 Open Access Article
Open Access ArticleVisible-light-initiated metal-free Csp3–Csp3 to Csp3–N conversion in homobenzylic sulfonamides with N-iodoimides†
Guillermo
Morales-Ortega
 ab,
Estíbaliz
Merino
ab,
Estíbaliz
Merino
 ab and
Javier
Carreras
ab and
Javier
Carreras
 *ab
*ab
aDepartamento de Química Orgánica y Química Inorgánica, Instituto de Investigación Química “Andrés M. del Río” (IQAR), Universidad de Alcalá, Alcalá de Henares, Madrid, 28805, Spain
bInstituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Madrid 28034, Spain. E-mail: javier.carreras@uah.es
First published on 19th May 2025
Abstract
We report a simple protocol for the transformation of Csp3–Csp3 to Csp3–N in homobenzylic sulfonamides in combination with N-iodoimides to form gem-diamino derivatives. The reaction exhibits a wide functional group tolerance and allows the incorporation of bioactive-derived fragments. The mechanistic insights provided by control experiments and DFT calculations suggest that an imine intermediate is formed after C–C bond cleavage.
Introduction
Visible-light-promoted reactions have become in recent years a powerful tool for both the formation and cleavage of chemical bonds and generate molecular complexity.1 The cleavage of C–C bonds is of particular interest,2,3 due to their ubiquity in organic molecules and the inherent challenge caused by their high dissociation energies. Similarly, the formation of C–N bonds under mild reaction conditions is highly relevant given the widespread presence of nitrogen-containing substructures in bioactive compounds and materials science.4Nitrogen-centered radicals have proven to be valuable intermediates for the formation of new C–N bonds.5 In recent years, numerous visible-light-promoted methodologies have been developed to provide aminyl, amidyl, iminyl or aminium radicals as key intermediates.5 Sulfonamidyl radicals promoted by visible-light have been employed in the literature in reactions involving the addition to unsaturated systems,6 such as aromatic rings or alkenes, in Hofmann–Löffler reactions via 1,5-HAT mechanism,7 as well as cycloadditions.8
Notably, iminyl and aminium radicals have been exploited as transient species in tandem C–C bond cleavage/functionalisation processes mediated by visible light.2c This reactivity can be divided into two groups, (i) the use of cyclic ketone-derived oximes, in which an iminyl radical intermediate cleaves a Csp2–Csp3 bond, leading to the formation of a nitrile group and the subsequent generation of a new Csp3–C or Csp3–X bond;9 and (ii) the formation of radical aminium cations, which trigger Csp3–Csp3 bond cleavage followed by the formation of a different Csp3–Csp/sp3 bond10 (Scheme 1a). Cycloadditions have also been reported from cyclopropyl or cyclobutylanilides.11 Meanwhile Csp3–N cleavage/Csp3–Csp3 bond formation has been described in the literature by photoredox procedures,12 to the best of our knowledge no tandem Csp3–Csp3 bond cleavage/Csp3–N formation has been reported from nitrogen-centered radicals.
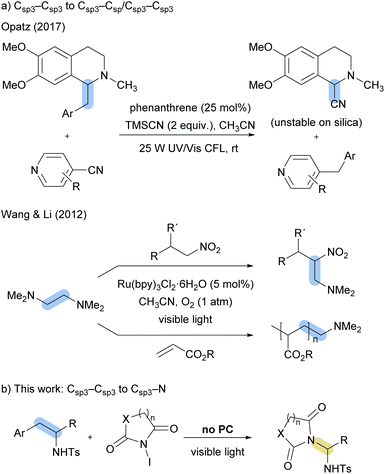 | ||
| Scheme 1 Tandem Csp3–Csp3 bond cleavage/functionalisation processes initiated by N-centered radicals mediated by visible-light. | ||
Herein we describe the reactivity of homobenzylic sulfonamides with N-iodoimides, leading to the formation of sulfonamidyl radicals which results in the Csp3–Csp3 bond cleavage followed by amination with the imide group (Scheme 1b). Under visible light irradiation, no photocatalyst is required,13 and the reaction produces compounds featuring a gem-diamino motif,14 found in several bioactive molecules, and an N-substituted imides,15 substructure with applications in medicinal chemistry or agrochemical.
