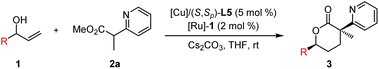Open Access Article
Open Access ArticleStereodivergent assembly of δ-valerolactones with an azaarene-containing quaternary stereocenter enabled by Cu/Ru relay catalysis†
Kui
Tian‡
a,
Zhuan
Jin‡
a,
Xin-Lian
Liu
a,
Ling
He
a,
Hong-Fu
Liu
a,
Pin-Ke
Yu
a,
Xin
Chang
a,
Xiu-Qin
Dong
 *a and
Chun-Jiang
Wang
*a and
Chun-Jiang
Wang
 *ab
*ab
aHubei Research Center of Fundamental Science-Chemistry, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei 430072, China. E-mail: xiuqindong@whu.edu.cn; cjwang@whu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Shanghai, 230021, China
First published on 3rd December 2024
Abstract
Developing methodologies for the expedient construction of biologically important δ-valerolactones bearing a privileged azaarene moiety and a sterically congested all-carbon quaternary stereocenter is important and full of challenges. We present herein a novel multicatalytic strategy for the stereodivergent synthesis of highly functionalized chiral δ-valerolactones bearing 1,4-nonadjacent quaternary/tertiary stereocenters by orthogonally merging borrowing hydrogen and Michael addition between α-azaaryl acetates and allylic alcohols followed by lactonization in a one-pot manner. Enabled by Cu/Ru relay catalysis, this cascade protocol offers the advantages of atom/step economy, redox-neutrality, mild reaction conditions, and broad substrate tolerance. Scale-up experiments and synthetic transformations further demonstrated the potential for synthetic applications. Mechanistic experiments support the envisioned bimetallic relay catalytic mechanism, and the key role of Cs2CO3 in promoting lactonization was also revealed.
Introduction
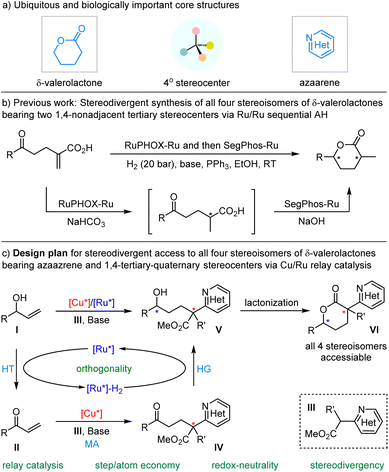
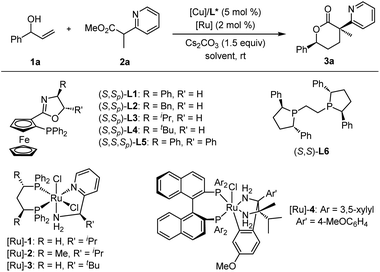
The stereoselective incorporation of all-carbon quaternary stereocenters into privileged heterocyclic molecular architectures to enhance three-dimensional complex geometries represents a significant objective in organic and medicinal synthesis as increased saturation and complexity often lead to increased biological activity and benefit drug discovery.1 Ubiquitous δ-valerolactones (Scheme 1a), especially in enantioenriched forms, are crucial subunits of numerous natural products2 and various molecules of pharmaceutical interest that display a wide range of biological properties, including antibacterial,3 antiviral,4 anticancer,5 and cholesterol-lowering agents (HMGR inhibitors).6 Two of the world's best-selling statin drugs, Lipitor and Zocor, contain a β-hydroxy-δ-valerolactone skeleton or its ring-opening δ-hydroxy carboxylate moiety.7 In addition, δ-valerolactones are also synthetically useful building blocks, such as the Prelog-Djerassi lactone, for the preparation of important bioactive compounds.8 Due to the pharmacological importance and synthetic utility of optically active δ-valerolactones bearing multiple stereocenters, there has been a growing interest in asymmetric preparation of such compounds in the last few decades. Various strategies have been developed to synthesize chiral δ-valerolactones;9 however, stoichiometric amounts of chiral auxiliaries and/or multiple-step sequences are generally required to achieve such chiral molecules as only one stereoisomer or its enantiomer.10 It is well-known that different stereoisomers of chiral molecules always exhibit different biological activities, which are closely related to their stereochemical configurations. Therefore, it is necessary to develop convenient and step-economical methods for the stereodivergent synthesis11 of all stereoisomers of chiral δ-valerolactones bearing multiple stereocenters, which is conducive to elucidating the relationship between molecular configurations and biological activities and discovering new drugs.12 Most recently, Zhang and co-workers developed a creative bimetallic Ru/Ru sequential catalysis for asymmetric hydrogenation of α-methylene δ-keto carboxylic acids, and achieved the only example of stereodivergent synthesis of chiral δ-valerolactones containing 1,4-non-adjacent tertiary/tertiary stereocenters13 (Scheme 1b). On the other hand, pyridine, as a privileged azaarene skeleton, is the most common nitrogen heterocycle among the 321 unique new small-molecule drugs approved by FDA (2013–2023).14 We envisioned that combining two biologically important δ-valerolactone and azaarene motifs into a single new three-dimensional molecule with saturation and complexity through a linchpin of all-carbon quaternary stereocenters would introduce some unprecedented benefits to enhance potency, selectivity, and other drug-like properties.1b To our knowledge, there is no efficient method that enables stereodivergent construction of δ-valerolactones with an azaarene group contained within a α-quaternary stereocenter in a convenient and step-economical manner.Borrowing-hydrogen (BH) catalysis has emerged as a convenient and atom-economic relay catalytic protocol for preparing structurally important chiral molecules starting from easily available alcohols in recent years,15 and this process generally includes three steps: hydrogen borrowing, the reaction of the activated intermediate, and hydrogen returning. In continuation of our interest in stereodivergent synthesis16 and recent achievements in BH-involved bimetallic relay catalysis,17,18 we envisaged that orthogonally merging