Open Access Article
Open Access ArticlePreparation of a lanthanum-modified flocculant and its removal performance towards phosphorus and fluoride in yellow phosphorus wastewater†
Boxuan Liabc,
Shaoxin Wenabc,
Jiacheng Liabc,
Dedong Heabc,
Yongming Luoabc,
Xiangqian Zheng*abcd and
Dingkai Chen *abc
*abc
aFaculty of Chemical Engineering, Kunming University of Science and Technology, Kunming 650500, P. R. China. E-mail: 371249656@qq.com; cdk684983@163.com
bThe Innovation Team for Volatile Organic Compounds Pollutants Control and Resource Utilization of Yunnan Province, Kunming 650500, P. R. China
cThe Higher Educational Key Laboratory for Odorous Volatile Organic Compounds Pollutants Control of Yunnan Province, Kunming 650500, P. R. China
dInstitute for Inspection and Certification of Xishuangbanna Dai Autonomous Prefecture, Jinghong 666100, P. R. China
First published on 2nd January 2025
Abstract
Polysilicate-ferric-calcium-lanthanum (PSFCL) was synthesized through a co-polymerization method in order to treat the yellow phosphorus wastewater. Its morphology, composition and functional group were analyzed by X-ray Diffraction (XRD), Fourier Transform-Infrared Spectroscopy (FTIR), Scanning Electron Microscopic (SEM) and X-ray Photoelectron Spectroscopy (XPS), respectively. The optimization of the flocculant was also investigated, including La/Si molar ratio, pH, agitation time, dosage and sedimentation time. Results showed that PSFCL has reached an excellent removal efficiency of 95% and 97% towards phosphorus and fluoride, respectively. It could be inferred that charge neutralization, bridging effect and ligand exchange were the main coagulation mechanisms. As a whole, after the introduction of lanthanum, PSFCL was found to be a promising flocculant in yellow phosphorus wastewater treatment owing to its high removal efficiency and simple synthesis route.
1. Introduction
In the past few decades, with the development of the chemical industry, a tremendous amount of phosphorus and fluoride have been produced and released into natural waterbodies via sewage discharge. The untreated wastewater can cause severe environmental issues. Excessive amounts of phosphorus can cause eutrophication problems which subsequently exacerbates the problem of algal bloom.1 Moreover, excessive intake of fluoride by humans could be harmful to the oral system, skeleton, digestive systems, and other health issues.2 Based on that, the aforementioned pollution problems have attracted much attention and become a worldwide environmental issue. Commonly used technologies towards phosphorus and fluoride removal include chemical precipitation,3 coagulation,4 adsorption,5 advanced oxidation,6 biological treatment,7 membrane filtration,8 etc. Among these treatment technologies, coagulation has been well adopted due to its cost-effective,9 easy operation10 and high removal efficiency.11 Zhang et al., applied a combined process of chemical precipitation and flocculation for TP removal. After adding calcium chloride, cPAM and adjusting pH value, the total removal efficiencies of TP and COD was 99%, 80%, respectively.12 Macherzyński et al., synthesized iron(II) sulfate(VI) and other pre-hydrolyzed coagulants for comparative studies of phosphorus removal, results showed that the iron coagulant (PIX-110) has reached a high phosphorus removal efficiency which is 99.5%.13 Ozairi et al., conducted a comparative study by using coagulation–flocculation process towards fluoride removal. The aluminum sulfate, poly-aluminum chloride and ferric chloride were used for quantitative elimination of fluoride from wastewater. Results showed that the maximum removal efficiency of fluoride (83%) was obtained at 30 mg L−1 of aluminum sulfate.14At present, the most widely used coagulants contain conventional aluminum or iron salt, poly-aluminum chloride (PAC),15 poly-ferric sulfate (PFS),16 and poly-ferric chloride (PFC).17 Nevertheless, the residual coagulants are harmful to the human nervous system, and ferric and aluminum ions can cause secondary pollution problems which may need further treatment processes and more capital investment.18 Based on the above situation, polyciliate composite flocculants were developed in order to solve these problems. Polysilicate is an anionic flocculant which does not show great health risk. A polysilicate composite flocculant combines the stability of polysilicate and the high-efficiency flocculation ability of metal salts. Compared with the low molecular weight and traditional high molecular weight inorganic flocculants, polysilicate composite metal salt flocculants show excellent removal performance, including higher average molecular weight and enhanced effect in charge neutralization, adsorption and bridging effect.19 Liu et al. synthesized a high molecular polysilicate composite flocculant by adding metal magnesium salt and silicate into the traditional PAC, the results showed that its decolourization efficiency is more than 90% which is better than other traditional coagulants.20 Liu et al. investigated an alginate grafted polysilicate aluminum calcium in drinking water treatment, the introduction of alginate and calcium has greatly enhanced the turbidity removal efficiency and color removal efficiency to 97% and 98%, respectively.21 Wu et al. synthesized a polysilicate aluminum ferric (PSAF) for oil wastewater treating. The results indicated that when the (Al + Fe)/Si ratio was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and Al/Fe ratio was 5
4 and Al/Fe ratio was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the PSAF reached a prefect turbidity removal rate and oil removal rate of 99%, 88%, respectively.22 All of the above results have shown the great potential of polysilicate composite metal salts in practical application.