Results and discussion
We began our investigation studying the reactivity of N-tosyl L-phenylalanine ethyl ester as model substrate in the presence of N-iodosuccinimide (NIS), upon irradiation with visible light. Initial experiments showed a clean transformation to the 1,1-diamine 2a, by formal substitution of the benzyl group by the succinimide moiety. Optimisation parameters included solvent, concentration, NIS equivalents, as well as light source (see ESI for more details†). The best results were obtained with CH2Cl2 as solvent, comparing with a variety of polar and apolar solvents (Table 1, entries 2 and 3, see ESI†). Changes in the concentration had minor effect on the outcome of the reaction. An excess of 2 equivalents of NIS were necessary to achieve full conversion, while N-bromosuccinimide (NBS) gave no product (entries 4 and 5). White LED showed the best results comparing with different wavelengths (entry 6, see ESI†), and the irradiation was essential for the reaction to proceed (entry 7). In summary, the optimized reaction parameters involved 2 equiv. of NIS in CH2Cl2 (0.1 M) under 40 W white LED irradiation for 24 h to get the product in 96% isolated yield. Other sulfonamides were tested, while mesylate gave a good result (81%, entry 8), triflimide showed low conversion (14%, see ESI†). When scaling up the reaction to 1 mmol under the same conditions we isolated diamine 2a in 70% yield (entry 9).| Entry | Deviation from above | Conv. [%] |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), NIS (2 equiv.), CH2Cl2 (0.1 M), 34 °C, white Kessil® (40 W), EvoluChem PhotoRedOx Box™ photoreactor, 24 h. Conversion measured by 1H NMR. b In parenthesis, isolated yield. | ||
| 1 | None | 100 (96)b |
| 2 | CH3NO2 instead of CH2Cl2 | 82 |
| 3 | CH3CN instead of CH2Cl2 | 30 |
| 4 | NIS (1.5 equiv.) | 78 |
| 5 | NBS (1.5 equiv.) | — |
| 6 | Blue instead of white LED | 86 |
| 7 | No light irradiation (40 °C) | — |
| 8 | NHMs instead of NHTs | 100 (81)b |
| 9 | 1 mmol scale | 82 (70)b |
Next, the scope of the transformation was explored (Scheme 2). Several carbonyl derivatives successfully led to the corresponding 1,1-diamines. Methyl ester (2b) was obtained from the O-tosyl tyrosine derivative and photoactive N-hydroxyphthalimide ester (2c) was also compatible with the reaction conditions. Different amides provided the products in good yields (49–72%, 2d–2f). A chiral amide, from (S)-1-(1-naphthyl)ethylamine, was tested to obtain a moderate diastereoselectivity in the process (2g). Ketones were unsuccessful under these reaction conditions, whereas nitrile derivative had a moderate reactivity to the corresponding product (2h). Remarkably, simple homobenzyl p-toluenesulfonamide led to the formation of the corresponding gem-diamine (2i) in moderate yield. The result could be improved by using diphenylmethyl chain as formal leaving group (80%). A range of functionalities were also included as substituents, such as phenyl, sulfonamide, azide, ether, ester or bromine (2j–2o). Moderate to good yields were obtained in all the cases (19–65%). The presence of an additional sulfonamide resulted in the competitive formation of the tetrahydroquinoline ring,16 decreasing the isolated yield of the 1,1-diamine 2k. We have also incorporated bioactive structures in the molecule, such as saccharine or ibuprofen (2p, 2q). Unfortunately, functional groups like alcohol, acetal, sulfide or sulfone led to decomposition or no reaction under the reaction conditions (see ESI†).
 | ||
| Scheme 2 Substrate scope. Reaction conditions: 1 (0.2 mmol), N-iodoimide (2 equiv.), CH2Cl2 (0.1 M), 34 °C, white Kessil® (40 W), EvoluChem PhotoRedOx Box™ photoreactor, 24 h. Isolated yields. a(S)-Methyl-2-(4-methylphenylsulfonamido)-3-(4-(tosyloxy)phenyl)propanoate employed as substrate. bR* = (S)-1-(1-naphthyl)ethylamine. cN-(2,2-Diphenylethyl)-4-methylbenzenesulfonamide employed as substrate. d(S)-4-Methyl-N-(1-tosyl-1,2,3,4-tetrahydroquinolin-3-yl)benzensulfonamide was obtained as the major product (see ESI†). | ||
Subsequently, we explored the substrate scope with respect to N-iodoimides. The reaction was tested with different phthalimide and succinimide derivatives with good isolated yields (71–85%, 2aa–2ac). The structure of compound 2aa was unambiguously established by single crystal X-ray diffraction (XRD) analysis. Different heterocycles, such as oxazolidine-2,4-dione or hydantoin are also compatible with this transformation (2ad–2af). Some bioactive imides, such as ethosuximide or thalidomide were successfully incorporated into the products (2ac, 2ag).
To illustrate the application of resulting gem-diamines, some transformations are presented in Scheme 3. The bromine derivative 2o was subjected to nucleophilic substitution by thiophenol in good yield. Furthermore, a carbonyl group of the phthalimide on compounds 2aa was partially reduced to obtain hydroxy and acetyl isoindolinone derivatives, substructure present in bioactive compounds.17
To establish a mechanistic proposal several experiments were performed. Initially, methylated derivatives 1r and 1s were subjected to the standard conditions (Scheme 4a). The reaction did not occur, indicating the transformation is initiated with a nitrogen-centred radical, and the alpha position cannot be a quaternary centre to allow the C–C bond cleavage. Different groups have also been placed at the alpha position of the sulfonamide to compare different formal leaving groups (Scheme 4b). Allyl or tert-butyl substituted glycine derivatives 1t and 1u, which can also stabilise radicals, led to moderate yields of the corresponding gem-diamino derivatives. Protected glycine derivative 1v only provides a low conversion to the product, while phenyl glycine derivative 1w does not react. These results suggest the C–C bond cleavage generates a carbon radical as byproduct and are consistent with the improved yields observed for product 2i when using diphenyl methyl instead of benzyl as the formal leaving group. This was confirmed in the reaction with O-tosyl tyrosine (1b), as benzyl iodide derivative 3 was isolated (Scheme 4c). When sulfonamide 1x was subjected to standard conditions, imine 4 was obtained as the sole product (Scheme 4d), rather than the expected gem-diamine. This suggests that the imine acts as a reaction intermediate. For this substrate, stabilisation by conjugation to the aromatic ring probably prevents diamine formation.18
Based on these set of results we proposed the mechanism outlined in Fig. 1, supported by DFT calculations. Initially, NIS is activated in the presence of light, generating the succinimide radical, which abstracts a hydrogen atom from phenyl alanine derivative 1a, forming sulfonamidyl radical A through an exothermic process (see ESI†). These species have been identified by Muñiz et al. using EPR spectroscopy.19 Subsequently, tosyl imine is generated via a transition state with a ΔG‡ of +11.1 kcal mol−1, with the release of the benzyl radical, which would couple with iodine radical. An imine was the product obtained in the reaction with 1w, with phenyl as a substituent, and a benzyl iodide derivative (3) was obtained by reacting 1b (Scheme 4), giving experimental support to these steps. Next, the attack of the succinimide radical leads to sulfonamidyl radical Bvia a transition state with a ΔG‡ of +29.8 kcal mol−1. Finally, hydrogen abstraction results in the formation of 2a through an exothermic process.20
Conclusions
In summary, we have developed a tandem Csp3–Csp3 bond cleavage/Csp3–N bond formation for homobenzylic sulfonamides in the presence of N-iodoimides under visible-light irradiation and metal-free conditions. The reaction features a wide scope in both reagents and led to a new method to prepare functionalized compounds that incorporate both gem-diamine and N-substituted imide motifs. Additionally, further functionalisation has also been demonstrated. Mechanistic study suggests that sulfonamidyl radicals initially generated provide imine intermediates through the loss of the benzyl group. These imines subsequently react with imide radicals to yield the products. This study extends the reactivity described for sulfanimidyl radicals, limited to addition to unsaturated systems, Hofmann–Löffler reactions and cycloadditions.Data availability
All experimental and characterization data, as well as NMR spectra are available in the ESI.† Crystallographic data for compound 2aa has been deposited at the Cambridge Crystallographic Data Centre under accession number CCDC 2431275.Author contributions
G. M.-O. carried out the experimental work and E. M. the computational study. J. C. designed the project and supervised the work. J. C. wrote the paper and all the authors discussed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support of this work by Ministerio de Ciencia, Innovación y Universidades of Spain (Agencia Estatal de Investigación, grants: CNS2022-135208 and CNS2022-135304 financed by MCIN/AEI/10.13039/501100011033 and EU NextGenerationEY/PRTR), Instituto de Salud Carlos III and EU (RICORS2040, Kidney Disease, RD24/0004/0008) and Universidad de Alcalá (Plan Excelencia: EPU-INV-UAH/2023/002 and EPU-INV-UAH/2023/005). G.M-O. thanks Comunidad de Madrid & MICIU for predoctoral contracts. Computational resources for the DFT calculations were provided by the HERMES Cluster at the University of Zaragoza. We also thank Marina Manzanares for initial experiments and Dr. R. Martín Romero for fruitful discussions.Notes and references
- (a) Y. Yin, M. You, X. Li and Z. Jiang, Chem. Soc. Rev., 2025, 54, 2246 RSC; (b) L. Candish, K. D. Collins, G. C. Cook, J. J. Douglas, A. Gómez-Suárez, A. Jolit and S. Keess, Chem. Rev., 2022, 122, 2907 CrossRef CAS PubMed; (c) S. P. Pitre and L. E. Overman, Chem. Rev., 2022, 122, 1717 CrossRef CAS PubMed; (d) K. P. S. Cheung, S. Sarkar and V. Gevorgyan, Chem. Rev., 2022, 122, 1543 CrossRef PubMed; (e) R. Cannalire, S. Pelliccia, L. Sancineto, E. Novellino, G. C. Tron and M. Giustiniano, Chem. Soc. Rev., 2021, 50, 766 RSC; (f) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034 CrossRef CAS PubMed.
- (a) Q. Ni, Y. Zhou, L. Chen and Y. Liu, Org. Chem. Front., 2025, 12, 975 RSC; (b) J. Vanderghinste and S. Das, Synthesis, 2022, 54, 3383 CrossRef CAS; (c) X.-Y. Yu, J.-R. Chen and W.-J. Xiao, Chem. Rev., 2021, 121, 506 CrossRef CAS PubMed; (d) S. P. Morcillo, Angew. Chem., Int. Ed., 2019, 58, 14044 CrossRef CAS PubMed.
- For recent examples in Csp3–Csp3 cleavage: (a) L. Wu, B. N. McIntyre, S. Wu, Z. Jiao, C. B. Fox, N. D. Schley and A. W. Schuppe, J. Am. Chem. Soc., 2025, 147, 11080 CrossRef CAS PubMed; (b) Y. Xu, J. Wang, Q. Zhang, X. Hu, C. Lv, H. Yang, B. Sun and C. Jin, Angew Chem Int Ed, 2025, e202500561 CAS; (c) Y. Ao, N. Wang, S.-Y. Tang, Z.-J. Wang, L.-H. Zou and H.-M. Huang, ACS Catal., 2025, 15, 2212 CrossRef CAS; (d) Y. Xu, W. Chen, R. Pu, J. Ding, Q. An, Y. Yang, W. Liu and Z. Zuo, Nat. Commun., 2024, 15, 9366 CrossRef CAS PubMed; (e) K. Liao, C. Y. Chan, S. Liu, X. Zhang, J. Chen and Y. Huang, J. Am. Chem. Soc., 2023, 145, 12284 CrossRef CAS PubMed , for Csp2–Csp2 cleavage: ; (f) R. Li, R. Zhan, Y. Lang, C.-J. Li and H. Zeng, Chem. Sci., 2024, 15, 12900 RSC.
- (a) H.-N. Yin, P.-C. Wang and Z. Liu, Bioorg. Chem., 2024, 144, 107108 CrossRef CAS PubMed; (b) R. Dorel, C. P. Grugel and A. M. Haydl, Angew. Chem., Int. Ed., 2019, 58, 17118 CrossRef CAS PubMed; (c) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- (a) M. F. Boselli, F. Medici and F. Franco, SynOpen, 2024, 8, 273 CrossRef CAS; (b) K. Kwon, R. T. Simons, M. Nandakumar and J. L. Roizen, Chem. Rev., 2022, 122, 2353 CrossRef CAS PubMed; (c) C. Pratley, S. Fenner and J. A. Murphy, Chem. Rev., 2022, 122, 8181 CrossRef CAS PubMed; (d) M. D. Kärkäs, ACS Catal., 2017, 7, 4999 CrossRef.
- See for example: (a) Y. Wang, J. Liu, Z. Chen, J. Chen, X. Peng, Z. Wang and Y. Zeng, Adv. Synth. Catal., 2024, 366, 1517 CrossRef CAS; (b) C. Pan, Z. Yang, X. Wu, J.-T. Yu and C. Zhu, Org. Lett., 2023, 25, 494 CrossRef CAS PubMed; (c) A. L. G. Kanegusuku, T. Castanheiro, S. K. Ayer and J. L. Roizen, Org. Lett., 2019, 21, 6089 CrossRef CAS PubMed; (d) Z.-Y. Ma, L.-N. Guo, Y. You, F. Yang, M. Hu and X.-H. Duan, Org. Lett., 2019, 21, 5500 CrossRef CAS PubMed; (e) N. Lucchetti, A. Tkacheva, S. Fantasia and K. Muñiz, Adv. Synth. Catal., 2018, 360, 3889 CrossRef CAS; (f) E. Ito, T. Fukushima, T. Kawakami, K. Murakami and K. Itami, Chem, 2017, 2, 383 CrossRef CAS; (g) T. W. Greulich, C. G. Daniliuc and A. Studer, Org. Lett., 2015, 17, 254 CrossRef CAS PubMed.
- See for example: (a) C. Wang, Z. Chen, J. Sun, L. Tong, W. Wang, S. Song and J. Li, Nat. Commun., 2024, 15, 5087 CrossRef CAS PubMed; (b) R. T. Simons, M. Nandakumar, K. Kwon, S. K. Ayer, N. M. Venneti and J. L. Roizen, J. Am. Chem. Soc., 2023, 145, 3882 CrossRef CAS PubMed; (c) L. M. Stateman, R. M. Dare, A. N. Paneque and D. A. Nagib, Chem, 2022, 8, 210 CrossRef CAS PubMed; (d) J. H. Herbort, T. N. Bednar, A. D. Chen, T. V. RajanBabu and D. A. Nagib, J. Am. Chem. Soc., 2022, 144, 13366 CrossRef CAS PubMed; (e) D. Bafaluy, Z. Georgieva and K. Muñiz, Angew. Chem., Int. Ed., 2020, 59, 14241 CrossRef CAS PubMed; (f) M. A. Short, M. F. Shehata, M. A. Sanders and J. L. Roizen, Chem. Sci., 2019, 11, 217 RSC; (g) E. Del Castillo, M. D. Martínez, A. E. Bosnidou, T. Duhamel, C. Q. O'Broin, H. Zhang, E. C. Escudero-Adán, M. Martínez-Belmonte and K. Muñiz, Chem.–Eur. J., 2018, 24, 17225 CrossRef CAS PubMed; (h) T. Duhamel, M. D. Martínez, I. K. Sideri and K. Muñiz, ACS Catal., 2019, 9, 7741 CrossRef CAS; (i) C. Q. O'Broin, P. Fernández, C. Martínez and K. Muñiz, Org. Lett., 2016, 18, 436 CrossRef PubMed; (j) E. A. Wappes, S. C. Fosu, T. C. Chopko and D. A. Nagib, Angew. Chem., Int. Ed., 2016, 55, 9974 CrossRef CAS PubMed; (k) C. Martínez and K. Muñiz, Angew. Chem., Int. Ed., 2015, 54, 8287 CrossRef PubMed; (l) N. R. Paz, D. Rodríguez-Sosa, H. Valdés, R. Marticorena, D. Melián, M. B. Copano, C. C. González and A. J. Herrera, Org. Lett., 2015, 17, 2370 CrossRef CAS PubMed.
- (a) J. Qiu, W. Li, X. Li, Y. Cao, C.-X. Pan and H. Li, Org. Lett., 2023, 25, 8000 CrossRef CAS PubMed; (b) A. Allen, A. Tharp and C. Stephenson, ChemRxiv, 2022, preprint, DOI:10.26434/chemrxiv-2022-42h36.