asymmetric borrowing-hydrogen catalysis and chiral Lewis acid catalysis with allylic alcohols and α-azaaryl acetates, to execute a one-pot dehydrogenation(HT)/Michael addition(MA)/hydrogenation(HG)/lactonization cascade process,19 could offer the opportunity to achieve stereodivergent synthesis of chiral δ-valerolactones with an azaazrene-containing α-quaternary stereocenter and a tertiary stereocenter. As shown in Scheme 1c, the racemic branched allylic alcohol substrate I undergoes oxidative dehydrogenation with the aid of a chiral borrowing-hydrogen Ru-catalyst to form the active α,β-unsaturated ketone II and chiral Ru-hydride species, and the former reacts with the nucleophilic α-azaaryl acetate III ligated by chiral Cu-Lewis acid to give the corresponding Michael addition intermediate IV; the ensuing chiral Ru-hydride-enabled asymmetric reduction of the carbonyl group followed by spontaneous δ-lactonization delivers enantioenriched α-azaaryl-containing δ-valerolactones VI bearing 1,4-nonadjacent tertiary and quaternary stereocenters in a stereodivergent manner. Although theoretically feasible, there are still some challenges in this design: (1) the potential coordination of the N-heteroarene group in the α-azaaryl acetate substrate may cause deactivation of the borrowing-hydrogen Ru-catalyst; (2) undesired background reactions in the Michael addition step, the annulation efficiency of lactonization, and the potential overreduction20 of the resulting δ-valerolactones into 1,5-diols; (3) the feasibility of stereoselectivity control enabled by this Cu/Ru relay catalysis, since the sterically congested quaternary stereocenter formed in the preceding Michael addition step has potential impact on the asymmetric induction of the following reductive hydrogenation.
Herein, we uncover a concise methodology enabled by bimetallic copper/ruthenium relay catalysis, which orthogonally merges borrowing hydrogen and Michael addition followed by lactonization. This cascade process circumvents substrate preactivation, intermediate isolation and purification to realize the stereodivergent and rapid synthesis of highly functionalized chiral δ-valerolactones with an azaazrene-containing α-quaternary and a tertiary stereocenter in good yields with excellent stereoselectivity control. This protocol features step economy, redox-neutrality, high atom economy,21 and the precise synthesis of all four stereoisomers of otherwise inaccessible chiral δ-valerolactones with 1,4-nonadjacent stereocenters from the same set of readily available starting materials.
Results and discussion
Optimization of reaction conditions
To verify the feasibility of the designed protocol, we began to investigate the cooperative Cu/Ru bimetallic relay catalysis for the cascade dehydrogenation/1,4-Michael addition/hydrogenation/lactonization process between the model substrates phenyl allylic alcohol 1a and methyl 2-(pyridin-2-yl)propanoate 2a. To our delight, the expected δ-valerolactone product 3a was obtained in good yield with high diastereoselectivity and excellent enantioselectivity using the Cu(I)/L1 and [Ru]-1 catalytic system with THF as the solvent and Cs2CO3 as the base (76% yield, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, >99% ee, Table 1, entry 1). A series of chiral ferrocene-based P,N-ligands (L2–L5) and the chiral diphosphine ligand (S,S)-Ph-BPE L6 for the copper complex were then examined, and ligand L5 derived from chiral 1,2-diphenyl amino alcohol provided the best results, giving an 80% yield with >20
1 dr, >99% ee, Table 1, entry 1). A series of chiral ferrocene-based P,N-ligands (L2–L5) and the chiral diphosphine ligand (S,S)-Ph-BPE L6 for the copper complex were then examined, and ligand L5 derived from chiral 1,2-diphenyl amino alcohol provided the best results, giving an 80% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 99% ee (Table 1, entries 2–6). The evaluation of other chiral borrowing-hydrogen catalysts was continued, but no further improvement was achieved (Table 1, entries 7–9). With the combination of [Cu]/L5 and [Ru]-1 as catalysts, several bases, including inorganic bases K3PO4 and Na2CO3, as well as organic bases DBU and Et3N, were then applied, and K3PO4 provided the desired product 3a with similar results (Table 1, entry 10). Further solvent screening showed that 1,4-dioxane gave comparable results (Table 1, entries 14–16). Ester group variation in other α-methyl pyridinyl acetates, such as ethyl ester and tert-butyl ester, was also investigated; product 3a could be obtained with decreasing yield and stereoselectivity (Table 1, entries 17 and 18).
1 dr and 99% ee (Table 1, entries 2–6). The evaluation of other chiral borrowing-hydrogen catalysts was continued, but no further improvement was achieved (Table 1, entries 7–9). With the combination of [Cu]/L5 and [Ru]-1 as catalysts, several bases, including inorganic bases K3PO4 and Na2CO3, as well as organic bases DBU and Et3N, were then applied, and K3PO4 provided the desired product 3a with similar results (Table 1, entry 10). Further solvent screening showed that 1,4-dioxane gave comparable results (Table 1, entries 14–16). Ester group variation in other α-methyl pyridinyl acetates, such as ethyl ester and tert-butyl ester, was also investigated; product 3a could be obtained with decreasing yield and stereoselectivity (Table 1, entries 17 and 18).
