Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
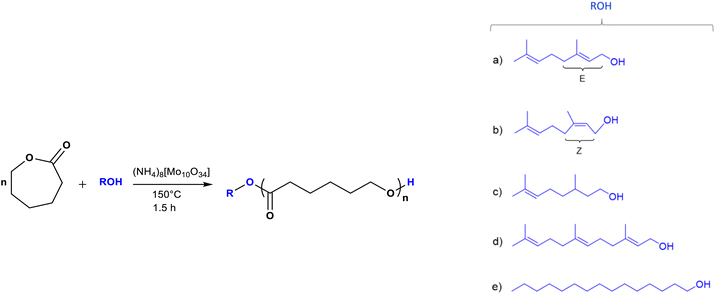
Monodisperse oligo(ε-caprolactones) with terpenes and alkyl end-groups: synthesis, isolation, characterization, and antibacterial activity†
María Guadalupe Ortiz-Aldaco a,
Miriam Estévez
a,
Miriam Estévez b,
Beatriz Liliana España-Sánchez
b,
Beatriz Liliana España-Sánchez c,
José Bonilla-Cruz
c,
José Bonilla-Cruz d,
Eloy Rodríguez-deLeón
d,
Eloy Rodríguez-deLeón e and
José E. Báez
e and
José E. Báez *a
*a
aDepartment of Chemistry, University of Guanajuato (UG), Noria Alta S/N, 36050 Guanajuato, Gto, Mexico. E-mail: jebaez@ugto.mx
bCentro de Fisica Aplicada y Tecnología Avanzada (CFATA), UNAM, Juriquilla, Qro., Mexico
cCentro de Investigación y Desarrollo Tecnológico en Electroquímica (CIDETEQ), Pedro Escobedo, Qro., Mexico
dCentro de Investigación en Materiales Avanzados S.C. (CIMAV), Unidad Monterrey, Mexico
eUniversidad Autónoma de Querétaro (UAQ), Querétaro, Qro., Mexico
First published on 2nd January 2025
Abstract
Linear aliphatic oligoesters derived from ε-caprolactone (CL) were synthesized by ring-opening polymerization (ROP) using terpene alcohols that have antibacterial activity as initiators (nerol, geraniol, β-citronellol and farnesol). Ammonium decamolybdate (NH4)8[Mo10O34] was used as a catalyst. From previous oligoesters, monodisperse species of monomers, dimers, and trimers were isolated by flash column chromatography (FCC). Poly(ε-caprolactone) (PCL) oligoesters [oligo(CLs)] and monodisperse oligomeric species were characterized by different analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy, electrospray ionization quadrupole time-of-flight mass spectrometry (ESI/MS-QTOF), and Fourier-transform infrared (FTIR) spectroscopy to determine the chemical nature of the samples. The thermal properties were analyzed by differential scanning calorimetry (DSC), which showed significant differences between the olefin and alkyl terminal groups. The end-groups affected crystalline domains according to the crystallization temperatures (Tc), melting temperatures (Tm), and glass transition temperature (Tg) of the oligo(CLs) and monodisperse oligomeric species. In addition, the results of thermogravimetric analysis (TGA) suggest that the thermal degradation in the case of the monomer and dimer species with olefin terminal groups is similar compared to that with the alkyl terminal group. Due to the antimicrobial properties of olefinic initiators, microbiological tests were carried out on the monodisperse oligomeric species through studies of the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and antibiograms. This is the first time in the literature that monodisperse oligomers derived from PCL functionalized with terpenes and alkyl end-groups were tested in terms of their antibacterial properties. The results indicated that these monodisperse species could lead to new antibiotic compounds with potential applications.
1. Introduction
Biodegradable polyesters such as poly(ε-caprolactone) (PCL) are of great interest due to their biocompatibility and biodegradability.1 PCL can be prepared by ring-opening polymerization (ROP) of ε-caprolactone (CL) using an alcohol (R-OH)2–4 [or diol (HO-R-OH)]5–9 as an initiator. The alkyl substituent becomes a terminal group and leads to α-hydroxyl-ω-alkyl-PCL (HO-PCL-R). This phenomenon is also applied to other monomers such as δ-valerolactone (δ-VL),2 glycolide (GA),4 and L-lactide (L-LA).4,10,11 A homopolymer of CL is often used in biomedicine due to its hydrophobic character when used in biodegradable stents12 in soft tissues and drug delivery.13 There is growing academic and industrial interest in biodegradable antimicrobial oligomeric polyesters, but few studies have been carried out on oligomers based on PCL.14 However, in recent years, efforts have been made to separate monodisperse species, including PCL.Stepwise synthesis methods (linear and exponential growth) have been very successful in the production of these oligomers, but these methods result in reduced separation with higher-molecular-weight oligomers and decreased statistical yield of any one oligomer length at a higher degree of polymerization (DP). Furthermore, the multi-step nature is inefficient for preparing large libraries of discrete oligomers and can be challenging. For such purposes, separation methods are used to isolate individual oligomers from dispersed materials, such as flash column chromatography (FCC).2,15–19
Terpenes are the most abundant family of organic natural products in nature and are the main constituents of essential oils. They have a skeleton that is formed by isoprene and oxygen units, and they are produced and secreted by specialized plant tissues.20 Terpenes may present various organic functional groups, such as alcohols, ketones, ethers, esters, and aldehydes,21 and they have biological activities that are related to their functional groups, arrangements, and structures.22 These natural hydrocarbons are made up of five carbon isoprene units with different configurations, various degrees of unsaturation, oxidation, functional groups, and rings.23,24 For example, nerol and geraniol are aliphatic monoterpene structures (C10H18O) produced by the combination of two isoprene units (C5H8) that have a functional alcohol group in their organic composition, while farnesol (C15H26O) is a sesquiterpene alcohol with three isoprene units.
Nerol is extracted from Damask rose (Rosa damascena), Lavandula stoechas, Lavandula multifida, and lemongrass (Cymbopogon citratus).25 β-Citronellol is a part several volatile oils in plants of the genus Cymbopogon (such as Cymbopogon nardus).26–28 Geraniol is a part of various volatile oils and a principle product of Cymbopogon martinii (66.2–76.9%),29 Pelargonium graveolens (21.08%),30 R. damascena (18.7–21.2%), Rosa centifolia (7.4–11.3%),31 and Cymbopogon nardus (22.77%).32 This phytoconstituent has been reported to have biological and pharmacological properties, such as antibacterial activity.33,34 The mechanism of this activity is based on its lipophilic character and is explained by the ability to adhere to cell membrane lipids of the microorganism. This allows it to interact with the organism's components, make it more permeable, bind essential intracellular sites, and thus destroy its structures.33,35 These terpene compounds exhibit good antimicrobial activity against Staphylococcus aureus and Escherichia coli.23,36–39
One of the main drawbacks of PCL is its lack of functional groups with antimicrobial activity, which is why it has been possible to synthesize biodegradable antimicrobial PCL.40–54 To improve the antibacterial behavior of PCL, natural compounds have been incorporated as additives, such as resveratrol,55 polyhexamethylene guanidine derivatives,56 and essential oils such as cinnamaldehyde and allyl isothiocyanate.57 Jummes et al. synthesized PCL nanoparticles that entrapped palmarosa essential oil and its majoritarian compound, geraniol, resulting in antimicrobial activity against E. coli and S. aureus.58
PCL is a biodegradable polyester with oligomers that can be recognized as a carbon source or degraded by enzymes of different microorganisms. Thus, we have been examining the fusion between two different types of chemical species: biodegradable polyesters, such as PCL, and organic molecules with biological properties, such as terpenes. Some of these terpenes have an alcohol functional group such as nerol, geraniol, β-citronellol, or farnesol and can act as initiators in the ROP of CL.
We examined what roles terpenic molecules have as a terminal group in a monodisperse oligoester derived from PCL, as well as the effect of the end-group on the physical properties of the monodisperse oligomer. We also examined whether a monodisperse oligomer with a terpenic end-group can retain the biological properties of its terpene precursor, as well as the ability of such oligomers to act as a “trojan horse” against bacteria. We report the synthesis, isolation, characterization, and antimicrobial evaluation of monodisperse oligomeric species derived from PCL and functionalized terpene alcohols that have antibacterial activity (nerol, geraniol, β-citronellol, and farnesol).
The terpenes were inserted as end-groups by ROP of the CL. Monodisperse oligomeric species from monomer to trimer were isolated by FCC and analyzed by a range of characterization techniques to examine the chemical nature and the effect of DP on the physical properties of the oligomer. Additionally, we compared the differences between the terpene farnesol (C15) and aliphatic 1-pentadecanol (C15) as a terminal group of monodisperse PCL. Microbiological tests were also carried out using Gram-positive bacteria S. aureus and Gram-negative Pseudomonas aeruginosa.