5, the PSAF reached a prefect turbidity removal rate and oil removal rate of 99%, 88%, respectively.22 All of the above results have shown the great potential of polysilicate composite metal salts in practical application.
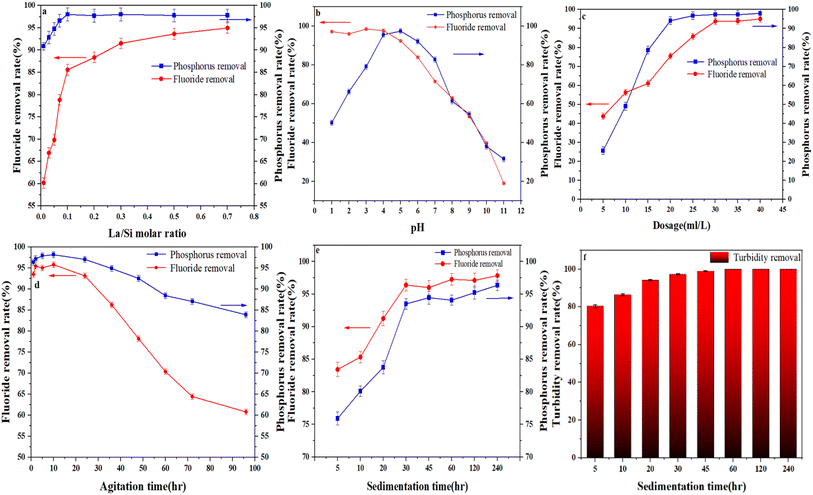
In this study, a polysilicate composite flocculant was synthesized via co-polymerization to measure its performance towards yellow phosphorus wastewater. After the polysilicate was prepared, the polysilicate doped with ferric (PSF), ferric-calcium (PSFC) and ferric-calcium-lanthanum (PSFCL) were added to the wastewater to remove the phosphorus and fluoride, respectively. Besides, its removal performance was also optimized by adjusting pH and SiO2 content of polysilicate, La/Si molar ratio, agitation time, pH, dose and sedimentation time. This study could provide a new perspective for the removal of phosphorus and fluoride from wastewater by coagulation and optimization of the reaction conditions for further practical application.
2. Material and methods
2.1 Materials
Sodium silicate (Na2SiO3·9H2O), lanthanum nitrate hexahydrate (La(NO3)2·6H2O), and ferric chloride hexahydrate (FeCl3·6H2O) were purchased from Zhiyuan Chemical Reagent Co., Ltd (Tianjin, China), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) was purchased from Shanghai Aladdin Chemistry Co., Ltd (Shanghai, China). Hydrochloric acid (36%) was provided by Kelong Chemical Co., Ltd (Chengdu, China). Ultrapure water (R = 18.3 MΩ cm−1) was used for all the experiments. Yellow phosphorus wastewater was collected from a local yellow phosphorus chemical plant in Kunming, the characteristics were shown in Table 1. All of these reagents were analytical reagent without further purification.| COD (mg L−1) | TSS (mg L−1) | Turbidity (NTU) | TF (mg L−1) | TP (mg L−1) | pH | Zeta potential (mV) |
|---|---|---|---|---|---|---|
| 61 | 545 | 10 | 103 | 3268 | 2.1 | −24.45 |
2.2 Preparation of flocculants
Flocculants were prepared as the following procedure at room temperature. Firstly, sodium silicate solution was dropwise added to the hydrochloric acid to polymerize and form polysilicic acid solution (PSI). To ensure an ideal degree of PSI polymerization, several pre-experiments were carried out, the pH was adjusted with 36 wt% (1 + 1) hydrochloric acid. As shown in Fig. S1 and S2,†the pH and silica content can significantly affect the polymerization time, an extremely short polymerization time could inhibit the metal salts bonding with PSI. However, when the polymerization time is too long, the PSI preparation process will be prolonged and consequently affect the degree of polymerization. In a word, the optimal pH of synthesized PSI and silica content was determined to be 2 and 2%, respectively. The obtained solution was then agitated for two hours to form PSI. A certain amount of ferric chloride hexahydrate FeCl3·6H2O, calcium nitrate tetrahydrate Ca(NO3)2·4H2O and lanthanum nitrate hexahydrate La(NO3)2·6H2O solution were slowly added into the PSI independently under constant magnetic stirring for 1 hour to form PSF, PSFC and PSFCL. The polysilicate-ferric-calcium-lanthanum (PSFCL) flocculant was obtained by agitating for another 12 h. PSF and PSFC could be obtained via adding ferric salt, calcium lanthanum salt, respectively. After 12 h continuously agitation, the formation of solid-state flocculants was achieved.2.3 Coagulation experiments
The coagulation experiments were carried out in several plexiglass beakers using a magnetic stirrer via a 3-step procedure, i.e., high rate stirring, coagulation and sedimentation. The wastewater was diluted 20 times before the experiments were conducted. In the high rate stirring step, the stirring rate was set in 200 rpm, the dose of each experiment was 6 ml/200 ml, and the pH of each solution was maintained at 5 by using hydrochloric acid and sodium hydroxide. The aliquots were extracted by syringes and then filtered through a 0.45 μm filter. In the end, the zeta potential of each flocs was measured. In the coagulation step, the stirring rate was decreased to 60 rpm for 5 min before the sedimentation step. All of aliquots were taken below 2–3 cm of the surface after 30 min sedimentation for phosphorus, fluoride, and turbidity test. The removal percent (R%) for turbidity, phosphorus and fluoride was calculated by eqn (1).
