenyl)-5-methyl-1H-1,2,3-triazol4-yl)-3-oxoprop-1-en-1-yl)-4-chloro-2H-chromen-2-one was found to be more effective against DU-145 and PC-3 cell lines with IC50 values of 9.845 ± 0.6 μM and 10.538 ± 0.3, respectively, with minimum toxicity at 10 μM. To obtain the desired product, conventional heating requires 3–4 h with 70% yield, while microwave-assisted protocol requires lesser reaction time (25–30 minutes) and 65% yield.113
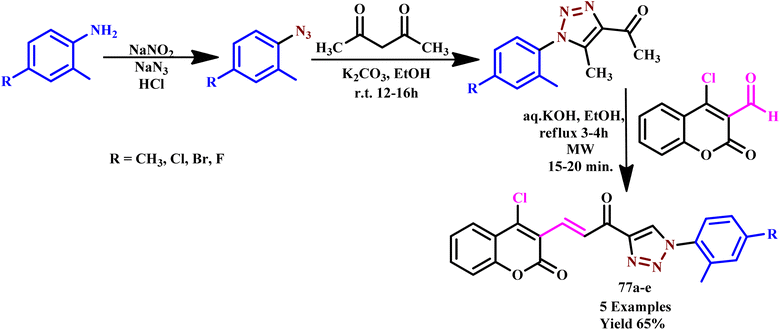 | ||
| Scheme 66 Microwave-assisted synthesis of coumarin-1,2,3-triazole conjugates (77a–e).113 | ||
Vani et al. used both conventional and MW irradiation techniques for the synthesis of dimers of 1,2,3-triazole-benzofuran bearing alkyl spacer derivatives (78a–j) via intermediate bis-1,2,3-triazole-carrying alkyl spacer compounds under quick reaction times and mild reaction conditions (Scheme 67). All the synthesized compounds were evaluated for their antioxidant and antimicrobial properties. Compound 78b exhibits strong antibacterial action against Gram-positive bacteria, while compound 78j demonstrated strong antioxidant activity using the DPPH technique. It was observed that in the microwave, the reaction required only 5–7 minutes at 180 W with a good yield compared to the conventional method (7–10 h, 49–59 yields %).114
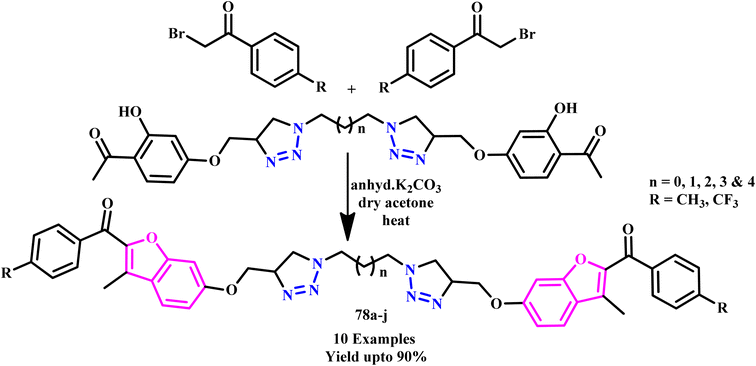 | ||
| Scheme 67 Microwave-assisted synthesis of (6,6′-(1,1′-(propane-1,3-diyl)bis(1H-1,2,3-trizole-4,1-diyl)bis(methylene)bis (oxy)(3-methylbenzofuran-6,2-diyl)bis(4-substituted phenylmethanone))) (78a–j).114 | ||
A highly active, easily recoverable synthesis of 1,2,3-triazole derivatives (79a–l) was reported by Xiong and Cai by employing a one-pot multi-component copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction in H2O. The terminal alkynes readily interacted with benzyl/alkyl azides produced in situ from corresponding sodium azide and primary halides under the MW irradiation conditions (MW, 480 W). In comparison to standard methods, microwave irradiation significantly reduces the reaction time while simultaneously improving yields up to 96% and the purity of 1,2,3-triazoles (Scheme 68).115
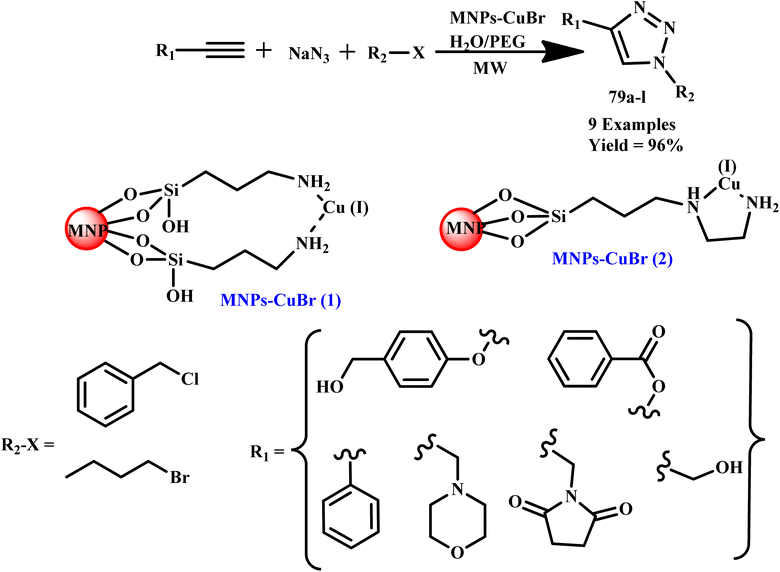 | ||
| Scheme 68 Microwave-assisted one-pot synthesis of 1,4 disubstituted 1,2,3-triazoles (79a–l) using the MNPs–CuBr catalyst.115 | ||
In situ generated aryl azides through the reaction of sodium azide and aryldiazonium silica sulfates, followed by coupling with a terminal alkyne in the presence of copper catalyst, were explored in the context of microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles (80a–r) by Zarei and his coworker (Scheme 69). It was reported that without the need for additional ligands, these reactions were carried out in water under mild, heterogeneous circumstances. It was found that all the compounds were synthesized within 15 minutes at 65 °C in a microwave-assisted process with 90% yield, while the same synthesis was carried out using the traditional method for 1–6 hours at room temperature to 70 °C with 76–86% yield.116