- For some representative examples: (a) Z. Li, R. O. Torres-Ochoa, Q. Wang and J. Zhu, Nat. Commun., 2020, 11, 403 CrossRef CAS PubMed; (b) M.-M. Zhang, S.-H. Li, J.-L. Tu, Q.-Q. Min and F. Liu, Org. Chem. Front., 2020, 7, 622 RSC; (c) T. Wang, Y.-N. Wang, R. Wang, B.-C. Zhang, C. Yang, Y.-L. Li and X.-S. Wang, Nat. Commun., 2019, 10, 5373 CrossRef PubMed; (d) P.-Z. Wang, B.-Q. He, Y. Cheng, J.-R. Chen and W.-J. Xiao, Org. Lett., 2019, 21, 6924 CrossRef CAS PubMed; (e) E. M. Dauncey, S. U. Dighe, J. J. Douglas and D. Leonori, Chem. Sci., 2019, 10, 7728 RSC; (f) B.-Q. He, X.-Y. Yu, P.-Z. Wang, J.-R. Chen and W.-J. Xiao, Chem. Commun., 2018, 54, 12262 RSC; (g) X. Yu, Q. Zhao, J. Chen, J. Chen and W. Xiao, Angew. Chem., Int. Ed., 2018, 57, 15505 CrossRef CAS PubMed; (h) F. L. Vaillant, M. Garreau, S. Nicolai, G. Gryn'ova, C. Corminboeuf and J. Waser, Chem. Sci., 2018, 9, 5883 RSC; (i) X. Yu, J. Chen, P. Wang, M. Yang, D. Liang and W. Xiao, Angew. Chem., Int. Ed., 2018, 57, 738 CrossRef CAS PubMed; (j) E. M. Dauncey, S. P. Morcillo, J. J. Douglas, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2018, 57, 744 CrossRef CAS PubMed.
- (a) B. Lipp, A. Lipp, H. Detert and T. Opatz, Org. Lett., 2017, 19, 2054 CrossRef CAS PubMed; (b) S. Cai, X. Zhao, X. Wang, Q. Liu, Z. Li and D. Z. Wang, Angew. Chem., Int. Ed., 2012, 51, 8050 CrossRef CAS PubMed.
- See for example: (a) S. Li and L. Zhou, Org. Lett., 2024, 26, 3294 CrossRef CAS PubMed; (b) Y. Dai, H. Huang, S. Liang, Y. Yin, X. Ban, X. Zhao and Z. Jiang, Org. Lett., 2023, 25, 4551 CrossRef CAS PubMed; (c) L. Chen, Y. Li, M. Han, Y. Peng, X. Chen, S. Xiang, H. Gao, T. Lu, S.-P. Luo, B. Zhou, H. Wu, Y.-F. Yang and Y. Liu, J. Org. Chem., 2022, 87, 15571 CrossRef CAS PubMed; (d) Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 23685 CrossRef CAS PubMed; (e) Y. Dai, S. Liang, G. Zeng, H. Huang, X. Zhao, S. Cao and Z. Jiang, Chem. Sci., 2022, 13, 3787 RSC; (f) L. Mollari, M. A. Valle-Amores, A. M. Martínez-Gualda, L. Marzo, A. Fraile and J. Aleman, Chem. Commun., 2022, 58, 1334 RSC; (g) Y. Yin, Y. Li, T. P. Gonçalves, Q. Zhan, G. Wang, X. Zhao, B. Qiao, K.-W. Huang and Z. Jiang, J. Am. Chem. Soc., 2020, 142, 19451 CrossRef CAS PubMed; (h) D. Staveness, T. M. Sodano, K. Li, E. A. Burnham, K. D. Jackson and C. R. J. Stephenson, Chem, 2019, 5, 215 CrossRef CAS PubMed; (i) Y. Cai, J. Wang, Y. Zhang, Z. Li, D. Hu, N. Zheng and H. Chen, J. Am. Chem. Soc., 2017, 139, 12259 CrossRef CAS PubMed; (j) J. Wang and N. Zheng, Angew. Chem., Int. Ed., 2015, 54, 11424 CrossRef CAS PubMed; (k) S. Maity, M. Zhu, R. S. Shinabery and N. Zheng, Angew. Chem., Int. Ed., 2012, 51, 222 CrossRef CAS PubMed.
- For recent examples: (a) T. Ju, Z.-H. Wang, A.-L. Lu, J. Sun, K. Huang, W. Xiong, Y. Han and C.-G. Yan, J. Org. Chem., 2025, 90, 5487 CrossRef CAS PubMed; (b) R.-G. Tian, M. Yu, B. Ni, M. Zhang and S.-K. Tian, Org. Biomol. Chem., 2025, 23, 3841 RSC; (c) H. Li, S. Li, H. Hu, R. Sun, M. Liu, A. Ding, X. Liu, W. Luo, Z. Fu, S. Guo and H. Cai, Chem. Commun., 2023, 59, 1205 RSC; (d) L. Zheng, Q. Jiang, H. Bao, B. Zhou, S.-P. Luo, H. Jin, H. Wu and Y. Liu, Org. Lett., 2020, 22, 8888 CrossRef CAS PubMed; (e) M. Miao, L.-L. Liao, G.-M. Cao, W.-J. Zhou and D.-G. Yu, Sci. China Chem., 2019, 62, 1519 CrossRef CAS.
- Recent reviews on photocatalyst-free transformations: (a) V. Murugesh and S. P. Singh, Chem. Commun., 2025, 61, 5899 RSC; (b) S. Ghara, P. Barik, S. Ghosh, S. Ghosh, A. Mandal, C. Pramanik, M. Ikbal, S. Dhara and S. Samanta, Org. Chem. Front., 2025, 12, 2790 RSC; (c) Y. Lang, C.-J. Li and H. Zeng, Org. Chem. Front., 2021, 8, 3594 RSC; (d) W. Liu, J. Li, C. Huang and C. Li, Angew. Chem., Int. Ed., 2020, 59, 1786 CrossRef PubMed; (e) Y. Wei, Q.-Q. Zhou, F. Tan, L.-Q. Lu and W.-J. Xiao, Synthesis, 2019, 51, 3021 CrossRef CAS.
- (a) R. Cao and J. C. Antilla, Org. Lett., 2020, 22, 5958 CrossRef CAS PubMed; (b) X. Fang, Z. Deng, W. Zheng and J. C. Antilla, ACS Catal., 2019, 9, 1748 CrossRef CAS; (c) S. Y. Park, Y. Liu, J. S. Oh, Y. K. Kweon, Y. B. Jeong, M. Duan, Y. Tan, J. Lee, H. Yan and C. E. Song, Chem.–Eur. J., 2018, 24, 1020 CrossRef CAS PubMed; (d) E. Aresu, S. Fioravanti, S. Gasbarri, L. Pellacani and F. Ramadori, Amino Acids, 2013, 44, 977 CrossRef CAS PubMed; (e) S. Zhu, J. Dong, S. Fu, H. Jiang and W. Zeng, Org. Lett., 2011, 13, 4914 CrossRef CAS PubMed; (f) Y. Liang, E. B. Rowland, G. B. Rowland, J. A. Perman and J. C. Antilla, Chem. Commun., 2007, 4477 RSC; (g) F. Palacios, A. M. Ochoa De Retana and J. M. Alonso, J. Org. Chem., 2005, 70, 8895 CrossRef CAS PubMed; (h) M. Yamato, J. Horiuchi and Y. Takeuchi, Chem. Pharm. Bull., 1981, 29, 3124 CrossRef CAS.
- F. A. Luzzio, Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry, Elsevier, 2019 Search PubMed.
- (a) H. Togo, Y. Hoshina, T. Muraki, H. Nakayama and M. Yokoyama, J. Org. Chem., 1998, 63, 5193 CrossRef CAS , see also: ; (b) D. Han, Q. He and R. Fan, Nat. Commun., 2018, 9, 3423 CrossRef PubMed; (c) S. C. Cosgrove, J. M. C. Plane and S. P. Marsden, Chem. Sci., 2018, 9, 6647 RSC.
- (a) R. K. Bhatia, Curr. Top. Med. Chem., 2017, 17, 189 CrossRef CAS PubMed; (b) K. Speck and T. Magauer, Beilstein J. Org. Chem., 2013, 9, 2048 CrossRef PubMed.
- Imine 4 does not react with either succinimide or NIS under visible-light irradiation or thermal conditions (see ESI† for details).
- (a) A. E. Bosnidou, T. Duhamel and K. Muñiz, Eur. J. Org Chem., 2020, 2020, 6361 CrossRef CAS; (b) A. E. Bosnidou, T. Duhamel and K. Muñiz, Eur. J. Org Chem., 2020, 2020, 6368 CrossRef CAS.
- For a more detailed discussion of the reaction mechanism see ESI†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2431275. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02168e |
| This journal is © The Royal Society of Chemistry 2025 |