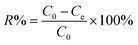 | (1) |
2.4 Characterizations of flocculants
The residue concentration of phosphorus present in the solution was measured by molybdenum blue spectrophotometric method via a UV spectrophotometer (723PC, China). The concentration of fluoride was determined by a fluorine ion meter (DWS-51, China). The solid samples of the flocculants were analyzed by Fourier transform infrared spectroscopy (FT-IR) with the Thermos Scientific Nicolet Is-50 spectrometer in the range of 4000–400 cm−1. X-ray diffraction (XRD) was measured by XRDynamic 500 (Anton Paar, Austria) for the determination of crystalline with Cu Kalpha radiation in the 2θ range of 20–80° at a scan rate of 4° min−1. The microstructure of flocculants and elemental compositions was examined by a Scanning Electron Microscope (SEM) (Zeiss Sigma 300, Germany). The XPS spectra were analyzed using X-ray photoelectron spectroscopy (Thermos Scientific K-Alpha, USA) with Al Kα radiation (hv = 1486.6 eV). Zeta potential for wastewater samples and flocculants was obtained by a nanoparticle size and zeta potential analyzer (Anton Paar Litesizer 500, Austria). The pH values and turbidity of solutions were measured by a pH meter (PHS-3C, China) and a turbidimeter (WGZ-200BS, China). Data processing was conducted via Origin 2021 software.2.5 Statistical analysis
One-way analysis of variance (ANOVA) with Fisher's least significant difference (LSD) post hoc (α – 0.05) tests was used to determine the significant differences in investigated pollutants removal from phosphorus and fluoride using different molar ratio; pH values; dosages; agitation time and sedimentation time. All calculations were carried out using Origin 2021 software.3. Results and discussion
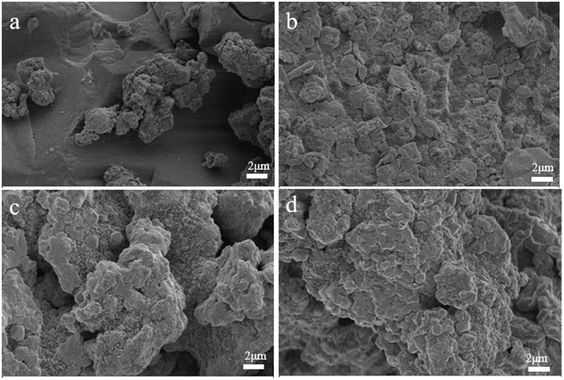
3.1 Morphology and structure
The distribution of PSFCL elements after coagulation was determined by EDS, and specific atomic percentage and corresponding elements distribution were shown in Fig. S3.† The result showed that each element was uniformly dispersed, and phosphorus and fluoride were successfully loaded after coagulation process.