2. Experimental
2.1. Materials
All reagents, ε-caprolactone (CL), nerol, geraniol, β-citronellol, farnesol and 1-pentadecanol, ammonium decamolybdate [(NH4)8(Mo10O34)] and deuterated chloroform (CDCl3) were purchased from Sigma Aldrich Co. (St Louis, MO, USA) and used as received. Thin-layer chromatography (TLC) was performed on percolated silica gel plates and using a Seebach staining reagent. Flash column chromatography (FCC) was conducted using 230–400 mesh silica gel. Toluene and ethyl acetate were used as the mobile phase during FCC. For the antibacterial assays, strains of Staphylococcus aureus #6538 and Pseudomonas aeruginosa #13388 from American Type Culture Collection (ATCC) were growth in Mueller Hinton broth (BD Bioxon).2.2. Instruments
Nuclear magnetic resonance (NMR) spectroscopy. Solution state 1H and 13C spectra were recorded at room temperature or above on a 500 MHz Bruker Avance III HD instrument, using CDCl3 as a solvent. Chemical shifts are reported as δ in parts per million (ppm) and referenced to the chemical shift of the residual solvent (13C at δ 77.16, and 1H at δ 7.26, for CDCl3). FT-IR spectra were recorded on a PerkinElmer Spectrum 100 FTIR spectrophotometer with attenuated total reflectance spectroscopy (ATR) accessory. Size exclusion chromatography (SEC). All polyester samples were dissolved in THF (5 mg/5 mL) heating at 37 °C for one hour and filtered with an 0.45 μm Acrodisc®. The SEC instrument (Agilent) was equipped with a refractive index detector. Measurements were determined using a single column PLgel 5 μm Mixed-D (Agilent) at a flow rate of 1.0 mL min−1 with HPLC-grade THF. Polystyrene standards (Polymer Laboratories) were used for calibration. Thermograms were performed in two different instruments, the first a Differential Scanning Calorimetry (DSC) Q200 V24.11 Build 124 instrument (an intracooler at −30 °C), the second similar to the first one but with an intracooler at −90 °C. For the oligomers: three scans were obtained with two heating scans (25 to 170 °C and −30 to 170 °C) and one cooling scan (170 to −30 °C) between them. For the monodisperses species: three scans were obtained with two heating scans (25 to 80 °C and −85 to 75 °C) and one cooling scan (80 to −85 °C) between them. The rate of heating/cooling was 10 °C min−1 and was performed under a nitrogen purge. The glass transition temperature (Tg) is given as an inflection point, and the melting points (Tm) are given as the minimum of the endothermic transition, and the data presented are taken from the second heating scan. Electrospray ionization quadrupole mass (ESI/MS-QTOF) spectroscopy in positive ionization mode using ESI-Q-TOF-MS (Waters – Synapt G1) equipped. The carrier gas was nitrogen with a flow rate of 1.5 mL min−1. The sample volume injected was 250 μL. The temperature of the injector was held at 120 °C and the transfer line at 300 °C. The mass spectrometer was operated at 2 V ionization energy, the spectra were recorded in scan mode in the range 50–1200 m/z. The spectra obtained were visualized in the program Mass Lynx V4.1 (Waters), and the ions produced are inspected. Gas Chromatography-Mass Spectrometry (GC-MS), using an Agilent model 6850 gas chromatograph coupled to a 5973N mass spectrometer with a single quadrupole detector (Agilent technologies, Palo Alto, CA, USA). Chromatographic separation was performed on a HP-5 capillary column Agilent (30 m × 20 mm × 0.25 μm). The carrier gas was helium with a flow rate of 1 mL min−1. The sample volume injected was 1 μL and the split ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The oven temperature started at 50 °C for 1 minute, then increased to 250 °C at 10 °C min−1. The temperature of the injector was held at 150 °C and the transfer line at 250 °C. The mass spectrometer was operated at 70 eV ionization energy, the spectra were recorded in scan mode in the range 100–280 m/z. Polarized optical microscopy (POM). POM micrographs were obtained using a Nikon ECLIPSE E200 optical microscope; photographs were taken using an iPhone 13 mini. All samples were collected with a magnification of 40×. Thermal and decomposition characteristics of the monodisperses species (C15F-CL1, and C151P-CL1, Fig. 12; C15F-CL2, and C151P-CL2, Fig. S27†) were determined by thermogravimetric analysis (TGA), conducted on a STARe SW 13.00 TGA/DSC 2 of METTLER TOLEDO, in the temperature range of 35–595 °C with a heating rate of 10 °C min−1 under a flow of nitrogen at 40 mL min−1.
50. The oven temperature started at 50 °C for 1 minute, then increased to 250 °C at 10 °C min−1. The temperature of the injector was held at 150 °C and the transfer line at 250 °C. The mass spectrometer was operated at 70 eV ionization energy, the spectra were recorded in scan mode in the range 100–280 m/z. Polarized optical microscopy (POM). POM micrographs were obtained using a Nikon ECLIPSE E200 optical microscope; photographs were taken using an iPhone 13 mini. All samples were collected with a magnification of 40×. Thermal and decomposition characteristics of the monodisperses species (C15F-CL1, and C151P-CL1, Fig. 12; C15F-CL2, and C151P-CL2, Fig. S27†) were determined by thermogravimetric analysis (TGA), conducted on a STARe SW 13.00 TGA/DSC 2 of METTLER TOLEDO, in the temperature range of 35–595 °C with a heating rate of 10 °C min−1 under a flow of nitrogen at 40 mL min−1.
2.3. Synthesis of oligo(CLs) (DPtheo = 1)
Polymerization was performed in a previously dried 25 mL round-bottom flask. ε-Caprolactone (CL) (10 mmol, 1.1414 g), ammonium heptamolybdate tetrahydrate (NH4)6[Mo7O24]·4H2O (Hep, 1.21 × 10−3 mmol, 1.5 mg), and an initiator were charged and heated to aluminum block at 150 °C for 1.5 hour. By thermal decomposition in situ of ammonium heptamolybdate (NH4)6[Mo7O24], ammonium decamolybdate (NH4)8[Mo10O34] was obtained in the solid-state.59 The oligo(CLs) obtained and analyzed were isolated without purification (yield = 96–99%). The different initiators: geraniol (C10) (10 mmol, 1.54 g), nerol (C10) (10 mmol, 1.54 g), β-citronellol (C10) (10 mmol, 1.56 g), farnesol (C15) (10 mmol, 2.22 g), and 1-pentadecanol (10 mmol, 2.28 g) [CL/initiator = 1]. Isolation of oligomers by flash column chromatography: 600 mg of oligo(CL) (DPtheo = 1) was dissolved in the minimum volume of toluene (5 mL) and added to a silica gel column with toluene used as mobile phase. Gradually, the fraction of toluene/ethyl acetate increased to 90/10, 80/20 and 75/25. All the fractions were analyzed by thin-layer chromatography (TLC mobile phase: toluene/ethyl acetate = 80/20) and visualizing the spots using the Seebach staining reagent. The fractions were collected in test tubes, the solvent was evaporated by a rotary evaporator, and the resulting oil (or solid) was dried overnight under vacuum. Fractions: monomer C10C-CL1 [226.0 mg, wt% = 34.27%, mol% = 11.83% (mol% C10OH = 41.20%, mol% CL = 58.8%)], dimer C10C-CL2 [181.2 mg, wt% = 27.48%, mol% = 20.48% (mol% C10OH = 29.68%, mol% CL = 70.32%)], and trimer C10C-CL3 [71.1 mg, wt% = 10.78%, mol% = 67.69% (mol% C10OH = 22.89%, mol% CL = 77.11%)]. NMR data at room temperature: 1H NMR (500 MHz, CDCl3, ppm). C10C-CL1 (Fig. S9†): δ 5.08 (t, 1H, [k,![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–], C10), 4.10 (quintet, 2H, [f, –CH2–O–], C10), 3.65 (t, 2H, [a, –CH2–OH], CL1), 2.31 (t, 2H, [d, –CH2–CO–], CL1), 1.98 (quintet, 2H, [j, –CH2–CH
CH–], C10), 4.10 (quintet, 2H, [f, –CH2–O–], C10), 3.65 (t, 2H, [a, –CH2–OH], CL1), 2.31 (t, 2H, [d, –CH2–CO–], CL1), 1.98 (quintet, 2H, [j, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ], C10), 1.68 (quintet, 3H, [l, CH3–], C10), 1.65 (quintet, 2H, [i, –CH2–CH2–], C10), 1.64 (quintet, 2H, [b, –CH2–CH2–], CL), 1.60 (quintet, 3H, [n, CH3–], C10), 1.40 (quintet, 2H, [g, –CH2–(CH)–], C10), 1.33 (quintet, 2H, [c, –CH2–CH2–], CL1), 1.19 (quintet, 1H, [h, –CH–(CH3)–CH2–], C10), 0.91 (quintet, 1H, [m, CH3–(CH)–], C10). FT-IR: C10C-CL1 (Fig. S19†).
], C10), 1.68 (quintet, 3H, [l, CH3–], C10), 1.65 (quintet, 2H, [i, –CH2–CH2–], C10), 1.64 (quintet, 2H, [b, –CH2–CH2–], CL), 1.60 (quintet, 3H, [n, CH3–], C10), 1.40 (quintet, 2H, [g, –CH2–(CH)–], C10), 1.33 (quintet, 2H, [c, –CH2–CH2–], CL1), 1.19 (quintet, 1H, [h, –CH–(CH3)–CH2–], C10), 0.91 (quintet, 1H, [m, CH3–(CH)–], C10). FT-IR: C10C-CL1 (Fig. S19†).