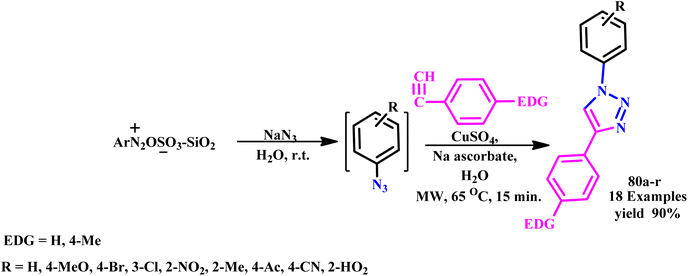 | ||
| Scheme 69 MW-assisted synthesis of 1,4-disubstituted 1,2,3-triazoles (80a–l).116 | ||
Ashok et al. developed a series of N-substituted 1,2,3-triazolylmethyl indole derivatives using MW irradiation to improve reaction efficiency. This synthesis involved the N-alkylation of tetrahydro-1H-carbazoles, followed by a copper-catalyzed Huisgen [3 + 2] cycloaddition with various aromatic azides using copper sulfate and sodium ascorbate as catalysts (Scheme 70). MW irradiation significantly enhanced yields (72–96%) and reduced reaction times compared to conventional heating (64–94%) showing the efficiency of the method in promoting cycloaddition reaction. Characterization of all new compounds was completed through 1HNMR and 13CNMR, mass, and IR spectroscopy and biological evaluation, revealing notable antimicrobial, antioxidant and anticancer properties. Molecular docking studies further confirmed that these compounds effectively fit into the active sites of caspase-3 and 17-beta-hydroxy steroid dehydrogenase type 1, suggesting their potential as bioactive agents.117
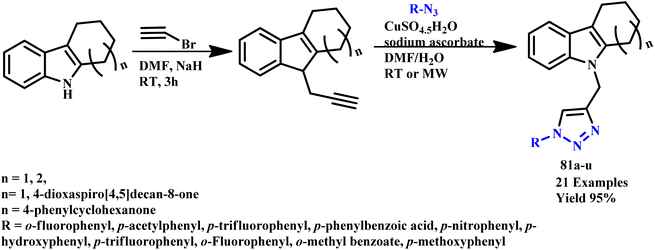 | ||
| Scheme 70 MW-assisted synthesis of substituted 1,2,3-triazolylmethyl indole derivatives (81a–u).117 | ||
Mitchell et al. developed an efficient synthesis of 1,4-disubstituted bis-1H-1,2,3-triazoles using an NHC–Cu catalyst under MW conditions, achieving yields between 87% and 93% (Scheme 71). This protocol offers the advantages of high yields and shorter reaction times, where conventional heating in water requires a longer reaction time and low yield, which shows the time-saving potential of MW-assisted synthesis.118
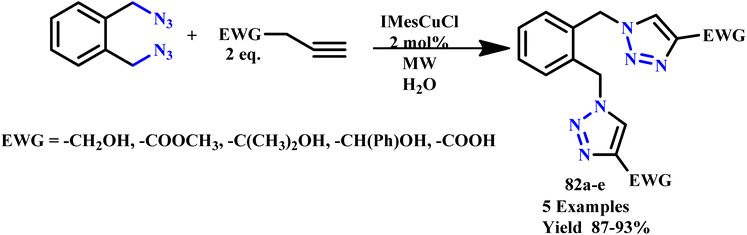 | ||
| Scheme 71 MW-assisted synthesis of 1,4-disubstituted bis-1H-1,2,3-triazoles (82a–e) using the NHC–Cu catalyst.118 | ||
In a recent study, NiO/Cu2O nanocomposites (NCs) were employed as heterogeneous nanocatalysts Ni–CuAAC to synthesize 1,4-disubstituted 1,2,3-triazoles with a high product yield under microwave irradiation (Scheme 72). The NiO/Cu2O NCs were prepared via coprecipitation of NiO nanoparticles onto a Cu2O surface generating a robust composite with enhanced catalytic capabilities. This catalyst facilitated reactions between benzoyl azides and phenylacetylene under optimized conditions in which NiO/Cu2O NCs demonstrated superior catalytic activity compared to Cu2O nanoparticles. Additionally, the use of an ethanol–water solvent system under microwave irradiation improved yields (89–95%) with simplified product separation.119 A comparative analysis of microwave-assisted 1,2,3-triazoles synthesis with conventional methods is tabulated in Table 2.