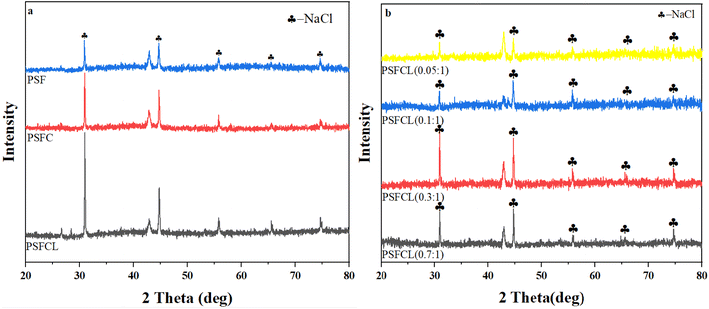 | ||
| Fig. 2 (a) XRD spectra of PSF, PSFC and PSFCL; (b) XRD spectra of PSFCL under different La/Si molecular ratio after flocculation. | ||
The XRD pattern of PSFCL under different La/Si molecular ratio after flocculation was shown in Fig. 2b. The result indicated that after flocculation there was almost no crystal phase difference between each flocculant, and the peak strength for each flocculant with different La/Si ratio was fluctuant. Moreover, with the increase of the La/Si ratio, the crystal phases of each flocculant seem be identical compared with PSFCL pattern in Fig. 2a, which guarantee an excellent stability of the flocculant. Polysilicate with excellent charge neutralization and adsorption bridging abilities is a promising coagulation agent.25 The introduction of polysilicic acid has large particle size and molecular weight which leads to strong adsorption bridging effect.26 In a word, polysilicate can act as skeleton materials and form hydrophobic channels to improve wastewater coagulation performance. If macromolecular substances were dominant in the composition of coagulants, the coagulation efficiency towards target pollutant was usually higher. The macromolecular substances could stimulate the bridge effect which was conductive for the formation of flocs, and these flocs could be easily settled for the subsequent separation.27
3.2 Evaluation of coagulation performance
3.3 Comparison of the removal efficiencies of various flocculants
The removal efficiency of PSF, PSFC and PSFCL in the diluted yellow phosphorus wastewater was investigated, as shown in Fig. 4. Each flocculant at identical dosages (PSF: 30 ml L−1, PSFC: 30 ml L−1, PSFCL: 30 ml L−1) was added into yellow phosphorus wastewater sample at room temperature (25 °C). After the introduction of lanthanum, the removal efficiency of the composite flocculant PSFCL significantly enhanced compared with that of PSF and PSFC, with the phosphorus and fluoride removal efficiency of 97% and 95%, respectively. Compared with other reported flocculants in Table 2, PSFCL has shown great performance towards simultaneously phosphorus and fluoride removal.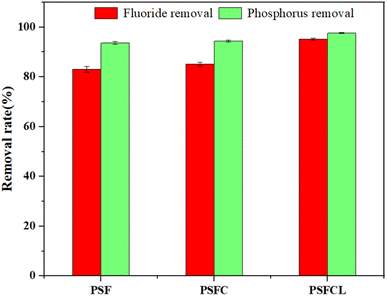 | ||
| Fig. 4 Comparison of removal efficiency of three flocculants under optimized conditions (La/Si molar ratio = 0.3, pH = 5, dosage = 30 ml L−1, agitation time = 10 h, sedimentation time = 30 min). | ||
| Samples | pH | Dose (mg L−1) | Stir rapidly | Stir slowly | Settling time (min) | TP removal rate (%) | TF removal rate (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a PFASiC, polyaluminium ferric silicate chloride; PAC, polyaluminium chloride; FD-PSAF, polysilicate aluminum ferric from foundry dust; PFS, polymerized ferrous sulfate; PSFCL, polysilicate ferric calcium lanthanum; TP, total phosphorus; TF, total fluoride. | ||||||||