2.4. Antibacterial assays
The quantitative in vitro antibacterial activity of monodisperse oligomeric species was performed by broth microdilution, according to the CLSI M 07 method (methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically) in contact with inoculum Gram-positive S. aureus and Gram-negative P. aeruginosa. For this purpose, different amount of each sample (4, 8, 16, 32, 64, 128, and 256 μg mL−1) were suspended in dimethyl sulfoxide (DMSO) to improve the oligomer dispersion. Ciprofloxacin was used as reference for antimicrobial agent. Inoculum of each microorganism were growth in MH broth for 16 h/37 °C and adjusted to 1 × 105 colony unit forming (CFU mL−1). An equal volume (500 μL) of bacteria/oligomer suspension was placed in a sterile Eppendorf tube and stirred at 37 °C for 24 h. After interaction, an aliquot (50 μL) was plated in MH agar and the minimum inhibitory concentration (MIC) and the bactericidal concentration (CMB) was calculated, compared with a positive control (bacterial growth).3. Results and discussion
3.1. Oligomers derived from oligo(ε-caprolactone) with olefinic and aliphatic end-groups
Oligomers were synthesized by ROP of CL using ammonium decamolybdate (NH4)8[Mo10O34] as a catalyst and alcohols (ROH) as initiators: prenol, nerol, geraniol, β-citronellol, farnesol, and 1-pentadecanol (Scheme 1). To examine the effect of the terminal group on the properties of the oligo(CLs), the feed molar ratio of CL to ROH was set to 1. The main idea was to obtain a blend of different types of oligomers such as monomers, dimers, and trimers after the polymerization reaction.Table 1 shows the results obtained for the oligo(CLs) synthesized after a reaction time of 1.5 h at 150 °C. These oligo(CLs) were successfully obtained with high conversion (from 96 to 99%). The experimental values of number-average molecular weight (Mn) were obtained by nuclear magnetic resonance (NMR) spectroscopy and size exclusion chromatography (SEC). The Mn(NMR) values were similar to those of Mn(calcd). On the other hand, Mn(SEC) was between 600 and 800 g mol−1 and was higher than Mn(calcd). The Mn(NMR)/Mn(SEC) ratio was between 0.3 and 0.5.
| No. | Sample | Initiator | MWa | Mnb (NMR) | Mnc (SEC) | DPb (NMR) | ĐMc,d | Conv.b (%) | Re (%) | Ratiof | Tcg (°C) | Tmg (°C) | ΔHmg (J g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Theoretical value (g mol−1).b Determined by 1H NMR in CDCl3.c Determined by size-exclusion chromatography (SEC) analysis.d ĐM: dispersity.e Obtained from the equation R (%) = (MWinitiator/Mn(NMR)) × 100, R = olefin or alkyl. Where MWinitiator is the molecular weight of initiator or alkyl diol (HOROH).f Mn(NMR)/Mn(SEC) ratio.g Obtained by DSC analysis. | |||||||||||||
| 1 | C10G-PCL | Geraniol | 268 | 275 | 809 | 1.06 | 1.10 | 97 | 56 | 0.34 | — | — | — |
| 2 | C10N-PCL | Nerol | 268 | 268 | 688 | 1.0 | 1.08 | 98 | 57 | 0.39 | −48 | −31, −5 | 19 |
| 3 | C10C-PCL | β-Citronellol | 270 | 272 | 756 | 1.02 | 1.09 | 99 | 57 | 0.36 | — | — | — |
| 4 | C15F-PCL | Farnesol | 336 | 338 | 665 | 1.02 | 1.15 | 99 | 65 | 0.50 | — | — | — |
| 5 | C151P-PCL | 1-Pentadecanol | 342 | 342 | — | 1.0 | — | 96 | 66 | — | — | 8, 16, 27, 31 | 141 |
In previous studies, Mn(SEC) was found to overestimate the real Mn value,59–61 and Mn(NMR) is usually more accurate for oligomers than Mn(SEC). This effect is attributed to the differences in the hydrodynamic radius between polystyrene standards and PCL samples. The experimental DP detected by NMR showed similar numbers to the feed molar values of CL/ROH, which indicated the control of DP. All oligo(CLs) exhibited a high end-group values (wt% = 56–66%).
Fig. 1 shows the 1H NMR spectrum of the oligo(CL) synthetized using nerol as an initiator (C10N-PCL). The spectrum showed characteristic peaks for methylene attached to hydroxyl [a, –CH2OH, δ 3.63], and methyl end groups [k, –CH3, δ 1.75]. The repetitive units are attributed to the methylenes of the main chain of the polymer [e, –CH2–O−, δ 4.05 and d, –CH2–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O−, δ 2.30], and the vinyl groups of the nerol [g′, j, –CH
O)–O−, δ 2.30], and the vinyl groups of the nerol [g′, j, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, δ 5.34, 5.08] were clearly visible. Furthermore, signals corresponding to unreacted nerol were observed ([f, –CH2–, δ 4.08] and [g, –CH
C–, δ 5.34, 5.08] were clearly visible. Furthermore, signals corresponding to unreacted nerol were observed ([f, –CH2–, δ 4.08] and [g, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, δ 5.43]). There was no evidence of the oxidation of olefinic groups (epoxy or secondary alcohol).
C–, δ 5.43]). There was no evidence of the oxidation of olefinic groups (epoxy or secondary alcohol).
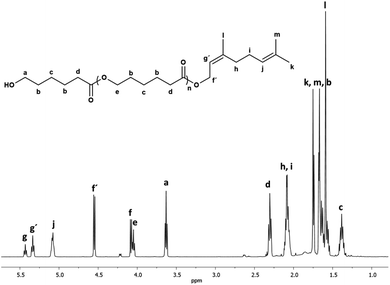 | ||
Fig. 1 1H NMR (500 MHz) spectrum of oligo(CL) synthetized using nerol as initiator (C10N-PCL, Table 1) in CDCl3 at 40 °C. Signal f[methylene, HO–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C] and g[methine, HO–CH2–CH C] and g[methine, HO–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C] are from unreacted nerol. C] are from unreacted nerol. | ||
Another oligomer obtained with farnesol as the initiator showed the same pattern of peaks for the PCL except for additional signals assigned to the longer olefin end-group (Fig. 2).
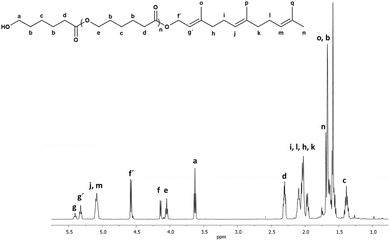 | ||
Fig. 2 1H NMR (500 MHz) spectrum of oligo(CL) synthetized using farnesol as initiator (C15F-PCL, Table 1) in CDCl3 at 40 °C. Signal f[methylene, HO–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C] and g[methine, HO–CH2–CH C] and g[methine, HO–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C] are from unreacted nerol. C] are from unreacted nerol. | ||
Thermal properties such as the glass transition temperature (Tg), crystallization temperature (Tc), and melting temperature (Tm) were studied by differential scanning calorimetry (DSC) to examine the effect of olefin and alkyl groups on the oligo(CLs) (Table 1). The oligo(CLs) produced using geraniol, β-citronellol, and farnesol as initiators did not show any transition in the range of −85 to 75 °C, which suggests that the samples were amorphous. This effect is due to branched methyl and vinyl carbons that favored steric hindrance and rigidity, inducing an amorphous domain in the oligomer chains.
In the case of oligo(CL) with the olefin nerol as the initiator (C10N-PCL, Tc = −48, and Tm = −31, −5 °C), an unusual result was observed in comparison to the rest of the oligo(CLs) with olefinic terminal groups.
Nerol and geraniol are geometric isomers with Z and E configurations, respectively. It is likely that the branched methyl attached to the alcohol in the nerol is sterically less exposed in comparison to geraniol. Consequently, when nerol becomes a terminal group in the oligo(CL), it results in less steric hindrance of the blend of different chains of oligo(CL), which leads to a semicrystalline domain at low temperature (Tm = −31, −5 °C).
In contrast, an oligo(CL) with an alkyl terminal group (initiated with 1-pentadecanol, C151P-PCL), there was an intense endothermic transition of Tm (8, 16, 27, 31 °C). Comparison between C151P-PCL (alkyl end-group) and C15F-PCL (olefin end-group) showed different environments in terms of their morphologies, which were semicrystalline and mainly amorphous, respectively. It is evident that an alkyl terminal group such as pentadecyl (C15, C151P-PCL) with anti conformation favored the crystallization of the oligo(CL). On the other hand, the olefinic end-group (C15, C15F-PCL) with sp2 carbons and branched methyl promotes amorphous domains.
3.2. Monodisperse species derived from oligo(ε-caprolactone) [oligo(CLs)]
The main purpose of the synthesis in this study is the possibility that the species examined could be the precursors of monodisperses oligomers (monomers, dimers, and trimers). The monodisperse species were gradually isolated from the crude reaction using thin layer chromatography (TLC). For this method, a mixture of toluene/ethyl acetate solvents (Experimental section) was effective in the separation of spots, and then flash column chromatography (FCC) was used to isolate monodisperse oligomeric species derived from oligo(CLs) (Table 2).| No. | Sample | Initiator | Species | MW(theo) (g mol−1) | Mn(NMR) | Tga (°C) | Tma (°C) |
|---|---|---|---|---|---|---|---|
| a Obtained by differential scanning calorimetry (DSC), second scan. | |||||||
| 1 | C10G-CL1 | Geraniol | Monomer | 268.39 | 257.22 | −38 | — |
| 2 | C10G-CL2 | Geraniol | Dimer | 382.53 | 397.36 | −45 | — |
| 3 | C10G-CL3 | Geraniol | Trimer | 496.67 | 570.86 | −51 | — |
| 4 | C10N-CL1 | Nerol | Monomer | 268.39 | 255.83 | −39 | — |
| 5 | C10N-CL2 | Nerol | Dimer | 382.53 | 390.52 | −48 | — |
| 6 | C10N-CL3 | Nerol | Trimer | 496.67 | 572.64 | −50 | — |
| 7 | C10C-CL1 | β-Citronelol | Monomer | 270.41 | 282.96 | −38 | — |
| 8 | C10C-CL2 | β-Citronelol | Dimer | 384.55 | 389.11 | −45 | — |
| 9 | C10C-CL3 | β-Citronelol | Trimer | 498.69 | 516.95 | −50 | — |
| 10 | C15F-CL1 | Farnesol | Monomer | 336.51 | 337.65 | −27 | — |
| 11 | C15F-CL2 | Farnesol | Dimer | 450.65 | 454.07 | −36 | — |
| 12 | C15F-CL3 | Farnesol | Trimer | 564.79 | 613.09 | −38 | — |
| 13 | C151P-CL1 | 1-Pentadecanol | Monomer | 342.56 | 347.11 | — | 43 |
| 14 | C151P-CL2 | 1-Pentadecanol | Dimer | 456.70 | 474.95 | — | 34 |
| 15 | C151P-CL3 | 1-Pentadecanol | Trimer | 570.84 | 583.38 | — | 36 |
In the case of the oligo(CL) functionalized with the olefinic end-group farnesyl (C15F-PCL; Table 1), a family of three different monodisperse oligomers was isolated after FCC: monomer (C15F-CL1), dimer (C15F-CL2), and trimer (C15F-CL3).