| Entry | [Ru] | L* | Solvent | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a All reactions were carried on with [Cu]/L* (5 mol%), [Ru*] (2 mol%), 0.6 mmol 1a, 0.2 mmol 2a and 0.3 mmol base in 2 mL of solvent for 24–36 h. Cu(I) = Cu(MeCN)4PF6. b Yields refer to isolated yields after chromatography. c dr was determined by crude 1H NMR analysis. d ee was determined by HPLC analysis. e K3PO4 was used as the base. f Na2CO3 was used as the base. g DBU was used as the base. h Et3N was used as the base. i Ethyl 2-(pyridin-2-yl)propanoate was used instead of 2a. j tert-Butyl 2-(pyridin-2-yl)propanoate was used instead of 2a. | ||||||
| 1 | [Ru]-1 | L1 | THF | 76 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 2 | [Ru]-1 | L2 | THF | 55 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 3 | [Ru]-1 | L3 | THF | 42 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 4 | [Ru]-1 | L4 | THF | 40 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 5 | [Ru]-1 | L5 | THF | 80 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 6 | [Ru]-1 | L6 | THF | 52 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| 7 | [Ru]-2 | L5 | THF | 74 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 8 | [Ru]-3 | L5 | THF | 36 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 9 | [Ru]-4 | L5 | THF | 30 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 10e | [Ru]-1 | L5 | THF | 72 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 11f | [Ru]-1 | L5 | THF | NR | — | — |
| 12g | [Ru]-1 | L5 | THF | NR | — | — |
| 13h | [Ru]-1 | L5 | THF | NR | — | — |
| 14 | [Ru]-1 | L5 | Toluene | 65 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 15 | [Ru]-1 | L5 | 1,4-Dioxane | 78 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 16 | [Ru]-1 | L5 | DCM | 76 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 17i | [Ru]-1 | L5 | THF | 75 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 18j | [Ru]-1 | L5 | THF | 70 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
Substrate scope study
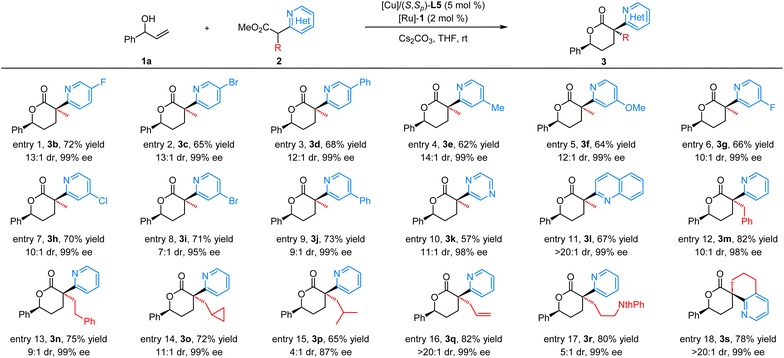
With the optimal reaction conditions in hand, we focused on the exploration of the substrate scope and generality of this Cu/Ru relay catalyzed cascade reaction. As summarized in Table 2, a variety of α-methyl azaaryl acetates 2 were employed to react with allylic alcohol 1a. α-Methyl pyridinyl acetates bearing diverse substituents (F, Cl, Br, Ph, and MeO) at different positions on the pyridinyl ring are well tolerated in this transformation, giving the corresponding products 3b–3j in good yields (62–73%) with high diastereoselectivity (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–14
1–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and generally 99% ee (Table 2, entries 1–9). Other α-methyl azaaryl acetate nucleophiles with pyrazinyl or quinolinyl units were also investigated; the expected products 3k–3l were generated with excellent reaction outcomes (57% yield, 11
1 dr) and generally 99% ee (Table 2, entries 1–9). Other α-methyl azaaryl acetate nucleophiles with pyrazinyl or quinolinyl units were also investigated; the expected products 3k–3l were generated with excellent reaction outcomes (57% yield, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 98% ee and 67% yield, >20
1 dr, and 98% ee and 67% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 99% ee; respectively, Table 2, entries 10 and 11). Moreover, a wide range of α-pyridinyl acetates bearing various alkyl substituted groups on the α-carbon, including aryl, cyclopropyl, alkenyl, and imide groups, were subjected to this transformation, giving the desired products 3m–3r in good yields with a high level of stereoselective control (65–82% yields, 4
1 dr, and 99% ee; respectively, Table 2, entries 10 and 11). Moreover, a wide range of α-pyridinyl acetates bearing various alkyl substituted groups on the α-carbon, including aryl, cyclopropyl, alkenyl, and imide groups, were subjected to this transformation, giving the desired products 3m–3r in good yields with a high level of stereoselective control (65–82% yields, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–>20
1–>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 87–99% ee, Table 2, entries 12–17). Remarkably, the cyclic azaaryl acetate 2s was well tolerated as the reaction partner, giving the desired product 3s containing a unique α-spiro-quaternary stereocenter in good yield with excellent diastereo/enantioselectivity (78% yield, >20
1 dr, and 87–99% ee, Table 2, entries 12–17). Remarkably, the cyclic azaaryl acetate 2s was well tolerated as the reaction partner, giving the desired product 3s containing a unique α-spiro-quaternary stereocenter in good yield with excellent diastereo/enantioselectivity (78% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 99% ee, Table 2, entry 18), which is an important scaffold in medicinal chemistry.22 Methyl 2-(pyridin-3-yl) and methyl 2-(pyridin-4-yl)propanoate were examined under the standard reaction conditions, and poor conversion was observed without formation of the desired products, demonstrating that the 2-(pyridin-2-yl) moiety in substrate 2 played a key role in enhancing the reactivity.