| PFASiC | 5–9 | 50 | 150 rpm, 3 min | 30 rpm, 10 min | 30 | >95 | — | 33 |
| PAC | 6–8 | 50 | 150 rpm, 3 min | 30 rpm, 10 min | 30 | 94 | — | 33 |
| ZrCl4 | 4–6 | 24 | 200 rpm, 1 min | 40 rpm, 15 min | 20 | — | 85 | 34 |
| Al2(SO4)3 | 8–10 | 16 | 200 rpm, 1 min | 40 rpm, 15 min | 20 | — | 63 | 34 |
| FD-PSAF | 2 | 10 | 120 rpm, 2 min | 60 rpm, 7 min | 20 | 99.7 | — | 35 |
| PFS | 2 | 13 | 120 rpm, 2 min | 60 rpm, 7 min | 20 | 98 | — | 35 |
| PSFCL | 5 | 30 (ml L−1) | 200 rpm, 2 min | 60 rpm, 5 min | 30 | 97.5 | 95.1 | The present study |
3.4 Flocculation mechanism
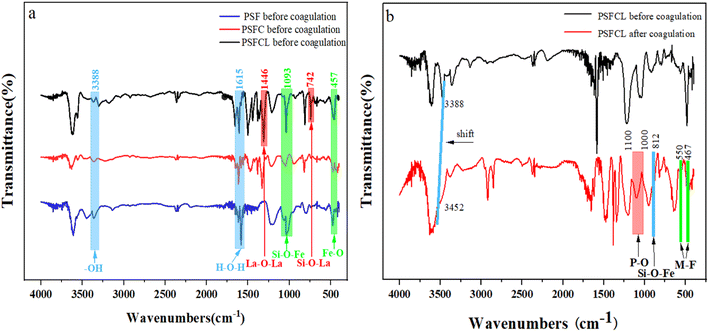 | ||
| Fig. 5 (a) FT-IR spectra of three flocculants before coagulation; (b) FT-IR spectra of PSFCL flocculants before and after coagulation. | ||
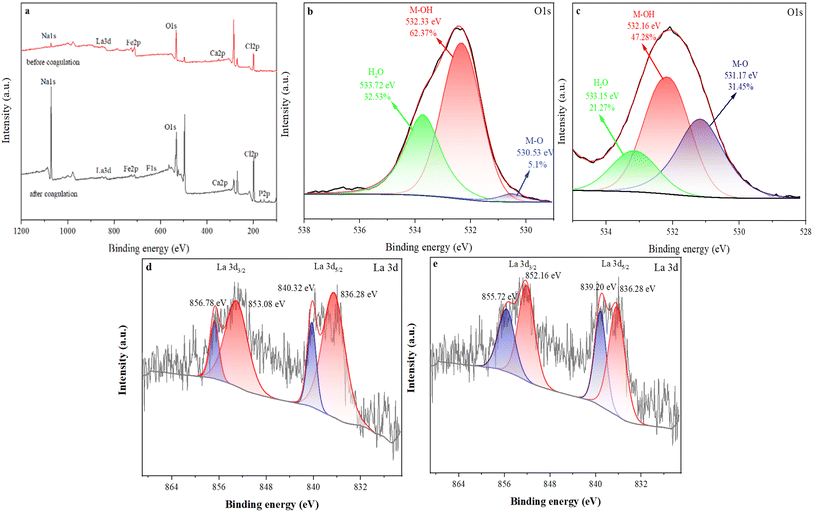
Fig. 5b showed the comparison of PSFCL spectra before and after flocculation. The characteristic peaks at 1073 cm−1 and 812 cm−1 were assigned to the bending vibration of Si–O–Si and Si–O–Fe, respectively. After coagulation, the band at 3388 cm−1 shifted to a higher frequency at 3452 cm−1, this indicated that –OH group interacted with fluorine ions which was conductive to removal of fluoride and phosphorus. The broadband at 1000–1100 cm−1 corresponded to P–O stretching vibration, which demonstrated a successful load of phosphorus on PSFCL. Moreover, the peaks at 1060 cm−1, 668 cm−1 and 457 cm−1 disappeared, which indicated that La and Fe were involved in the coagulation process. The absorption peak at 467 cm−1 and 550 cm−1 after adsorption demonstrated that the metal interacted with fluorine ions via complexation to form M–F bonds (M = La or Fe).39
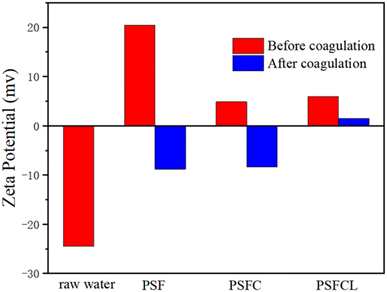
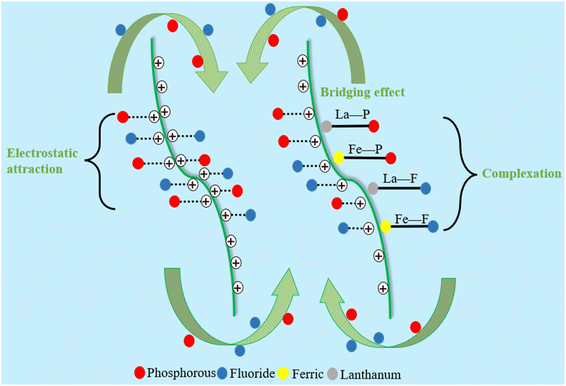
Finally, the possible mechanism schematic diagram of flocculation over PSFCL was shown in Fig. 8. The yellow phosphorus wastewater was negative in the analysis of zeta potential. After adding the positively charged composite flocculants PSFCL, it could bond with the colloid particles via charge neutralization and subsequently accelerate the formation of small flocs. PSFCL was a macromolecular substance due to the polymerization of polysilicic acid. This could consequently enhance the molecular weight of the composite and generate a strong bridging effect. PSFCL could also form a net-shaped structure within the water body, which could effectively capture the small flocs, leading to larger flocs and compact aggregates. Moreover, during the coagulation process, the loading positively charged metal ions could interact with phosphorus and fluoride ions through complexation, ligand exchange and co-precipitation process. As a whole, charge neutralization, bridging effect and ligand exchange were the main flocculation mechanisms.
4. Conclusion
In this study, a polysilicate-ferric-calcium-lanthanum flocculant (PSFCL) was successfully prepared through co-polymerization method and employed to investigate its performance for yellow phosphorus wastewater treatment. The results shown that PSFCL possessed a better coagulation performance, and the removal efficiency towards phosphorus and fluoride was 95% and 87%, respectively, compared with PSF and PSFC. The flocculation conditions were set up by single-factor variable tests. The optimum conditions for removal of phosphorus and fluoride were confirmed, namely, the dosage was 6 ml L−1, the sedimentation time was 30 min, the pH for flocculation was 5, the agitation time was less than 24 h, and the La/Si molar ratio was 0.3. The results from XPS and FTIR demonstrated that charge neutralization, bridging effect, ligand exchange and complexation existed in the flocculation process. In summary, the current work not only provided a new design for composite flocculants, but also exhibited an important significance in the optimization and future practical application for wastewater treatment.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from Yunnan Major Scientific and Technological Projects (Grant No. 202202AG050001-5).References
- K. Y. Koh, S. Zhang and J. Paul Chen, Hydrothermally synthesized lanthanum carbonate nanorod for adsorption of phosphorus: material synthesis and optimization, and demonstration of excellent performance, Chem. Eng. J., 2020, 380, 122153 CrossRef CAS.
- M. Chaudhary and A. Maiti, Defluorination by highly efficient calcium hydroxide nanorods from synthetic and industrial wastewater, Colloids Surf., A, 2019, 561, 79–88 CrossRef CAS.
- T. Robshaw, S. Tukra, D. B. Hammond, G. J. Leggett and M. D. Ogden, Highly efficient fluoride extraction from simulant leachate of spent potlining via La-loaded chelating resin. An equilibrium study, J. Hazard. Mater., 2019, 361, 200–209 CrossRef CAS.
- W. X. Gong, J. H. Qu, R. P. Liu and H. C. Lan, Effect of aluminum fluoride complexation on fluoride removal by coagulation, Colloids Surf., A, 2012, 395, 88–93 CrossRef CAS.
- S. Raghav and D. Kumar, Comparative kinetics and thermodynamic studies of fluoride adsorption by two novel synthesized biopolymer composites, Carbohydr. Polym., 2019, 203, 430–440 CrossRef CAS.
- B. Rajasekhar, U. Venkateshwaran, N. Durairaj, G. Divyapriya, I. M. Nambi and A. Joseph, Comprehensive treatment of urban wastewaters using electrochemical advanced oxidation process, J. Environ. Manage., 2020, 266, 110469 CrossRef CAS.
- H. Zhang, Z. Zhang, K. Jiang, Z. Li, K. Zhang, J. Ma and Y. Qian, Salt effect on MUCT system performance of nitrogen and phosphorus removal, Green Energy Environ., 2021, 6(5), 670–677 CrossRef CAS.
- W. J. Xia, L. X. Guo, L. Q. Yu, Q. Zhang, J. R. Xiong, X. Y. Zhu, X. C. Wang, B. C. Huang and R. C. Jin, Phosphorus removal from diluted wastewaters using a La/C nanocomposite-doped membrane with adsorption-filtration dual functions, Chem. Eng. J., 2021, 405, 126924 CrossRef CAS.
- C. Y. Teh, P. M. Budiman, K. P. Y. Shak and T. Y. Wu, Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment, Ind. Eng. Chem. Res., 2016, 55(16), 4363–4389 CrossRef CAS.
- X. Yu, C. Wei, H. Wu, Z. Jiang and R. Xu, Improvement of biodegradability for coking wastewater by selective adsorption of hydrophobic organic pollutants, Sep. Purif. Technol., 2015, 151, 23–30 CrossRef CAS.
- T. Sun, C. H. Sun, G. L. Zhu, X. J. Miao, C. C. Wu, S. B. Lv and W. J. Li, Preparation and coagulation performance of poly-ferric-aluminum-silicate-sulfate from fly ash, Desalination, 2011, 268(1–3), 270–275 CrossRef CAS.
- Z. Zhang, H. Zheng, Y. Sun, C. Zhao, Y. Zhou, X. Tang and C. Zhao, A combined process of chemical precipitation and flocculation for treating phosphating wastewater, Desalin. Water Treat., 2016, 57(53), 25520–25531 CrossRef CAS.
- B. Macherzyński, M. Wszelaka-Rylik, M. Włodarczyk-Makuła, M. Osiak, A. Pietrzak, B. Bień and A. Poniatowska, Comparative efficiency of phosphorus removal from supernatants by coagulation process, Desalin. Water Treat., 2023, 301, 209–215 CrossRef.
- N. Ozairi, S. A. Mousavi, M. T. Samadi, A. Seidmohammadi and D. Nayeri, Removal of fluoride from water using coagulation–flocculation process: a comparative study, Desalin. Water Treat., 2020, 180, 265–270 CrossRef CAS.
- C. Zhao, J. Zhang, Z. Luan, X. Peng and X. Ren, Preparation of high concentration polyaluminum chloride with high content of Alb or Alc, J. Environ. Sci., 2009, 21(10), 1342–1346 CrossRef CAS PubMed.
- Z. P. Xing and D. Z. Sun, Treatment of antibiotic fermentation wastewater by combined polyferric sulfate coagulation, fenton and sedimentation process, J. Hazard. Mater., 2009, 168(2–3), 1264–1268 CrossRef CAS.
- G. Lei, J. Ma, X. Guan, A. Song and Y. Cui, Effect of basicity on coagulation performance of polyferric chloride applied in eutrophicated raw water, Desalination, 2009, 247(1–3), 518–529 CrossRef CAS.
- A. Campbell, D. Hamai and S. C. Bondy, Differential toxicity of aluminum salts in human cell lines of neural origin: implications for neurodegeneration, Neurotoxicology, 2001, 22(1), 63–71 CrossRef CAS.
- R. F. Packham, Some studies of the coagulation of dispersed clays with hydrolyzing salts, J. Colloid Sci., 1965, 20(1), 81–92 CrossRef CAS.
- Z. M. Liu, Y. M. Sang, Z. G. Tong, Q. H. Wang and T. C. Sun, Decolourization performance and mechanism of leachate secondary effluent using poly-aluminium(III)–magnesium(II) sulphate, Water Environ. J., 2012, 26(1), 85–93 CrossRef CAS.
- Y. Liu, J. Ma, L. Lian, X. Wang, H. Zhang, W. Gao and D. Lou, Flocculation performance of alginate grafted polysilicate aluminum calcium in drinking water treatment, Process Saf. Environ. Prot., 2021, 155, 287–294 CrossRef CAS.
- L. Wu, Y. Gao, X. Xu, J. Deng and H. Liu, Excellent coagulation performance of polysilicate aluminum ferric for treating oily wastewater from Daqing gasfield: responses to polymer properties and coagulation mechanism, J. Environ. Manage., 2024, 356, 120642 CrossRef CAS PubMed.
- Q. Lin, H. Peng, S. Zhong and J. Xiang, Synthesis, characterization, and secondary sludge dewatering performance of a novel combined silicon–aluminum–iron–starch flocculant, J. Hazard. Mater., 2015, 285, 199–206 CrossRef CAS.
- W. Chen, H. Zheng, J. Zhai, Y. Wang, W. Xue, X. Tang, Z. Zhang and Y. Sun, Characterization and coagulation–flocculation performance of a composite coagulant: poly-ferric-aluminum-silicate-sulfate, Desalin. Water Treat., 2015, 56(7), 1776–1786 CrossRef CAS.
- J. Yi, Z. Chen, D. Xu, D. Wu and A. Howard, Preparation of a coagulant of polysilicate aluminum ferric from foundry dust and its coagulation performance in treatment of swine wastewater, J. Cleaner Prod., 2024, 434, 140400 CrossRef CAS.
- Y. Sun, C. Zhu, H. Zheng, W. Sun, Y. Xu, X. Xiao, Z. You and C. Liu, Characterization and coagulation behavior of polymeric aluminum ferric silicate for high-concentration oily wastewater treatment, Chem. Eng. Res. Des., 2017, 119, 23–32 CrossRef CAS.
- J. Li, J. Li, X. Liu, Z. Du and F. Cheng, Effect of silicon content on preparation and coagulation performance of poly-silicic-metal coagulants derived from coal gangue for coking wastewater treatment, Sep. Purif. Technol., 2018, 202, 149–156 CrossRef CAS.
- K. You, W. Yang, P. Song, L. Fan, S. Xu, B. Li and L. Feng, Lanthanum-modified magnetic oyster shell and its use for enhancing phosphate removal from water, Colloids Surf., A, 2022, 633, 127897 CrossRef CAS.
- C. Liu, B. Gao, S. Wang, K. Guo, X. Shen, Q. Yue and X. Xu, Synthesis, characterization and flocculation performance of a novel sodium alginate-based flocculant, Carbohydr. Polym., 2020, 248, 116790 CrossRef CAS.
- T. Krahnstöver and T. Wintgens, Optimizing the flocculation of powdered activated carbon in wastewater treatment by dosing iron salt in single- and two-stage processes, J. Water Process. Eng., 2017, 20, 130–137 CrossRef.
- T. Kim, The effects of polyaluminum chloride on the mechanical and microstructural properties of alkali-activated slag cement paste, Cem. Concr. Compos., 2019, 96, 46–54 CrossRef CAS.
- T. Chen, M. Niu, X. Wang, W. Wei, J. Liu and Y. Xie, Synthesis and characterization of poly-aluminum silicate sulphate (PASS) for ultra-low density fiberboard (ULDF), RSC Adv., 2015, 5(113), 93187–93193 RSC.
- X. Niu, X. Li, J. Zhao, Y. Ren and Y. Yang, Preparation and coagulation efficiency of polyaluminium ferric silicate chloride composite coagulant from wastewater of high-purity graphite production, J. Environ. Sci., 2011, 23(7), 1122–1128 CrossRef CAS PubMed.
- Y. Gan, X. Wang, L. Zhang, B. Wu, G. Zhang and S. Zhang, Coagulation removal of fluoride by zirconium tetrachloride: performance evaluation and mechanism analysis, Chemosphere, 2019, 218, 860–868 CrossRef CAS.
- J. Yi, Z. Chen, D. Xu, D. Wu and A. Howard, Preparation of a coagulant of polysilicate aluminum ferric from foundry dust and its coagulation performance in treatment of swine wastewater, J. Cleaner Prod., 2024, 434, 140400 CrossRef CAS.
- B. Zhang, Z. Chang, J. Li, X. Li, Y. Kan and Z. Gao, Effect of kaolin content on the performances of kaolin-hybridized soybean meal-based adhesives for wood composites, Composites, Part B, 2019, 173, 106919 CrossRef.
- M. Li, S. Kuang, Y. Kang, H. Ma, J. Dong and Z. Guo, Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment, Sci. Total Environ., 2022, 819, 153157 CrossRef CAS PubMed.
- B. Zhang, L. Xu, Z. Zhao, S. Peng, C. Yu, X. Zhang, Y. Zong and D. Wu, Enhanced phosphate removal by nano-lanthanum hydroxide embedded silica aerogel composites: superior performance and insights into specific adsorption mechanism, Sep. Purif. Technol., 2022, 285, 120365 CrossRef CAS.
- P. K. Raul, R. R. Devi, I. M. Umlong, S. Banerjee, L. Singh and M. Purkait, Removal of Fluoride from Water Using Iron Oxide-Hydroxide Nanoparticles, J. Nanosci. Nanotechnol., 2012, 12(5), 3922–3930 CrossRef CAS.
- G. P. Lam, E. K. Zegeye, M. H. Vermuë, D. M. M. Kleinegris, M. H. M. Eppink, R. H. Wijffels and G. Olivieri, Dosage effect of cationic polymers on the flocculation efficiency of the marine microalga Neochloris oleoabundans, Bioresour. Technol., 2015, 198, 797–802 CrossRef.
- J. He, K. Zhang, S. Wu, X. Cai, K. Chen, Y. Li, B. Sun, Y. Jia, F. Meng, Z. Jin, L. Kong and J. Liu, Performance of novel hydroxyapatite nanowires in treatment of fluoride contaminated water, J. Hazard. Mater., 2016, 303, 119–130 CrossRef CAS PubMed.
- L. Chen, K. S. Zhang, J. Y. He, W. H. Xu, X. J. Huang and J. H. Liu, Enhanced fluoride removal from water by sulfate-doped hydroxyapatite hierarchical hollow microspheres, Chem. Eng. J., 2016, 285, 616–624 CrossRef CAS.
- M. Yi, K. Wang, H. Wei, D. Wei, X. Wei, B. Wei, L. Shao, T. Fujita and X. Cui, Efficient preparation of red mud-based geopolymer microspheres (RM@GMs) and adsorption of fluoride ions in wastewater, J. Hazard. Mater., 2023, 442, 130027 CrossRef CAS PubMed.
- Y. Jia, B. S. Zhu, K. S. Zhang, Z. Jin, B. Sun, T. Luo, X. Y. Yu, L. T. Kong and J. H. Liu, Porous 2-line ferrihydrite/bayerite composites (LFBC): fluoride removal performance and mechanism, Chem. Eng. J., 2015, 268, 325–336 CrossRef CAS.
- X. Wu, Y. Zhang, X. Dou, B. Zhao and M. Yang, Fluoride adsorption on an Fe–Al–Ce trimetal hydrous oxide: characterization of adsorption sites and adsorbed fluorine complex species, Chem. Eng. J., 2013, 223, 364–370 CrossRef CAS.
- P. Koilraj and K. Sasaki, Selective removal of phosphate using La-porous carbon composites from aqueous solutions: batch and column studies, Chem. Eng. J., 2017, 317, 1059–1068 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07237e |
| This journal is © The Royal Society of Chemistry 2025 |