Different characterization techniques were used to illustrate the chemical nature of C15F-CL1. Fig. 3a shows the 1H NMR spectrum of C15F-CL1, which indicates characteristic peaks of both terminal groups, such as methylene adjacent to the hydroxyl group [a, –CH2–OH, δ 3.64] and methine of the vinyl groups in the farnesyl group [g, j, m, –(CH3)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, δ 5.33, 5.09].
CH–, δ 5.33, 5.09].
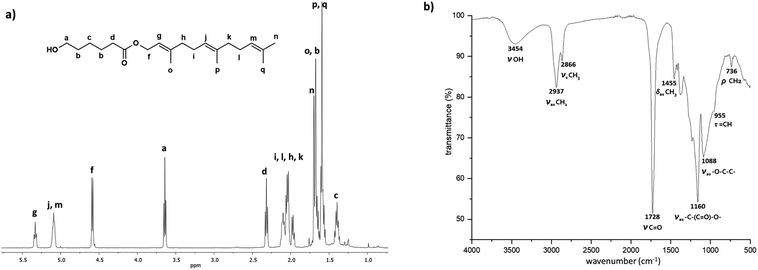 | ||
| Fig. 3 Spectra of a monomer C15F-CL1 (monodisperse oligomeric specie, Table 2) isolated by FCC from C15F-PCL (Table 1). (a) 1H NMR (500 MHz) in CDCl3, and (b) FTIR. | ||
The signals attributed to two methines showed an overlap of two triplets (j and m), where the relative ratio of j and m with respect to the third methine (g) is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A ratio of 2
1. A ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was obtained for the two signals assigned to methylenes attached to the hydroxyl group (a) and ester terminal group [f, –CH2–O–(C
2 was obtained for the two signals assigned to methylenes attached to the hydroxyl group (a) and ester terminal group [f, –CH2–O–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–, δ 4.58]. Thus, the integral values of both end-groups confirm the presence of C15F-CL1 as a monomer species.
O)–, δ 4.58]. Thus, the integral values of both end-groups confirm the presence of C15F-CL1 as a monomer species.
Additionally, the 1H NMR spectrum of the monodisperse species C15F-CL1 (Fig. 3a) lacks the characteristic signal of the ester group [e, –CH2–O–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–, δ 4.05] in the repetitive unit of a typical oligomer such as C15F-PCL (Fig. 2). On the other hand, as shown in Fig. 3b, the Fourier-transform infrared (FTIR) spectrum of C15F-CL1 contained a band at 3454 cm−1, which was attributed to the hydroxyl group, as well as a band characteristic of ester carbonyl (C
O)–, δ 4.05] in the repetitive unit of a typical oligomer such as C15F-PCL (Fig. 2). On the other hand, as shown in Fig. 3b, the Fourier-transform infrared (FTIR) spectrum of C15F-CL1 contained a band at 3454 cm−1, which was attributed to the hydroxyl group, as well as a band characteristic of ester carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1728 cm−1, another stretching vibration at 1160 cm−1 corresponding to the ester group [–(C
O) at 1728 cm−1, another stretching vibration at 1160 cm−1 corresponding to the ester group [–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–], and a band at 955 cm−1 that was attributable to olefinic (C
O)–O–], and a band at 955 cm−1 that was attributable to olefinic (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) group vibration. The NMR and FTIR results suggest that C15F-CL1 is a monodisperse species. To confirm this, a mass spectrometry analysis was carried out.
CH) group vibration. The NMR and FTIR results suggest that C15F-CL1 is a monodisperse species. To confirm this, a mass spectrometry analysis was carried out.
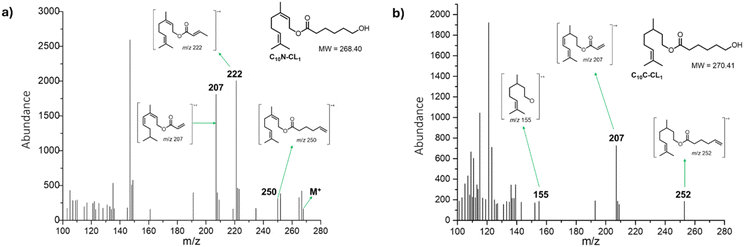
Fig. 4 (top, left) shows the electrospray ionization quadrupole time-of-flight mass spectrometry (ESI/MS-QTOF) spectrum of the monodisperse monomer C15F-CL1 (Table 2) in positive mode after doping with sodium (Na+). The results confirmed the expected molecular weight. Compared with simulated spectrum, the difference was less than 0.30 g mol−1, indicating the presence of this monodisperse species.
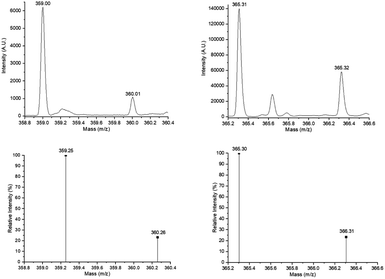 | ||
| Fig. 4 Experimental ESI/MS-QTOF spectrum in positive ionization mode of the monomer species [C15F-CL1Na+ (top, left) (Fig. 3a) and C151P-CL1Na+ (top, right) (Fig. 7a)] and isotopic distribution calculated for DP = 1 [C21H36O3Na+ (C15F-CL1Na+, bottom, left) and C21H42O3Na+ (C151P-CL1Na+, bottom, right)] in ref. 62 and 63 http://www.chemcalc.org/.64 | ||
Other monodisperse monomers initiated by nerol (C10N-CL1) and citronellol (C10C-CL1) and isolated using FCC were analyzed by electron impact (EI) mass spectra (Fig. 5). In the mass spectrum in Fig. 5a, the molecular ion of C10N-CL1 at m/z 268 was observed, but there was a peak at m/z 250 corresponding to M-18 from loss of water, as well as a peak at m/z 222 resulting from allylic cleavage from the peak at m/z 250. The rationalized structure corresponding to the peak at m/z 207 is formed by double bond isomerization, resulting in increased conjugation. Similarly, the EI mass spectrum confirmed the presence of monodisperse species of C10C-CL1 (Fig. 5b). The peak at m/z 252 corresponds to the dehydration product, and the peak at m/z 207 is formed by dehydrogenation that increases the system conjugation and partial loss of the alkyl chain on the caprolactone side. Finally, the peak at m/z 155 corresponds to citronellol with one less proton.
Another interesting species in the sequences of the DP is a dimer derived from β-citronellol and 2-CL called C10C-CL2. Fig. 6 shows the 1H NMR spectrum of the C10C-CL2 isolated from the oligomer synthesized with β-citronellol (C10C-PCL).
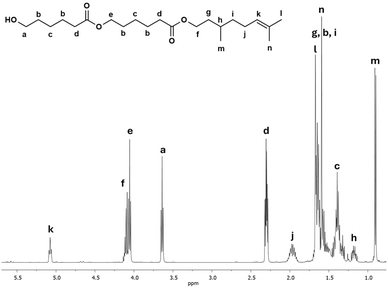 | ||
| Fig. 6 1H NMR (500 MHz) spectrum of a dimer derived from β-citronellol as initiator C10C-CL2 (monodisperse specie, Table 2) in CDCl3 isolated by FCC from C10C-PCL (Table 1). | ||
The characteristic peaks show the presence of dimeric species, such as methylene adjacent to the hydroxyl group [a, –CH2–OH, δ 3.64], a signal for the vinyl group of the terpene [k, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, δ 5.08], and a d signal composed of two triplets at the same chemical shift indicating two α-methyles into the dimer. Additionally, a new signal e is observed, which indicates the presence of a second monomeric unit of CL. This signal was is absent in the previous 1H NMR spectrum of the monodisperse monomer of C15F-CL1 (Fig. 3a). The methylene groups of the signals a and d [–CH2–(C
CH–, δ 5.08], and a d signal composed of two triplets at the same chemical shift indicating two α-methyles into the dimer. Additionally, a new signal e is observed, which indicates the presence of a second monomeric unit of CL. This signal was is absent in the previous 1H NMR spectrum of the monodisperse monomer of C15F-CL1 (Fig. 3a). The methylene groups of the signals a and d [–CH2–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–, δ 4.10] adjacent to the ester group had a relative ratio of 2
O)–O–, δ 4.10] adjacent to the ester group had a relative ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, which is evidence of a dimer species.
4, which is evidence of a dimer species.