1 dr, and 99% ee, Table 2, entry 18), which is an important scaffold in medicinal chemistry.22 Methyl 2-(pyridin-3-yl) and methyl 2-(pyridin-4-yl)propanoate were examined under the standard reaction conditions, and poor conversion was observed without formation of the desired products, demonstrating that the 2-(pyridin-2-yl) moiety in substrate 2 played a key role in enhancing the reactivity.
| a All reactions were carried on with [Cu]/(S,Sp)-L5 (5 mol%), [Ru]-1 (2 mol%), 0.6 mmol 1a, 0.2 mmol 2 and 0.3 mmol Cs2CO3 in 2 mL of THF for 36 h. Cu(I) = Cu(MeCN)4PF6. Yields refer to isolated yields after chromatography. dr was determined by crude 1H NMR analysis. ee was determined by HPLC analysis. |
|---|
|
Having investigated the substrate generality of α-azaaryl acetates, we turned our attention to the examination of this Cu/Ru relay catalysis with regard to allylic alcohols (Table 3). The racemic allylic alcohols 1 bearing different electron-deficient (F, Br, CF3, and CO2Me) or electron-donating (Me and MeO) substituted groups on the phenyl ring underwent this cascade reaction smoothly, giving the corresponding δ-valerolactone products (3t–3D) in 55–73% yields with 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–19
1–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and generally with 99% ee (Table 3, entries 1–11). It was found that the position of the substituted groups has a negligible effect on the reaction results. Encouraged by these promising results, sterically constrained 2-naphthyl and 1-naphthyl substituted allylic alcohols were also tested in this protocol as suitable reaction partners, providing the corresponding products 3E and 3F in good yields with excellent stereoselectivities (Table 3, entries 12 and 13). The allylic alcohols containing heteroaromatic rings served as compatible substrates, generating the desired products 3G and 3H with satisfactory results (Table 3, entries 14 and 15). The challenging alkyl substituted allylic alcohols were further examined, and the expected product 3I was obtained in good yield with acceptable diastereoselectivity and excellent enantioselectivity (Table 3, entry 16). The absolute configuration of product 3t was unequivocally determined to be (3R,6S) by X-ray diffraction analysis (CCDC 2375149).23
1 dr and generally with 99% ee (Table 3, entries 1–11). It was found that the position of the substituted groups has a negligible effect on the reaction results. Encouraged by these promising results, sterically constrained 2-naphthyl and 1-naphthyl substituted allylic alcohols were also tested in this protocol as suitable reaction partners, providing the corresponding products 3E and 3F in good yields with excellent stereoselectivities (Table 3, entries 12 and 13). The allylic alcohols containing heteroaromatic rings served as compatible substrates, generating the desired products 3G and 3H with satisfactory results (Table 3, entries 14 and 15). The challenging alkyl substituted allylic alcohols were further examined, and the expected product 3I was obtained in good yield with acceptable diastereoselectivity and excellent enantioselectivity (Table 3, entry 16). The absolute configuration of product 3t was unequivocally determined to be (3R,6S) by X-ray diffraction analysis (CCDC 2375149).23
| Entry | R | 3 | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|
| a All reactions were carried out with [Cu]/(S,Sp)-L5 (5 mol%), [Ru]-1 (2 mol%), 0.6 mmol 1, 0.2 mmol 2a and 0.3 mmol Cs2CO3 in 2 mL of THF for 36 h. Cu(I) = Cu(MeCN)4PF6. b Yields refer to isolated yields after chromatography. c dr was determined by crude 1H NMR analysis. d ee was determined by HPLC analysis. | |||||
| 1 | 4-FC6H4 | 3t | 65 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 2 | 4-BrC6H4 | 3u | 68 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 3 | 4-CF3C6H4 | 3v | 55 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 4 | 4-CO2MeC6H4 | 3w | 60 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 5 | 4-MeC6H4 | 3x | 73 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 6 | 4-OMeC6H4 | 3y | 67 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 7 | 3-MeC6H4 | 3z | 62 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 8 | 3-FC6H4 | 3A | 57 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 9 | 2-MeC6H4 | 3B | 61 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 10 | 2-FC6H4 | 3C | 64 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 11 | 3,5-Cl2C6H3 | 3D | 66 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 12 | 2-Naphthyl | 3E | 81 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 13 | 1-Naphthyl | 3F | 71 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 14 | 3-Thienyl | 3G | 70 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 15 | 3-Furyl | 3H | 65 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 16 | Cyclohexyl | 3I | 60 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
Stereodivergent synthesis
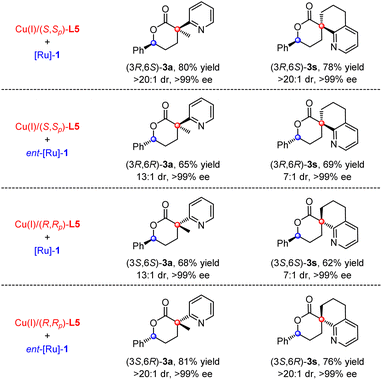
The stereodivergence of this cascade protocol was then demonstrated by the orthogonal permutation of the chiral copper catalysts and chiral ruthenium catalysts. As demonstrated in Scheme 2, when the reaction of α-methyl pyridinyl acetate 2a was conducted with four different catalyst combinations, all four stereoisomers of (3R,6S)-, (3R,6R)-, (3S,6S)-, and (3S,6R)-3a could be readily obtained in good yields with high diastereoselectivities and excellent enantioselectivity, respectively. Likewise, the precise synthesis of all four stereoisomers of spiro heterocyclic 3s could also be realized in good yields with decent diastereoselectivities and nearly perfect enantioselectivity. These promising stereodivergence results provide strong evidence to support that each chiral catalyst in this bimetallic Cu/Ru relay catalytic system can independently control the stereochemistry of both the proceeding Michael addition and the subsequent reductive hydrogenation in this cascade protocol.Scale-up experiments and synthetic application
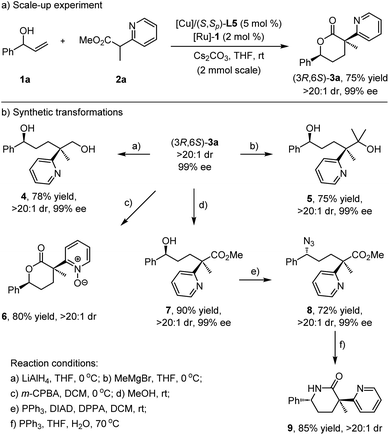
To showcase the synthetic utility of this protocol, the scale-up experiment and synthetic elaborations were then performed. As depicted in Scheme 3a, the reaction between model substrates phenyl allylic alcohol 1a and α-methyl pyridinyl acetate 2a at a 2 mmol scale was carried out under standard conditions, and the chiral product (3R,6S)-3a was obtained in high yield with preserved diastereoselectivity and enantioselectivity (75% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 99% ee). The lactone group in (3R,6S)-3a could be reduced by LiAlH4 to afford chiral 1,5-diol 4 in 78% yield with >20
1 dr, and 99% ee). The lactone group in (3R,6S)-3a could be reduced by LiAlH4 to afford chiral 1,5-diol 4 in 78% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 99% ee. Treatment of (3R,6S)-3a with the methyl Grignard reagent delivered another chiral 1,5-diol 5 in good yield without loss of stereoselectivity. The pyridinyl moiety in 3a was easily oxidized by m-chloroperoxybenzoic acid (m-CPBA), affording pyridine-N-oxide 6 in 80% yield as a single stereoisomer. Alcoholysis of (3R,6S)-3a in methanol occurred smoothly at room temperature, and the corresponding ring-opening δ-hydroxyl α-methyl azaaryl acetate 7 could be readily isolated as a stable compound in 90% yield without any loss of diastereoselectivity and enantioselectivity. The Mitsunobu reaction of 7 provided enantioenriched azide derivative 8 in a highly stereospecific manner, and the subsequent cascade Staudinger reduction/lactamization delivered chiral δ-valerolactam249 in high yield (Scheme 3b).
1 dr and 99% ee. Treatment of (3R,6S)-3a with the methyl Grignard reagent delivered another chiral 1,5-diol 5 in good yield without loss of stereoselectivity. The pyridinyl moiety in 3a was easily oxidized by m-chloroperoxybenzoic acid (m-CPBA), affording pyridine-N-oxide 6 in 80% yield as a single stereoisomer. Alcoholysis of (3R,6S)-3a in methanol occurred smoothly at room temperature, and the corresponding ring-opening δ-hydroxyl α-methyl azaaryl acetate 7 could be readily isolated as a stable compound in 90% yield without any loss of diastereoselectivity and enantioselectivity. The Mitsunobu reaction of 7 provided enantioenriched azide derivative 8 in a highly stereospecific manner, and the subsequent cascade Staudinger reduction/lactamization delivered chiral δ-valerolactam249 in high yield (Scheme 3b).
Mechanistic studies
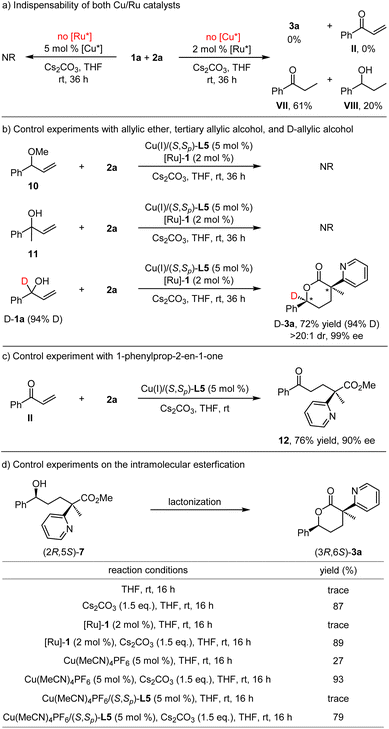
A series of control experiments were carried out to gain some mechanistic insights into this dual Cu/Ru relay catalysis. As displayed in Scheme 4a, no reaction occurred when the model reaction was conducted in the absence of the chiral redox ruthenium catalyst. On the other hand, when the reaction was carried out without the chiral copper complex, the process became messy, and the redox process occurred on vinyl phenyl carbinol to afford a mixture of propiophenone VII and 1-phenyl-1-propanol VIII. These experimental results verified that these two chiral metal catalysts were indispensable in this cascade transformation. Control experiments with allyl methyl ether 10 and tertiary allylic alcohol 11 were conducted, and no conversion was observed under standard reaction conditions (Scheme 4b). Furthermore, the deuterium labeling experiment using allylic alcohol D-1a as the substrate was further performed under standard reaction conditions, and the target product D-3a containing 94% deuterium at the tertiary carbon stereocenter was observed. The similar deuterated ratio, with preserved diastereoselectivity and enantioselectivity control, supported the designed relay catalysis via orthogonally merging borrowing hydrogen and Michael addition. In addition, the Michael addition between 1-phenylprop-2-en-1-one II and α-methyl pyridinyl acetate 2a was conducted in the presence of Cu/(S,Sp)-L5, and the corresponding adduct 12 could be obtained in 76% yield with 90% ee (Scheme 4c), which demonstrated that 1-phenylprop-2-en-1-one II was the active intermediate in this redox neutral process. As for the subsequent lactonization, which was supposed to occur spontaneously, the isolation of δ-hydroxyester 7 as a stable ring-opening molecule from δ-hydroxyester 3a (vide supra) forced us to scrutinize this intramolecular transesterification. The control experimental results tabulated in Scheme 4d revealed that the base Cs2CO3 plays a key role in this lactonization, serving as an efficient promoter to convert the intermediacy of δ-hydroxyester to the final chiral δ-valerolactone.Conclusions
In conclusion, we have successfully developed a novel multicatalytic strategy for the expedient synthesis of biologically important chiral δ-valerolactones bearing 1,4-nonadjacent stereocenters with key features such as atom/step economy and redox-neutrality. The current method relies on orthogonally merging hydrogen borrowing and Michael addition reactions between α-azaaryl acetates and allylic alcohols, enabled by Cu/Ru-relay catalysis, followed by base-promoted lactonization in a one-pot manner, while tolerating a broad substrate scope with the highly functionalized δ-valerolactones being modularly assembled in good to high yields with excellent stereoselective control. The cascade protocol provides a conceptually novel pathway for the stereodivergent synthesis of all four stereoisomers of otherwise inaccessible δ-valerolactones bearing a unique azaarene-containing α-quaternary and a tertiary stereocenter. Considering the prominence of δ-valerolactones, all-carbon quaternary stereocenters, and pyridine moieties in medicinal chemistry, we anticipate that this stereodivergent cascade protocol will be useful for the preparation of the related complex molecules.Data availability
All experimental procedures, characterisation data, mechanistic investigations, NMR spectra and HPLC spectra can be found in the ESI.†Author contributions
C. J. W. conceptualized the project. C. J. W. and X. Q. D. supervised the investigation. K. T., Z. J., X. L. L., L. H., H. F. L., P. K. Y., and X. C. performed the research. C. J. W., X. Q. D. and K. T. co-wrote the paper. All authors analyzed the data, discussed the results, and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2023YFA1506700), NSFC (22071186, 22071187, 22101216, 22271226, and 22371216), and National Youth Talent Support Program. The authors also thank the team at the Core Research Facilities of CCMS (WHU) for assistance in X-ray analysis.Notes and references
- (a) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS; (b) T. T. Talele, J. Med. Chem., 2020, 63, 13291–13315 CrossRef CAS PubMed; (c) M. Aldeghi, S. Malhotra, D. L. Selwood and A. W. Chan, Chem. Biol. Drug Des., 2014, 83, 450–461 CrossRef CAS; (d) W. R. Galloway, A. Isidro-Llobet and D. R. Spring, Nat. Commun., 2010, 1, 80 CrossRef.
- (a) T. Haffner, A. Nordsieck and R. Tressl, Helv. Chim. Acta, 1996, 79, 2088–2099 CrossRef CAS; (b) B. Zhang, Y. Zhao, Z. Huang, K. Zhang, X. Xu, Q. Zhao and X. Zhang, Nat. Prod. Res., 2023, 37, 2144–2150 CrossRef CAS; (c) S. Saito, Y. Xiaohanyao, T. Zhou, J. Nakajima-Shimada, E. Tashiro, D. W. Triningsih, E. Harunari, N. Oku and Y. Igarashi, J. Nat. Prod., 2022, 85, 1697–1703 CrossRef CAS PubMed.
- Y. Kato, Y. Ogawa, T. Imada, S. Iwasaki, N. Shimazaki, T. Kobayashi and T. Komai, J. Antibiot., 1991, 44, 66–75 CrossRef CAS.
- F. E. Boyer, J. V. N. Vara Prasad, J. M. Domagala, E. L. Ellsworth, C. Gajda, S. E. Hagen, L. J. Markoski, B. D. Tait, E. A. Lunney, A. Palovsky, D. Ferguson, N. Graham, T. Holler, D. Hupe, C. Nouhan, P. J. Tummino, A. Urumov, E. Zeikus, G. Zeikus, S. J. Gracheck, J. M. Sanders, S. VanderRoest, J. Brodfuehrer, K. Iyer, M. Sinz, S. V. Gulnik and J. W. Erickson, J. Med. Chem., 2000, 43, 843–858 CrossRef CAS.
- (a) X.-P. Fang, J. E. Anderson, C.-J. Chang, J. L. McLaughlin and P. E. Fanwick, J. Nat. Prod., 1991, 54, 1034–1043 CrossRef CAS; (b) D. S. Lewy, C.-M. Gauss, D. R. Soenen and D. L. Boger, Curr. Med. Chem., 2002, 9, 2005–2032 CrossRef CAS PubMed; (c) S. J. Shaw, K. F. Sundermann, M. A. Burlingame, D. C. Myles, B. S. Freeze, M. Xian, I. Brouard and A. B. Smith, J. Am. Chem. Soc., 2005, 127, 6532–6533 CrossRef CAS PubMed; (d) M. Kobayashi, K. Higuchi, N. Murakami, H. Tajima and S. Aoki, Tetrahedron Lett., 1997, 38, 2859–2862 CrossRef CAS.
- A. Endo, J. Med. Chem., 1985, 28, 401–405 CrossRef CAS PubMed.
- E. S. Istvan and J. Deisenhofer, Science, 2001, 292, 1160–1164 CrossRef CAS PubMed.
- (a) S. R. Martin and D. E. Guinn, Synthesis, 1991, 245–262 CrossRef CAS; (b) J. Mulzer and E. Öhler, Chem. Rev., 2003, 103, 3753–3786 CrossRef CAS PubMed.
- (a) I. Collins, J. Chem. Soc., Perkin Trans. 1, 1999, 1377–1396 RSC; (b) V. Boucard, G. Broustal and J. M. Campagne, Eur. J. Org Chem., 2007, 225–236 CrossRef CAS.
- (a) P. S. Tiseni and R. Peters, Angew. Chem., Int. Ed., 2007, 46, 5325–5328 CrossRef CAS PubMed; (b) G. Bluet, B. Bazán-Tejeda and J.-M. Campagne, Org. Lett., 2001, 3, 3807–3810 CrossRef CAS PubMed; (c) D. Enders, J. Vázquez and G. Raabe, Eur. J. Org Chem., 2000, 893–901 CrossRef CAS; (d) D. Enders and J. Vázquez, Synlett, 1999, 629–631 CrossRef CAS; (e) J. H. Smitrovich, G. N. Boice, C. Qu, L. DiMichele, T. D. Nelson, M. A. Huffman, J. Murry, J. McNamara and P. J. Reider, Org. Lett., 2002, 4, 1963–1966 CrossRef CAS PubMed; (f) Y.-Y. Hua, H.-Y. Bin, T. Wei, H.-A. Cheng, Z.-P. Lin, X.-F. Fu, Y.-Q. Li, J.-H. Xie, P.-C. Yan and Q.-L. Zhou, Org. Lett., 2020, 22, 818–822 CrossRef CAS; (g) R. Doran and P. J. Guiry, Synthesis, 2014, 46, 761–770 CrossRef; (h) A. Díaz-Rodríguez, W. Borzęcka, I. Lavandera and V. Gotor, ACS Catal., 2014, 4, 386–393 CrossRef; (i) H.-J. Zhang and L. Yin, J. Am. Chem. Soc., 2018, 140, 12270–12279 CrossRef CAS PubMed.
- (a) L. Lin and X. Feng, Chem.–Eur. J., 2017, 23, 6464–6482 CrossRef CAS PubMed; (b) G. Zhan, W. Du and Y.-C. Chen, Chem. Soc. Rev., 2017, 46, 1675–1692 RSC; (c) S. Krautwald and E. M. Carreira, J. Am. Chem. Soc., 2017, 139, 5627–5639 CrossRef CAS; (d) I. P. Beletskaya, C. Nájera and M. Yus, Chem. Rev., 2018, 118, 5080–5200 CrossRef CAS PubMed; (e) L. Wei and C.-J. Wang, Chin. J. Chem., 2021, 39, 15–24 CrossRef CAS; (f) X. Huo, G. Li, X. Wang and W. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202210086 CrossRef CAS PubMed; (g) D. Moser, T. A. Schmidt and C. Sparr, JACS Au, 2023, 3, 2612–2630 CrossRef CAS PubMed; (h) L. Wei, X. Chang, Z. Zhang, Z.-F. Wang and C.-J. Wang, Chin. Sci. Bull., 2023, 68, 3956–3968 Search PubMed; (i) H. Wang, Q. Zhang and W. Zi, Acc. Chem. Res., 2024, 57, 468–488 CAS; (j) L. Wei, C. Fu, Z.-F. Wang, H.-Y. Tao and C.-J. Wang, ACS Catal., 2024, 14, 3812–3844 CrossRef CAS.
- (a) K. Jozwiak, W. J. Lough and I. W. Wainer, Drug Stereochemistry: Analytical Methods and Pharmacology, CRC Press, Boca Raton, 3rd edn, 2012 CrossRef; (b) J. Caldwell, J. Clin. Pharmacol., 1992, 32, 925–929 CrossRef CAS PubMed; (c) K. M. Rentsch, J. Biochem. Biophys. Methods, 2002, 54, 1–9 CrossRef CAS PubMed; (d) B. Waldeck, Chirality, 1993, 5, 350–355 CrossRef CAS PubMed.
- J. He, Z. Li, R. Li, X. Kou, D. Liu and W. Zhang, Adv. Sci., 2024, 11, 2400621 CrossRef CAS.
- (a) C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2024, 67, 11622–11655 CrossRef CAS; (b) Pyridines: From lab to Production, ed. E. F. V. Scriven, Academic Press, Oxford, 2013 Search PubMed.
- For reviews, see: (a) Q. Yang, Q. Wang and Z. Yu, Chem. Soc. Rev., 2015, 44, 2305–2329 RSC; (b) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed; (c) T. Irrgang and R. Kempe, Chem. Rev., 2019, 119, 2524–2549 CrossRef CAS PubMed; (d) B. G. Reed-Berendt, D. E. Latham, M. B. Dambatta and L. C. Morrill, ACS Cent. Sci., 2021, 7, 570–585 CrossRef CAS; (e) Y. Gao, G. Hong, B.-M. Yang and Y. Zhao, Chem. Soc. Rev., 2023, 52, 5541–5562 RSC , For selected recent examples, see: ; (f) B. Lainer, S. Li, F. Mammadova and P. Dydio, Angew. Chem., Int. Ed., 2024, 63, e202408418 CrossRef CAS; (g) Y. Liu, H. Diao, G. Hong, J. Edward, T. Zhang, G. Yang, B.-M. Yang and Y. Zhao, J. Am. Chem. Soc., 2023, 145, 5007–5016 CrossRef CAS; (h) F. Li, L. Long, Y. M. He, Z. Li, H. Chen and Q. H. Fan, Angew. Chem., Int. Ed., 2022, 61, e202202972 CrossRef CAS.
- (a) L. Wei, Q. Zhu, S.-M. Xu, X. Chang and C.-J. Wang, J. Am. Chem. Soc., 2018, 140, 1508–1513 CrossRef CAS; (b) S.-M. Xu, L. Wei, C. Shen, L. Xiao, H.-Y. Tao and C.-J. Wang, Nat. Commun., 2019, 10, 5553 CrossRef PubMed; (c) L. Xiao, L. Wei and C.-J. Wang, Angew. Chem., Int. Ed., 2021, 60, 24930–24940 CrossRef CAS PubMed; (d) C. Fu, Q. Xiong, L. Xiao, L. He, T. Bai, Z. Zhang, X.-Q. Dong and C.-J. Wang, Chin. J. Chem., 2022, 40, 1059–1065 CrossRef CAS; (e) L. Xiao, X. Chang, H. Xu, Q. Xiong, Y. Dang and C.-J. Wang, Angew. Chem., Int. Ed., 2022, 61, e202212948 CrossRef CAS PubMed; (f) L. Xiao, B. Li, F. Xiao, C. Fu, L. Wei, Y. Dang, X.-Q. Dong and C.-J. Wang, Chem. Sci., 2022, 13, 4801–4812 RSC; (g) C. Che, Y. N. Lu and C. J. Wang, J. Am. Chem. Soc., 2023, 145, 2779–2786 CrossRef CAS PubMed; (h) B.-K. Zhu, H. Xu, L. Xiao, X. Chang, L. Wei, H. Teng, Y. Dang, X.-Q. Dong and C.-J. Wang, Chem. Sci., 2023, 14, 4134–4142 RSC; (i) K. Tian, X. Chang, L. Xiao, X.-Q. Dong and C.-J. Wang, Fundam. Res., 2024, 4, 77–85 CrossRef CAS.
- For reviews, see: (a) T. L. Lohr and T. J. Marks, Nat. Chem., 2015, 7, 477–482 CrossRef CAS PubMed; (b) F. Rudroff, M. D. Mihovilovic, H. Gröger, R. Snajdrova, H. Iding and U. T. Bornscheuer, Nat. Catal., 2018, 1, 12–22 CrossRef; (c) F. Romiti, J. del Pozo, P. H. S. Paioti, S. A. Gonsales, X. Li, F. W. W. Hartrampf and A. H. Hoveyda, J. Am. Chem. Soc., 2019, 141, 17952–17961 CrossRef CAS PubMed; (d) S. Martínez, L. Veth, B. Lainer and P. Dydio, ACS Catal., 2021, 11, 3891–3915 CrossRef; (e) D. F. Chen, Z. Y. Han, X. L. Zhou and L. Z. Gong, Acc. Chem. Res., 2014, 47, 2365–2377 CrossRef CAS PubMed; (f) D. F. Chen and L. Z. Gong, J. Am. Chem. Soc., 2022, 144, 2415–2437 CrossRef CAS PubMed , For selected recent examples, see: ; (g) Y. Liu, Y. Chen, A. Yihuo, Y. Zhou, X. Liu, L. Lin and X. Feng, ACS Catal., 2022, 12, 1784–1790 CrossRef CAS; (h) X. Sang, Y. Mo, S. Li, X. Liu, W. Cao and X. Feng, Chem. Sci., 2023, 14, 8315–8320 RSC; (i) W. Wang, F. Zhang, Y. Liu and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202208837 CrossRef CAS PubMed; (j) H. Wang, Y. Xu, F. Zhang, Y. Liu and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202115715 CrossRef CAS PubMed; (k) Z. Wu, X. Yang, F. Zhang, Y. Liu and X. Feng, Chem. Sci., 2024, 15, 13299–13305 RSC; (l) M. Chen, L. Yang, Y. Li, Y. Qu, G. Pan, X. Feng and X. Liu, Sci. China:Chem., 2024, 67, 542–550 CrossRef CAS; (m) H. Zheng, Y. Wang, C. Xu, Q. Xiong, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2019, 58, 5327–5331 CrossRef CAS.
- (a) X. Chang, X. Cheng, X.-T. Liu, C. Fu, W.-Y. Wang and C.-J. Wang, Angew. Chem., Int. Ed., 2022, 61, e202206517 CrossRef CAS; (b) C. Fu, L. He, X. Chang, X. Cheng, Z.-F. Wang, Z.-P. Zhang, V. A. Larionov, X.-Q. Dong and C.-J. Wang, Angew. Chem., Int. Ed., 2024, 63, e202315325 CrossRef CAS PubMed; (c) H.-R. Yang, X. Cheng, X. Chang, Z.-F. Wang, X.-Q. Dong and C.-J. Wang, Chem. Sci., 2024, 15, 10135–10145 RSC . For selected examples from other groups, see: ; (d) J. Masson-Makdissi, L. Prieto, X. Abel-Snape and M. Lautens, Angew. Chem., Int. Ed., 2021, 60, 16932–16936 CrossRef CAS PubMed; (e) D.-X. Zhu, J.-G. Liu and M.-H. Xu, J. Am. Chem. Soc., 2021, 143, 8583–8589 CrossRef CAS PubMed; (f) J.-H. Xie, Y.-M. Hou, Z. Feng and S.-L. You, Angew. Chem., Int. Ed., 2023, 62, e202216396 CrossRef CAS.
- P. F. Xu and W. Wang, Catalytic Cascade Reactions, Wiley-VCH, Weinheim, 2014 Search PubMed.
- (a) X.-H. Yang, K. Wang, S.-F. Zhu, J.-H. Xie and Q.-L. Zhou, J. Am. Chem. Soc., 2014, 136, 17426–17429 CrossRef CAS PubMed; (b) N. Arai, T. Namba, K. Kawaguchi, Y. Matsumoto and T. Ohkuma, Angew. Chem., Int. Ed., 2018, 57, 1386–1389 CrossRef CAS.
- (a) B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed; (b) P. A. Wender, V. A. Verma, T. J. Paxton and T. H. Pillow, Acc. Chem. Res., 2008, 41, 40–49 CrossRef CAS PubMed; (c) N. Z. Burns, P. S. Baran and R. W. Hoffmann, Angew. Chem., Int. Ed., 2009, 48, 2854–2867 CrossRef CAS.
- K. Hiesinger, D. Dar'in, E. Proschak and M. Krasavin, J. Med. Chem., 2021, 64, 150–183 CrossRef CAS PubMed.
- Deposition Number CCDC 2375149 (3R,6S)-3t contains the supplementary crystallo-graphic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service..
- (a) P. A. Reddy, K. E. Woodward, S. M. McIlheran, B. C. H. Hsiang, T. N. Latifi, M. W. Hill, S. M. Rothman, J. A. Ferrendelli and D. F. Covey, J. Med. Chem., 1997, 40, 44–49 CrossRef CAS PubMed; (b) X. Zhao, Y. Wu, T. Feng, J. Shen, H. Lu, Y. Zhang, H. H. Chou, X. Luo and J. D. Keasling, Metab. Eng., 2023, 77, 89–99 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2375149. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc05852f |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |