Open Access Article
Open Access ArticleA new P3N ligand for Pd-catalyzed cross-couplings in water†‡
Erfan
Oftadeh
 ,
Max
Baumann
,
Max
Baumann
 ,
Marco
Ortiz
,
Marco
Ortiz
 ,
Kirubel
Mamo
,
Kirubel
Mamo
 ,
Eduam
Boeira
,
Eduam
Boeira
 ,
Esveidy
Oceguera Nava
,
Esveidy
Oceguera Nava
 ,
Monica S.
Lopez Lemus
,
Monica S.
Lopez Lemus
 ,
Shili
Fang
,
Shili
Fang
 ,
Donald H.
Aue
,
Donald H.
Aue
 * and
Bruce H.
Lipshutz
* and
Bruce H.
Lipshutz
 *
*
Department of Chemistry & Biochemistry, University of California, Santa Barbara, CA 93106, USA
First published on 4th June 2025
Abstract
A new “P3N” ligand, (n-Bu2N)3P, derived from PCl3 and three equivalents of n-Bu2NH is reported. When complexed to palladium, it readily participates in homogeneous (copper-free) Heck–Cassar–Sonogashira and Suzuki–Miyaura couplings. Reliance on relatively low loadings of Pd is documented under aqueous micellar conditions; i.e., in water containing micelles using the well-established and inexpensive anionic surfactant SDS. Comparisons are shown with several commonly used, representative ligands which, by contrast, are made typically in a number of steps that involve environmentally egregious conditions, and can typically be quite expensive. These issues can now be avoided for these very important reactions using this P3N ligand. Quantum calculations showed the conformational, steric, and electronic nature of P3N ligands and the energetics of their binding to Pd.
Introduction
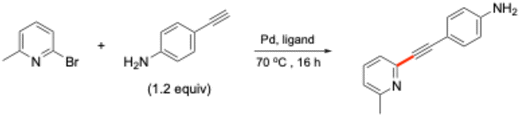
Phosphine ligands are a ubiquitous part of homogeneous catalysis, serving as fundamental catalytic components in key transformations such as coupling reactions.1 While there are occasional examples of Pd catalysis proceeding under “ligandless” conditions, the vast majority of reactions, particularly cross-couplings, rely on the careful selection of an appropriate ligand.2–17 These reactions are not just academically significant but also serve as essential tools in the synthesis of pharmaceuticals, complex organic molecules, and advanced materials.18–20 In particular, cross-couplings such as Suzuki–Miyaura (SM) and Heck–Cassar–Sonogashira21–23 have played a pivotal role in the development of innovative pharmaceuticals and bioactive natural products, further emphasizing the need for efficient metal–ligand systems24 (Fig. 1). Triphenylphosphine is an example of a ligand with a rich history of use in organic synthesis. While it remains widely available and inexpensive, it is an example whose synthesis involves resource-intensive steps, generating a significant environmental burden25,26 (Fig. 2). Performance-wise, it is by today's standards limited in effectiveness.27,28 This has driven research toward the development of more electronically and sterically controlled phosphines, which have significantly advanced the field by improving several features associated with the catalytic processes involved.29–35 | ||
| Fig. 2 Conventional synthetic and sustainability issues involving triphenylphosphine ligands composed of P–N bonds, as originally disclosed,47 especially those containing three such arrays (i.e., “P3N” ligands, or (R2N)3P) have led to extensive research in attempts to understand their structure and reactivity.48 Since the first synthesis of an aminophosphine by Michaelis and Luxembourg in 1895,49 significant advances have enhanced both their applicability and practical value. Their unique electronic properties and steric arrangements have made them valuable ligands in transition metal complexes, in addition to being important intermediates in various transformations.50–55 | ||
The complexity of synthesizing these advanced ligands raises concerns not only about their scalability and cost, but also about the broader implications associated with their production. Thus, while extensive progress has been made over the past several decades to fine-tune ligands for catalytic performance, far less attention has been given to the chemistry required for their synthesis. As a result, many ligands are designed with a singular focus in mind: achieving a targeted reaction outcome, oftentimes forgoing environmental and practical constraints associated with their preparation. The preparation of these ligands oftentimes relies on harsh reagents, multi-step procedures, and hazardous waste generation, raising concerns about their long-term sustainability.36–39 However, times have changed, and there is now a growing awareness of the processes behind reagent, and especially ligand development, the analyses of which include sustainability benchmarks such as Life Cycle Assessments (LCA) and “cradle-to-grave” evaluations.25,34,40–43 In this context, aminophosphines have resurfaced as a potential alternative to traditional phosphines. Their status is reflected by their expanding roles across several disciplines, such as in coordination chemistry,44 catalysis,45 and to a limited extent, organic synthesis.46 This versatility can be attributed to their unique molecular structure, as aminophosphines are characterized by phosphorus being directly attached to each nitrogen (P–N), setting them apart from both “P,N-ligands” as well as conventional phosphines that primarily contain phosphorus–carbon (P–C) bonds.
Aminophosphine ligands, however, have only recently gained attention in catalysis. Thus, while their origins can be traced back to the mid-1990s (Fig. 4), a foundational study by Petersen introduced examples of aminophosphines employing N-pyrrolylphosphines as a class of phosphorus–nitrogen ligands characterized by unusual steric features and uncommon π-acceptor properties (Fig. 3).44 Although initially limited to coordination studies, P–N ligands were later investigated in metal-catalyzed transformations; e.g., Verkade and co-workers showed that bicyclic triaminophosphines (see Fig. 4) serve as ligands in Pd-catalyzed Suzuki–Miyaura cross-couplings,56 work that coincided with the emergence of mono- and di-aminophosphine variants.53,57,58 In some cases, water in place of traditional organic solvents led to improved selectivity in SM reactions by suppressing homocouplings.59 A report on 1,3-diaminobenzene-based aminophosphine pincer complexes from Frech and co-workers then followed, which included a limited number of P–N ligands applied to Pd catalysis.60–62 Subsequently, the same group described aminophosphine ligands with flexible coordination geometries, likewise being utilized in Pd-catalyzed cross-couplings and other metal-catalyzed reactions.63–66 Notwithstanding these noteworthy advances, significant challenges remained regarding their use as ligands in modern Pd catalysis, in particular from the sustainability perspective.
Herein, we describe the preparation and use of a triaminophosphine ligand, (n-Bu2N)3P, designed to address these factors via a combination of synthetic simplicity, reaction efficiency, and environmental compatibility. Thus, P3N ligands, in general, can be prepared in a single step utilizing (in several cases) essentially cost-free, commercially available precursors under mild and safety-conscious conditions and hence, suitable for scale up. This simple approach, which readily accommodates single solvent recovery, therefore also leads to a low E-Factor (e.g., cEF), especially when compared to ligands made decades ago.67 When complexed to Pd, catalysis of both Suzuki–Miyaura and Heck–Cassar–Sonogashira reactions take place under environmentally respectful conditions in aqueous micellar media, and with low catalyst loadings.