It is well known in organic and polymer chemistry that aliphatic and olefinic groups have different properties. Therefore, aliphatic and olefinic groups were compared as parts of terminal groups in a monodisperse species. The main idea was to contrast aliphatic and olefinic terminal groups with the same number of carbons (C15). Using a similar procedure to that described previously (Experimental section), a family of monodisperse species was derived from CL and functionalized with an aliphatic terminal group such as a pentadecyl (C15) group and isolated (C15-1P-CL1, C15-1P-CL2, and C15-1P-CL3, Table 2).
Fig. 7 shows 1H NMR spectra of the monomer (C15-1P-CL1), dimer (C15-1PCL2), and trimer (C15-1PCL3) functionalized with 1-pentadecyl. There were characteristic peaks of two terminal groups: the methyl group of the C15 moiety [i, CH3, δ 0.89] and the methylene adjacent to the hydroxyl group [a, CH2, δ 3.66].
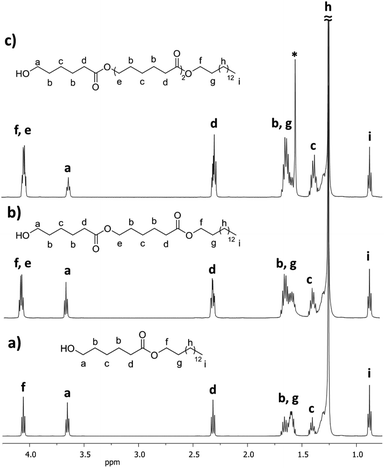 | ||
| Fig. 7 1H NMR (500 MHz) spectra in CDCl3 of the monodisperse oligomers derived from ε-caprolactone (CL) functionalized with 1-pentadecanol, isolated by flash column chromatography (FCC), (a) monomer C151P-CL1, (b) dimer C151P-CL2, and (c) trimer C151P-CL3 (Table 2) (* signal of the water). | ||
The relative ratio of terminal groups i to a was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Additionally, the proportion of signals between a and methylene close to ester [f and e, CH2, δ 4.10] in the monomer (a to f; Fig. 7a), dimer (a to f,e; Fig. 7b) and trimer (a to f,e; Fig. 7c) was 2
2. Additionally, the proportion of signals between a and methylene close to ester [f and e, CH2, δ 4.10] in the monomer (a to f; Fig. 7a), dimer (a to f,e; Fig. 7b) and trimer (a to f,e; Fig. 7c) was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 2
4, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, respectively, confirming the monodisperse oligomers. The 13C NMR spectrum also confirmed both terminal groups of C151P-CL1, α-hydroxyl (δ 62.7 ppm, a) and ω-methyl (δ 22.6 ppm, n) (Fig. S12†). Fig. 4 (top, right) shows the ESI/MS-QTOF spectrum used to validate the chemical nature of C15-1P-CL1 by another technique. The theoretical and experimental signals correspond to the monomer functionalized with a pentadecyl terminal group doped with sodium (Na+). Three different monodisperse species (monomer, dimer, and trimer) were derived with an olefinic farnesyl end-group, isolated, and characterized. The monomer was previously illustrated in Fig. 3.
6, respectively, confirming the monodisperse oligomers. The 13C NMR spectrum also confirmed both terminal groups of C151P-CL1, α-hydroxyl (δ 62.7 ppm, a) and ω-methyl (δ 22.6 ppm, n) (Fig. S12†). Fig. 4 (top, right) shows the ESI/MS-QTOF spectrum used to validate the chemical nature of C15-1P-CL1 by another technique. The theoretical and experimental signals correspond to the monomer functionalized with a pentadecyl terminal group doped with sodium (Na+). Three different monodisperse species (monomer, dimer, and trimer) were derived with an olefinic farnesyl end-group, isolated, and characterized. The monomer was previously illustrated in Fig. 3.
Table 2 shows the thermal properties of the monodisperse species. No melting point (Tm) was observed for all oligomeric monodisperse species derived from CL and olefinic terpenes (no. 1–12), indicating that the samples are amorphous with a liquid translucent appearance and viscosity. However, the glass transition temperature (Tg) was observed in the entire family of oligoesters with the olefinic end-group (Fig. 8) with a characteristic pattern from monomer to trimer. In the case of oligomers with C10 (geraniol) terminal group (Fig. 9), Tg decreased from −38 (monomer) to −51 °C (trimer), indicating that the olefinic and methyl branch of the terminal group induced a disruption of order in the monodisperse oligo(CL) chain. This effect increased with the DP, with increases of DP producing an amorphous domain with an olefinic end-group. The same effect was observed for the rest of the family of monodisperse oligo(CLs) with an olefinic terminal group (Table 2, no. 1–12).
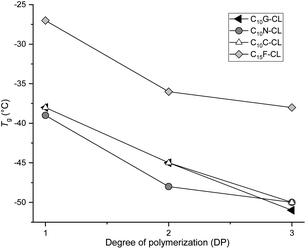 | ||
| Fig. 8 Plots of the family of monodisperse oligo(CL) with olefinic end-group. Effect of DP on the glass transition temperature (Tg). | ||
 | ||
| Fig. 9 DSC thermograms [glass transition temperature (Tg)] for species derived from monodisperse oligo(CL) from geraniol: monomer (C15G-CL1), dimer (C15G-CL2) and trimer (C15G-CL3). | ||
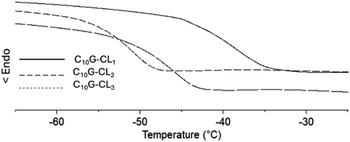
Monodisperse species with an aliphatic pentadecyl (C15) end-group formed a unique family that exhibited a melting temperature (Tm). The monomer (C15-1P-CL1), dimer (C15-1P-CL2), and trimer (C15-1P-CL3) had Tm values of 34 to 43 °C. Olefinic and aliphatic terminal groups had an opposite effect on the PCL monodisperse oligomers. To illustrate both effects, Fig. 10 shows the DSC thermograms of two types of families with different end-groups (aliphatic C15-1P-CL and olefinic C15F-CL).
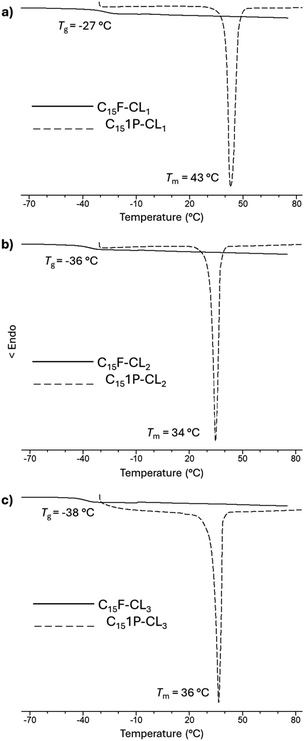 | ||
| Fig. 10 DSC thermograms for species derived from farnesol (C15F-CLx) and 1-pentadecanol (C151P-CLx) (where x = 1, 2, 3): (a) monomer, (b) dimer and (c) trimer. | ||
An amorphous domain was induced by the olefinic end-group farnesyl, and the semicrystalline domain was favored by the aliphatic terminal group pentadecyl. Both terminal groups induced monodisperse oligomers (monomer, dimer, and trimer) with the same physical properties as those of the alcohols or initiators [farnesol (C15H26O, liquid), and 1-pentadecanol (C15H32O, solid)].
These results suggest the importance of the alcohols (R-OH) as initiators and that the hybridization (sp2, olefin vs. sp3, aliphatic) and the substituents (methyl branch, olefin vs. linear, aliphatic) are the key to the physical properties of monodisperse monomer, dimer, and trimer species. To illustrate the physical properties of both monodisperse monomers with different terminal groups (C15F-CL1 and C151P-CL1), Fig. 11 shows the POM micrography results obtained in different environments. C15F-CL1 appears as an amorphous liquid, but C151P-CL1 exhibits a spherulite showing a semicrystalline domain.
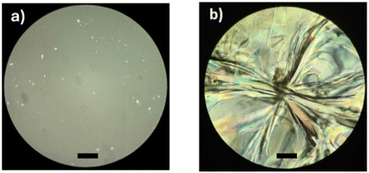 | ||
| Fig. 11 Polarized optical microscopy (POM, magnification: 40×) of (a) C15F-CL1 and (b) C151P-CL1 (Table 2). Black bar = 50 μm. | ||
To examine the thermal stability, the initiators were characterized by thermogravimetric analysis (TGA) (Fig. 12a and b). The thermograms show differences in the thermal stability between farnesol and 1-pentadecanol with a very different thermal decomposition temperatures (Td) of 296 and 250 °C, respectively. However, the monodisperse monomers functionalized with farnesyl (C15F-CL1) and pentadecyl (C151P-CL1) end-groups (Fig. 12c and d) showed an increase in Td relative to their initiators. Thus, the addition of a caprolactone unit provides an effect of thermal stability toward pyrolysis.