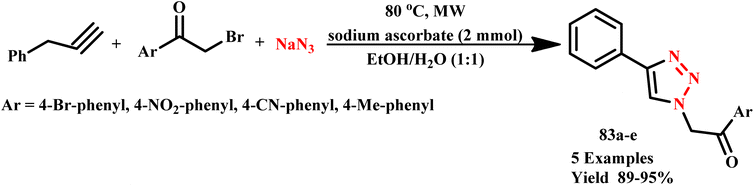 | ||
| Scheme 72 MW-assisted synthesis of 1,4-disubstituted 1H-1,2,3-triazoles (83a–e) using NiO/Cu2O nanocomposite catalyst.119 | ||
| Entry | Structure | Name | Conventional method | Microwave method | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Temp (°C) | Yield (%) | Time (min) | Temp. (°C) | Yield (%) | ||||
| 1 |  |
Imidazole linked 1,2,3-triazole hybrid compounds | 4–5 | 80 | 60–64 | 3–5 | 180 W | 90–95 | 65 |
| 2 |  |
Substituted 1,2,3-triazoles | 6–7 | 200 | 28 | 6–7 hours | 200 | 55–99 | 66 |
| 3 |  |
1,2,3-Triazole derivative | 3 | rt | 86 | 1 | 300 W | 93 | 67 |
| 4 | 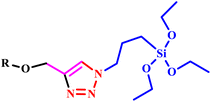 |
Silicon-based multidentate triazole scaffold | 16 | 25 | 71–92 | 21 | 60 | 86–98 | 68 |
| 5 |  |
1,2,3-Triazole-based pyrazole aldehydes and their benzimidazole derivatives | 5–8 | 100 | 55–65 | 7–9 | 300 W | 77–89 | 69 |
| 6 |  |
Fused 1,2,3-triazoles | 20–24 | 100–140 | 47 | 30–40 | 150 | 88 | 70 |
| 7 |  |
Acridone-based 1,2,3-triazole hybrid derivatives | 10 | rt | 60–75 | 10 | 40–100 | 65–88 | 71 |
| 8 | 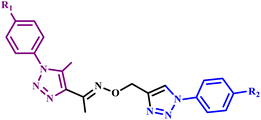 |
1-(5-Methyl-1-aryl-1H-1,2,3-triazol-4-yl)ethanone O-((1-aryl-1H-1,2,3-triazol-4-yl)methyl) oxime derivative | 8 | 80 | 56 | 12 | 130 W | 92 | 9 |
| 9 |  |
1-Monosubstituted 1,2,3-triazole derivative | 12 | 80 | 82 | 15 | 80 | 89 | 72 |
| 10 | 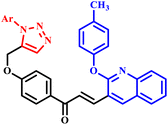 |
p-Tolyloxyquinoline-triazole hybrid derivatives | 8–10 | rt | 75 | 5–6 | 180 W | 93 | 4 |
| 11 | 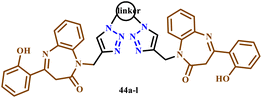 |
N-bis-1,2,3-triazolo-linked-1,5-benzodiazepin-2-one (BZD) derivative | 24 | rt or reflux | 30–45 | 6–7 | 400 W | 67–92 | 73 |
| 12 | 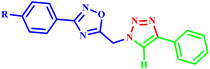 |
1,2,4-Oxadiazol-5-ylmethyl-1,2,3-triazole derivatives | 16 | 65 | 73 | 2.5 | 65 | 75–99 | 64 |
| 13 |  |
1,4-Disubstituted 1,2,3-triazole derivatives | 6–12 | 50–60 | 67 | 12 | 120 | 98 | 74 |
| 14 |  |
Acridone-derived 1,2,3-triazole derivatives | 4 | 80 | 48–62 | 10 | 200 W | 75–90 | 75 |
| 15 |  |
1,4-Disubstituted 1,2,3-triazole derivative | 5 | 50 | — | 6 | 70 | 98 | 76 |
| 16 | 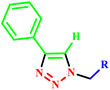 |
1,4-Disubstituted 1,2,3-triazole derivative | — | — | — | 10–15 | 125 | 93 | 77 |
| 17 | 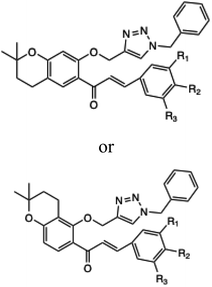 |
1-[7-(1-Benzyl-1H-[1,2,3]triazol-4-yl-methoxy)-2,2-dimethyl-chroman-6-yl]-3-aryl-2-propen-1-ones and 1-[5-(1-benzyl-1H-[1,2,3]triazol-4-ylmethoxy)-2,2-dimethylchroman-6-yl]-3-aryl-propen-1-ones derivative | 24–26 | rt | 30–66 | 8–15 | 180 W | 85–94 | 78 |
| 18 | 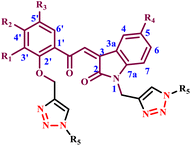 |
2-Indolinone-based bis-1,2,3-triazole derivatives | 8–11 h | 80 | 59–70 | 3–5 | 180 W | 85–93 | 79 |
| 19 |  |
Coumarin-1,2,3-triazole hybrids | 24 | 80 | 43–52 | 10 | 180 W | 79–86 | 80 |
| 20 | 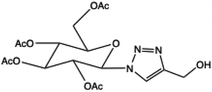 |
1,2,3-Triazole derivative | — | — | — | 120 | 100 | 92 | 81 |
| 21 |  |
a-[4-(1-substituted)-1,2,3-triazol-4-yl]-benzylacetamide derivative | 2 | rt | — | 10 | 125 | 92 | 82 |
| 22 |  |
1,2,3-Triazole derivative | — | — | — | 15 | 90 | 98 | 83 |
| 23 | 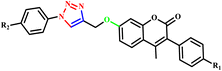 |
Coumarin-based 1,4-disubstituted 1,2,3-triazole derivatives | 4–6 | — | 68–79 | 5–8 | 80–90 | 84 | |
| 24 |  |
1,4-Dihydrochromene triazoles | 2.5 | 80 | 86 | 20–55 | 80 | 83 | 85 |
| 25 |  |
1,2,3-Triazole 10-homo-30–isoazanucleoside derivative | 22 | 70 | 70 | 30 | 70 | 92 | 87 |
| 26 |  |
4-Aryl-NH-1,2,3-triazole derivative | 2–3 | 130 | 91 | 5 | 130 | 94 | 88 |
| 27 |  |
Triazole-linked amino acid–glucoside conjugates | 14 | 55 | 97 | 89 | |||
| 28 |  |
Arylating 1,4-disubstituted 1,2,3-triazoles | 24 | Reflux | 58 | 15 | 250 | 99 | 90 |
| 29 |  |
1,4-Disubstituted 1,2,3-triazole-linked glycodendrimers derivative | 20 | 80 | 99 | 91 | |||
| 30 |  |
(E)-N′ -((1,3-diphenyl-1H-pyra zol-4-yl)methylene)-3-(4-phenyl-1H-1,2,3-triazol-1-yl)benzohydrazide derivative | 1–2 | 80–100 | 65–80 | 4–6 | 300 W | 88–98 | 92 |
| 31 |  |
1,2,3-Triazoles bearing isoxazole ether derivative | 6–10 | 50 | 45–84 | 10 | 50 | 80–95 | 93 |