Results and discussion
The investigation began by screening several bench-mark ligand-containing Pd catalysts currently in use to effect copper-free Heck–Cassar–Sonogashira reactions.68 Only an aminophosphine ligand bearing three diethylamino groups (bis(diethylamino)phosphine; L1; Scheme 1) led to detectable product formation (see ESI and Table S1‡). The performance of XPhos aligned with previous reports demonstrating its utility in Sonogashira reactions conducted in aqueous media.68 The modest activity observed with this Pd-complexed P3N ligand L1 suggested that, with further refinement, a more effective catalyst matched to the new rules69 associated with chemistry in water might be identified. | ||
| Scheme 1 (a) Different variations of synthesized symmetrical aminophosphines. (b) Unsymmetrical variation of aminophosphines. (c) Scaled-up synthesis of L4. | ||
The commercially available aminophosphine, tris(dimethylamino)phosphine (HMPT; L2) was not pursued given its precursor status to its oxidized version, HMPA. Using L1, therefore, a limited screening was performed which revealed that improvements could be achieved using triethylamine as base under aqueous micellar medium, albeit by increasing the reaction temperature. Reducing the Pd loading to 0.5 mol% had minimal impact on efficiency (Table 1, entries 1 and 2; also see ESI, Table S2‡). Increasing the ligand lipophilicity was also anticipated to assist the process by enhancing the binding constant between the Pd-containing catalyst and inner cores of the nanomicelles in which the coupling is taking place.70,71
Inspired by the earliest known aminophosphine ligand, tris(piperidinyl)phosphine, used by Frech and co-workers in Heck–Cassar–Sonogashira couplings,49,62 the analog tris(morpholino)phosphine (L3) was prepared and then tested in the same coupling. This ligand was chosen as a more polar variant with the aim of improving solubility and performance under aqueous micellar conditions. However, when evaluated under the same optimized reaction conditions, L3 afforded an unacceptable level of conversion and hence, isolated yield (Table 1, entry 3).
In order to evaluate new ligands featuring increased steric bulk and lipophilicity in Heck–Cassar–Sonogashira reactions in micellar media, two variants were prepared by simply modifying the sequence. Hence, starting with bis(diethylamino)phosphorus monochloride, 1-methylbenzyl alcohol or dibenzylamine was added to arrive at L5 and L6, respectively (Scheme 1b). Evaluation of L4vs.L5, and L6 as ligands complexed to Pd revealed notable differences in reactivity (Table 1). Phosphine ligand L4 led to full conversion and thus, the highest yield, likely benefiting from its steric bulk, which could enhance ligand stability and allow greater temperature tolerance, as well as facilitating reductive elimination.72,73 Its increased lipophilicity improved micellar compatibility and likely contributes to the observed improvement. Unsymmetrical aminophosphine L5 derived from dibenzylamine also performed well. Mixed ligand L6, incorporating a P–O bond showed no catalytic activity, possibly due to an unfavorable coordination profile or more likely, its electronic nature. These results highlight the significant role that both structure and electronics can play in modulating reactivity in derived P3N-Pd catalysts towards cross-couplings in aqueous micellar media.
Using [Pd(allyl)Cl]2 as the palladium source, a variety of surfactants were investigated, although the best results were anticipated to be found using either nonionic vitamin E-based TPGS-750-M74 or Savie.75 However, as the results in Table 2 show, in fact it was the micellar array (and not a nanoemulsion) derived from anionic surfactant sodium dodecylsulfate (SDS)76,77 that gave the highest yield of acetylenic product. Switching to Pd(OAc)2 (see ESI, Table S7‡) led to the same outcome: SDS outperformed the other surfactants screened (Table 2). Closer examination of the palladium source using two aryl bromides with different electronic properties; 4-bromoanisole and 2-bromobenzonitrile indicated that Pd(OAc)2 is preferred, and hence, it was used in all subsequent studies (see ESI, Tables S4 and S5‡).
Organic co-solvents were then evaluated in combination with SDS in order to address the likelihood of limited solubility of advanced, highly functionalized substrates (i.e., “brick dust”) in the aqueous micellar medium. A sulfonamide derivative, notorious for its poor aqueous solubility,78 was selected as a test case. Significant improvements in the extent of conversion were observed, relative to SDS in water alone, screening various ether-based solvents, with cyclopentyl methyl ether (CPME)79 providing the best outcome (see ESI, Table S6‡).
Bases of both the organic and inorganic types was examined under micellar conditions. Organic bases generally performed best, and between triethylamine and those derived from guanidine affording the best results, the former was selected for use due to its low cost, availability, and consistent performance (see ESI, Table S8‡).
Several other reaction parameters were studied in an effort to determine optimum conditions for use of this P3N ligand in Pd-catalyzed Heck–Cassar–Sonogashira couplings. Variations in global concentration had minimal impact on the outcome (see ESI, Table S8‡). The loading of palladium was determined to be 0.25 mol% of dimeric catalyst (thus 0.5 mol% Pd) (see ESI, Tables S9 and S10‡), with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of this Group 10 metal to P3N ligand (see ESI, Tables S11 and S12‡). Also, while the reaction temperature varied by substrate (see ESI, Table S13‡), reaction times can vary from minutes to usually two hours, although this depends upon both the electronic configuration of the aryl/heteroaryl halide and substrate complexity (see ESI, Tables S14 and S15‡).
4 ratio of this Group 10 metal to P3N ligand (see ESI, Tables S11 and S12‡). Also, while the reaction temperature varied by substrate (see ESI, Table S13‡), reaction times can vary from minutes to usually two hours, although this depends upon both the electronic configuration of the aryl/heteroaryl halide and substrate complexity (see ESI, Tables S14 and S15‡).
As illustrated in Table 3, a wide variety of substrate types readily participate in Heck–Cassar–Sonogashira couplings using this new catalytic system derived from a P3N ligand (n-Bu2N)3P complexed to (low loadings of) Pd, thereby forming a catalyst that functions well under aqueous micellar conditions. These included unprotected products containing amines (1, 12), secondary amides (3, 4, 8), and sulfonamides (3). Additionally, several alkyne-containing heteroaromatics such as products containing a pyridine (7, 9), oxazole (4), quinoline (10), benzothiophene (12), oxadiazole (6), and indole (2) were all readily formed. Acetylenes bearing ester groups (1, 5, 11) were also obtained, as were several bearing carbamates such as Boc-protected amines (2, 11) and Cbz-derivatized amine (5). Aldehyde 9 could also be isolated in modest yield. Notably, some highly functionalized aryl bromides from the Merck informer library (X4 and X8) were well tolerated, leading in excellent yields to products 5 and 11. Single-crystal X-ray diffraction of products 1 and 7 (see ESI, Tables S31 and S32‡) confirmed their structure.
The comparative performance of the P3N aminophosphine L4 to other well-established phosphine ligands was investigated for Heck–Cassar–Sonogashira couplings under identical conditions. Thus, in addition to their simplicity of preparation and very modest cost, as well as their use under aqueous micellar conditions in the total absence of an organic solvent, the results are shown in Table 4. These were obtained using 0.5 mol% of Pd(OAc)2 in the presence of ligand L4, indicating that comparable or better yields (by qNMR) are to be expected relative to those screened, including ferrocenyl, N-heterocyclic carbenes, and biaryl phosphine ligands.
| Entry | Ligand | Yieldb (%) |
|---|---|---|
| a Reaction conditions: Pd(OAc)2 (0.50 mol%), L4 (2 mol%), triethylamine (3 equiv.), 2 wt% SDS/H2O (0.5 M) 70 °C, 16 h. b By quantitative NMR using 1,3,5-trimethoxybenzene as the internal standard. | ||
| 1 | SPhos | 77 |
| 2 | XPhos | 58 |
| 3 | dppf | 79 |
| 4 | DTBPF | 87 |
| 5 | CyPPh2 | 90 |
| 6 | P(NBu 2 ) 3 ( L4) | 92 |
| 7 | IPr | 15 |
Using the optimized coupling conditions applied to Heck–Cassar–Sonogashira couplings (see Table 3), various ligands were screened in this case for use in Suzuki–Miyaura cross-coupling reactions.80 Aminophosphine L4 again displayed good performance (see ESI, Table S15‡). Lowering the Pd loading to 0.125 mol% had minimal effect on reaction outcome (see ESI, Table S16‡). A surfactant screening confirmed that SDS provided considerably higher yields compared to nonionic surfactants, revealing a potential benefit from the sodium counterion in SDS, which may play a role in the transmetalation step81 (Table 5).
Here, too, relatively inexpensive Pd(OAc)2 was found to be an excellent source of this Group 10 metal. It is worth noting that using the alternative (albeit more costly) commercially available, pre-ligated Pd(PPh)2Cl2, along with ligand L4 led to a nearly quantitative yield of the desired product, indicative of a lack of interference of Ph3P given its presence in the medium (see ESI, Table S18‡).
Finding a compatible co-solvent with potential solubility challenges in mind did not lead to a clear choice (see ESI, Table S19‡), indicating a potential dependence dictated by the substrate. Reaction temperatures of 70–80 °C consistently afforded high catalytic activity. And while use of lower temperatures may suffice, more complex cases appear to require these somewhat elevated temperatures, especially given the low loadings of catalyst (see ESI, Table S24‡).
The scope shown in Table 6 indicates, as with Heck–Cassar–Sonogashira coupling products (see Table 3), that a wide range of functionality is tolerated in these SM reactions using L4 as the ligand on palladium. Heterocyclic aryl bromides leading to products containing pyridine (16, 18, 23, 30), pyrimidine (17), quinoline (with leaving group at positions 2 and 4; 19, 28), pyrazole (30), indole (32) and isoindoline (26) are all preserved under these reaction conditions. Unprotected amines present in products 16, 23, and 29 are also compatible. Additionally, poorly water-soluble substrates worked efficiently, except in a few cases where a minimal amount of co-solvent was needed to increase the levels of conversion and hence, maintain efficiency (19, 23, 26). Most, however, worked well in the absence of any co-solvent, leading to products 18, 20, 21, 29, and 32. Several representative cases demonstrated that a bromide reacts selectively in the presence of an aryl chloride, which remains for further functionalization (23–25, 28). Other functional groups such as products containing a methyl ketone (16–19, 27, 30), an unprotected benzylic alcohol (24), fluorides (22 and 23), methyl esters (21 and 26), a sulfonamide (20), carbamate (16), phenol (25), and internal alkyne (23) were all well-tolerated. On the other hand, product 27 was unexpectedly obtained in low yield. Importantly, both boronic acids and esters all appeared to be compatible with this methodology (see products 28, 35, and 37). The X-ray crystal structure of 37 further confirmed a successful coupling (see ESI and Table S33‡).
By way of comparison of various ligands commonly associated with Suzuki–Miyaura couplings (Table 7), all led to the product biaryls in high yields using the standard 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of Pd
2 ratio of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand, with a loading of only 0.125 mol% Pd(OAc)2. However, considering the complexity and the number of required steps to synthesize traditional phosphines, P3N ligand L4 still deserves serious consideration as an inexpensive, easy-to-make, and effective ligand that appears to be competitive with other common phosphine ligands used on palladium for SM cross-coupling reactions.
ligand, with a loading of only 0.125 mol% Pd(OAc)2. However, considering the complexity and the number of required steps to synthesize traditional phosphines, P3N ligand L4 still deserves serious consideration as an inexpensive, easy-to-make, and effective ligand that appears to be competitive with other common phosphine ligands used on palladium for SM cross-coupling reactions.
| Entry | Ligand | Yieldb (%) |
|---|---|---|
| a Reaction conditions: aryl bromide (0.25 mmol), boronic acid (1.2 equiv.; 0.3 mmol), Pd(OAc)2 (0.125 mol%), L4 (0.25 mol%), triethylamine (1.5 equiv.), 2 wt% SDS/H2O (0.5 M), 80 °C, 16 h. b By quantitative NMR using 1,3,5-trimethoxybenzene as internal standard. | ||
| 1 | SPhos | Quant |
| 2 | Xphos | Quant |
| 3 | dppf | 94 |
| 4 | CyPPh2 | 95 |
| 5 | (n-Bu2N)3P (L4) | 92 |
Focus then shifted towards use of aminophosphine L4 as a ligand on Pd as catalyst in a late-stage application, selecting synthesis of the unsymmetrical alkyne ponatinib, an FDA-approved tyrosine-kinase inhibitor (Scheme 2).82 To obtain terminal alkyne intermediate 35, imidazo[1,2-b]pyridazine 33 was initially treated with iodine/NIS to obtain iodinated product 34.83 (Triethylsilyl)acetylene was then coupled to form the corresponding protected alkyne 35. Deprotection afforded terminal alkyne 36.84 This 3-step sequence (Scheme 2a) led to an overall yield of 61%. Analysis of 36via ICP-MS revealed the presence of only 7.7 ppm Pd to be present, notwithstanding the lack of any “clean up” involved that is required under typical Heck–Cassar–Sonogashira conditions where higher loadings of catalyst are utilized in organic solvents. Since the FDA limit for Pd is only 10 ppm/dose/d, use of low loadings of a Pd complexed by a very low-cost ligand may translate into considerable catalyst cost savings on scale up, especially when this is a major factor in drug synthesis earmarked for distribution in low-income countries. Synthesis of the coupling partner 39 was straightforword (2-steps; 93% overall yield; Scheme 2b).85 The final Heck–Cassar–Sonogashira coupling between fragments 36 and 39 (Scheme 2c) afforded ponatinib (40) (78% isolated yield). The overall 6-step sequence took place in 44% yield, while the ICP-MS on the API itself showed it to contain only 5.8 ppm of palladium.
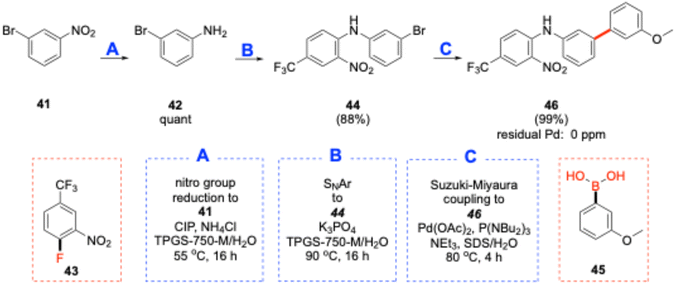
An additional 3-step sequence highlighting a SM coupling catalyzed by the same Pd catalyst containing ligand L4 is shown in Scheme 3. Thus, starting with meta-bromonitrobenzene (41), treatment with carbon iron powder (CIP)/ammonium chloride led to aniline 42. Simple filtration to remove the CIP followed by an SNAr reaction using crude material with 43 afforded 44. SM coupling of 44 with boronic acid 45 gave biaryl 46, which was obtained in an overall yield of 87% over three steps. As expected, when using low loadings of a Pd catalyst under aqueous micellar conditions, ICP-MS analysis in this case failed to show any residual Pd to be present in final product 46.
Terminal alkynes, as either targets or reaction partners, are readily fashioned in 1-pot using this new methodology. Heck–Cassar–Sonogashira couplings using TES-acetylene (as employed en route to ponatinib; see Scheme 2a) followed by an in-flask desilylation with TBAF at 0 °C-rt arrives at each of the terminal acetylenic products 47–50 (Scheme 4). This sequence included the biaryl containing alkyne 47, 14 grams of which was prepared in 85% isolated yield suggesting that scaling up these couplings should be straightforward.
Complete environmental factors (cEF) is a common metric used to assess a methodology's greenness.86 For the synthesis of compound 13 (Scheme 5) a cEF was calculated to be 21.5 (see the ESI,‡ page 42 for calculations). Recycling the aqueous medium containing SDS twice was accompanied by a moderate decrease in conversion, and hence, isolated yield, to the desired product.
Ligand studies
The reasons for the efficacy of the P3N ligands are not immediately obvious. The nature of these ligands has been explored with quantum calculations. The electronic properties of the P–N bond and the lone pair on phosphorus are unique and are revealed, for example, in calculated gas-phase electronic proton affinities (PAs) of the very simple model ligand, P(NH2)3 shown in Table 8 for protonation on phosphorus along with related molecules for comparison. To compensate for well-known polarization effects of substituents in the gas phase87 the PAs of the model ligands P(NH2)3 and N(NH2)3 are compared with those of PMe3 and NMe3, respectively. Where the methyl substituents are of comparable polarizability to the amino substituents. Thus, the relative electronic PAs in Table 8 will reflect the remaining effects on the basicities, such as inductive and other effects, after the polarization effects from charge-induced dipole stabilization of the phosphonium and ammonium ions are subtracted out. In the case of N(NH2)3, the interpretation seems simple: the 17 kcal mol−1 difference in electronic PA relative to that of trimethylamine in Table 8 is almost surely due to a quite strong short-range inductive effect of the three amino substituents. , for protonation at the central phosphorus (or nitrogen) at the G4 level of theory. Energies in kcal mol−1
, for protonation at the central phosphorus (or nitrogen) at the G4 level of theory. Energies in kcal mol−1
For P(NH2)3, interpreting the PA data is more complicated. Its electronic PA is remarkably much higher, by almost 20 kcal mol−1, than that for N(NH2)3, in spite of the fact that protonation on N is slightly favored over P in trimethylamine versus trimethylphosphine. Why? The 4.33 kcal mol−1 higher basicity relative to PMe3, must result from more than one effect. The strong 17 kcal mol−1 inductive effect in N(NH2)3, should be felt in P(NH2)3 in near equal force. So, there must be another, even stronger, base-strengthening effect(s) of 21 kcal mol−1 operating! Looking at the calculated structures gives a hint of what this might be (see Fig. S1 in ESI-2‡ for structures). The computed PNH bond angles in P(NH2)3 at the amino nitrogens lie well above 109.5°, up to 116.3° and 119.1°, and the computed natural bond orbital analysis shows sp1.98 and sp2.84 hybridizations at N for the localized orbitals. The structure for N(NH2)3 shows no such behavior. It has near-tetrahedral geometry at the amino nitrogens and computed hybridizations near sp3. This seems to point directly to d-orbital involvement in the phosphorus-containing model ligand, though, interestingly, little documentation of such a large d-orbital effect seems to appear in the aminophosphine literature.88 Although one might expect the three amino substituents would exert a strong inductive electron withdrawal from the phosphorus, as in N(NH2)3, it would appear that considerable electron density may be donated back from the nitrogen lone pair to appropriately-oriented empty d-orbital(s) on P via a P–N pi bond, a type of “back bonding”. This would explain the shift toward sp2 hybridization on the amino nitrogens to provide a nearly p lone-pair orbital on N. Looking at the computed charge on phosphorus and P-31 chemical shifts of aminophosphines, however, it does not appear that the inductive effect of the amino groups has fully been mitigated by p–d back bonding in P(NH2)3. Looking at the computed structure of the protonated aminophosphine, HP+(NH2)3, shows another, more remarkable, bond-angle change. There the amino nitrogens have assumed nearly trigonal geometry and a nearly 0.1 Å shorter P–N bond length than in P(NH2)3.88a Thus, the large effect of d-orbitals is not just in the neutral P(NH2)3, but appears to exert a much stronger effect on the stabilization of the protonated species HP+(NH2)3. This is not unexpected since P now has a full formal plus charge that can be very strongly stabilized by electron donation from three N lone-pair p orbitals. One would expect this to also affect the Pd–P bonding as electrons are donated from P to Pd in catalysts derived from P3N ligands. We are actively exploring this question.
The structure of the tris-(di-n-butylamino)phosphine ligand in Fig. 1 was optimized at the B3LYPD3BJ/6-31+G(d,p) level of theory with an unsymmetrical C1 conformation A and an all-anti arrangement of the n-butyl groups chosen to have the groups arranged to have a minimum steric energy and keep the n-butyl groups away from the face of the phosphine to allow binding to palladium and it's oxidative addition substrate. A second reasonable conformation B like this was of C3 symmetry, but a bit less hindered sterically on the face of the phosphine, had about a 1.7 kcal mol−1 higher free energy at 298 K in the gas phase and in toluene solvent, which mimics the solvation environment in the micelles (see ESI-2‡). The n-butyl groups could adopt numerous other conformations, but conformations A and B both appear to be reasonably low energy and representative. Other conformations were not explored. Reaction energies for various reactions of A and B are shown in Table 9 (see Fig. 5 for structures).
| Structure | B3LYP | B3LYPD3BJ | |||
|---|---|---|---|---|---|
| A to B | −0.57 | 0.88 | 0.95 | 1.42 | 1.68 |
| PdA to PdB | −0.23 | 2.96 | 3.14 | 3.04 | 3.25 |
| PdPhBrA to PdPhBrB | −1.86 | −0.94 | −0.68 | −0.85 | −1.04 |
| Pd + B to PdB | −41.92 | −50.74 | −52.52 | −43.36 | −43.55 |
| PdB + B to PdB2 | −34.90 | −52.03 | −53.47 | −37.08 | −34.39 |
| PdB + PhBr to PdPhBrB | −38.55 | −53.91 | −52.17 | −37.83 | −31.19 |
| PdB2 + PhBr to PdPhBrB2 | −11.00 | −33.52 | −28.80 | −11.52 | −6.30 |
| PdPhBrB + B to PdPhBrB2 | −7.35 | −31.63 | −30.10 | −10.77 | 2.05 |
Attachment of Pd to conformers A and B led to formation of PdA and PdB, which were optimized at the B3LYP/6-31G(d)/SDD(Pd,Br) and B3LYPD3BJ/6-31+G(d,p)/SDD(Pd,Br) levels of theory, in this case, with the PdA conformer more stable by 3.3 kcal mol−1 in toluene. The ligand addition has a free energy of reaction of about −44 kcal mol−1. A second ligand could be added on the PdB to give PdB2, with a free energy of −35 kcal mol−1. Oxidative addition of bromobenzene to PdB gave the intermediate PdPhBrB that is strongly downhill by −39 kcal mol−1. In spite of a good deal of steric hindrance, we find that PdB2 might also undergo oxidative addition with bromobenzene with a somewhat negative free energy of −12 kcal mol−1 in the gas phase and −6 kcal mol−1 in toluene. That product PdPhBrB2, however, is predicted to be vulnerable to lose one ligand in toluene, though more stable in the gas phase. Thus, it appears that both the di-ligated and mono-ligated palladium molecules are candidates for catalytic activity with substrates that are not too sterically demanding, in which case the monoligated catalyst should be preferred.
Conclusions
A new P3N ligand has been shown to be effective when complexed to Pd in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, forming a very inexpensive catalyst useful for both Sonogashira and Suzuki–Miyaura couplings run under environmentally respectful conditions using water as the reaction medium. This ligand, (n-Bu2N)3P, is formed by adding the secondary amine n-Bu2NH to PCl3 at ambient temperatures, a trivial process amenable to scale-up, thereby allowing for its straightforward multi-gram preparation. Both types of couplings using this P3N ligand-derived catalyst led to excellent isolated yields of desired products, relying on relatively low loadings of palladium. Comparisons under aqueous micellar conditions with other commonly used ligands (normally performed in waste-generating organic solvents) indicate that (n-Bu2N)3P is equally effective, or even more so, for these couplings. Two sequences illustrative of these commonly used Pd-catalyzed cross-couplings are presented: one that results in a streamlined route to the unsymmetrical alkyne-containing pharmaceutical ponatinib, while the second features a Suzuki–Miyaura coupling as part of an overall high-yielding sequence, leading to a product containing an undetectable level of residual Pd. Calculations of E-Factors document the attention to “greenness” associated with this ligand development.
1 ratio, forming a very inexpensive catalyst useful for both Sonogashira and Suzuki–Miyaura couplings run under environmentally respectful conditions using water as the reaction medium. This ligand, (n-Bu2N)3P, is formed by adding the secondary amine n-Bu2NH to PCl3 at ambient temperatures, a trivial process amenable to scale-up, thereby allowing for its straightforward multi-gram preparation. Both types of couplings using this P3N ligand-derived catalyst led to excellent isolated yields of desired products, relying on relatively low loadings of palladium. Comparisons under aqueous micellar conditions with other commonly used ligands (normally performed in waste-generating organic solvents) indicate that (n-Bu2N)3P is equally effective, or even more so, for these couplings. Two sequences illustrative of these commonly used Pd-catalyzed cross-couplings are presented: one that results in a streamlined route to the unsymmetrical alkyne-containing pharmaceutical ponatinib, while the second features a Suzuki–Miyaura coupling as part of an overall high-yielding sequence, leading to a product containing an undetectable level of residual Pd. Calculations of E-Factors document the attention to “greenness” associated with this ligand development.
Ab initio calculated structures and proton affinities of the model ligands P(NH2)3 and N(NH2)3 give strong evidence of a previously little-known phenomenon in aminophosphines. The aminophosphine has p–d pi bonding and donation of lone-pair electrons into empty d orbitals on phosphorus that especially stabilizes the phosphonium ion after protonation. This effect should make the aminophosphine particularly effective as a donor ligand on palladium. DFT calculations on the P3N ligand (n-Bu2N)3P gives a set of structures and relative energies for the ligand, its palladium binding complexes, and oxidative-addition products. These results reveal possible intermediates in the catalytic pathways of this P3N ligand. Many structures are sterically crowded and include moderately stable candidates as intermediates with two (n-Bu2N)3P ligands on Pd.
Data availability
There are 2 files containing ESI-1 and ESI-2‡ that contain all the data.Author contributions
E. O. designed the project and performed the ligands synthesis and characterizations along with experiments related to H–C–S with the assistance of M. S. L. L. and S. F. and M. B. designed, optimized and characterized the ponatinib synthesis and the intermediates with the assistance of S. F. M. O. contributed to the experimental, characterization and optimization of S.-M. with the assistance of E. B. and K. M. contributed to the optimization work on H–C–S. E. B. contributed to the synthesis and characterization of terminal alkynes. E. O. N. designed, optimized and characterized the 3-Step sequence and the intermediates. D. H. A. conducted the computational study. E. O. and D. H. A. wrote the ms. E. O. wrote the ESI-1‡ with the assistance of M. B., M. O. and E. O. N. DHA carried out all computations, wrote the ESI-2,‡ and contributed to the writing of the manuscript B. H. L. supervised the work and assisted in revising the ms and ESI-1.‡ All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support for the experimental work was provided by Anthem Biosciences, PHT International, and the US NSF (CHE 2152566), for which we are most appreciative. Computational facilities used were purchased with funds from the National Science Foundation (CNS-1725797) and administered by the Center for Scientific Computing (CSC). The CSC is supported by the California NanoSystems Institute and the Materials Research Science and Engineering Center (MRSEC; NSF DMR 1720256) at UCSB. The software used for 3-D drawings was from CYLview, 1.0b; Legault, C. Y., Université de Sherbrooke, 2009 (http://www.cylview.org). E. Oceguera Nava is supported by the National Science Foundation, California, LSAMP Bridge to Doctorate Fellowship under Grant No. EES- 2404971.References
- S. Handa, The ligand: an overlooked element in sustainable catalysis, Trends Chem., 2024, 428–431 CrossRef CAS
.
- T. I. Wallow and B. M. Novak, Highly efficient and accelerated Suzuki aryl couplings mediated by phosphine-free palladium sources, J. Org. Chem., 1994, 59, 5034–5037 CrossRef CAS
.
- E. M. Campi, W. R. Jackson, S. M. Marcuccio and C. G. M. Naeslund, High yields of unsymmetrical biaryls via cross coupling of arylboronic acids with haloarenes using a modified Suzuki-Beletskaya procedure, J. Chem. Soc.,Chem. Commun., 1994, 2395, 10.1039/c39940002395
.
- S. Darses, T. Jeffery, J. P. Genet, J. L. Brayer and J. P. Demoute, Cross-coupling of arenediazonium tetrafluoroborates with arylboronic acids catalysed by palladium, Tetrahedron Lett., 1996, 37, 3857–3860 CrossRef CAS
.
- N. A. Bumagin and V. V. Bykov, Ligandless palladium catalyzed reactions of arylboronic acids and sodium tetraphenylborate with aryl halides in aqueous media, Tetrahedron, 1997, 53, 14437–14450 CrossRef CAS
.
- D. Badone, M. Baroni, R. Cardamone, A. Ielmini and U. Guzzi, Highly efficient palladium-catalyzed boronic acid coupling reactions in water: Scope and limitations, J. Org. Chem., 1997, 62, 7170–7173 CrossRef CAS PubMed
.
- F. E. Goodson, T. I. Wallow and B. M. Novak, Accelerated Suzuki coupling via a ligandless palladium catalyst:: 4-methoxy-2′-methylbiphenyl, Org. Synth., 1998, 75, 61–68 CrossRef CAS
.
- C. G. Blettner, W. A. König, W. Stenzel and T. Schotten, Microwave-assisted aqueous Suzuki cross-coupling reactions, J. Org. Chem., 1999, 64, 3885–3890 CrossRef CAS
.
- J. C. Bussolari and D. C. Rehborn, Preparation of 5-aryl furfurals and aryl thiophene-2-carboxaldehydes via palladium-catalyzed C-C bond formation in aqueous media, Org. Lett., 1999, 1, 965–967 CrossRef CAS
.
- G. W. Kabalka, R. M. Pagni and C. M. Hair, Solventless Suzuki Coupling Reactions on Palladium-Doped KF/Al2O3, Org. Lett., 1999, 1, 1423–1425 CrossRef CAS
.
- G. A. Molander and B. Biolatto, Efficient ligandless palladium-catalyzed Suzuki reactions of potassium aryltrifluoroborates, Org. Lett., 2002, 4, 1867–1870 CrossRef CAS PubMed
.
- G. A. Molander and B. Biolatto, Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions of potassium aryl- and heteroaryltrifluoroborates, J. Org. Chem., 2003, 68, 4302–4314 CrossRef CAS PubMed
.
- G. Lu, R. Franzén, Q. Zhang and Y. Xu, Palladium charcoal-catalyzed, ligandless Suzuki reaction by using tetraarylborates in water, Tetrahedron Lett., 2005, 46, 4255–4259 CrossRef CAS
.
- A. Decottignies, A. Fihri, G. Azemar, F. Djedaini-Pilard and C. Len, Ligandless Suzuki–Miyaura reaction in neat water with or without native β-cyclodextrin as additive, Catal. Commun., 2013, 32, 101–107 CrossRef CAS
.
- J. Qiu, L. Wang, M. Liu, Q. Shen and J. Tang, An efficient and simple protocol for a PdCl2-ligandless and additive-free Suzuki coupling reaction of aryl bromides, Tetrahedron Lett., 2011, 52, 6489–6491 CrossRef CAS
.
- B. Bandgar and A. Patil, A rapid, solvent-free, ligandless and mild method for preparing aromatic ketones from acyl chlorides and arylboronic acids via a Suzuki–Miyaura type of coupling reaction, Tetrahedron Lett., 2005, 46, 7627–7630 CrossRef CAS
.
- G. Hamasaka, D. Roy, A. Tazawa and Y. Uozumi, Arylation of terminal alkynes by aryl iodides catalyzed by a parts-per-million loading of palladium acetate, ACS Catal., 2019, 9, 11640–11646 CrossRef CAS
.
- T. Schlatzer and R. Breinbauer, Synthesis of Hydrophilic Phosphorus Ligands and Their Application in Aqueous-Phase Metal-Catalyzed Reactions, Adv. Synth. Catal., 2021, 363, 668–687 CrossRef CAS PubMed
.
- W. Chen, P. Cai, H. C. Zhou and S. T. Madrahimov, Bridging Homogeneous and Heterogeneous Catalysis: Phosphine-Functionalized Metal-Organic Frameworks, Angew. Chem., 2024, 136, e202315075 CrossRef
.
- D. Van der Westhuizen, A. C. Castro, N. Hazari and A. Gevorgyan, Bulky, electron-rich, renewable: analogues of Beller's phosphine for cross-couplings, Catal. Sci. Technol., 2023, 13, 6733–6742 RSC
.
- We thank a referee for pointing out the accurate history associated with “copper-free Sonogashira” couplings; that is, the original work by both Heck and Cassar describing the use of Sonogashira coupling conditions that were in the absence of copper, while Sonogashira couplings, technically, have copper present in the reaction medium. In this report, both types are combined into “copper-free Heck–Cassar–Sonogashira” couplings since this expression should capture all contributors to what continues to be generally referred to as “Sonogashira couplings”. See: H. Dieck and F. Heck, Palladium catalyzed synthesis of aryl, heterocyclic and vinylic acetylene derivatives, J. Organomet. Chem., 1975, 93, 259–263 CrossRef CAS
.
- See also: L. Cassar, Synthesis of aryl-and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes, J. Organomet. Chem., 1975, 93, 253–257 CrossRef CAS
.
- See also: F. Valentini, F. Ferlin, E. Tomarelli, H. Mahmoudi, M. Bagherzadeh, M. Calamante and L. Vaccaro, A Waste-Minimized Approach to Cassar-Heck Reaction Based on POLITAG-Pd0 Heterogeneous Catalyst and Recoverable Acetonitrile Azeotrope, ChemSusChem, 2021, 14, 3359–3366 CrossRef CAS PubMed
.
- M. J. Buskes and M.-J. Blanco, Impact of cross-coupling reactions in drug discovery and development, Molecules, 2020, 25, 3493 CrossRef CAS PubMed
.
- J. L. Osorio-Tejada, F. Ferlin, L. Vaccaro and V. Hessel, The sustainability impact of Nobel Prize Chemistry: life cycle assessment of C–C cross-coupling reactions, Green Chem., 2023, 25, 9760–9778 RSC
.
- R. A. Breitenmoser, T. Fink and S. Abele, Safety Assessment for the Scale-up of an Oxime Reduction with Melted Sodium in Standard Pilot-Plant Equipment, Org. Process Res. Dev., 2012, 16, 2008–2014 CrossRef CAS
.
- M. Nishimura, M. Ueda and N. Miyaura, Palladium-catalyzed biaryl-coupling reaction of arylboronic acids in water using hydrophilic phosphine ligands, Tetrahedron, 2002, 58, 5779–5787 CrossRef CAS
.
- V. Polshettiwar, A. Decottignies, C. Len and A. Fihri, Suzuki–Miyaura Cross-Coupling Reactions in Aqueous Media: Green and Sustainable Syntheses of Biaryls, ChemSusChem, 2010, 3, 502–522 CrossRef CAS PubMed
.
- A. Sekiya and N. Ishikawa, The cross-coupling of aryl halides with Grignard reagents catalyzed by iodo (phenyl) bis (triphenylphosphine) palladium (II), J. Organomet. Chem., 1976, 118, 349–354 CrossRef CAS
.
- I. Fenger and C. Le Drian, Reusable polymer-supported palladium catalysts: An alternative to tetrakis (triphenylphosphine) palladium in the Suzuki cross-coupling reaction, Tetrahedron Lett., 1998, 39, 4287–4290 CrossRef CAS
.
- B. Soberats, L. Martinez, M. Vega, C. Rotger and A. Costa, Conventional Tetrakis (triphenylphosphine) palladium-Copper (I) Iodide-Catalyzed Sonogashira Coupling of Free and BOC-Protected Propargylic Amines “On Water”, Adv. Synth. Catal., 2009, 351, 1727–1731 CrossRef CAS
.
- C. M. Crawforth, S. Burling, I. J. Fairlamb, R. J. Taylor and A. C. Whitwood, Bromobis (triphenylphosphine)(N-succinimide) palladium (ii) as a novel catalyst for Stille cross-coupling reactions, Chem. Commun., 2003, 2194–2195 RSC
.
- R. Tanimoto, S. Suzuki, M. Kozaki and K. Okada, Nitronyl nitroxide as a coupling partner: Pd-mediated cross-coupling of (nitronyl nitroxide-2-ido)(triphenylphosphine) gold (I) with aryl halides, Chem. Lett., 2014, 43, 678–680 CrossRef CAS
.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, Catalysts for Suzuki− Miyaura coupling processes: scope and studies of the effect of ligand structure, J. Am. Chem. Soc., 2005, 127, 4685–4696 CrossRef CAS PubMed
.
- S.-Z. Bai, C. Xu, H.-M. Li, Z.-Q. Wang and W.-J. Fu, Synthesis and Characterization ofTriphenylphosphine Adducts of Ferrocene-Based Palladacycles and Their Performance in the Suzuki and Sonogashira Reactions with Bromo-and Chloroarenes, Molecules, 2012, 17, 5532–5543 CrossRef CAS PubMed
.
- N. Kamiya, T. Kuroda, Y. Nagata, T. Yamamoto and M. Suginome, Single-Handed Helical Polymer-Based Polycarboxylate with Achiral Triarylphosphine Pendants as Chiral Catalysts for Asymmetric Cross-Coupling Reactions in Pure Water, J. Am. Chem. Soc., 2025, 147, 8534–8547 CrossRef CAS PubMed
.
- S. Baweja, A. Kazimir, P. Lönnecke and E. Hey-Hawkins, Modular Synthesis of Phosphino Hydrazones and Their Use as Ligands in
a Palladium-Catalysed Cu-Free Sonogashira Cross-Coupling Reaction, ChemPlusChem, 2023, 88, e202300163 CrossRef CAS PubMed
.
- H. Yang, X. Yang and W. Tang, Transition-metal catalyzed asymmetric carbon–carbon cross-coupling with chiral ligands, Tetrahedron, 2016, 72, 6143–6174 CrossRef CAS
.
- Y. Jiang, K. W. Cheng, H. Zhang, Z. Yang and J. Wang, Palladium/Azaphos-Catalyzed Asymmetric Suzuki–Miyaura Coupling, Synthesis, 2023, 55, 2537–2542 CrossRef CAS
.
- B. S. Kote, M. A. Mohite, D. Mondal, M. K. Pandey and M. S. Balakrishna, Sterically Demanding Novel Triazole Based Tertiary Phosphines: Synthesis, Transition Metal Chemistry and Catalytic Applications, Eur. J. Inorg. Chem., 2023, 26, e202300291 CrossRef CAS
.
- H. S. Kunchur, L. Radhakrishna, M. K. Pandey and M. S. Balakrishna, Novel approach to benzo-fused 1, 2-azaphospholene involving a Pd (II)-assisted tandem P–C bond cleavage and P–N bond formation reaction, Chem. Commun., 2021, 57, 4835–4838 RSC
.
- G. Wernet, S. Conradt, H. P. Isenring, C. Jiménez-González and K. Hungerbühler, Life cycle assessment of fine chemical production: a case study of pharmaceutical synthesis, Int. J. Life Cycle Assess., 2010, 15, 294–303 CrossRef CAS
.
- E. Von Schneidemesser, P. S. Monks, J. D. Allan, L. Bruhwiler, P. Forster, D. Fowler, A. Lauer, W. T. Morgan, P. Paasonen and M. Righi, Chemistry and the linkages between air quality and climate change, Chem. Rev., 2015, 115, 3856–3897 CrossRef CAS PubMed
.
- K. G. Moloy and J. L. Petersen, N-pyrrolyl phosphines: an unexploited class of phosphine ligands with exceptional. pi.-acceptor character, J. Am. Chem. Soc., 1995, 117, 7696–7710 CrossRef CAS
.
- R. Jackstell, H. Klein, M. Beller, K. D. Wiese and D. Röttger, Synthesis of Pyrrolyl-, Indolyl-, and Carbazolylphosphanes and Their Catalytic Application as Ligands in the Hydroformylation of 2-Pentene, Eur. J. Org Chem., 2001, 2001, 3871–3877 CrossRef
.
- A. V. Alexandrova, T. Mašek, S. M. Polyakova, I. Císařová, J. Saame, I. Leito and I. M. Lyapkalo, Synthesis of Electron-Rich Sterically Hindered P1 Phosphazene Bases by the Staudinger Reaction, Eur. J. Org Chem., 2013, 2013, 1811–1823 CrossRef CAS
.
- B. Lipshutz, E. Oftadeh, M. Baumann, M. Ortiz, K. Mamo, E. Boeira, S. Nguyen and D. Aue, Following Nature's Lead. Designing a Very Simple yet Effective Ligand System Applicable to Pd-Catalyzed Cross Couplings… in Water, ChemRxiv, 2024, preprint, DOI:10.26434/chemrxiv-2024-mj25f.
- J. Gopalakrishnan, Aminophosphines: their chemistry and role as ligands and synthons, Appl. Organomet. Chem., 2009, 23, 291–318 CrossRef CAS
.
- A. Michaelis and K. Luxembourg, Ueber n-Phosphine und n-Phosphoniumverbindungen, Ber. Dtsch. Chem. Ges., 1895, 28, 2205–2211 CrossRef
.
- Y. Minko, T. V. Fetrow, S. Sharma, B. K. Cashman and A. M. Tondreau, Flexible interactions of the rare-earth elements Y, La, and Lu with phosphorus in metallacyclohexane rings, Chem. Sci., 2024, 15, 12138–12147 RSC
.
- C. Zheng, Y. Tang and B. Yu, Tri (N-carbazolyl) phosphine Gold (I) Complexes: Structural and Catalytic Activity Studies, Inorg. Chem., 2022, 61, 16874–16886 CrossRef CAS PubMed
.
- F. Buß, M. B. Röthel, J. A. Werra, P. Rotering, L. F. Wilm, C. G. Daniliuc, P. Löwe and F. Dielmann, Tris (tetramethylguanidinyl) phosphine: The Simplest Non-ionic Phosphorus Superbase and Strongly Donating Phosphine Ligand, Chem.–Eur. J., 2022, 28, e202104021 CrossRef PubMed
.
- J. Cheng, Y. Sun, F. Wang, M. Guo, J.-H. Xu, Y. Pan and Z. Zhang, A copper-and amine-free Sonogashira reaction employing aminophosphines as ligands, J. Org. Chem., 2004, 69, 5428–5432 CrossRef CAS PubMed
.
- S. Breeden and M. Wills, ESPHOS and SEMI-ESPHOS: A new family of mono-and bidentate diazaphospholidine ligands for asymmetric catalysis, J. Org. Chem., 1999, 64, 9735–9738 CrossRef CAS
.
- J. Ansell and M. Wills, Enantioselective catalysis using phosphorus-donor ligands containing two or three P–N or P–O bonds, Chem. Soc. Rev., 2002, 31, 259–268 RSC
.
- S. Urgaonkar, M. Nagarajan and J. Verkade, Pd/P (i-BuNCH2CH2)3N: an efficient catalyst for Suzuki cross-coupling of aryl bromides and chlorides with arylboronic acids, Tetrahedron Lett., 2002, 43, 8921–8924 CrossRef CAS
.
- J. Cheng, F. Wang, J.-H. Xu, Y. Pan and Z. Zhang, Palladium-catalyzed Suzuki–Miyaura reaction using aminophosphine as ligand, Tetrahedron Lett., 2003, 44, 7095–7098 CrossRef CAS
.
- T. Schareina and R. Kempe, Combinatorial Libraries with P-Functionalized Aminopyridines: Ligands for the Preparation of Efficient C (Aryl)− Cl Activation Catalysts, Angew. Chem., Int. Ed., 2002, 41, 1521–1523 CrossRef CAS PubMed
.
- Z.-Y. Peng, J.-P. Wang, J. Cheng, X.-m. Xie and Z. Zhang, Water works: an efficient palladium-catalyzed cross-coupling reaction between boronic acids and bromoacetate with aminophosphine ligand, Tetrahedron, 2010, 66, 8238–8241 CrossRef CAS
.
- J. L. Bolliger and C. M. Frech, The 1,3-Diaminobenzene-Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance, Adv. Synth. Catal., 2010, 352, 1075–1080 CrossRef CAS
.
- J. L. Bolliger, O. Blacque and C. M. Frech, Short, Facile, and High-Yielding Synthesis of Extremely Efficient Pincer-Type Suzuki Catalysts Bearing Aminophosphine Substituents, Angew. Chem., Int. Ed., 2007, 46, 6514–6517 CrossRef CAS PubMed
.
- J. L. Bolliger and C. M. Frech, Highly Convenient, Clean, Fast, and Reliable Sonogashira Coupling Reactions Promoted by Aminophosphine-Based Pincer Complexes of Palladium Performed under Additive-and Amine-Free Reaction Conditions, Adv. Synth. Catal., 2009, 351, 891–902 CrossRef CAS
.
- M. Oberholzer and C. M. Frech, Mizoroki–Heck reactions catalyzed by palladium dichloro-bis (aminophosphine) complexes under mild reaction conditions. The importance of ligand composition on the catalytic activity, Green Chem., 2013, 15, 1678–1686 RSC
.
- R. Gerber and C. M. Frech, Negishi Cross-Coupling Reactions Catalyzed by an Aminophosphine-Based Nickel System: A Reliable and General Applicable Reaction Protocol for the High-Yielding Synthesis of Biaryls, Chem.–Eur. J., 2011, 17, 11893–11904 CrossRef CAS PubMed
.
- R. Gerber and C. M. Frech, Alkyne hydrothiolation catalyzed by a dichlorobis (aminophosphine) complex of palladium: selective formation of cis-configured vinyl thioethers, Chemistry, 2012, 18, 8901–8905 CrossRef CAS PubMed
.
- J. L. Bolliger and C. M. Frech, Dichloro-Bis (aminophosphine) Complexes of Palladium: Highly Convenient, Reliable and Extremely Active Suzuki–Miyaura Catalysts with Excellent Functional Group Tolerance, Chem.–Eur. J., 2010, 16, 4075–4081 CrossRef CAS PubMed
.
- X. Huang, K. W. Anderson, D. Zim, L. Jiang, A. Klapars and S. L. Buchwald, Expanding Pd-catalyzed C− N bond-forming processes: the first amidation of aryl sulfonates, aqueous amination, and complementarity with Cu-catalyzed reactions, J. Am. Chem. Soc., 2003, 125, 6653–6655 CrossRef CAS PubMed
.
- E. Ghiglietti, E. A. Incarbone, S. Mattiello and L. Beverina, Efficient Copper-Free Sonogashira Coupling in Water and under Ambient Atmosphere, Eur. J. Org Chem., 2024, 27, e202400223 CrossRef CAS
.
- D. J. Lippincott, E. Landstrom, M. Cortes-Clerget, B. H. Lipshutz, K. Buescher, R. Schreiber, C. Durano, M. Parmentier, N. Ye and B. Wu, Surfactant technology: with new rules, designing new sequences is required, Org. Process Res. Dev., 2019, 24, 841–849 CrossRef
.
- J. K. Virdi, A. Dusunge and S. Handa, Aqueous micelles as solvent, ligand, and reaction promoter in catalysis, JACS Au, 2024, 4, 301–317 CrossRef CAS PubMed
.
- P. Hauk, S. Trienes, F. Gallou, L. Ackermann and J. Wencel-Delord, Next-generation functional surfactant for mild C− H arylation under micellar conditions, Chem Catal., 2024, 4, 101146 CrossRef CAS
.
- S. M. Vanden Broeck, F. Nahra and C. S. Cazin, Bulky-yet-flexible carbene ligands and their use in palladium cross-coupling, Inorganics, 2019, 7, 78 CrossRef
.
- J. F. Hartwig, S. Richards, D. Barañano and F. Paul, Influences on the relative rates for C− N bond-forming reductive elimination and β-hydrogen elimination of amides. A case study on the origins of competing reduction in the palladium-catalyzed amination of aryl halides, J. Am. Chem. Soc., 1996, 118, 3626–3633 CrossRef CAS
.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, TPGS-750-M: a second-generation amphiphile for metal-catalyzed cross-couplings in water at room temperature, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed
.
- J. R. Kincaid, M. J. Wong, N. Akporji, F. Gallou, D. M. Fialho and B. H. Lipshutz, Introducing Savie: A biodegradable surfactant enabling chemo-and biocatalysis and related reactions in recyclable water, J. Am. Chem. Soc., 2023, 145, 4266–4278 CrossRef CAS PubMed
.
-
D. Myers, Surfactant Science and Technology, John Wiley & Sons, Hoboken, NJ, 3rd edn, 2020. See on page 35 therein the statement: “it is, in fact, the most extensively studied and best understood surfactant known to science-sodium dodecylsulfate (SDS)” Search PubMed
.
- At reaction temperature of 70–80 °C, SDS remains in micellar form, although its critical micelle concentration (CMC) is known to increase; see: B. Hammouda, Temperature effect on the nanostructure of SDS micelles in water, J. Res. Natl. Inst. Stand. Technol., 2013, 118, 151 CrossRef CAS PubMed
.
- E. Rahimpour, W. E. Acree Jr and A. Jouyban, Prediction of sulfonamides' solubilities in the mixed solvents using solvation parameters, J. Mol. Liq., 2021, 339, 116269 CrossRef CAS
.
- K. Watanabe, N. Yamagiwa and Y. Torisawa, Cyclopentyl methyl ether as a new and alternative process solvent, Org. Process Res. Dev., 2007, 11, 251–258 CrossRef CAS
.
- D. G. Brown and J. Bostrom, Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? Miniperspective, J. Med. Chem., 2016, 59, 4443–4458 CrossRef CAS PubMed
.
- H. Zhang, F. Y. Kwong, Y. Tian and K. S. Chan, Base and cation effects on the Suzuki cross-coupling of bulky arylboronic acid with halopyridines: synthesis of pyridylphenols, J. Org. Chem., 1998, 63, 6886–6890 CrossRef CAS PubMed
.
- W.-S. Huang, C. A. Metcalf, R. Sundaramoorthi, Y. Wang, D. Zou, R. M. Thomas, X. Zhu, L. Cai, D. Wen and S. Liu, Discovery of 3-[2-(imidazo [1, 2-b] pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl) methyl]-3-(trifluoromethyl)phenyl} benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant, J. Med. Chem., 2010, 53, 4701–4719 CrossRef CAS PubMed
.
- S. Yoshida, T. Morita and T. Hosoya, Synthesis of diverse benzotriazoles from aryne precursors bearing an azido group via inter-and intramolecular cycloadditions, Chem. Lett., 2016, 45, 726–728 CrossRef CAS
.
- H. K. Akula and M. K. Lakshman, Synthesis of deuterated 1, 2, 3-triazoles, J. Org. Chem., 2012, 77, 8896–8904 CrossRef CAS PubMed
.
- Y. Jian, Y.-J. He, C. Hu, X. Li and P.-N. Liu, Catalyst-free [4+ 1] annulation of α-imidoyl sulfoxonium ylides and diazo compounds enabling the modular synthesis of 2-indanones and 3 (2 H)-furanones, Org. Lett., 2024, 26, 8492–8497 CrossRef CAS PubMed
.
- R. A. Sheldon, The E factor 25 years on: the rise of green chemistry and sustainability, Green Chem., 2017, 19, 18–43 RSC
.
-
(a)
D. H. Aue and M. T. Bowers, Stabilities of positive ions from equilibrium gas-phase basicity measurements, in Gas Phase Ion Chemistry, ed. M. T. Bowers, Academic Press, New York, 1979 Search PubMed
; (b) D. H. Aue, H. M. Webb and M. T. Bowers, Quantitative Proton Affinities, Ionization Potentials, and Hydrogen Affinities of Alkylamines, J. Am. Chem. Soc., 1976, 78, 311–317 CrossRef
; (c) D. H. Aue, Gas-Phase Basicities, Experiment and Theory, Encyclopedia of Mass Spectrometry, ed. N. M. M. Nibbering, Elvevier, Oxford, 2005 Search PubMed
.
- While there is mention by Woollins
of structural evidence for this effect, we find little else, for example in the Gopalakrishnan review article, and no prior evidence on the large energetic consequences.
(a) J. Gopalakrishnan, Aminophosphines: their chemistry and role as ligands and synthons, J. Appl. Organometal. Chem., 2009, 23, 291–318 CrossRef CAS
; (b) M. L. Clarke, D. J. Cole-Hamilton, A. M. Z. Slawin and J. D. Woollins, P-N bond formation as a route to highly electron rich phosphine ligands, Chem. Commun., 2000, 2065 Search PubMed
; (c) M. L. Clarke, D. J. Cole-Hamilton and J. D. Woollins, Synthesis of bulky, electron rich hemilabile phosphines and their application in the Suzuki coupling reaction of aryl chlorides, J. Chem. Soc., Dalton Trans., 2001, 2721 Search PubMed
; (d) S. Arumugam, D. McLeod and J. G. Verkade, Use of the Nonionic Superbase P(MeNCH2CH2)3N in the Selective Monoalkylation of Active-Methylene Compounds, J. Org. Chem., 1998, 63, 3677–3679 CrossRef CAS
; (e) S. T. Howard and J. A. Platts, Relationship between Phosphine Proton Affinities and Lone Pair Density Properties, J. Phys. Chem., 1995, 99, 9027–9033 CrossRef CAS
; (f) J. W. Gilje and K. Seff, The Crystal and Molecular Structure of Tris(dimethylhydrazino)bis(phosphine oxide), OP(NCH3NCH3)3PO, Inorg. Chem., 1972, 11, 1643 CrossRef CAS
; (g) P. E. Baskakova, A. V. Belyakova, T. Colacotb, L. K. Krannichb, A. Haalandc, H. V. Voldenc and O. Swangd, The molecular structures of tris(dimethylamino)-phosphane, -arsane and -stibane, E(NMe2)3, E = P, As or Sb and Me = CH3, by gas electron diffraction and ab initio molecular orbital calculations, J. Molec. Struct., 1998, 445, 311–317 CrossRef CAS
; (h) A. H. Cowley, R. E. Davis and K. Remadna, Conformation of ligated tris(dimethylamino)phosphne, J. Am. Chem. Soc., 1979, 101, 5090–5092 CrossRef CAS
.
Footnotes |
| † This contribution is dedicated to the memory of Aunt Shahla and Dr Alex B. Wood. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02923f |
| This journal is © The Royal Society of Chemistry 2025 |