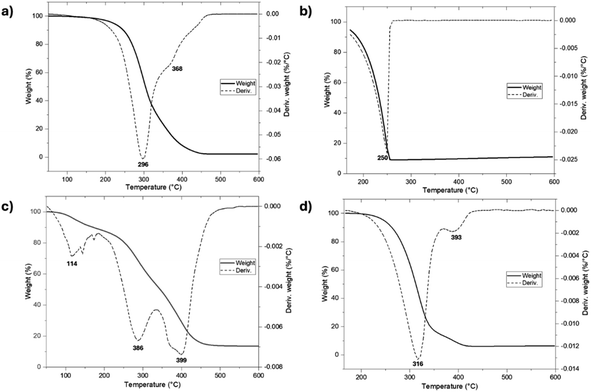 | ||
| Fig. 12 Thermal degradation (TGA) of (a) farnesol, (b) 1-pentadecanol, (c) C15F-CL1, and (d) C151P-CL1. | ||
3.3. Biological properties of monodisperse oligo(CL)
Terpenes such as β-citronellol, geraniol, nerol, and farnesol, have been studied due to their antibacterial activities.34,39,64–70 One of the goals in this work was to preserve the biological effects and properties of terpenes as part of terminal groups in monodisperse oligomeric species. First, a conventional antibiotic, ciprofloxacin, and a monodisperse oligomer, C10C-CL1, were compared using antibiograms (Fig. 13). There were dramatic differences in antibacterial activity and effective concentrations between ciprofloxacin and C10C-CL1. The C10C-CL1 inhibited the growth of S. aureus (Gram positive), but the inhibition decreased at low concentration.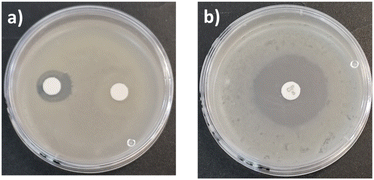 | ||
| Fig. 13 Antibiograms of S. aureus ATCC # 6538 in presence of (a) C10C-CL1 at 33 mg mL−1 (left), and 512 μg mL−1 (right). (b) Ciprofloxacin at 0.005 mg mL−1. | ||
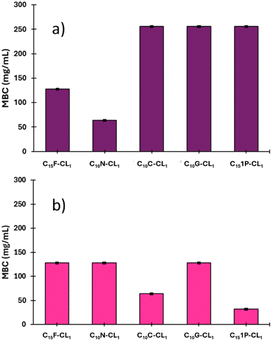
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were studied using the NCCLS method71 and two types of bacteria: S. aureus (Gram positive) and P. aeruginosa (Gram negative) (Table 3). All alcohols previously used as initiators in the polymerizations (β-citronellol, nerol, geraniol, farnesol, and 1-pentadecanol) showed antibacterial activity, as reported previously.34,39,64–70
| S. aureus ATCC #6538 | P. aeruginosa ATCC # 13388 | ||||
|---|---|---|---|---|---|
| MIC (95%) | MBC | MIC (95%) | MBC | ||
| Reference | Ciprofloxacin | 0.125 ± 0.01 | 0.25 ± 0.01 | ≤0.06 ± 0.01 | 0.06 ± 0.01 |
| Series 1 | Farnesol | 32 ± 0.01 | 64 ± 0.01 | 64 ± 0.01 | 128 ± 0.01 |
| C15F-CL1 | 64 ± 0.01 | 128 ± 0.02 | 64 ± 0.05 | 128 ± 0.05 | |
| C15F-CL2 | 64 ± 0.02 | 128 ± 0.02 | 64 ± 0.01 | 128 ± 0.01 | |
| C15F-CL3 | 64 ± 0.05 | 128 ± 0.05 | 64 ± 0.06 | 128 ± 0.06 | |
| Series 2 | Nerol | 64 ± 0.02 | 128 ± 0.02 | 32 ± 0.01 | 64 ± 0.01 |
| C10N-CL1 | 32 ± 0.01 | 64 ± 0.01 | 64 ± 0.05 | 128 ± 0.05 | |
| C10N-CL2 | 64 ± 0.05 | 128 ± 0.05 | 64 ± 0.05 | 128 ± 0.05 | |
| C10N-CL3 | 64 ± 0.05 | ≥256 ± 0.06 | 64 ± 0.05 | 128 ± 0.05 | |
| Series 3 | β-Citronellol | 32 ± 0.02 | 64 ± 0.01 | 64 ± 0.04 | 128 ± 0.05 |
| C10C-CL1 | 64 ± 0.05 | 256 ± 0.05 | 32 ± 0.01 | 64 ± 0.01 | |
| C10C-CL2 | 32 ± 0.02 | 64 ± 0.01 | 32 ± 0.02 | 64 ± 0.01 | |
| C10C-CL3 | 32 ± 0.01 | 64 ± 0.01 | 64 ± 0.05 | 128 ± 0.05 | |
| Series 4 | Geraniol | 64 ± 0.06 | 256 ± 0.05 | 32 ± 0.04 | 64 ± 0.01 |
| C10G-CL1 | 64 ± 0.04 | ≥256 ± 0.05 | 64 ± 0.02 | 128 ± 0.01 | |
| C10G-CL2 | 64 ± 0.02 | ≥256 ± 0.05 | 64 ± 0.05 | 128 ± 0.01 | |
| C10G-CL3 | 32 ± 0.01 | ≥256 ± 0.05 | 64 ± 0.06 | 128 ± 0.01 | |
| Series 5 | 1-Pentadecanol | 64 ± 0.04 | ≥256 ± 0.05 | 32 ± 0.01 | 64 ± 0.04 |
| C151P-CL1 | 64 ± 0.04 | ≥256 ± 0.06 | 16 ± 0.01 | 32 ± 0.01 | |
| C151P-CL2 | 64 ± 0.02 | ≥256 ± 0.05 | 32 ± 0.04 | 64 ± 0.05 | |
| C151P-CL3 | 64 ± 0.04 | ≥256 ± 0.05 | 32 ± 0.02 | 64 ± 0.05 | |
In the case of S. aureus, an MIC of 32 μg mL−1 was obtained for β-citronellol and farnesol. The same result was found for geraniol, nerol, and 1-pentadecanol with P. aeruginosa.
The antimicrobial activity of terpenes against Gram-positive bacteria is influenced by their lipophilicity and hydrophobicity, as well as the presence of hydroxyl groups.70,72 The bactericidal behavior of oligomers can be regulated through the incorporation of terpene alcohols, which modulate the membrane stability of S. aureus or potentiate their bacterial loss integrity.73 According to Lopez-Romero et al.,74 the bactericidal mechanisms of essential oils such as β-citronellol include surface-charge alteration and K+ leakage, which improve the disruption of S. aureus membranes.
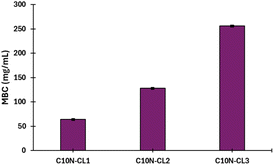
As shown in Table 3, the monodisperse oligomers functionalized with terpenes had antibacterial activity and preserved properties similar to those of their precursors. For instance, the length of the oligomers (C10 or C15) and the unsaturation degree are independent of the bactericidal response, which is mainly attributed to each terpene as terminal group. However, higher bactericidal response is observed in specific monomers, including C10N-CL1, and C10C-CL2–3 against S. aureus, and C101P-CL1 against P. aeruginosa (Fig. 14). This behavior indicates that the size of monodisperse oligomers, for example, mainly monomers plays a crucial role in the antibacterial properties associated with the availability of the terminal chain of terpenes, which can perform the ionic imbalance through the cell membrane and interfere with glycan synthesis, resulting in cell death.75,76 For example, for series 1, the results with S. aureus showed that farnesol had half the values of MIC (32 μg mL−1) and MBC (64 μg mL−1) with respect to the monomer (C15F-CL1), dimer (C15F-CL2), and trimer (C15F-CL3). This indicated a decreased activity of oligomers, which is probably due to the repetitive unit of CL. However, in the case of P. aeruginosa, farnesol and the oligomers exhibited an MIC and MBC of 64 μg mL−1 and 128 μg mL−1, respectively. C15F-CL1, C15F-CL2, and C15F-CL3 exhibited the same antibacterial activity (MIC and MBC) for both S. aureus and P. aeruginosa. On the other hand, comparing two types of geometric isomers as terminal groups in terms of MBC, such as, derivatives of nerol [Z isomer or cis, (C10N-CLx)] vs. geraniol [E isomer or trans, (C10G-CLx)] (Table 3), the effect on the P. aeruginosa was negligible. However, with the bacterium S. aureus the case was dramatically different, where C10N-CLx monodisperse species showed a pattern on the antibacterial activity, where monomer > dimer > trimer (Fig. 15). In contrast, C10G-CLx species had non-significant effect. So, this result suggests that Z isomer (or cis) end group in the C10N-CLx can induce a significant disruption, probably, in the cell membrane of S. aureus, and this effect is directly proportional to the weight percent (wt%) of the nerol as a terminal group, from trimer (31%) to monomer (57%) (Fig. 15). In this sense, the exploration of organic molecules with Z isomers (or cis isomers as end-groups) as a significant factor against S. aureus will be worked in a future contribution.
Although the monodisperse species (Table 3) demonstrated antibacterial activity, there was a significant gap from that of the conventional antibiotic ciprofloxacin, which has 512 times higher activity in terms of MIC for S. aureus. This is attributed to the direct inhibition of the DNA-gyrase and prevention of bacterial DNA replication. It is important to note that 1-pentadecanol oligomers demonstrate slight bactericidal behavior against S. aureus. However, the MIC value of C151P-CL1 against P. aeruginosa is 16 μg mL−1 with the pentadecyl end-group. The key point was the difference with P. aeruginosa, for which MIC and MBC were low compared to S. aureus. These results suggest that an aliphatic terminal group tends to act more as an antibiotic on the Gram-negative bacterium such as P. aeruginosa compared to the Gram-positive bacterium S. aureus. Thus, the bacterial membrane composition (particularly peptidoglycan) plays a key role in the oligomer interaction.
It is well known that the molecules as terpenes and aromatic compound has been explored in terms of their antifungal77,78 and antibacterial79 activity, for example, nerol,77 citral,78 trans-anethole79 and estragole.79 In these cases, the inherent hydrophobicity to the terpenes play a significant role, where the affinity and accumulation of those in the cell membrane produce a loss of membrane integrity;79 this phenome was detected with an increase in the extracellular conductivity and extracellular pH,77,78 which indicates rapid leakage of ions; complementary, using scanning electron microscopy (SEM) was observed severe effects on the cell wall and cytoplasmic membrane.79 In the case of monodisperse oligomers (Table 3), probably, the mechanism of antibacterial activity involves a significant membrane disruption against bacteria, however, more studies will be explored in our laboratory to validate the damage.
4. Conclusions
In this work, a series of oligomers derived from PCL were synthesized by ring-opening polymerization (ROP) of ε-caprolactone (CL) using terpenes as initiators called oligo(CLs). The oligo(CLs) had specific terminal groups derived from terpenes. Using a flash column chromatography (FCC) a family of fifteen monodisperse species such as monomer, dimer, and trimer were isolated. The thermal properties of monodisperse species derived from olefinic terpenes included a unique glass transition temperature (Tg), which decreased from the monomer to the trimer and increased the amorphous domain. However, monodisperse oligomers derived from aliphatic pentadecyl exhibited a semicrystalline domain with characteristic melting temperature (Tm).There were remarkable differences in physical properties between monodisperse oligomeric species with farnesyl and pentadecyl end-groups, which both have the same number of carbons (C15) but different functionality. Farnesyl produced liquid oligomers, and pentadecyl produced semicrystalline powders. This is the first report of using a family of terpenes to functionalize monodisperse oligomeric species. Due to the antibacterial properties of terpenes and their use as initiators, the monodisperse species showed antibacterial activity, especially for Gram-positive S. aureus. These monodisperse species could lead to new antibiotic compounds with potential applications. Studies on the mechanisms of action are currently underway in our laboratory.
Data availability
The data supporting this article have been included as part of ESI.†Author contributions
María Guadalupe Ortiz-Aldaco: investigation, validation, formal analysis, writing – original draft. Miriam Estévez: funding acquisition. Beatriz Liliana España-Sánchez: biological properties, supervision. José Bonilla-Cruz: thermal properties. Eloy Rodríguez-deLeón: mass spectrometry. José E. Báez: conceptualization, supervision, formal analysis, writing – original draft, writing – review, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
José E. Báez would like to thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) Ciencia Básica for the grant “Proyecto SEP/284893”, “CF-2023-I-2233”, and “CONACYT-SENER 177007”, the Sistema Nacional de Investigadores (SNI), the Dirección de Apoyo a la Investigación y al Posgrado (DAIP), and the University of Guanajuato (UG) Mexico, for financial support. José E. Báez would like to thank Dr Alberto Toxqui-Terán (DSC), M. en I. Gerardo Antonio Fonseca Hernández (TGA), Dr Claudia Leticia Mendoza Macías (Biological properties), and Q. Daniel Ruiz Plaza (NMR). The authors thank the Laboratorio Nacional de Caracterización de Propiedades Fisicoquímicas y Estructura Molecular (UG-UAA-CONACYT) of the University of Guanajuato (UG).References
- A. D. Jenkins, P. Kratochvíl, R. F. T. Stepto and U. W. Suter, Pure Appl. Chem., 1996, 68, 2287–2311 CAS.
- J. E. Báez, K. J. Shea, P. R. Dennison, A. Obregón-Herrera and J. Bonilla-Cruz, Polym. Chem., 2020, 11, 4228–4236 RSC.
- P. D. Falcón-Torres, A. G. Morales-Segoviano, A. A. Martínez-Salazar, M. G. Ortiz-Aldaco, R. Navarro, A. Marcos-Fernández, A. Ramírez-Hernández, K. J. Moreno and J. E. Báez, Chem. Pap., 2021, 75, 5587–5598 CrossRef.
- J. E. Báez and A. Marcos-Fernández, Int. J. Polym. Anal. Charact., 2015, 20, 637–644 CrossRef.
- M. P. Barrera-Nava, R. Navarro, A. Marcos-Fernández and J. E. Báez, RSC Adv., 2024, 14, 27241–27251 RSC.
- E. Rodriguez-deLeón, M. Bah, J. E. Báez, M. T. Hernández-Sierra, K. J. Moreno, A. Nuñez-Vilchis, J. Bonilla-Cruz and K. J. Shea, RSC Adv., 2022, 12, 30851–30859 RSC.
- E. Rodríguez-deLeón, M. Bah, J. O. C. Jiménez-Halla, J. Bonilla-Cruz, M. Estevez and J. E. Báez, Polym. Chem., 2019, 10, 6580–6587 RSC.
- J. E. Báez, A. Marcos-Fernández, R. Navarro, C. García, A. Ramírez-Hernández and K. J. Moreno, J. Polym. Res., 2019, 26, 32 CrossRef.
- J. E. Báez, A. Marcos-Fernández and R. Navarro, Chem. Pap., 2019, 73, 1287–1299 CrossRef.
- O. F. González-Belman, J. O. C. Jiménez-Halla, G. González and J. E. Báez, RSC Adv., 2024, 14, 34733–34745 RSC.
- M. G. Ortiz-Aldaco, J. E. Báez and J. O. C. Jiménez-Halla, RSC Adv., 2020, 10, 30815–30824 RSC.
- A. Guerra, P. Cano, M. Rabionet, T. Puig and J. Ciurana, Materials, 2018, 11, 1679–1691 CrossRef PubMed.
- M. Vert, Eur. Polym. J., 2015, 68, 516–525 CrossRef CAS.
- C. Gong, Y. Gu, X. Wang and C. Yi, Macromolecules, 2022, 55, 5342–5352 CrossRef CAS.
- J. De Neve, J. J. Haven, S. Harrison and T. Junkers, Angew. Chem., Int. Ed., 2019, 58, 13869–13873 CrossRef CAS PubMed.
- J. J. Haven and T. Junkers, Polym. Chem., 2019, 10, 679–682 RSC.
- J. Lawrence, S. H. Lee, A. Abdilla, M. D. Nothling, J. M. Ren, A. S. Knight, C. Fleischmann, Y. Li, A. S. Abrams, B. V. K. J. Schmidt, M. C. Hwaker, L. A. Connal, A. J. McGrath, P. G. Clark, W. R. Gutekunst and C. J. Hawker, J. Am. Chem. Soc., 2016, 138, 6306–6310 CrossRef CAS PubMed.
- J. M. Ren, J. Lawrence, A. S. Knight, A. Abdilla, R. B. Zerdan, A. E. Levi, B. Oschmann, W. R. Gutekunst, S. H. Lee, Y. Li, A. J. McGrath, C. M. Bates, G. G. Qiao and C. J. Hawker, J. Am. Chem. Soc., 2018, 140, 1945–1951 CrossRef CAS PubMed.
- N. S. Vail, C. Stubbs, C. Biggs and M. I. Gibson, ACS Macro Lett., 2017, 6, 1001–1004 CrossRef CAS PubMed.
- M. Iriti, G. Colnaghi, F. Chemat, J. Smadja, F. Faoro and F. A. Visinoni, Flavour Fragrance J., 2006, 21, 704–712 CrossRef CAS.
- C. B. Silva, E. Simionatto, S. C. Hess, M. T. L. P. Peres, A. W. Júnior, N. R. Poppi, O. Faccenda, A. C. S. Candido and S. P. Q. Scalon, Quim. Nova, 2009, 32(9), 2373–2376 CrossRef.
- J. C. Romero, H. González-Ríos, A. Borges and M. Simões, J. Evidence-Based Complementary Altern. Med., 2009, 1–9 Search PubMed.
- E. Breitmaier, Appl. Organomet. Chem., 2007, 21, 377 CrossRef.
- J. D. Connolly and R. A. Hill, Nat. Prod. Rep., 2010, 27, 79–132 RSC.
- H. S. Nunes and M. G. Miguel, Trends Phytochem. Res., 2017, 1(3), 111–128 CAS.
- R. G. Brito, P. L. dos Santos, J. S. S. Quintans, W. de Lucca-Júnior, A. A. S. Araújo, S. Saravanan, I. R. A. Menezes, H. D. M. Coutinho and L. J. Quintans-Júnior, Chem.-Biol. Interact., 2015, 239, 111–117 CrossRef CAS PubMed.
- V. Y. Ma, L. Chan and K. J. Carruthers, Arch. Phys. Med. Rehabil., 2014, 95(5), 986–995 CrossRef.
- D. P. De Sousa, J. C. R. Goncalves, L. Quintans-Júnior, J. S. Cruz, D. A. M. Araújo and R. N. Almeida, Neurosci. Lett., 2006, 401, 231–235 CrossRef PubMed.
- S. Kakaraparthi, K. V. N. S. Srinivas, J. K. Kumar, A. N. Kumar, D. K. Rajput and S. Anubala, Ind. Crops Prod., 2015, 69, 348–354 CrossRef.
- R. Essid, M. Hammami, D. Gharbi, I. Karkouch, T. B. Hamouda, S. Elkahoui, F. Limam and O. Tabbene, Appl. Microbiol. Biotechnol., 2017, 101(18), 6993–7006 CrossRef CAS.
- C. Labadie, C. Ginies, M. H. Guinebretiere, C. M. G. C. Renard, C. Cerutti and F. Carlin, Int. Food Res. J., 2015, 76(3), 576–586 CrossRef CAS PubMed.
- L. G. Toledo, M. A. S. Ramos, L. Spósito, E. M. Castilho, F. R. Pavan, E. O. Lopes, G. J. Zocolo, F. A. N. Silva, T. H. Soares, A. G. Santos, T. M. Bauab and Almeida M. T. G., Int. J. Mol. Sci., 2016, 17(8), 1252–1268 CrossRef.
- H. D. M. Coutinho, M. A. Freitas, C. N. F. L. Gondim, R. S. Albuquerque, J. V. A. Ferreira and J. C. Andrade, Rev. Cubana Plant. Med., 2015, 20(1), 98–105 Search PubMed.
- M. H. Pereira-de Lira, F. P. de Andrade-Júnior, G. F. Queiroga-Moraes, G. da Silva-Macena, F. de Oliveira-Pereira and I. Oliveira-Lima, J. Essent. Oil Res., 2020, 32, 187–197 CrossRef.
- G. I. Marei, M. A. A. Rasoul and S. A. M. Abdelgaleil, Pestic. Biochem. Physiol., 2012, 103, 56–61 CrossRef CAS.
- M. Arputha, P. Selvamani and S. Latha, Int. J. Environ. Sci., 2012, 3, 583–590 Search PubMed.
- A. Panggabean and G. E. Willem, J. Akad. Kim., 2013, 10(2), 74–79 Search PubMed.
- W. Nurcholis, M. Takene, R. Puspita, L. Tumanggor and E. N. Qomaliyah, Curr. Biochem., 2019, 6(2), 86–89 Search PubMed.
- L. Jirovetz, G. Buchbauer, E. Schmidt, A. S. Stoyanova, Z. Denkova, R. Nikolova and M. Geissler, J. Essent. Oil Res., 2007, 19(3), 288–291 CrossRef CAS.
- Y. Xu, K. Zhang, S. Reghu, Y. Lin, M. B. Chan-Park and X. W. Liu, Biomacromolecules, 2019, 20, 949–958 CrossRef CAS.
- J. J. Haven, J. De Neve, A. C. Villavicencio and T. Junkers, Polym. Chem., 2019, 10, 6540–6544 RSC.
- S. Singh, M. M. Alrobaian, N. Molugulu, N. Agrawal, A. Numan and P. Kesharwani, ACS Omega, 2020, 5(21), 11935–11945 CrossRef CAS PubMed.
- F. G. Santos, L. C. Bonkovoski, F. P. Garcia, T. S. P. Cellet, M. A. Witt, C. V. Nakamura, A. F. Rubira and E. C. Muniz, ACS Appl. Mater. Interfaces, 2017, 9, 9304–9314 CrossRef CAS.
- Y. Xu, K. Zhang, S. Reghu, Y. Lin, M. B. Chan-Park and X. W. Liu, Biomacromolecules, 2019, 20, 949–958 CrossRef CAS PubMed.
- W. Yuan, J. Wei, H. Lu, L. Fan and J. Du, Chem. Commun., 2012, 48, 6857–6859 RSC.
- X. Zhou, J. He and C. Zhou, Polym. Chem., 2019, 10, 945–953 RSC.
- C. Sardo, B. Nottelet, D. Triolo, G. Giammona, X. Garric, J. P. Lavigne, G. Cavallaro and J. Coudane, Biomacromolecules, 2014, 15, 4351–4362 CrossRef CAS PubMed.
- M. Wang, C. Zhou, J. Chen, Y. Xiao and J. Du, Bioconjugate Chem., 2015, 26, 725–734 CrossRef CAS PubMed.
- F. Huang, Y. Gao, Y. Zhang, T. Cheng, H. Ou, L. Yang, J. Liu, L. Shi and J. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 16880–16889 CrossRef CAS.
- Y. Xi, T. Song, S. Tang, N. Wang and J. Du, Biomacromolecules, 2016, 17, 3922–3930 CrossRef CAS PubMed.
- S. Haas, N. Hain, M. Raoufi, S. Handschuh-Wang, T. Wang, X. Jiang and H. Schönherr, Biomacromolecules, 2015, 16, 832–841 CrossRef CAS PubMed.
- B. Hisey, P. J. Ragogna and E. R. Gillies, Biomacromolecules, 2017, 18, 914–923 CrossRef CAS PubMed.
- M. H. Xiong, Y. Bao, X. Z. Yang, Y. C. Wang, B. Sun and J. Wang, J. Am. Chem. Soc., 2012, 134, 4355–4362 CrossRef CAS PubMed.
- R. Ghosh, M. Malhotra, R. R. Madhuri-Sathe and M. Jayakannan, Biomacromolecules, 2020, 21(7), 2896–2912 CrossRef CAS PubMed.
- A. Karakucuk and S. Tort, Pharm. Dev. Technol., 2020, 25, 1216–1225 CrossRef CAS PubMed.
- M. Swiontek-Brzezinska, M. Walczak, U. Jankiewicz and M. Pejchalová, J. Polym. Environ., 2018, 26, 589–595 CrossRef.
- A. Martínez-Abad, G. Sánchez, V. Fuster, J. M. Lagaron and M. J. Ocio, Food Control, 2013, 34, 214–220 CrossRef.
- B. Jummes, W. G. Sganzerla, C. Gonçalves-da Rosa, C. Montanheiro-Noronha, M. Ramos-Nunes, F. Cleber-Bertoldi and P. L. Manique-Barreto, Biocatal. Agric. Biotechnol., 2020, 23, 101499 CrossRef.
- J. E. Báez, M. Martínez-Rosales and A. Martinez-Richa, Polymer, 2003, 44, 6767–6772 CrossRef.
- J. E. Báez, A. Marcos-Fernández and A. Martinez-Richa, Macromolecules, 2005, 38, 1599–1608 CrossRef.
- J. E. Báez, A. Marcos-Fernández, R. Lebrón-Aguilar and A. Martinez-Richa, Polymer, 2006, 47, 8420–8429 CrossRef.
- L. Patiny and A. Borel, ChemCalc: A building block for tomorroẃs chemical infrastructure, J. Chem. Inf. Model., 2013, 53(5), 1223–1228 CrossRef CAS.
- J.S. Desport, G. Franche and L. Patiny, Rapid Commun. Mass Spectrom., Rapid Commun. Mass Spectrom., 2019, 34(S2), e8652 CrossRef.
- S. K. Bhattamisra, C. H. Kuean, L. B. Chieh, V. L. Y. Yan, C. K. Lee, L. P. Hooi, L. P. Shyan, Y. K. Liew, M. Candasamy and P. S. Sahu, Nat. Prod. Commun., 2018, 13(7), 7791–793 CrossRef.
- W. R. Li, T. H. Zeng, Z. Q. Zhang, Q. S. Shi and X. B. Xie, Front. Microbiol., 2023, 14, 1–10 CAS.
- R. L. Silva-Pereira, F. Ferreira-Campina, M. d. S. Costa, R. Pereira-da Cruz, T. Sampaio-de Freitas, A. T. Lucas-dos Santos, B. Gonçalves-Cruz, D. M. de Sena-Júnior, I. K. Campos-Lima, M. Regivânia-Xavier, A. M. Rodrigues-Teixeira, I. R. Alencar-de Menezes, L. J. Quintans-Júnior, A. A. de S. Araújo and H. D. Melo-Coutinho, Microb. Pathog., 2021, 156, 1–7 CrossRef.
- M. Nagaki, T. Narita, H. Ichikawa, J. Kawakami and A. Nakane, Trans. Mater. Res. Soc. Jpn., 2011, 36(1), 55–58 CrossRef CAS.
- M. A. Jabra-Rizk, T. F. Meiller, C. E. James and M. E. Shirtliff, Antimicrob. Agents Chemother., 2006, 50(4), 1463–1469 CrossRef CAS PubMed.
- M. Kaneko, N. Togashi, H. Hamashima, M. Hirohara and Y. Inoue, J. Antibiot., 2011, 64, 547–549 CrossRef CAS.
- A. Fajdek-Bieda, J. Pawlińska, A. Wróblewska and A. Łuś, Molecules, 2024, 29, 950 CrossRef CAS.
- National Committee for Clinical Laboratory Standards, Approved standards M7-A3, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, National Committee for Clinical Laboratory Standards, Villanova, PA. 1993 Search PubMed.
- H. Zengin and A. Baysal, Molecules, 2014, 11, 17773–17798 CrossRef PubMed.
- N. Togashi, Y. Inoue, H. Hamashima and A. Takano, Molecules, 2008, 13, 3069–3076 CrossRef CAS.
- J. C. Lopez-Romero, H. González-Ríos, A. Borges and M. Simões, J. Evidence-Based Complementary Altern. Med., 2015, 1–9 Search PubMed.
- L. Lian, C. Pang, H. Wei and L. Hong, Macromol. Biosci., 2023, 23, 2300169 CrossRef CAS PubMed.
- A. Ivanova, K. Ivanova, L. Fiandra, P. Mantecca, T. Catelani, M. Natan, E. Banin, G. Jacobi and T. Tzanov, Int. J. Mol. Sci., 2022, 23, 7527 CrossRef CAS PubMed.
- Y. Wang, X. Zeng, Z. Zhou, K. Xing, A. Tessema, H. Zeng and J. Tian, Food Control, 2015, 55, 54–61 CrossRef CAS.
- N. Tao, Q. OuYang and L. Jia, Food Control, 2014, 41, 116–121 CrossRef CAS.
- W.-R. Diao, Q.-P. Hu, H. Zhang and J.-G. Xu, Food Control, 2014, 35, 109–116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08104h |
| This journal is © The Royal Society of Chemistry 2025 |