| 32 | — | N,N-metal-binding ligands into peptoid oligomer | 15–72 | rt | 35 | 60 | 94 | ||
| 33 | 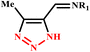 |
NH-1,2,3-triazoloimines | 10 days | rt | 85 | 10 | 100 | 92 | 95 |
| 34 | 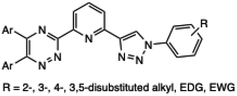 |
Pyridyl-1,2,3-trizole derivative | — | — | — | 12 | 160 | 97 | |
| 35 | 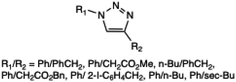 |
1,4-Dibustituted-1,2,3-triazole derivatives | — | — | — | 60–120 | 45–80 | 55–98 | 98 |
| 36 | 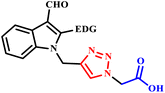 |
3-Formyl-indole clubbed 1,2,3-triazole derivatives | — | — | — | 15 | 70 | 92–93 | 99 |
| 37 | 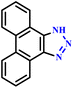 |
1H-phenanthro[9,10-d][1,2,3]triazole | — | — | — | 45 seconds | 700 W | 62 | 100 |
| 38 | 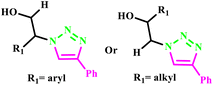 |
β-Hydroxy-1,2,3-triazole derivative | — | — | — | 6 | 277 W | 97.54 | 101 |
| 39 |  |
1,2,3-Triazole–sucrose derivatives | 3 | 70 | 91 | 5 | 70 | 82–94 | 102 |
| 40 |  |
Fused 1,2,3-triazole | 20 | 140 | 45 | 30 | 150 | 81 | 103 |
| 41 | 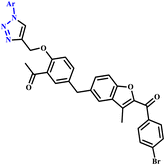 |
Benzofuran-based 1,2,3-triazole derivatives | 23–24 | rt | 62–71 | 6–8 | 180 W | 82–89 | 104 |
| 42 |  |
1,2,3-Triazole linked flavonol hybrids | 12–24 | 110 | 55–65 | 3–5 | 250 W | 78–93 | 105 |
| 43 |  |
1,4-Disubstituted 1,2,3-triazoles | 4 | Reflux | 80 | 8–10 | 65 | 95 | 106 |
| 44 |  |
Triazole-linked phthalocyanine–peptide derivative | 20 | rt | 120 | 60 | 86 | 107 | |
| 45 |  |
1,2,3-Triazole-functionalized polysulfone | — | — | — | 10 | 120 | >80 | 108 |
| 46 |  |
Imine-1,4-disubstituted 1,2,3-triazoles derivative | 10 | 120–180 | 80 | 57–83 | 109 | ||
| 47 |  |
2-Amino-1H-imidazole/triazole conjugates | 24 | rt | 24 | 2 | 100 | 90 | 110 |
| 48 |  |
1,2,3-Triazole derivative | 10 | 130–140 | 25 | 30 | 125 | 92 | 111 |
| 49 | 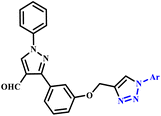 |
Pyrazole-based 1,2,3-triazole derivatives | 12–18 | rt | 65–73 | 4–6 | 180 W | 80–90 | 112 |
| 50 | 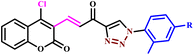 |
Coumarin–triazole hybrid derivative | 3–4 | rt | 62–70 | 15–30 | 160–320 W | 58–65 | 113 |
| 51 |  |
1,2,3-Triazole-benzofuran bearing alkyl spacer derivatives | 7–10 | Refluxed | 49–59 | 5–7 | 180 W | 87–96 | 114 |
| 52 |  |
1,4-Disubstituted 1,2,3-triazole derivative | — | — | — | 15–28 | 480 W | 89–96 | 115 |
| 53 |  |
1,4-Disubstituted 1,2,3-triazoles | 6 | rt | 78 | 15 | 65 | 90 | 116 |
| 54 |  |
N-substituted 1,2,3-triazolylmethyl indole | 8–10 | rt | 64–94 | 6 | 100 W | 72–96 | 117 |
| 55 |  |
1,4-Disubstituted bis-1H-1,2,3-triazoles | 12–24 | rt | 56–98 | 15 | 80 | 59–98 | 118 |
| 56 |  |
1,4-Disubstituted 1,2,3-triazoles | 24 | 80 | 62 | 10 | 80 | 94 | 119 |
Conclusion
In conclusion, microwave synthesis has emerged as a transformative and environmentally friendly laboratory method for synthesizing various triazole derivatives. This technique boasts rapid reaction rates and remarkably short reaction times, typically ranging from few seconds to minutes. Such efficiency enhances yields and promotes superior atom economy in chemical processes. By minimizing hazards and demonstrating compatibility with a wide array of reactants, microwave synthesis ensures a safer pathway for synthesizing triazole derivatives. Its energy-efficient nature and scalability highlight its significant environmental advantages. Beyond these benefits, microwave synthesis breaks new ground in triazole synthetic chemistry by facilitating reactions that are traditionally difficult or unachievable with conventional heating methods. Thus, it represents a pivotal advancement in sustainable triazole synthesis, promising broader applications and innovations in the field.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
N. S and R. S are grateful thanks to Manipal UniverSity Jaipur for providing laboratory facilities. SS appreciated the efforts driven by NS and GS for this work. All the authors are highly thankful to Chandigarh University, Punjab for the e-resources.References
- J. K. Sahu, S. Ganguly and A. Kaushik, Chin. J. Nat. Med., 2013, 11, 456–465 CAS.
- J.-R. Duan, H.-B. Liu, P. Jeyakkumar, L. Gopala, S. Li, R.-X. Geng and C.-H. Zhou, MedChemComm, 2017, 8, 907–916 RSC.
- Y. H. Lau, P. J. Rutledge, M. Watkinson and M. H. Todd, Chem. Soc. Rev., 2011, 40, 2848–2866 RSC.
- M. Vishnuvardhan, M. Pradeep and T. Gangadhar, Chem. Data Collect., 2021, 31, 100612 CrossRef CAS.
- V. K. Tripathi, J. Singh, T. Ara, S. Koul, S. Farooq and A. Kaul, Bioorg. Med. Chem. Lett., 2014, 24, 4243–4246 CrossRef PubMed.
- A. Majumder, R. Gupta and A. Jain, Green Chem. Lett. Rev., 2013, 6, 151–182 CrossRef CAS.
- M. Zaheer, M. Zia-ur-Rehman, R. Munir, N. Jamil, S. Ishtiaq, R. S. Zaib Saleem and M. R. Elsegood, ACS Omega, 2021, 6, 31348–31357 CrossRef CAS PubMed.
- R. M. Kumbhare, U. B. Kosurkar, M. J. Ramaiah, T. L. Dadmal, S. Pushpavalli and M. Pal-Bhadra, Bioorg. Med. Chem. Lett., 2012, 22, 5424–5427 CrossRef CAS PubMed.
- D. Ashok, M. R. Reddy, R. Dharavath, K. Ramakrishna, N. Nagaraju and M. Sarasija, J. Chem. Sci., 2020, 132, 1–9 CrossRef.
- K. M. Al-Zaydi, Ultrason. Sonochem., 2009, 16, 805–809 CrossRef CAS PubMed.
- A. El-Saghier, H. Abd El-Halim, L. H. Abdel-Rahman and A. Kadry, Appl. Organomet. Chem., 2019, 33, e4641 CrossRef.
- A. H. Abdelmonsef, A. M. El-Saghier and A. M. Kadry, Green Chem. Lett. Rev., 2023, 16, 2150394 CrossRef.
- M. Boominathan, N. Pugazhenthiran, M. Nagaraj, S. Muthusubramanian, S. Murugesan and N. Bhuvanesh, ACS Sustain. Chem. Eng., 2013, 1, 1405–1411 CrossRef CAS.
- M. Nasrollahzadeh, S. M. Sajadi and Y. Mirzaei, J. Colloid Interface Sci., 2016, 468, 156–162 CrossRef CAS PubMed.
- S. Deswal, R. K. Tittal, P. Yadav, K. Lal, G. Vikas D and N. Kumar, ChemistrySelect, 2019, 4, 759–764 CrossRef CAS.
- S. Bijani, D. Iqbal, S. Mirza, V. Jain, S. Jahan, M. Alsaweed, Y. Madkhali, S. A. Alsagaby, S. Banawas and A. Algarni, Life, 2022, 12, 519 CrossRef CAS PubMed.
- J. A. Mokariya, D. P. Rajani and M. P. Patel, Arch. Pharm., 2023, 356, 2200545 CrossRef CAS PubMed.
- A. J. Bañon Gomis, M. Guillén Parra, W. M. Hoffman and R. E. McNulty, Bus. Soc. Rev., 2011, 116, 171–191 CrossRef.
- J. A. Dixon and L. A. Fallon, Soc. Nat. Resour., 1989, 2, 73–84 CrossRef.
- A. Loupy, C. R. Chim., 2004, 7, 103–112 CrossRef CAS.
- D. Patel and B. Patel, J. Pharm. Res., 2011, 4, 2090–2092 CAS.
- V. Ahluwalia, Laboratory Techniques in Organic Chemistry, IK International Pvt Ltd, 2013 Search PubMed.
- C. O. Kappe, A. Stadler and D. Dallinger, Microwaves in Organic and Medicinal Chemistry, John Wiley & Sons, 2012 Search PubMed.
- A. De la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- S. Sadler, A. R. Moeller and G. B. Jones, Expert Opin. Drug Discovery, 2012, 7, 1107–1128 CrossRef CAS PubMed.
- F. Gao, T. Wang, J. Xiao and G. Huang, Eur. J. Med. Chem., 2019, 173, 274–281 CrossRef CAS PubMed.
- Z. Karczmarzyk, M. Swatko-Ossor, W. Wysocki, M. Drozd, G. Ginalska, A. Pachuta-Stec and M. Pitucha, Molecules, 2020, 25, 6033 CrossRef CAS PubMed.
- J. Xu, Y. Cao, J. Zhang, S. Yu, Y. Zou, X. Chai, Q. Wu, D. Zhang, Y. Jiang and Q. Sun, Eur. J. Med. Chem., 2011, 46, 3142–3148 CrossRef CAS PubMed.
- R. Paprocka, M. Wiese, A. Eljaszewicz, A. Helmin-Basa, A. Gzella, B. Modzelewska-Banachiewicz and J. Michalkiewicz, Bioorg. Med. Chem. Lett., 2015, 25, 2664–2667 CrossRef CAS PubMed.
- S. El-Sebaey, ChemistrySelect, 2020, 5, 11654–11680 CrossRef CAS.
- S. Ceylan, H. Bayrak, S. Basoglu Ozdemir, Y. Uygun, A. Mermer, N. Demirbas and S. Ulker, Heterocycl. Commun., 2016, 22, 229–237 CrossRef CAS.
- M. Shiradkar, U. Pandit, K. C. Akula, A. Maheta and G. V. S. Kumar, Arkivoc, 2006, 14, 141–154 Search PubMed.
- N. A. Virk, A. u. Rehman, M. A. Abbasi, S. Z. Siddiqui, J. Iqbal, S. Rasool, S. U. Khan, T. T. Htar, H. Khalid and S. J. Laulloo, J. Heterocycl. Chem., 2020, 57, 1387–1402 CrossRef CAS.
- M. Shaikh, D. Wagare, M. Farooqui and A. Durrani, Polycyclic Aromat. Compd., 2020, 40, 1315–1320 CrossRef CAS.
- R. Shcherbyna, J. Fac. Pharm. Ankara Univ., 2019, 43, 220–229 Search PubMed.
- N. A. Virk, M. A. Abbasi, S. Z. Siddiqui, U. Rashid, J. Iqbal, M. Saleem, M. Ashraf, W. Shahid and S. A. A. Shah, Pak. J. Pharm. Sci., 2018, 31(4), 1501–1510 CAS.
- S. B. Ozdemir, N. Demirbas, A. Demirbas, F. A. Ayaz and N. Çolak, J. Heterocycl. Chem., 2018, 55, 2744–2759 CrossRef.
- Y. A. Al-Soud, N. A. Al-Masoudi, T. Schuppler, E. D. Clercq and C. Pannecouque, Nucleosides, Nucleotides Nucleic Acids, 2008, 27, 469–483 CrossRef CAS PubMed.
- Y. Uygun Cebeci, S. Ceylan, S. A. Karaoglu and M. Altun, J. Heterocycl. Chem., 2023, 60, 47–62 CrossRef CAS.
- L.-J. Min, Y.-X. Shi, H.-K. Wu, Z.-H. Sun, X.-H. Liu, B.-J. Li and Y.-G. Zhang, Appl. Sci., 2015, 5, 1211–1220 CrossRef CAS.
- P. Banerjee, O. P. Pandey and S. K. Sengupta, Transit. Met. Chem., 2008, 33, 1047–1052 CrossRef CAS.
- F. A. Brede, J. Heine, G. Sextl and K. Müller-Buschbaum, Chem.–Eur. J., 2016, 22, 2708–2718 CrossRef CAS PubMed.
- A. Dandia, R. Singh, H. Sachdeva and K. Arya, J. Fluorine Chem., 2001, 111, 61–67 CrossRef CAS.
- S. Dengale, B. Karale, H. Akolkar, N. Darekar and K. Deshmukh, Russ. J. Gen. Chem., 2019, 89, 1535–1540 CrossRef CAS.
- E. El Ashry, L. Awad, H. Abdel Hamid and A. Atta, Nucleosides, Nucleotides Nucleic Acids, 2006, 25, 325–335 CrossRef CAS PubMed.
- K. Jaisankar, K. Kumaran, S. Raja Mohamed Kamil and T. Srinivasan, Res. Chem. Intermed., 2015, 41, 1975–1984 CrossRef CAS.
- A. Jha, Y. Murthy, G. Durga and T. Sundari, E-J. Chem., 2010, 7, 1571–1577 CAS.
- B. Kahveci, M. Özil, E. Menteşe, O. Bekircan and K. Buruk, Russ. J. Org. Chem., 2008, 44, 1816–1820 CrossRef CAS.
- G. K. Kantar, N. Baltaş, E. Menteşe and S. Şaşmaz, J. Organomet. Chem., 2015, 787, 8–13 CrossRef CAS.
- M. Kidwai, P. Sapra, P. Misra, R. Saxena and M. Singh, Bioorg. Med. Chem., 2001, 9, 217–220 CrossRef CAS PubMed.
- Y. Kumar, A. Matta, P. Kumar, V. S. Parmar, E. V. Van der Eycken and B. K. Singh, RSC Adv., 2015, 5, 1628–1639 RSC.
- G. M. Shelke, V. K. Rao, M. Jha, T. S. Cameron and A. Kumar, Synlett, 2015, 26, 404–407 CrossRef CAS.
- J. Lee, M. Hong, Y. Jung, E. J. Cho and H. Rhee, Tetrahedron, 2012, 68, 2045–2051 CrossRef CAS.
- F. P. L. Lim, L. M. Hu, E. R. Tiekink and A. V. Dolzhenko, Tetrahedron Lett., 2018, 59, 3792–3796 CrossRef CAS.
- X.-H. Liu, J.-Q. Weng, B.-L. Wang, Y.-H. Li, C.-X. Tan and Z.-M. Li, Res. Chem. Intermed., 2014, 40, 2605–2612 CrossRef CAS.
- J. Meng and P.-P. Kung, Tetrahedron Lett., 2009, 50, 1667–1670 CrossRef CAS.
- M. Xu, Y.-B. Zhu, M. Wang, I. A. Khan, X.-H. Liu and J.-Q. Weng, Lett. Drug Des. Discovery, 2018, 15, 347–352 CrossRef CAS.
- N.-B. Sun, J.-Q. Fu, J.-Q. Weng, J.-Z. Jin, C.-X. Tan and X.-H. Liu, Molecules, 2013, 18, 12725–12739 CrossRef CAS PubMed.
- A. Nas, S. Fandaklı, H. Kantekin, A. Demirbaş and M. Durmuş, Dyes Pigm., 2012, 95, 8–17 CrossRef CAS.
- A. R. Nesaragi, R. R. Kamble, P. K. Bayannavar, S. K. J. Shaikh, S. R. Hoolageri, B. Kodasi, S. D. Joshi and V. M. Kumbar, Bioorg. Med. Chem. Lett., 2021, 41, 127984 CrossRef CAS PubMed.
- M. Rahimizadeh, A. Davoodnia, M. Heravi and M. Bakavoli, Phosphorus, Sulfur, Silicon Relat. Elem., 2002, 177, 2923–2929 CrossRef CAS.
- M. R. Aouad, M. A. Almehmadi, F. F. Albelwi, M. Teleb, G. N. Tageldin, M. M. Abu-Serie, M. Hagar and N. Rezki, Bioorg. Chem., 2022, 105816 CrossRef PubMed.
- Q. Wei, C. Qiao, Z. Xia and S. Chen, Synth. Commun., 2013, 43, 3181–3191 CrossRef CAS.
- Y. Dürüst and H. Karakuş, Synth. Commun., 2017, 47, 907–912 CrossRef.
- N. Subhashini, E. P. Kumar, N. Gurrapu and V. Yerragunta, J. Mol. Struct., 2019, 1180, 618–628 CrossRef CAS.
- S. Roshandel, S. C. Suri, J. C. Marcischak, G. Rasul and G. S. Prakash, Green Chem., 2018, 20, 3700–3704 RSC.
- J. Krim, B. Sillahi, M. Taourirte, E. Rakib and J. Engels, Arkivoc, 2009, 8, 142–152 Search PubMed.
- G. Singh, P. Satija, A. Singh, D. Aulakh, M. Wriedt, C. E. Ruiz, M. A. Esteban, S. Sinha and R. Sehgal, Appl. Organomet. Chem., 2019, 33, e4695 CrossRef.
- D. Ashok, M. Ram Reddy, N. Nagaraju, R. Dharavath, K. Ramakrishna, S. Gundu, P. Shravani and M. Sarasija, Med. Chem. Res., 2020, 29, 699–706 CrossRef CAS.
- S. Narsimha, K. S. Battula, Y. N. Reddy and V. R. Nagavelli, Chem. Heterocycl. Compd., 2018, 54, 1161–1167 CrossRef CAS.
- M. Aarjane, S. Slassi, B. Tazi, M. Maouloua and A. Amine, J. Chem. Sci., 2019, 131, 1–11 CrossRef CAS.
- Z. Du, F. Li, L. Li and R. Li, J. Chem. Sci., 2020, 132, 1–7 CrossRef.
- Z. Jaafar, S. Chniti, A. B. Sassi, H. Dziri, S. Marque, M. Lecouvey, R. Gharbi and M. Msaddek, J. Mol. Struct., 2019, 1195, 689–701 CrossRef CAS.
- J. I. Sarmiento-Sánchez, A. Ochoa-Terán and I. A. Rivero, Arkivoc, 2011, 9, 177–188 Search PubMed.
- M. Aarjane, S. Slassi, B. Tazi and A. Amine, J. Chem., 2021, 2021, 1–10 Search PubMed.
- S. G. Agalave, S. G. Pharande, S. M. Gade and V. S. Pore, Asian J. Org. Chem., 2015, 4, 943–951 CrossRef CAS.
- P. Appukkuttan, W. Dehaen, V. V. Fokin and E. Van der Eycken, Org. Lett., 2004, 6, 4223–4225 CrossRef CAS PubMed.
- D. Ashok, D. Mohan Gandhi, G. Srinivas and A. Vikas Kumar, Med. Chem. Res., 2014, 23, 3005–3018 CrossRef CAS.
- D. Ashok, S. Gundu, V. K. Aamate and M. G. Devulapally, Mol. Diversity, 2018, 22, 57–70 CrossRef CAS PubMed.
- D. Ashok, S. Gundu, V. K. Aamate, M. G. Devulapally, R. Bathini and V. Manga, J. Mol. Struct., 2018, 1157, 312–321 CrossRef CAS.
- A. I. Mohammed, A. H. Dawood, K. F. Ali and M. Al-mosawi, Asian J. Chem., 2012, 24, 5592 CAS.
- D. Castagnolo, F. Dessì, M. Radi and M. Botta, Tetrahedron: Asymmetry, 2007, 18, 1345–1350 CrossRef CAS.
- I. L. Librando, A. G. Mahmoud, S. A. Carabineiro, M. F. C. Guedes da Silva, C. F. Geraldes and A. J. Pombeiro, Catalysts, 2021, 11, 185 CrossRef CAS.
- R. Dharavath, N. Nagaraju, M. R. Reddy, D. Ashok, M. Sarasija, M. Vijjulatha, T. Vani, K. Jyothi and G. Prashanthi, RSC Adv., 2020, 10, 11615–11623 RSC.
- T. M. Alves, G. A. Jardim and M. A. Ferreira, RSC Adv., 2021, 11, 10336–10339 RSC.
- B. Dutta, A. Garg, P. Phukan, A. Kulshrestha, A. Kumar and D. Sarma, New J. Chem., 2021, 45, 12792–12797 RSC.
- J. F. da Costa, X. García-Mera, O. Caamano, J. M. Brea and M. I. Loza, Eur. J. Med. Chem., 2015, 98, 212–220 CrossRef PubMed.
- A. Garg, D. Sarma and A. A. Ali, Curr. Res. Green Sustainable Chem., 2020, 3, 100013 CrossRef.
- X.-P. He, C. Li, X.-P. Jin, Z. Song, H.-L. Zhang, C.-J. Zhu, Q. Shen, W. Zhang, L. Sheng and X.-X. Shi, New J. Chem., 2011, 35, 622–631 RSC.
- M. Iwasaki, H. Yorimitsu and K. Oshima, Chem.–Asian J., 2007, 2, 1430–1435 CrossRef CAS PubMed.
- J. A. F. Joosten, N. T. H. Tholen, F. Ait El Maate, A. J. Brouwer, G. W. Van Esse, D. T. S. Rijkers, R. M. J. Liskamp and R. J. Pieters, Eur. J. Org. Chem., 2005, 15, 3182 CrossRef.
- K. Sravanthi, P. Snehalatha and N. Subhashini, J. Heterocycl. Chem., 2018, 55, 508–516 CrossRef CAS.
- J. Li, H. Liu, F. Meng, L. Yan, Y. Shi, Y. Zhang and Q. Gu, Chem. Res. Chin. Univ., 2018, 34, 197–202 CrossRef CAS.
- T. Zabrodski, M. Baskin, P. J. Kaniraj and G. Maayan, Synlett, 2015, 26, 461–466 Search PubMed.
- A. S. Medvedeva, M. M. Demina, T. V. Kon'kova, T. L. H. Nguyen, A. V. Afonin and I. A. Ushakov, Tetrahedron, 2017, 73, 3979–3985 CrossRef CAS.
- D. Ashok, G. Thara, R. Dharavath, B. Kirankumar, M. Sarasija and B. Bhima, Russ. J. Gen. Chem., 2022, 92, 718–724 CrossRef CAS.
- Z. Z. Gulledge, D. P. Duda, D. A. Dixon and J. D. Carrick, J. Org. Chem., 2022, 87, 12632–12643 CrossRef CAS PubMed.
- N. Mnasri, J. L. Nyalosaso, E. Colacino, G. Derrien, F. Lamaty, J. Martinez, J. Zajac and C. Charnay, ACS Sustain. Chem. Eng., 2015, 3, 2516–2525 Search PubMed.
- J. A. Mokariya, A. G. Kalola, P. Prasad and M. P. Patel, Mol. Diversity, 2021, 1–17 Search PubMed.
- A. A. Taherpour and M. Faraji, Molbank, 2008, 2008, M577 Search PubMed.
- H. Naeimi, V. Nejadshafiee and S. Masoum, Appl. Organomet. Chem., 2015, 29, 314–321 Search PubMed.
- T. M. Potewar, K. T. Petrova and M. T. Barros, Carbohydr. Res., 2013, 379, 60–67 CrossRef CAS PubMed.
- E. R. Sucharitha, T. M. Krishna, R. Manchal, G. Ramesh and S. Narsimha, Bioorg. Med. Chem. Lett., 2021, 47, 128201 Search PubMed.
- E. R. Reddy, S. Ramesh, K. Anitha, A. P. Reddy and V. P. Reddy, Chem. Data Collect., 2021, 34, 100730 CrossRef CAS.
- M. Znati, M. Horchani, L. Latapie, H. B. Jannet and J. Bouajila, J. Mol. Struct., 2021, 1246, 131216 CrossRef CAS.
- A. A. Saikia, R. N. Rao, S. Das, S. Jena, S. Rej, B. Maiti and K. Chanda, Tetrahedron Lett., 2020, 61, 152273 CrossRef CAS.
- N. V. Sokolova, T. Schotten, H. J. Berthold, J. Thiem and V. G. Nenajdenko, Synthesis, 2013, 45, 556–561 CrossRef CAS.
- R. Sood, A. Donnadio, S. Giancola, A. Kreisz, D. J. Jones and S. Cavaliere, ACS Appl. Mater. Interfaces, 2016, 8, 16897–16906 CrossRef CAS PubMed.
- F. B. Souza, D. C. Pimenta and H. A. Stefani, Tetrahedron Lett., 2016, 57, 1592–1596 CrossRef CAS.
- H. Steenackers, D. Ermolat'ev, T. T. T. Trang, B. Savalia, U. K. Sharma, A. De Weerdt, A. Shah, J. Vanderleyden and E. V. Van der Eycken, Org. Biomol. Chem., 2014, 12, 3671–3678 RSC.
- N.-N. Su, Y. Li, S.-J. Yu, X. Zhang, X.-H. Liu and W.-G. Zhao, Res. Chem. Intermed., 2013, 39, 759–766 CrossRef CAS.
- N. Subhashini, K. Sravanthi, C. Sravanthi and M. Reddy, Russ. J. Gen. Chem., 2016, 86, 2777–2784 CrossRef CAS.
- C. B. Vagish, K. Kumara, H. K. Vivek, S. Bharath, N. K. Lokanath and K. A. Kumar, J. Mol. Struct., 2021, 1230, 129899 CrossRef CAS.
- R. Sireesha, K.-K. Mak, P. M. Rao, K. R. S. Prasad and M. V. B. Rao, Chem. Data Collect., 2021, 31, 100605 CrossRef.
- X. Xiong and L. Cai, Catal. Sci. Technol., 2013, 3, 1301–1307 RSC.
- A. Zarei, L. Khazdooz, A. R. Hajipour, H. Aghaei and G. Azizi, Synthesis, 2012, 44, 3353–3360 CrossRef CAS.
- D. Ashok, G. Thara, B. K. Kumar, G. Srinivas, D. Ravinder, T. Vishnu, M. Sarasija and B. Sushmitha, RSC Adv., 2023, 13, 25–40 RSC.
- L. T. Mitchell, E. Barnett, M. Hexom, A. Ruiz and A. Schoffstall, Catalysts, 2024, 14, 702 CrossRef CAS.
- Y. A. Attia and Y. M. Mohamed, J. Chem. Technol. Biotechnol., 2024, 99, 2311–2319 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |