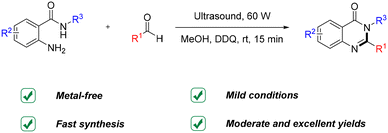
Ultrasound-assisted condensation cyclization reaction: fast synthesis of quinazolinones from o-aminobenzamides and aldehydes under ambient conditions†
Xuerou
Chen
,
Siqi
Li
,
Shilong
Sun
and
Wuji
Sun
 *
*
School of Pharmacy, North China University of Science and Technology, Tangshan, 063210, China. E-mail: sunwuji@yeah.net
First published on 9th December 2024
Abstract
In this work, a facile and efficient ultrasound-assisted fast synthesis of quinazolinones from o-aminobenzamides and aldehydes is reported. The reaction proceeds smoothly under ambient temperature and pressure conditions without the need for a metal catalyst in just 15 minutes. In addition, this approach exhibits broad substrate tolerance and provides a series of quinazolinones with moderate to excellent yields. The results reported here reveal an important new application of ultrasound-assisted synthesis in the fast synthesis of valuable organic products.
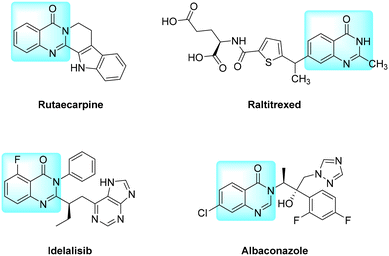
Quinazolinones are an important class of nitrogen-containing heterocycles that have been found to exhibit a broad spectrum of biological and pharmacological activity, including anti-inflammatory,1 antitumor,2 antimicrobial,3 antifungal4 and antiviral activities.5 They are core structures commonly existing in a variety of natural products and pharmaceutical candidates, and a number of quinazolinone-based drugs have been approved (Fig. 1). For example, rutaecarpine from a Chinese herbal drug is used to treat anti-inflammatory and gastrointestinal disorders, cholera and headache.6,7 Raltitrexed has played an important role in the treatment of advanced colorectal cancer where 5-fluorouracil (5-FU) is not tolerated or inappropriate, and has single-agent activity in a variety of advanced solid tumours.8,9 Idelalisib is a potent small-molecule PI3K inhibitor that abrogates PI3K/Akt signaling and promotes apoptosis.10,11 Albaconazole is a novel triazole antifungal drug with a potent broad spectrum of antifungal activity, favorable pharmacokinetics and high oral bioavailability.12,13
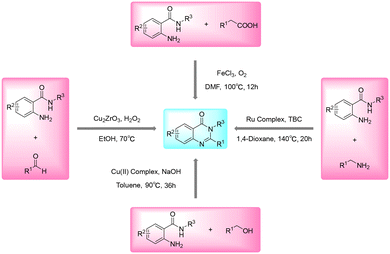
In view of their importance as privileged scaffolds, a number of different synthetic approaches have been developed to synthesize quinazolinones over the past several decades.14–21 Although many approaches have been used to synthesize quinazolinones to date, the most classical and versatile approach is the condensation reaction of o-aminobenzamides with aldehydes, alcohols, amines, or carboxylic acid derivatives (Scheme 1). Sreenivasa et al. revealed a protocol for the synthesis of quinazolinones via reaction between o-aminobenzamides and aldehydes using mesoporous ZrO2-supported Cu2O (Cu2ZrO3) as catalyst.22 Paul et al. reported a metal–ligand cooperative approach to achieve dehydrogenative functionalization of alcohols to quinazolinones.23 Yi et al. disclosed a ruthenium-catalyzed synthesis of quinazolinones from the dehydrogenative and deaminative coupling reactions of o-aminobenzamides with amines.24 Zhou et al. realized a strategy for the iron-catalyzed aerobic oxidative functionalization of sp3 C–H bonds for the construction of quinazolinones from o-aminobenzamides and carboxylic acid derivatives.25 Despite the merits of these approaches, some of them suffer from limitations such as corrosive or environmentally unfriendly catalysts, special prefunctionalized reagents, long reaction time and harsh reaction conditions. Therefore, the pursuit of alternative approaches for the synthesis of quinazolinones in a facile, efficient and green way is still urgently needed.
Over the past decades, several novel technologies have emerged as powerful operating tools for organic synthesis, with the following being the most commonly used: visible light irradiation,26–29 electrochemical technique,30,31 microwave irradiation32,33 and ultrasound irradiation.34,35 Due to the uniqueness of ultrasound irradiation in accelerating organic reactions, its application has been widely reported.36–38 Compared to conventional heating approaches, it not only reduces the reaction time but also improves the reaction rate and yield. In addition, ultrasound irradiation facilitates the reaction under ambient conditions, eliminating the need for high temperature, high pressure and metal catalyst.
Owing to these benefits, ultrasound irradiation offers an attractive alternative to achieve chemical transformations which are usually difficult to attain under mild conditions. Herein, we present an ultrasound-assisted fast synthesis of quinazolinones from the condensation cyclization reaction of o-aminobenzamides and aldehydes (Scheme 2). The reaction is carried out under ambient temperature and pressure conditions without the need for a metal catalyst. We believe that the present strategy not only represents a facile and efficient approach for the synthesis of quinazolinones but also exhibits promising prospects in organic synthesis.
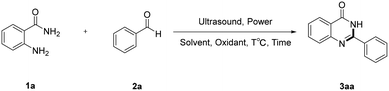
The model reaction was initiated by choosing o-aminobenzamide 1a and benzaldehyde 2a as substrates in CH3OH under ultrasound irradiation at 150 W and 60 °C for 30 min. As expected, the desired product 3aa was obtained with 55% yield (Table 1, entry 1). First, different solvents, such as C2H5OH, CH3CN, DMF, THF and H2O, were screened. Compared with CH3OH, a similar yield was obtained by using C2H5OH as the solvent (Table 1, entry 2), and CH3CN provided a lower yield (Table 1, entry 3). Besides, other solvents were not suitable for this reaction (Table 1, entries 4–6). Subsequently, various oxidants, such as DDQ, TBHP and DTBP, were evaluated and it was found that the best yield, as high as 80%, of the desired product 3aa was obtained when DDQ was used as the oxidant (Table 1, entry 7), while other oxidants such as TBHP or DTBP did not affect the yield of 3aa significantly (Table 1, entries 8 and 9).
| Entry | Solvent | Oxidant (equiv.) | Power (W) | Temp (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: o-aminobenzamide 1a (1.0 mmol) and benzaldehyde 2a (1.0 mmol) as substrates, oxidant based on 1a, solvent (10.0 mL), under ultrasound irradiation. b Isolated yield. | ||||||
| 1 | CH3OH | — | 150 | 60 | 30 | 55 |
| 2 | C2H5OH | — | 150 | 60 | 30 | 53 |
| 3 | CH3CN | — | 150 | 60 | 30 | 36 |
| 4 | DMF | — | 150 | 60 | 30 | Trace |
| 5 | THF | — | 150 | 60 | 30 | Trace |
| 6 | H2O | — | 150 | 60 | 30 | N.R. |
| 7 | CH3OH | DDQ (1.0) | 150 | 60 | 30 | 80 |
| 8 | CH3OH | TBHP (1.0) | 150 | 60 | 30 | 68 |
| 9 | CH3OH | DTBP (1.0) | 150 | 60 | 30 | 60 |
| 10 | CH3OH | DDQ (1.2) | 150 | 60 | 30 | 90 |
| 11 | CH3OH | DDQ (1.5) | 150 | 60 | 30 | 90 |
| 12 | CH3OH | DDQ (1.2) | 120 | 60 | 30 | 90 |
| 13 | CH3OH | DDQ (1.2) | 90 | 60 | 30 | 90 |
| 14 | CH3OH | DDQ (1.2) | 60 | 60 | 30 | 90 |
| 15 | CH3OH | DDQ (1.2) | 30 | 60 | 30 | 61 |
| 16 | CH3OH | DDQ (1.2) | 60 | 50 | 30 | 90 |
| 17 | CH3OH | DDQ (1.2) | 60 | rt | 30 | 90 |
| 18 | CH3OH | DDQ (1.2) | 60 | rt | 15 | 90 |
| 19 | CH3OH | DDQ (1.2) | 60 | rt | 10 | 83 |
Remarkably, the yield of 3aa increased from 80% to 90% with the DDQ loading increased to 1.2 equiv. (Table 1, entry 10). However, when the DDQ loading increased to 1.5 equiv., the yield of 3aa was not significantly improved (Table 1, entry 11). Next, the effect of different powers of ultrasound irradiation on the reaction was investigated. It was observed that the reaction afforded the best result with 90% yield under ultrasound irradiation with power of 60 W, and increase in the power from 60 W to 150 W did not affect the yield of the desired product 3aa (Table 1, entries 12–14). Further decrease in power to 30 W gave the desired product 3aa with only 61% yield (Table 1, entry 15). Then, the effect of temperature on the reaction was further explored. It was clear that increasing the reaction temperature had no effect on the reaction yield (Table 1, entry 16). We observed the formation of the desired product 3aa in an excellent yield of 90% when the reaction was carried out at room temperature (Table 1, entry 17). Finally, experiments with different reaction times were performed and it was found that the optimal reaction time could be reduced to 15 min (Table 1, entry 18). A decrease in yield was observed when the reaction time was reduced from 15 min to 10 min (Table 1, entry 19). In summary, the optimal reaction conditions were found to be o-aminobenzamide 1a (1.0 mmol), benzaldehyde 2a (1.0 mmol), DDQ (1.2 mmol), CH3OH (10 mL) under ultrasound irradiation at 60 W for 15 min at room temperature.
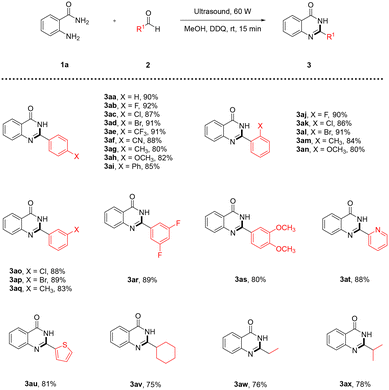
Having identified the optimal reaction conditions, we explored the applicability of the reaction scope of diversely substituted aldehydes (Table 2). First, monosubstituted aromatic aldehydes bearing groups with different electronic effects at the para-, ortho- or meta-position were successively subjected to the conditions, and the desired products were obtained in moderate to good yields (3aa–3aq). Generally, aromatic aldehydes with electron-withdrawing substituents (–F, –Cl, –Br, –CF3, –CN) provided higher yields of the desired products (3ab–3af, 3aj–3al, 3ao–3ap), mostly around 90%, while the desired products of electron-donating substituents (–CH3, –OCH3) were acquired in slightly lower yields of 80–85% (3ag–3ai, 3am–3an, 3aq). Also, the positions of the substituents on the aromatic aldehydes had little influence on the reaction. Multi-substituted aromatic aldehydes also worked well to afford the corresponding products 3ar and 3as in 89% and 80% yield, respectively. Next, heteroaromatic aldehydes with pyridinyl and thienyl groups were examined, and the corresponding products 3at and 3au were successfully acquired in 88% and 81% yield, respectively. In addition, it is important to note that aliphatic aldehydes were also tolerated and led to the corresponding products (3av–3ax) in acceptable yields of 75–78%.
| a Reaction conditions: o-aminobenzamide 1a (1.0 mmol), aldehyde 2 (1.0 mmol), DDQ (1.2 mmol), CH3OH (10 mL), ultrasound irradiation, 60 W, 15 min, rt. |
|---|
|
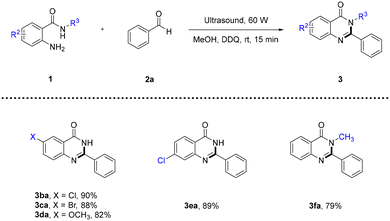
To further supplement the substrate scope, we investigated the scope of o-aminobenzamides with benzaldehyde 2a as the reaction partner under optimal reaction conditions. To our pleasure, a range of o-aminobenzamides bearing various substitution patterns were tolerated in this reaction, thus giving the corresponding products in moderate to good yields (Table 3). Primary o-aminobenzamides bearing either an electron-withdrawing group or an electron-donating group at different positions of the phenyl ring proceeded smoothly to afford the desired products (3ba–3ea) in 82–90% yields. Moreover, 2-amino-N-methylbenzamide 1f was also compatible and afforded the corresponding product 3fa in 79% yield under optimal reaction conditions.
| a Reaction conditions: o-aminobenzamide 1 (1.0 mmol), benzaldehyde 2a (1.0 mmol), DDQ (1.2 mmol), CH3OH (10 mL), ultrasound irradiation, 60 W, 15 min, rt. |
|---|
|
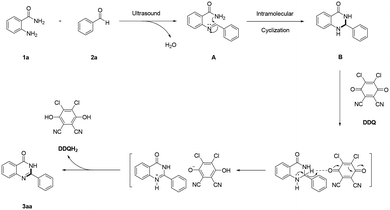
Based on the relevant literature,39–43 a plausible reaction mechanism for the ultrasound-assisted synthesis of quinazolinones from the reaction of o-aminobenzamides and aldehydes is presented in Scheme 3. Initially, the condensation reaction between o-aminobenzamide 1a and benzaldehyde 2a afforded the imine intermediate A under ultrasound irradiation. Subsequently, the intramolecular cyclization of intermediate A occurred to generate the aminal intermediate B. Finally, the aminal intermediate B was prone to be oxidized to the desired product 3aa in the presence of DDQ which may be converted to the by-product DDQH2. It is supposed that oxidation of the substrate occurs by hydride transfer from the substrate via anomeric-based oxidation to DDQ, thereby forming an ion pair adduct. The substrate-cation/DDQH ion pair may convert to the desired product and DDQH2.
Conclusions
In summary, we successfully developed a facile and efficient approach for the fast synthesis of quinazolinones via ultrasound-assisted condensation cyclization reaction of o-aminobenzamides and aldehydes. The approach was performed with CH3OH as the solvent and DDQ as the oxidant under ultrasound irradiation at 60 W for 15 min at room temperature. The reactions proceeded smoothly, affording the corresponding quinazolinones in moderate to excellent yields with good functional group tolerance. The present approach significantly improved conventional approaches, giving way to obvious advantages including ease of operation, short reaction time, metal-free and mild reaction conditions. Moreover, a plausible reaction mechanism for the ultrasound-assisted synthesis of quinazolinones was proposed based on the relevant literature. The application of this approach to the preparation of other valuable organic products is underway in our laboratory.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the support from the Tangshan Science and Technology Plan Project (No. 24130220C), the Basic Research Fund of Provincial Universities in Hebei Province (No. JJC2024037) and the Natural Science Foundation of Hebei Province of China (No. B2021209007).Notes and references
- A. Kumar and C. S. Rajput, Eur. J. Med. Chem., 2009, 44, 83–90 CrossRef CAS.
- M. A. Mohamed, R. R. Ayyad, T. Z. Shawer, A.-M. Alaa and A. S. El-Azab, Eur. J. Med. Chem., 2016, 112, 106–113 CrossRef CAS.
- S. K. Pandey, U. Yadava, A. Upadhyay and M. L. Sharma, Bioorg. Chem., 2021, 108, 104611 CrossRef PubMed.
- W. Ettahiri, R. Salim, M. Adardour, E. Ech-Chihbi, I. Yunusa, M. M. Alanazi, S. Lahmidi, A. E. Barnossi, O. Merzouki and A. Iraqi Housseini, Molecules, 2023, 28, 5340 CrossRef CAS.
- M. Ashraf-Uz-Zaman, X. Li, Y. Yao, C. B. Mishra, B. K. Moku and Y. Song, J. Med. Chem., 2023, 66, 10746–10760 CrossRef CAS PubMed.
- K. M. Tian, J. J. Li and S. W. Xu, Pharmacol. Res., 2019, 141, 541–550 CrossRef CAS.
- X. Li, J. Ge, Q. Zheng, J. Zhang and R. Liu, Phytomedicine, 2020, 68, 153180 CrossRef CAS PubMed.
- E. Van Cutsem, D. Cunningham, J. Maroun, A. Cervantes and B. Glimelius, Ann. Oncol., 2002, 13, 513–522 CrossRef CAS PubMed.
- C. Kelly, N. Bhuva, M. Harrison, A. Buckley and M. Saunders, Eur. J. Cancer, 2013, 49, 2303–2310 CrossRef CAS PubMed.
- D. A. Fruman and L. C. Cantley, N. Engl. J. Med., 2014, 370, 1061–1062 CrossRef.
- A. Markham, Drugs, 2014, 74, 1701–1707 CrossRef CAS PubMed.
- G. Aperis and E. Mylonakis, Expert Opin. Invest. Drugs, 2006, 15, 579–602 CrossRef CAS.
- R. M. Guillon, F. Pagniez, C. Picot, D. Hédou, A. Tonnerre, E. Chosson, M. Duflos, T. Besson, C. D. Logé and P. Le Pape, ACS Med. Chem. Lett., 2013, 4, 288–292 CrossRef CAS PubMed.
- F. Teng, T. Yu, Y. Peng, W. Hu, H. Hu, Y. He, S. Luo and Q. Zhu, J. Am. Chem. Soc., 2021, 143, 2722–2728 CrossRef CAS PubMed.
- X. Yang, G. Cheng, J. Shen, C. Kuai and X. Cui, Org. Chem. Front., 2015, 2, 366–368 RSC.
- X. Jiang, T. Tang, J. M. Wang, Z. Chen, Y. M. Zhu and S. J. Ji, J. Org. Chem., 2014, 79, 5082–5087 CrossRef CAS PubMed.
- Z. Xie, J. Lan, H. Zhu, G. Lei, G. Jiang and Z. Le, Chin. Chem. Lett., 2021, 32, 1427–1431 CrossRef CAS.
- Z. Bie, G. Li, L. Wang, Y. Lv, J. Niu and S. Gao, Tetrahedron Lett., 2016, 57, 4935–4938 CrossRef CAS.
- S. R. Vemula, D. Kumar and G. R. Cook, Tetrahedron Lett., 2018, 59, 3801–3805 CrossRef CAS.
- T. Ahl el haj, K. E. Mejdoubi, K. Sadraoui, B. C. El Idrissi and B. Sallek, Kinet. Catal., 2022, 63, 707–715 CrossRef.
- B. Dutta, R. P. Borah, L. Bahsis, A. Dutta, B. Sarma, K. Singh, A. Kumar and D. Sarma, Eur. J. Org. Chem., 2024, e202400789 CrossRef.
- L. Parashuram, S. Sreenivasa, S. Akshatha, V. U. Kumar and S. Kumar, Asian J. Org. Chem., 2017, 6, 1755–1759 CAS.
- S. Das, S. Sinha, D. Samanta, R. Mondal, G. Chakraborty, P. Brandao and N. D. Paul, J. Org. Chem., 2019, 84, 10160–10171 CAS.
- P. T. Kirinde Arachchige and C. S. Yi, Org. Lett., 2019, 21, 3337–3341 CAS.
- X. Chen, T. Chen, F. Ji, Y. Zhou and S. F. Yin, Catal. Sci. Technol., 2015, 5, 2197–2202 CAS.
- D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 Search PubMed.
- L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034–10072 CrossRef CAS PubMed.
- M. Zhang, Q. Y. Fu, G. Gao, H. Y. He, Y. Zhang, Y. S. Wu and Z. H. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 6175–6182 CrossRef CAS.
- J. Q. Di, M. Zhang, Y. X. Chen, J. X. Wang, S. S. Geng, J. Q. Tang and Z. H. Zhang, Green Chem., 2021, 23, 1041–1049 RSC.
- Y. Jiang, K. Xu and C. Zeng, Chem. Rev., 2017, 118, 4485–4540 CrossRef.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS.
- Á. Díaz-Ortiz, P. Prieto and A. De La Hoz, Chem. Rec., 2019, 19, 85–97 CrossRef.
- A. De la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- B. Banerjee, Ultrason. Sonochem., 2017, 35, 1–14 CrossRef CAS.
- G. Cravotto and P. Cintas, Chem. – Eur. J., 2007, 13, 1902–1909 CrossRef CAS.
- G. Brahmachari, N. Nayek, M. Mandal, A. Bhowmick and I. Karmakar, Curr. Org. Chem., 2021, 25, 1539–1565 CrossRef CAS.
- S. Saranya, S. Radhika, C. M. Afsina Abdulla and G. Anilkumar, J. Heterocyclic Chem., 2021, 58, 1570–1580 CrossRef CAS.
- S. Puri, B. Kaur, A. Parmar and H. Kumar, Curr. Org. Chem., 2013, 17, 1790–1828 CrossRef CAS.
- S. Rachakonda, P. S. Pratap and M. V. B. Rao, Synthesis, 2012, 44, 2065–2069 CrossRef CAS.
- S. H. Siddiki, K. Kon, A. S. Touchy and K.-i. Shimizu, Catal. Sci. Technol., 2014, 4, 1716–1719 RSC.
- Q. Wang, M. Lv, J. Liu, Y. Li, Q. Xu, X. Zhang and H. Cao, ChemSusChem, 2019, 12, 3043–3048 CrossRef CAS.
- N. Ghorashi, Z. Shokri, R. Moradi, A. Abdelrasoul and A. Rostami, RSC Adv., 2020, 10, 14254–14261 RSC.
- V. T. Nguyen, H. Q. Ngo, D. T. Le, T. Truong and N. T. Phan, Chem. Eng. J., 2016, 284, 778–785 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4re00479e |
| This journal is © The Royal Society of Chemistry 2025 |