Open Access Article
Open Access ArticleOverview of rough surface construction technology for cotton fabrics used in oil/water separation
Huanhuan
Bai
a,
Chengzhi
Song
b,
Limei
Zheng
b,
Tong
Shen
b,
Xu
Meng
 *a and
JinXing
Ma
*c
*a and
JinXing
Ma
*c
aSchool of Materials Science and Engineering, Changzhou University, Changzhou, China. E-mail: mengqiaoshen@163.com
bSchool of Textile Science and Engineering, Shaoxing University, Shaoxing, China
cShaoxing Shui Heung Textile Technology Co., Ltd., Shaoxing, China. E-mail: majx3881@163.com
First published on 8th January 2025
Abstract
The discharge of waste organic solvents, various oil/water mixtures and the frequent infiltration of oil into water bodies have created significant threats to the ecological environment. As a result, the separation and recovery of oil/water mixtures have been increasingly investigated by scholars. Many researchers have developed numerous separation materials with excellent separation efficiency and high separation flux, including filter materials, adsorption materials and smart materials with switchable wettability. Among them, natural cotton fabric has been widely studied as a separation material substrate due to its three-dimensional surface structure, porosity, excellent fiber adsorption capacity, recyclability, low cost, and biodegradability. As an oil/water separation material, it is essential for the substrate surface to have a micro–nano structure. Researchers typically use various methods to modify the surface of cotton fabrics with various kinds of micro–nano particles, which create a certain roughness on the fabric surface. These methods include dip-coating, spray-coating, and grafting reactions, followed by further modifications to obtain separation materials for various purposes. In this work, we review the technology of creating rough textures on the surface of cotton fabrics for oil/water separation.
Sustainability spotlightThe discharge of waste organic solvents, various oil–water mixtures, and frequent oil pollution infiltration into water pose significant threats to ecological and environmental safety. Many researchers have developed separation materials, including filter materials, adsorption materials and smart materials with switchable wettability. Among these, natural cotton fabrics have been widely studied as the matrix for separation materials due to their three-dimensional surface structure, porosity, recyclability, low cost, and biodegradability. As an oil–water separation material, the matrix surface must possess micro–nano structures. This work reviews the technology of creating rough textures on the surface of cotton fabric for oil/water separation. In this way, this work aligns with the United Nations' sustainable development goals, particularly Goal 6: Clean Water and Sanitation. |
1. Introduction
In recent decades, the oil industry has experienced rapid growth, significantly benefiting various aspects of daily life.1 However, the frequent leakage of oil from vessels at sea has resulted in immeasurable economic losses and inflicted severe damage to the water environment and aquatic organisms.2 Moreover, oily wastewater generated from domestic sewage systems has detrimental effects on the water environment and the planet as a whole.3–6 Therefore, to safeguard the environment and promote public health, an increasing number of researchers have devoted their efforts towards developing effective treatments for oil/water mixtures.7–9 To date, numerous oil/water separation materials with excellent properties have been developed, including sponges,10 foams,11 metal meshes,12 synthetic membranes,13 natural organic materials14 and others.15,16According to the superwetting theory of the lotus leaf, materials need to have a certain level of roughness and hydrophobicity on their surfaces.17 In line with this theory, researchers have utilized various techniques, such as deposition,18 dip drying,19 spray painting,20 layer-by-layer assembly,21in situ growth,22 external etching23 and other strategies,24 to create the required rough structures on material surfaces. By reducing the surface energy with hydrophobic modifiers, these materials can achieve excellent superhydrophobic and superlipophilic properties, enabling them to effectively separate oil from water.25 This type of material can achieve oil/water separation by removing oil. However, one issue with oil-absorbing materials is that the pores on their surfaces can be easily blocked by oil, greatly reducing their separation effectiveness.26 Accordingly, hydrophilic underwater superoleophobic materials provide a good solution to this problem, as they can improve the material's anti-fouling ability and durability.27
However, due to the fact that the surface free energy of water droplets is higher than that of oil, it is difficult for a material to exhibit both hydrophilic and oleophobic properties simultaneously.28 Currently, the common methods used to achieve both properties include modifying the surface of materials through the addition of rough and uneven structures, which can increase their surface area and improve their surface energy. Ultimately, these modifications can improve the hydrophilic and oleophobic properties of materials.29 The hydrophilic and oleophobic properties of fluorocarbon surfactants have been utilized for the chemical modification of materials.30 Intelligent controllable oil/water separation materials have been extensively studied due to their unique structures, which allow switchable wettability under specific conditions. These materials have been designed to respond to various stimuli such as pH, light, heat, electricity, and gas conversion, making them highly intelligent and efficient for separating oil and water.31 The distinctive characteristic of switchable wettability endows these materials with superior performances compared to conventional separation techniques in terms of separation efficiency, durability, and pollution resistance. Therefore, they are highly anticipated for the continuous treatment of oil/water separation.32,33
Compared to conventional separation materials, naturally grown biomass materials possess certain advantages.34 Firstly, the utilization of natural materials facilitates green environmental protection, given that these materials exhibit excellent degradation properties, and therefore do not pose a significant burden on the environment. Secondly, the low cost of biomass materials is highly conducive to their widespread adoption in separation applications.35 The utilization of natural fiber woven fabrics offers a plethora of advantages. These fabrics possess outstanding hygroscopicity and capillary effect, which are inherent properties of their fibers. The three-dimensional and multidimensional structure of fabrics is also favorable for the adhesion of other modified materials. Additionally, the overall aperture of fabrics is highly controllable.36 Moreover, with the increasing functionalization and diversification of cotton fabrics, there is a wider range of environments in which these fabrics can be applied in the field of oil/water separation.37–39
Herein, we introduce the fundamental theory of surface wettability and analyze three practical applications of oil/water separation fabrics, namely hydrophobic oil-wet, hydrophilic/underwater super oil-wet, and switching wettability fabrics. As shown in Fig. 1, the commonly used preparation methods, including dip drying, deposition, sol–gel, and spray methods, are analyzed and discussed. Finally, oil/water separation fabrics are summarized and their future prospects are provided.
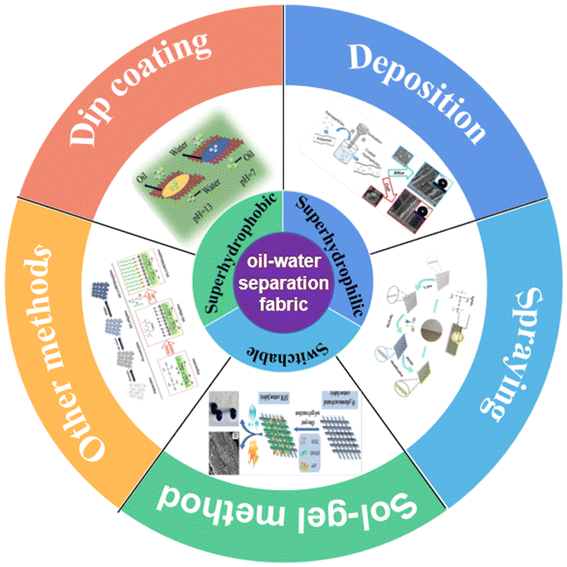 | ||
| Fig. 1 Schematic of the methods for the fabrication of oil/water separation cotton fabrics, such as dip coating,60 deposition,84 spraying,88 sol–gel method,132 and other methods.103 | ||
2. Surface wettability theory
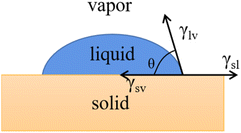
Water droplets in contact with solid surfaces form different ranges of contact angles (WCA), where the size of the contact angle represents the wettability of a material to water, with 10° < WCA < 90° and 90° < WCA < 150° representing hydrophilic and hydrophobic materials, respectively.40 To date, researchers have given more attention to the superhydrophilicity and superhydrophobicity of water droplets on the surface, where 0° < WCA < 10° and 150° < WCA < 180°, respectively, of materials.The most basic theoretical model of contact angle is Young's equation, which describes the equilibrium relationship of surface tension among solid, liquid and gas phases (Fig. 2). It was proposed based on rationalization and is applicable to solids without surface friction, with the conditions of uniform distribution of tension among these three phases. Young's equation is represented as follows:41
γlv![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = γsv − γsl θ = γsv − γsl | (1) |
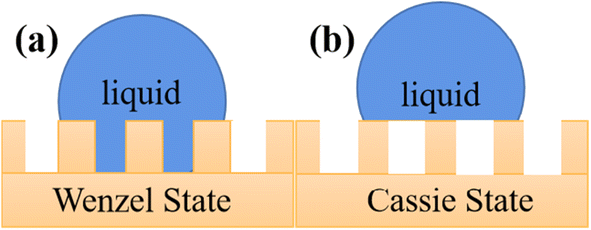
However, in reality, the roughness and tension of a solid surface will lead to a decrease in the contact angle.42 Accordingly, the Wenzel equation and Cassie–Baxter equation are used to correct Young's contact angle.43
The Wenzel equation considers the effects of the rough structure of a solid surface and the uneven interfacial tension and assumes that the liquid is completely filled in the microscopic raised structure of the solid surface, and there is no air between the liquid and the solid. The Wenzel equation describes the contact angle θw, as follows:44
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θw = r θw = r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (2) |
As shown in Fig. 3b, when there is air between the droplet and the contact uneven solid, and it is not completely paved and unfolded, and it is assumed that the area ratio of liquid and gas on the solid surface is f and f1 (f + f1 = 1) and θ and θ1 are the solid–liquid contact angle and gas–liquid contact angle, respectively, where θ1 = 180°. Then, the contact angle θc can be described by the Cassie–Baxter equation, as follows:45
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θc = f θc = f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + f1 θ + f1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ1 = f θ1 = f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + f − 1 θ + f − 1 | (3) |
It can be seen that the contact angle, θc, increases with a decrease in the liquid contact proportion.
The above-mentioned contact angle models have been widely used in many fields. Based on these theories,46 hydrophilic and hydrophobic materials can be prepared by changing the surface energy of materials and constructing micro–nano structures on their surface.47
3. Superhydrophobic and oleophilic fabrics
In recent years, researchers have combined various micro and nano ions on the surface of soft fabrics to form rough three-dimensional structures. Subsequently, effective chemical treatment agents are used to reduce their surface energy and obtain modified fabrics with superhydrophobicity and superoleophilicity.48 In addition, these modified fabrics also have excellent properties such as self-cleaning, anti-fouling, and antibacterial. The effectiveness of these fabric materials is affected by the firmness of their surface coating and the harsh environment of the sewage water body,49 and thus durability is an important indicator for measuring the effectiveness of fabric preparation, which is closely related to the method used to create roughness on the fabric surface. The common methods include dip drying, surface deposition, spraying, sol–gel, in situ growth, and free radical polymerization. Table 1 lists the materials, methods, and efficiency of typical hydrophobic oil-wet fabrics.| Substrate | Material | Method | Efficiency | Ref. |
|---|---|---|---|---|
| Cotton fabrics | Cu(NO3)2, NaOH, 1-dodecanethiol | Dipping | 96.0% | 65 |
| Cotton fabrics | Polydopamine, AgNO3, BPO | Deposition | 96% | 82 |
| Cotton fabrics | TiO2, VTMS, 3-MPTMS | Spraying | 96.7% | 86 |
| Cotton fabrics | TETA, TMC, Al2O3 | Crosslinking polymerization method | 99% | 133 |
| Cotton fabrics | PDA, DDT, FeCl3·6H2O | In situ | 98% | 134 |
| Cotton fabrics | MPTES, octadecyl methacrylate | Grafting reaction | 94% | 135 |
| Cotton fabrics | DMA, octadecyl acrylate | Grafting reaction | 94% | 136 |
| Cotton fabrics | Octadecyl methacrylate | Grafting reaction | 97% | 137 |
| Cotton fabrics | PDMS | Dipping | 95% | 138 |
| Cotton fabrics | PDMS | Dipping | 90% | 139 |
| Cotton fabrics | Palmitic acid | Grafting reaction | 95% | 140 |
| Cotton fabrics | NH4-HMP, LAP, hexadecyltrimethoxysilane | Finishing | — | 141 |
| Cotton fabrics | POSS, MPTES | Grafting reaction | — | 142 |
| Cotton fabrics | Polyacrylates | Dip-coating | — | 143 |
| Cotton fabrics | Lignin/metal ion | Dip-coating | 99.9% | 144 |
3.1. Dip coating
Dip coating is a commonly used technique for preparing modified materials due to its ease of operation and low difficulty. Typically, a bonding material is dissolved in a solution, and the fabric is fully immersed in the solution. Subsequently, the method of drying or curing is applied to form a rough and firm coating on the surface of the fabric.50Compared to other types of nanoparticles, silica nanoparticles have stable chemical properties, high optical transparency, lower toxicity, and environmentally friendly nature. As a result, they are widely applied to improve the self-cleaning performance of materials.51 Furthermore, their controllable size and large specific surface area enable them to be effectively integrated onto various fabric surfaces, resulting in a nanoscale structural effect, which enhances the hydrophobic abilities of materials.
Lin et al.52 successfully prepared a superhydrophobic/superoleophobic-modified composite fabric using cotton fabric as the substrate through a simple two-step dipping strategy. Firstly, the modified SiO2 was dipped on the fabric surface to obtain a micro/nano level rough structure, and then the fluoropolymer was combined by dipping to improve the durability of the composite material. The modified fabric possessed a two-dimensional hierarchical structure, which not only had excellent superhydrophobic/superlipophilic properties, but also maintained good washable durability and self-cleaning ability. However, although modified materials with excellent properties can be easily obtained using the two-step dipping method, and fluorine-containing substances have exceptionally good durability and hydrophobic effect, the use of fluoropolymers can still cause harm to the environment.
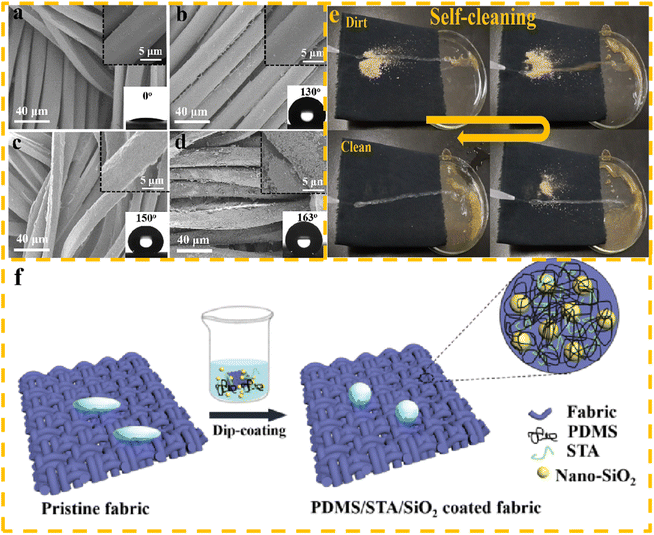
Liu et al.53 used a low-cost and environmentally friendly dipping method to coat polyester fabric with a combination of polydimethylsiloxane, stearic acid, and silicon dioxide. The resulting PDMS/STA/SiO2-coated fabric exhibited improved hydrophobicity, reduced surface energy, and increased surface roughness. The modified polyester exhibited excellent hydrophobic properties, with a water contact angle (WCA) of 163°. In addition, after 700 friction experiments, its contact angle remained above 150°, indicating its potential for practical applications as an environmentally friendly and durable material. Fig. 4 shows the SEM diagram, self-cleaning performance, and preparation process of superhydrophobic fabrics.
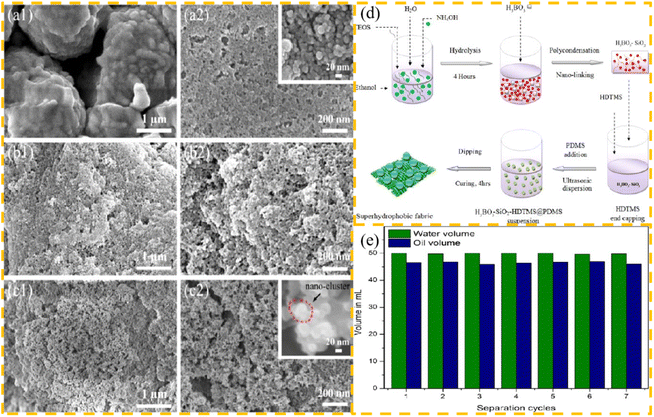
Jannatun et al.54 utilized eco-friendly materials, including boric acid, silica, and polyvinyl alcohol to modify cotton fabrics. By using a two-step dip coating and drying method and leveraging the unique cross-linking properties of three different materials, a dense, microporous, and rough structure was formed on the surface of the cotton fabric. Additionally, the use of PDMS helped to reduce the surface energy and resulted in the creation of a durable, environmentally friendly, and self-healing superhydrophobic cotton fabric. The preparation process is simple and rapid, providing a novel approach for the development of stable and durable superhydrophobic materials that can be applied to various substrates and easily produced on a large scale.
Based on the previous introduction, we learned that the process of combining nanoparticle materials with fabric surfaces often requires the use of adhesives. Unfortunately, many of the widely used adhesives are not environmentally friendly, such as vinyl chloride copolymer, polymethyl methacrylate (PMMA), and phenolic resin.55,56 For the sustainable development of ecology, the level of environmental friendliness of materials has attracted increasing attention. Cheng et al.57 prepared a superhydrophobic and environmentally friendly composite fabric using cheap and environmentally friendly materials, such as renewable fabric as the base and biodegradable diacid curable epoxidation soybean oil thermosetting material as the adhesive of ZnO nanoparticles attached to the fabric surface, combined with a two-step dip coating method. The modified material was not only environmentally friendly, but also exhibited excellent superhydrophobic properties through immersion in an oil/water mixture for up to a week. Inspired by the superhydrophobic properties of lotus leaves, He et al.58 successfully reduced the surface energy of stearic acid, a hydrophobic substance on the surface of lotus leaves, by grafting it onto the surface of cotton fabric. In addition, the pre-treated cotton fabric was immersed in a prepared non-toxic ZnO nanoparticle seed solution to obtain a micro–nano structure fabric surface. The prepared superhydrophobic fabric still maintained excellent separation efficiency even under harsh acid-alkaline conditions, and because of the combination of ZnO, the fabric exhibited a self-cleaning effect and could resist ultraviolet radiation.
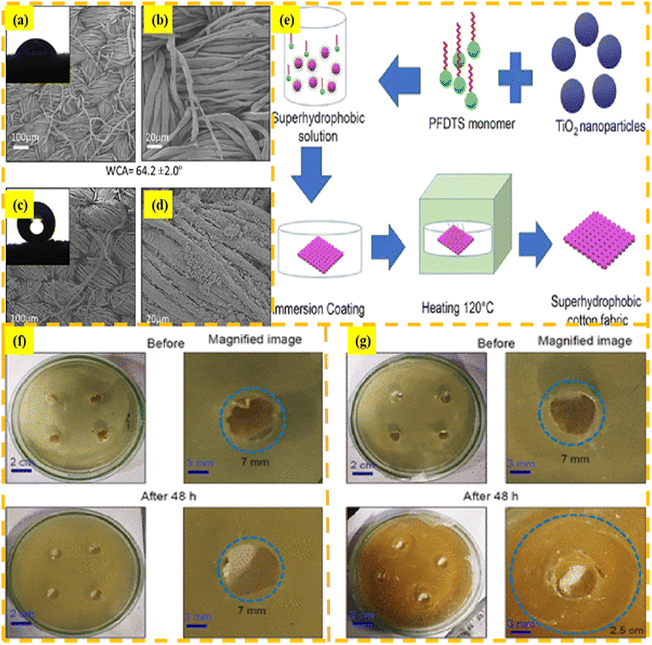
As is well known, TiO2-based materials possess self-cleaning ability, high acid and alkali resistance, and the ability to degrade pollutants in wastewater under light conditions. Therefore, they are widely used in composite materials for oil/water separation and wastewater treatment.59 In addition to oil/water mixtures, wastewater also contains many organic dyes. Feng et al.60 employed a fast and environmentally friendly dip coating approach to fabricate micro–nano level roughness of carboxylic acid-modified TiO2 on the surface of nonwoven fabric. The resulting coated fabric exhibited excellent superhydrophobic properties and displayed efficient degradation of organic pollutants under light conditions. Pal et al.61 utilized fluoride-free, environmentally friendly, and economical TiO2 and 3-(trimethoxysilyl)propyl methacrylate materials to impregnate the surface of cotton fabrics and successfully produced a highly efficient, strong acid and alkali-resistant, self-cleaning, and superhydrophobic cotton fabric. Tudu et al.62 developed a rapid method for creating superhydrophobic fabrics by applying a combination of TiO2 nanoparticles and silane coupling agents on the surface of cotton fabrics. The silane coupling agent used in this study was perfluorodecyl triethoxysilane (PFDTS), which not only reduced the surface energy of the fabric, but also imparted a certain degree of roughness. The presence of TiO2 on the surface of the coated fabric also brings high antibacterial properties. A schematic of the preparation and antibacterial performance of the modified superhydrophobic fabric is shown in Fig. 5.
CuO nanoparticles have been frequently utilized for air and water purification owing to their exceptional photocatalytic properties. With the rapid development of industry, many researchers have also combined the photocatalytic properties of CuO with oil/water separation materials for application in sewage treatment.63 Moreover, nanoparticles of CuO and Ag exhibit excellent antibacterial properties, are more cost-effective, and have good market potential.64 Cao et al.65 prepared a superhydrophobic nanocoated fabric using a low-cost impregnation strategy, which involved immersing the fabric in a Cu ion solution, binding CuO on the fabric surface, and reducing its surface energy using a silane coupling agent. This fabric not only exhibited excellent separation efficiency for a variety of oil/water and organic compound mixtures, but also had a good photocatalytic degradation effect on organic dyes present in sewage.
The bonding stability of crosslinkers is crucial for the adhesion of nanoparticles to fabric surfaces, but the environmental impact of most crosslinkers needs further consideration.66 Agrawal et al.67 adopted an environmentally friendly and simple dipping method to attach CuO nanoparticles to the surface of fabric using a fluorosilane-free coupling agent as a crosslinking agent, effectively improving the durability of the hydrophobic coating. Additionally, the successful combination of metal oxides imparts superhydrophobic, antibacterial, and anti-fouling properties to the fabric.
Despite the relatively high cost of silver ions compared to other nanoparticles, the outstanding antibacterial effect of silver-containing composites obvious. Additionally, their good stability ensures that the separation performance of silver-treated separation materials is not compromised even in harsh solution environments. Thus, silver ions have been widely employed in recent years to enhance the efficiency of oil/water separation by inhibiting the growth of bacteria and microorganisms in wastewater.68 Zhu et al.69 utilized a simple dipping process to densely and roughly coat the surface of a fabric with nano-silver, followed by fluorination to create a repairable and stable superhydrophobic fabric. Even when the hydrophobic properties of fabrics are lost after repeated use, they can be easily restored through simple repairs, which not only extends the lifespan of these materials, but also significantly reduces costs. Although modified fabrics require a certain amount of time and technology for subsequent maintenance and repair, they can still be widely used in many fields in the future. Considering the aforementioned limitations, Liu et al.70 successfully developed a superhydrophobic cotton fabric by combining Ag/AgCl particles on the fabric surface through dip coating and electrostatic adsorption, followed by modification with polydimethylsiloxane for hydrophobicity. The resulting fabric exhibited excellent mechanical stability even after undergoing 50 cycles of friction. Additionally, the fabric displayed excellent self-cleaning properties under ultraviolet irradiation. It is worth noting that the incorporation of Ag/AgCl particles on the fabric surface is known to enhance the anti-bacterial and anti-fungal properties of the fabric, making it useful for various applications in healthcare and the textile industry.
In general, the dip coating method is a cost-effective and efficient surface modification technology with a low entry barrier. However, achieving a uniform coating is crucial, which is challenging, given that the coating process requires careful adjustment of various parameters such as material concentration, temperature, and pH to achieve the desired coating effect.
3.2. Deposition
The deposition method has been widely used for the preparation of superhydrophobic and superoleophilic fabrics, and both chemical vapor deposition and solution deposition methods are commonly employed. The difference from the dip coating method is the various means of deposition, and the compounds are deposited onto the fabric surface through external conditions.71 The use of plasma technology enables the state of a substance to be altered, allowing uniform, controllable, and effective deposition on the surface of a material.72 Electrophoretic deposition is a process that enables the rapid and uniform deposition of charged particles on an electrode surface under the influence of an electric field.73 The chemical vapor deposition (CVD) method is primarily used for the preparation of thin films. This process occurs under high-temperature conditions, where molecules of the raw material in the gas phase react chemically on the surface of the material to form a coating.74In recent years, researchers have found that functionalizing material surfaces with SiO2 nanosol particles have effectively improved their friction resistance and stability during use. Also, this treatment can be combined with the excellent characteristics of fabrics, such as environmental protection and renewability. The SiO2 nanosol particles firmly combine with the fabric, reducing the possibility of loose particles falling off and improving the resistance of the fabric to washing.75
Wear resistance is an important consideration in the design of hydrophobic surfaces, given that the surface roughness of modified fabrics without fluorine materials is easily damaged by friction, leading to a reduction in their hydrophobic properties. Lahiri et al.76 used non-toxic and environmentally friendly materials, such as silicone polymer, to create micro–nano structures on the surface of a fabric using a deposition strategy involving boric acid, alkyl silane polymer, and silica composite material. Subsequently, they achieved a superhydrophobic cotton fabric with excellent durability through hydrophobic modification using PDMS. After being subjected to 40 rounds of sandpaper grinding and 80 rounds of tape bonding, the cotton fabric still retained its superhydrophobic properties. This cost-effective and eco-friendly approach holds great potential for industrial applications. The SEM image of the coated fabric, the process for preparing the fluorine-free superhydrophobic cotton fabric, and the volumes of oil and water after multiple cycles are shown in Fig. 6.
Shaheen et al.77 applied a chemical in situ deposition method to combine a SiO2/TiO2 nanoparticle sol mixture onto cotton fabric. They then treated the surface with octamethyltrisiloxane to create an ultra-hydrophobic cotton fabric with UV resistance and effective antibacterial properties. The experimental results showed that the modified material had a high bactericidal effect even against the most pathogenic Gram-positive bacteria at high nanosol concentrations.
Medical textiles are a common infrastructure for health care because of the low cost of fabric renewing, as well as their superior comfort. Furthermore, the medical field requires medical textiles with excellent antibacterial properties to prevent the spread of infection.78 By employing plasma deposition, Irfan et al.79 successfully fabricated a green ultra-hydrophobic medical cotton cloth by incorporating a silver nanoparticle coating onto the surface of the cloth. The deposition efficiency was high, and the issue of agglomeration and uneven dispersion of silver nanoparticles was effectively addressed. Consequently, the modified fabric exhibited long-lasting antibacterial properties.
As a bionic adhesive, polydopamine (PDA) exhibits strong adhesion ability and excellent durability when combined with various materials, making it an ideal candidate for fabric and nanoparticle binding. However, the efficiency of the commonly used polydopamine deposition methods is often low.80,81 Zhang et al.82 discovered that the deposition process of polydopamine on the surface of cotton fabrics could be accelerated by using external ultraviolet irradiation and photosensitizer treatment. The fabric was treated with silver nanoparticles, which have a rough structure, and modified with alkyl to achieve hydrophobicity. This resulted in the preparation of a photothermal-responsive superhydrophobic fabric. This strategy significantly reduced the reaction time and has strong practical application value.
Electrophoretic deposition is a commonly used deposition method with good results; however, it is significantly limited by the need for a conductive substrate. Kim et al.83 solved this issue by utilizing a combination of nanoparticle self-assembly and electrophoretic deposition. Through this approach, a multi-layer mixed structure of ZnO and SiO2 nanoparticles was formed on the surface of the fabric, which minimized the adhesion and survival of bacteria on the fabric surface. Then, by hydrophobic modification using a water repellent agent, a superhydrophobic cotton fabric with effective antibacterial properties was successfully prepared.
TiO2 and ZnO typically exhibit a rod-like morphology, whereas CuO tends to form a flower-like structure. Ming et al.84 utilized a simple and cost-effective acoustic chemical deposition method to deposit copper oxide nanoparticles onto polyester fabric surfaces, resulting in the creation of environmentally friendly and durable superhydrophobic textiles. The entire process utilized non-fluorinated, harmless coatings, maximized the use of environmentally friendly solvents, and boasted high efficiency in separating oil and water, as well as long-lasting durability.
The deposition method is highly efficient and produces satisfactory results in terms of quality. However, it often requires complex auxiliary acceleration means, which can be difficult to control, limiting its scope of application. As environmental protection requirements continue to evolve, there is a growing need for the development and design of more environmentally friendly deposition methods.
3.3. Spraying
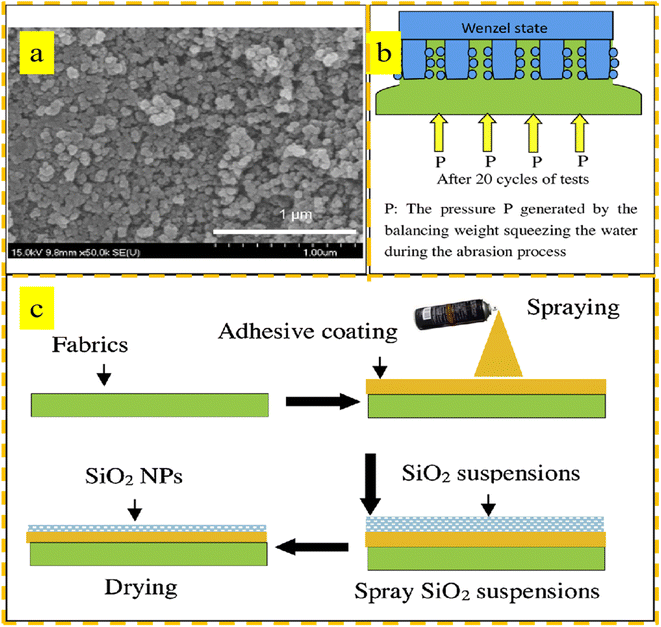
Spraying is considered a promising method for surface modification due to its simplicity, efficiency, and ability to be applied on a large scale. This process involves preparing a mixture of materials with both hydrophobic and lipophilic properties, which is then sprayed onto the surface of a fabric using a specialized device, resulting in a uniform and firmly bonded coating.However, the coating on a superhydrophobic surface is easy to fall off during use and has weak mechanical stability, hindering its practical application to a large extent. To address this issue, Kong et al.85 utilized a simple and efficient two-step spraying method to apply an elastic rubber adhesive as the first layer coating onto the surface of a fabric. Subsequently, hydrophobic-modified vapor SiO2 nanoparticles were sprayed onto the adhesive coating to prepare a superhydrophobic-modified fabric with strong resistance to wear and pollution. After various friction tests, the coating produced by the spraying method on the fabric surface was proven to be stable and durable. In the self-cleaning test, the coating showed an excellent anti-fouling performance, and it is believed that this fluoride-free, environmentally friendly coating has a good application market in the field of outdoor wear. The SEM images after wear and the process of preparing the coating on the polyester fabric surface are shown in Fig. 7.
It is difficult to bind TiO2 nanoparticles to the surface of fabrics by impregnation. Thus, He et al.86 used a more efficient method to modify these nanoparticles and fabric using a silane coupling agent in a two-step process. The modified nanoparticles were firmly bonded to the fabric by spraying, results in the preparation of a superhydrophobic TiO2 composite cotton fabric. This material could be used to decompose pollutants by photocatalysis, while separating sewage, which is of great significance for environmental restoration.
The application of circuits has penetrated all aspects of human life, but the electrical conductivity of aging circuits will decline in harsh environments. As is known, silver has excellent electrical conductivity and good chemical stability, and is widely used in the field of conductive films.87 Wang et al.88 adopted a spraying strategy, spraying rough silver nanoparticles on the surface of a fabric, and then using the polydimethylsiloxane bonding effect to make the nanoparticles bond more firmly. The water contact angle of the modified conductive composite fabric was as high as 163°, and it maintained an efficient ice-breaking performance and electrical conductivity even in a humid environment. The demonstrated excellent performance can be well developed and applied in electromagnetic shielding materials.
Although ZnO has excellent ultraviolet irradiation resistance and antibacterial properties, the durability of ZnO nanoparticle coatings is still an issue. Song et al.89 solved this problem of poor durability by studying different proportions of ZnO and APESP siloxane sprayed on the surface of a fabric. When the ratio of ZnO to APESP was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the modified fabric exhibited the lowest washing loss rate.
2, the modified fabric exhibited the lowest washing loss rate.
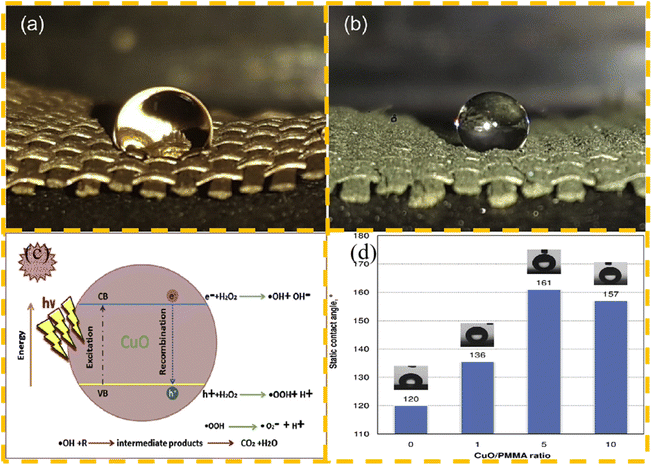
CuO nanoparticles have a high specific surface area and satisfactory activity for the photocatalytic degradation of pollutants. In recent years, CuO nanoparticles have been widely used in the field of pollutant removal and photocatalysis. Long chain fatty acids are commonly used as modifiers for the low surface energy modification of fabrics.90 Ghashghaee et al.91 first modified CuO nanoparticles with stearic acid, although this can already make the fabric have certain hydrophobic properties, and then formed a nanocomposite layer of polymethyl methacrylate and modified nanoparticles on the surface of the fabric through a simple spraying strategy, and prepared by mixing CuO/PMMA in different proportions. The resulting superhydrophobic-modified fabric with optimal properties exhibited a water contact angle of up to 161°. At the same time, the photocatalytic degradation effect of the fabric was good, but after multi-layer coating, the time for the degradation of pollutants became longer. The state of water droplets on the surface of modified fabrics, the mechanism of photocatalytic degradation, and effect of CuO/PMMA ratio on the contact angle of the textile surface are shown in Fig. 8.
Although the spraying method is the most widely used method because of its high efficiency, it still has some practical shortcomings. For example, the dispersion uniformity of nanomaterials in mixed solutions should focus on the stability of the particles. Durability is also worth considering, given that the modified fabric in the process of use cannot avoid the test of wind blowing and various harsh water environments.
3.4. Sol–gel method
The sol–gel method is often combined with other methods with the main purpose of inducing raw materials such as nanoparticles to form a sol–gel with excellent uniformity after a series of reactions in a liquid environment, which can then be combined with impregnation, spraying, coating and other methods on the fabric surface.92Hao et al.93 formed a thin film coating on the surface of a fabric using the sol–gel method. The surface had nano-scale roughness, and the material was re-treated with a new fluoroalkyl siloxane polymer as a hydrophobic agent, resulting in the successful preparation of a fluorine-containing wear-resistant superhydrophobic fabric. Yang et al.94 adopted a low-cost and environmentally friendly one-step sol–gel strategy. Firstly, a TiO2 sol was catalyzed by acetic acid, and then a micro–nano rough coating containing TiO2 sol was combined on the fabric surface. The coated fabric showed excellent self-cleaning performance through testing in harsh environments, expanding the application range of superhydrophobic materials.
In recent years, the performance synthesized nanomaterials has attracted attention from many scholars. For example, the combination of the excellent properties of at least two materials can overcome some defects of modified materials. For example, compared with a single nanoparticle, a composite nanoparticle-combined fabric exhibited a higher self-cleaning performance.95 However, it is difficult to adjust the content ratio of nanoparticles through ion and sputtering technology. Li et al.96 adopted a process combining sol–gel and impregnation with high efficiency and controllable composition to coat AgNO3 and SiO2 nanoparticles on the surface of cotton/linen fabric using a coating machine, which not only possessed a high contact angle after repeated friction, but also had a high contact angle due to the presence of silver ions. The antibacterial properties of the material were also surprising.
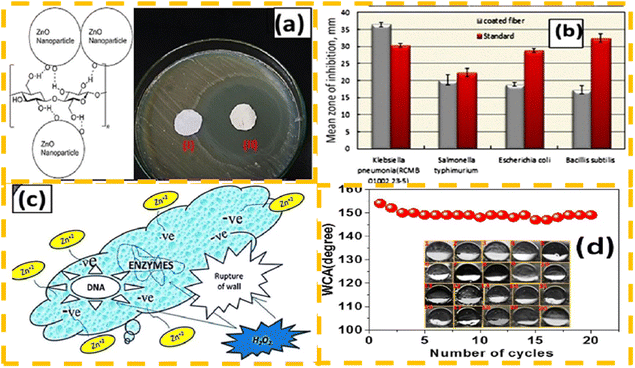
In the process of hydrophobic fabric oil water treatment, the fabric will be polluted and produce a bad smell due to the presence of bacteria and other microorganisms in sewage.97 Shaban et al.98 used a sol–gel strategy to prepare ZnO nanoparticles, which were then loaded on the surface of a fabric by means of coating. The micro and nano structures formed on the surface of the composite not only improved the hydrophobic properties of the fabric, but also endowed the fabric with an excellent antibacterial effect and self-cleaning ability. The photocatalytic antibacterial process is particularly effective in inhibiting Gram-positive and Gram-negative bacteria. The formula for the reaction structure of ZnO on the surface of fabrics, its inhibitory effect on different types of bacteria, antibacterial mechanism, and hydrophobic effect are shown in Fig. 9.
The annual consumption of medical textiles is very surprising, where antibacterial performance is the basis of medical textiles and the most critical link, copper metal has a low adverse reaction to human skin and is conducive to wound healing.99 Khani et al.100 first combined CuO and TiO2 nanoparticles on the surface of a fabric to build a rough structure through an easy-to-operate sol–gel strategy, and then used acids and alcohols to improve the bonding strength of the nanoparticles and reduce the adhesion of bacteria during the use of the material. The prepared medical antibacterial dressing possessed good hydrophobic properties and excellent durability.
The sol–gel strategy enables the preparation of materials that can be used under harsh conditions and are suitable for larger-scale production. However, because special materials and more processing equipment are often used in the production process, the preparation cost is higher, and solvents that are not conducive to environmental protection are used. Thus, in the future, the sol–gel process and the materials used need to be further designed and improved.
3.5. Other methods
In recent years, many scholars have made good progress in the field of antibacterial fabrics, where although the antibacterial performance of medical textiles is excellent, their durability is still an issue. When more bacteria are killed on the surface of the fabric, this will result in their accumulation, and thus the anti-fouling self-cleaning effect of the fabric needs to be improved. When the fabric has low surface energy and rough surface structure, it can effectively solve the accumulation and adhesion of bacteria.101Cheng et al.102 adopted a green economy preparation method using the strong adhesion properties of the biopolymer polydopamine to bind silver nanoparticles in situ on the surface of a fabric, and then grafted the hydrophobic octylamine on its surface through an addition reaction and Schiff base reaction. The modified fabric possessed hydrophobic and antibacterial properties, and the inhibition effect against Escherichia coli and Staphylococcus aureus reached 99%, and the PDA/AgNP/ODA coating showed excellent adhesion fastness after multiple wear tests and acid–base tests.
Fu et al.103 adopted the strategy of free radical polymerization combined with the sol–gel method. Firstly, a large number of silica nanoparticles was combined with tetraethyl orthosilicate and 3-mercaptopropyl triethoxysilane on the surface of a fabric through hydrolytic condensation reaction, and the micro–nano structures formed exhibited a preliminary hydrophobic effect. Subsequently, the surface energy was reduced by grafting 2,2,3,4,4,4-hexafluoromethacrylate on the rough surface. Also, the formed chemical bond energy was large, ensuring the stability of the polymer, making the fabric durable even under harsh conditions and after multiple oil/water mixture cycles, guaranteeing a separation efficiency of more than 98%.
Abd El-Hady et al.104 combined ZnO/SiO2 nanocomposites on the surface of a fabric through electrostatic layer-by-layer self-assembly technology, and the prepared composite materials possessed a multi-layer structure. Firstly, cotton fabric was cationized to facilitate the construction of a film layer by layer. Then, ZnO/SiO2 nanocomposites were deposited on the fabric surface by electrostatic adsorption, and the surface energy of the material was reduced by stearic acid. Using the UPF method, the modified fabric showed excellent UV resistance, and the tensile properties and air permeability of the treated material also improved.
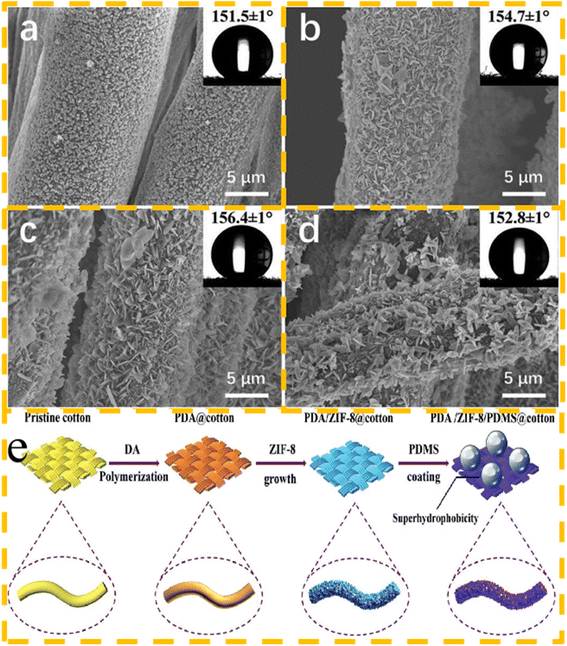
Due to their flexible and adjustable structural aperture, highly ordered structure, and excellent surface contact ability, metal–organic frameworks possess excellent adsorption capacity, and thus have been widely used in storage, filtration, separation and other fields in recent decades. However, the previous applications of MOFs have mainly been studied in powder form, which has many disadvantages in the performance and recycling of materials. In this case, the surface of fabrics can provide sites to facilitate the binding of MOFs.105 A fabric modified by MOFs could not only remove pollutants and harmful heavy metals, while separating them in water treatment, but also improve the reuse capacity of MOFs, facilitate the reuse of materials, and reduce the burden on the environment.106 Long et al.107 adopted an in situ growth strategy to generate compact ZIF-8 nanocrystals on the surface of a fabric. Firstly, PDA@cotton and ZIF-8 nanomaterials were prepared. On the one hand, the durability and impact resistance of the materials were further improved by PDA. On the other hand, the nanoparticles could be well bonded by PDA as an intermediate layer. The materials were immersed in a mixed solution and ZIF-8 nanocrystals were in situ bonded on the surface of PDA@cotton at room temperature. The overall material separation efficiency was remarkable, reaching up to 97%, and after repeated use and testing under harsh chemical conditions, it showed excellent durability and stability. The SEM image, corresponding contact angles, and preparation process of superhydrophobic fabrics are shown in Fig. 10.
4. Hydrophilic and oleophobic fabrics
Superhydrophobic and superoleophilic fabrics have certain anti-pollution and self-cleaning capabilities, but after repeated recycling, the oil stains and bacteria in the oil–water mixture can still block the surface of these fabrics to some extent, greatly reducing their oil/water separation efficiency. In this case, although subsequent surface treatment can restore their original performance, it also wastes a certain amount of human resources, and thus hydrophilic underwater superoleophobic fabrics can solve this problem.108Cotton fabrics have hydrophilic and oleophilic properties. To give cotton fabrics oil/water separation ability, they are modified to have hydrophilic and underwater superoleophobic properties. Inspired by the hydrophilic and oleophobic characteristics of the surface of fish and shrimp in water, it has been confirmed in many studies that hydrophilic/underwater oleophobic surfaces should have hydrophilic substances and a certain multi-dimensional structure.109 When hydrophilic/underwater superoleophobic fabrics are pre-wetted, they form a water film, which effectively blocks the adhesion of oil substances and reduces the risk of being clogged by oil. Thus far, researchers have also used nanoparticles and other substances to form micro and nano structures on the surface of fabrics by various means and modified them by hydrophilic chemical components to effectively improve their hydrophilic and oleophobic properties. They can be used for the separation of mixtures of light oil and water, where the denser water stays at the bottom and the light oil moves at the top. The water can pass through the fabric due to its hydrophilic and oleophobic properties, thus achieving oil/water separation. Table 2 lists the materials, methods, and efficiency of typical hydrophilic and oleophobic fabrics.
| Substrate | Material | Method | Efficiency | Ref. |
|---|---|---|---|---|
| Cotton fabrics | ZnCl2, ammonia | In situ | 99.3% | 110 |
| Basalt fibre fabric | CCl4, H2SO4, HCl, NaOH | Coating | 99.4% | 145 |
| MCC | PFOA, TEMPO | Spraying | 98% | 114 |
| Cotton fabrics | Chitosan, APS, MBA | Coating | 98% | 120 |
| Cotton fabrics | HDTMS, 12-aminodododecanedioic acid | Grafting reaction | 97.3% | 146 |
| Cotton fabrics | 1H,1H,2H,2H-Perfluorooctyltriethoxysilane | Grafting reaction | 97% | 147 |
| Cotton fabrics | PFPE | Dip-coating | — | 148 |
| Cotton fabrics | ABC miktoarm star terpolymers | Dip-coating | 99.4% | 149 |
| Cotton fabrics | STA, TiO2, Al2O3 | Dip-coating | — | 150 |
| Cotton fabrics | Copolymer of isopropylacrylamide and acrylic acid | Dip-coating | — | 151 |
| Cotton fabrics | Polyethyleneimine, perfluorooctanoic acid | Grafting reaction | 96.5% | 152 |
| Cotton fabrics | MOF | Dip-coating | 98.6% | 153 |
| Cotton fabrics | Anionic ammonium polyphosphate | Micro-dissolution | — | 154 |
| Cotton fabrics | Cellulose | Dip-coating | 93.2% | 155 |
| Cotton fabrics | Chitosan | In situ surface deposition | 99% | 156 |
ZnO nanoparticles have excellent surface area and photocatalytic properties and are often used in pollution treatment. Yang et al.110 used a zinc chloride aqueous solution as a micro-solubilizing agent and zinc source, ammonia gas as the base, and in situ growth strategy to uniformly bind zinc ions on the surface of fabric fibers. Under ultraviolet conditions, the ZnO semiconductor material produces holes, which can improve the ability to adsorb water molecules, thus optimizing the water absorption of the fabric. The modified cotton fabric was superhydrophilic/underwater superoleophobic, and its separation efficiency was still as high as 99.2% after multiple cycles of separation. It exhibited excellent degradation ability for dyes in oil/water mixtures, and its excellent oil resistance allowed the fabric to maintain durability, and thus this material is expected to be effectively applied in the field of separation.
Nowadays, most separation materials have excellent processing capacity for dispersed oil/water mixtures, but because an emulsion is formed when liquids of different particle sizes are mixed, these separation methods are difficult to have a good effect, requiring the use of a demulsifier to achieve the separation effect.111,112 Zhang et al.113 prepared a solution with aqueous glutaraldehyde as the crosslinking agent and H2SO4 as the pH, and immersed the fabric in the solution. Then, the PVA solution was poured onto the fabric, and the crosslinking reaction of PVA was controlled to ensure the firm combination of PVA and the fabric while retaining the hydrophilicity of PVA. In addition, because of the capillary effect of the fabric on water and the hydrophilic effect of the coating, the oil droplets in the emulsion contact and fuse with each other to form large oil droplets, resulting in the demulsification effect. The separation efficiency of an oil-in-water emulsion by the surface of the fabric could reach more than 96%. In addition, the composite material did not lose its effect in strong acid and alkali environments, and the hydrophilic and oleophobic ability ensured the anti-fouling and self-cleaning effect.
In view of the problem that some oleophobic materials need to be pre-treated before use, Li et al.,114 inspired by the special infiltration of natural insects, adopted a simple spraying strategy and constructed high and low surface energy coatings on the surface of ball-milled microcrystalline cellulose using perfluorooctanoic acid, successfully preparing fabrics with stable superhydrophilic and superoleophobic properties.
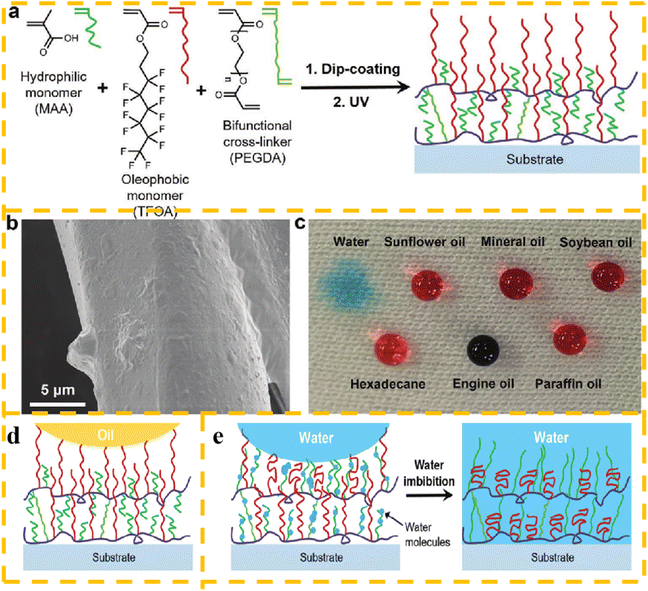
In the literature, a highly oleophobic and superhydrophilic coating was prepared on a fabric using a short fluoroalkyl acrylate.115,116 Chi et al.117 used a UV-induced polymerization strategy to prepare coatings on the surface of polyester fabrics using environmentally friendly short-chain fluorinated acrylates, hydrophilic monomers and crosslinkers as raw materials. The results showed that the coated fabric is superoleophobic and hydrophilic, where the contact angle of most oils is as high as 150°. This fabric quickly absorbed water droplets within 70 ms, effectively prevented the oil droplets from permeating, and showed an improved anti-fouling ability during the separation process. Also, this fabric possessed a good antistatic property, and thus the coated fabric is expected to be used in the field of oil cleanup. A schematic diagram of the formation of hydrophilic and hydrophobic coatings, SEM image of the fiber, photos of water and oil drops on the coated polyester fabric, the repellency of fluorinated chains to oil and the hydration of water molecules into the hydrophilic subsurface are shown in Fig. 11.
Hydrogels with a three-dimensional network structure have strong water absorption and water retention ability, which can be attributed to the abundant hydrophilic groups on their surface. Unlike traditional materials, which are difficult to recycle and have poor anti-pollution ability, the application of hydrogels in water-absorbing materials has been widely investigated.118,119 Kordjazi et al.120 successfully prepared oil/water separation and filtration materials covered by a hydrogel via the in situ synthesis of chitosan/acrylamide hydrogels on the surface of a fabric using the strategy of thermal polymerization. The whole experimental process is simple and green, and the results showed that this material has excellent acid-alkali resistance and stable separation efficiency, and its excellent cost and environmental benefits are considered to be the product of industrialization.
Fabrics have the advantages of natural environmental protection and low price. However, although oil/water separation materials based on fabrics have been widely studied and used in practical applications in recent years, they still have obvious disadvantages in terms of external force resistance and wear resistance compared with other rigid materials such as stainless-steel mesh. In this case, the mechanical properties of nonwovens can be greatly improved by mixing polyester fibers with different melting points.121 Sun et al.122 blended polypropylene PP and polyester fiber LPET in different proportions to effectively strengthen the tensile strength of nonwovens, and then modified the fabric surface hydrophilically with N-isopropylacrylamide (PNIPAM) via the dipping coating strategy. The modified nonwovens showed hydrophilic/underwater superhydrophobic properties and improved mechanical properties.
5. Intelligent response oil/water separation fabric
There are quite a few types of oil/water mixtures, such as suspended oil and emulsion, which are very complex and difficult to deal with. Besides, due to the uncontrollable external environment, separated materials with one-way processing capacity are faced with the limitation of low efficiency. Therefore, the research and development of switchable wettability materials is also expected to be a major trend and result of separated materials. At present, the research on intelligent controllable oil/water separation fabrics has made some progress.123Table 3 lists the materials, methods, and efficiency of typical intelligent response fabrics.Yan et al.124 adopted an easy-to-operate impregnation strategy, mixing Fe3O4 nanoparticles with TiO2 composites modified by lauric acid to make a solution. In the soaking process, the composite nanoparticles were combined on the surface of a fabric, and successfully preparing a fabric with switchable wettability under the influence of acid and alkali. This material exhibited excellent hydrophobic properties in an acidic environment. When the pH value exceeded 11 and the environment became alkaline, the wettability of this material changed from hydrophobic to hydrophilic. After repeated use of this material and exposure to the external condition of ultraviolet light, it still maintained a separation efficiency of up to 98%. In addition, the modified fabric was magnetic and easy to recycle after use.
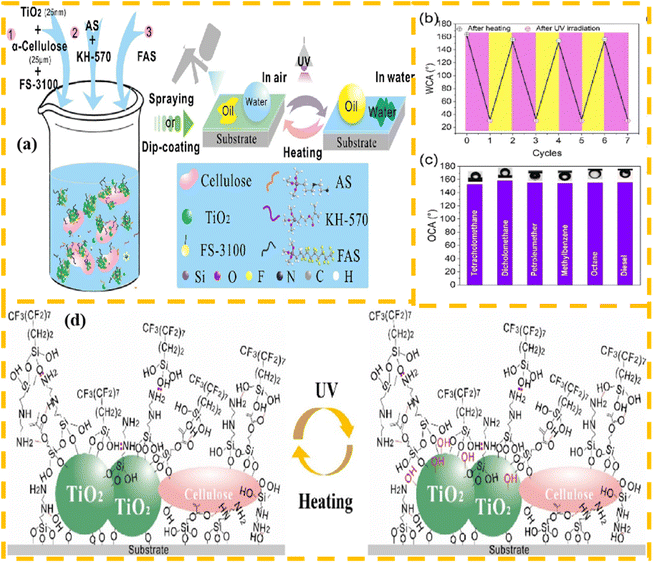
However, the preparation process of many switchable wettability materials is very complicated,125 and the excessive use of organic solvents during their preparation also brings a certain burden to the environment, which is not conducive to their wide market application.126 In this case, Liu et al.127 modified TiO2 particles and a cellulose mixture in water with a variety of silane coupling agents, and successfully prepared coated cotton fabrics with switching wettability under heat treatment and ultraviolet irradiation. Under normal air conditions, the modified fabric exhibited a super hydrophobic wetting effect, and after a period of ultraviolet irradiation, its adsorption property to water gradually increased. Also, this switch in wettability was reversible, where upon treatment at 120 °C, the wettability of the material could be restored to the original state. Due to the photocatalytic effect of TiO2 particles, the fabric could also effectively degrade pollutants in water during the separation process, which greatly improved its anti-fouling ability and durability. This ability to treat oil/water mixtures on demand greatly improves the separation efficiency of separated materials. A flow chart for the preparation of switchable wettability coatings, underwater OCA of different organic solutions, and mechanism diagram of wettability switching are shown in Fig. 12.
Intelligent separation membranes have a good separation efficiency for oil/water mixtures with stable surface activity and not induced to form a miscible oil/water emulsion, but intelligent response materials still encounter the issues of difficult migration and inadequate response.128 Compared to the trigger conditions of other responsive materials, changing the wettability of materials with gases is safer, cheaper, and does not produce additional products that harm the environment.129
Wang et al.130 adopted a self-assembly strategy, first using poly(diethylaminoethyl methacrylate)-methyl comethacrylate to prepare a CO2 and N2-responsive polymers, and then in situ self-assembly inside and on the surface of a fabric, successfully obtaining a switching wettable fabric with a gas response. This material was driven by capillary diffusion force and exhibited an excellent treatment efficiency, especially for unevenly dispersed emulsion mixtures. After CO2 treatment, the wettability of the material changed from superhydrophobic to superhydrophilic, and then CO2 was removed by N2, and its wettability returned to the original superhydrophobic state.
6. Conclusions
In this work, we reviewed three types of fabric-based oil/water separation applications that have emerged in recent years, including superhydrophobic and oleophilic fabrics, hydrophilic oleophobic fabric, and intelligent response oil/water separation fabrics. The majority of scholars have employed techniques that involve the integration of multiple nanoparticles onto the surface of fabrics to create micro and nanostructures with a rough texture. Subsequently, their hydrophobic properties are further enhanced through the application of hydrophobic materials.However, although these separation materials exhibit excellent oil/water treatment effects under laboratory conditions, it is challenging to obtain more comprehensive preparation results. The sustainable and green development of oil/water separation fabrics, including simple fabrication, rapid fabrication, low-cost fabrication; good adhesion, and the separation of emulsified oil/water samples by novel methods such as rapid in situ complexation between fatty acid ligands/metal ions/surface to form hierarchical rough and superhydrophobic fabric surfaces in a facile way, is the future development direction.131 Given that there is a risk of damage to the substances forming the rough structures on the fabric surface during actual use, which may lead to reduced oil–water efficiency, obtaining fabrics with good corrosion resistance and mechanical durability is also a future research direction.
Data availability
All data associated with this study are available upon request from the corresponding author.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors gratefully acknowledge the financial support of the Start-up Projects for Scientific Research of Changzhou University (Grant No. ZMF24020048).References
- Y. Zhai, X. Yuan, C. C. Weber, R. J. Varley and L. C. Henderson, Review of plant cellulose-based aerogel materials for oil/water mixture separation, J. Environ. Chem. Eng., 2024, 12(5), 113716 CrossRef CAS.
- A. MohammadAlizadeh and F. Elmi, Flame retardant and superoleophilic polydopamine/chitosan-graft (g)-octanal coated polyurethane foam for separation oil/water mixtures, Int. J. Biol. Macromol., 2024, 259, 129237 CrossRef CAS PubMed.
- I. Kanu and O. Achi, Industrial effluents and their impact on water quality of receiving rivers in Nigeria, J. Appl. Technol. Environ. Sanit., 2011, 1, 75–86 CAS.
- R. L. Singh and P. K. Singh, Global environmental problems, in Principles and Applications of Environmental Biotechnology for a Sustainable Future, ed. R. L. Singh, Springer Singapore, Singapore, 2017, pp. 13–41 Search PubMed.
- C. Zhao, H. Huang, Z. Li, J. Li, Y. Li, D. Xiang, Y. Wu, J. Chen, X. Chen and M. Qin, 3D superhydrophobic/superoleophilic sponge with hierarchical porous structure and robust stability for high-efficiency and continuous separation of oily wastewater, Sep. Purif. Technol., 2022, 299, 121820 CrossRef CAS.
- C. Zhao, J. Li, Z. Chen, H. Huang, J. Cheng, D. Xiang, Z. Li, Y. Li and Y. Wu, Simple preparation superhydrophobic melamine sponge via one-step emulsion polymerization for continuous oil/water separation in harsh environment, Colloids Surf., A, 2023, 676, 132267 CrossRef CAS.
- K. Tomkowiak, B. Mazela, Z. Szubert and W. Perdoch, Hydrophobic cellulose-based sorbents for oil/water separation, Molecules, 2024, 29(19), 4661 CrossRef CAS.
- K. P. Matabola, T. C. Mokhena, M. F. Bambo, T. H. Mokhothu, J. S. Modise and M. J. Mochane, PVDF-based electrospun nanofibers for oil/water separation: A review, Macromol. Mater. Eng., 2024, 309(8), 2300390 CrossRef CAS.
- A. Beagan, C. Chen and M. E. Mohamed, Bio-copper nanoparticle-based superhydrophobic membranes for sustainable oil/water separation, Water Sci. Technol., 2024, 89(3), 799–810 CrossRef CAS PubMed.
- Q. Li, K. Yan, S. Li, M. Wang, K. Liu, M. Xia, Q. Cheng, J. Xu, S. He, Y. Zhao, M. Li and Y. Wu, Robust and multifunctional 3D superhydrophilic/superoleophobic sponge for rapid oil/water separation and water purification, Prog. Org. Coat., 2024, 192, 108427 CrossRef CAS.
- X. Zhang, Z. Li, K. Liu and L. Jiang, Bioinspired multifunctional foam with self-cleaning and oil/water separation, Adv. Funct. Mater., 2013, 23(22), 2881–2886 CrossRef CAS.
- Y. Q. Liu, D. D. Han, Z. Z. Jiao, Y. Liu, H. B. Jiang, X. H. Wu, H. Ding, Y. L. Zhang and H. B. Sun, Laser-structured Janus wire mesh for efficient oil–water separation, Nanoscale, 2017, 9(45), 17933–17938 RSC.
- U. Baig, M. Faizan and M. A. Dastageer, Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: A review, Adv. Colloid Interface Sci., 2021, 297, 102525 CrossRef CAS.
- X. Bai, Z. Yuan, C. Lu, H. Zhan, W. Ge, W. Li and Y. Liu, Recent advances in superwetting materials for separation of oil/water mixtures, Nanoscale, 2023, 15(11), 5139–5157 RSC.
- N. Zhang, Y. Qi, Y. Zhang, J. Luo, P. Cui and W. Jiang, A review on oil/water mixture separation material, Ind. Eng. Chem. Res., 2020, 59(33), 14546–14568 CrossRef CAS.
- L. Zheng, X. Su, X. Lai, W. Chen, H. Li and X. Zeng, Conductive superhydrophobic cotton fabrics via layer-by-layer assembly of carbon nanotubes for oil-water separation and human motion detection, Mater. Lett., 2019, 253, 230–233 CrossRef CAS.
- M. Yamamoto, N. Nishikawa, H. Mayama, Y. Nonomura, S. Yokojima, S. Nakamura and K. Uchida, Theoretical explanation of the lotus effect: Superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf, Langmuir, 2015, 31(26), 7355–7363 CrossRef CAS PubMed.
- M. A. Gondal, M. S. Sadullah, M. A. Dastageer, G. H. McKinley, D. Panchanathan and K. K. Varanasi, Study of factors governing oil–water separation process using TiO2 films prepared by spray deposition of nanoparticle dispersions, ACS Appl. Mater. Interfaces, 2014, 6(16), 13422–13429 CrossRef CAS.
- L. Zhang, H. Li, X. Lai, X. Su, T. Liang and X. Zeng, Thiolated graphene-based superhydrophobic sponges for oil-water separation, Chem. Eng. J., 2017, 316, 736–743 CrossRef CAS.
- B. Chen, J. Qiu, E. Sakai, N. Kanazawa, R. Liang and H. Feng, Robust and superhydrophobic surface modification by a “paint + adhesive” method: applications in self-cleaning after oil contamination and oil–water separation, ACS Appl. Mater. Interfaces, 2016, 8(27), 17659–17667 CrossRef CAS.
- J. Wang and H. Wang, Multilayered chitosan/kaolin@calcium carbonate composite films with excellent chemical and thermal stabilities for oil/water filtration realized by a facile layer-by-layer assembly, Sep. Purif. Technol., 2022, 289, 120738 CrossRef CAS.
- F. Sun, T. T. Li, X. Zhang, B. C. Shiu, Y. Zhang, H. T. Ren, H. K. Peng, J. H. Lin and C. W. Lou, In situ growth polydopamine decorated polypropylen melt-blown membrane for highly efficient oil/water separation, Chemosphere, 2020, 254, 126873 CrossRef CAS PubMed.
- R. K. Upadhyay and P. R. Waghmare, Underwater oil drop storage, guided transport, and oil/water separation using surfaces with wettability contrast prepared through a vapor-based etching method, ACS Appl. Mater. Interfaces, 2020, 12(9), 11144–11154 CrossRef CAS PubMed.
- B. Wang and Z. Guo, Superhydrophobic copper mesh films with rapid oil/water separation properties by electrochemical deposition inspired from butterfly wing, Appl. Phys. Lett., 2013, 103(6), 063704 CrossRef.
- B. Qiao, Y. Liang, T. Wang and Y. Jiang, Surface modification to produce hydrophobic nano-silica particles using sodium dodecyl sulfate as a modifier, Appl. Surf. Sci., 2016, 364, 103–109 CrossRef CAS.
- M. Xu, G. Wang, Z. Zeng, J. Chen, X. Zhang, L. Wang, W. Song and Q. Xue, Diverse wettability of superoleophilicity and superoleophobicity for oil spill cleanup and recycling, Appl. Surf. Sci., 2017, 426, 1158–1166 CrossRef CAS.
- J. Hu, Y. Zhan, G. Zhang, Q. Feng, W. Yang, Y. H. Chiao, S. Zhang and A. Sun, Durable and super-hydrophilic/underwater super-oleophobic two-dimensional MXene composite lamellar membrane with photocatalytic self-cleaning property for efficient oil/water separation in harsh environments, J. Membr. Sci., 2021, 637, 119627 CrossRef CAS.
- B. P. Binks and J. H. Clint, Solid wettability from surface energy components: relevance to Pickering emulsions, Langmuir, 2002, 18(4), 1270–1273 CrossRef CAS.
- S. N. Wan Ikhsan, N. Yusof, F. Aziz, A. F. Ismail, J. Jaafar, W. N. Wan Salleh and N. Misdan, Superwetting materials for hydrophilic-oleophobic membrane in oily wastewater treatment, J. Environ. Manage., 2021, 290, 112565 CrossRef CAS.
- N. M. Kovalchuk, A. Trybala, V. Starov, O. Matar and N. Ivanova, Fluoro- vs. hydrocarbon surfactants: Why do they differ in wetting performance?, Adv. Colloid Interface Sci., 2014, 210, 65–71 CrossRef CAS PubMed.
- B. Xiang, Q. Sun, Q. Zhong, P. Mu and J. Li, Current research situation and future prospect of superwetting smart oil/water separation materials, J. Mater. Chem. A, 2022, 10(38), 20190–20217 RSC.
- Z. Xue, Y. Cao, N. Liu, L. Feng and L. Jiang, Special wettable materials for oil/water separation, J. Mater. Chem. A, 2014, 2(8), 2445–2460 RSC.
- H. Liu, L. Zhang, J. Huang, J. Mao, Z. Chen, Q. Mao, M. Ge and Y. Lai, Smart surfaces with reversibly switchable wettability: Concepts, synthesis and applications, Adv. Colloid Interface Sci., 2022, 300, 102584 CrossRef CAS PubMed.
- J. Yong, J. Huo, F. Chen, Q. Yang and X. Hou, Oil/water separation based on natural materials with super-wettability: recent advances, Phys. Chem. Chem. Phys., 2018, 20(39), 25140–25163 RSC.
- T. C. Lin, J. S. Chang and D. Lee, Cotton fabrics modified with tannic acid/1-eicosanamine grafting layer for oil/water separation, Chemosphere, 2024, 355, 141703 CrossRef CAS.
- G. Ramaiah, Z. Simeno, T. A. Negawo, S. Y. Baraki, R. Legese and D. Asfaw, Extraction of ensete fibers and its woven fabric green composite development for ceiling board applications, Ind. Crops Prod., 2025, 223, 120189 CrossRef CAS.
- M. H. Abu Elella, N. Y. Abu-Thabit, O. J. Uwaezuoke and A. K. Azad, Superwetting cotton textiles for separation of oil/water mixtures, Cellulose, 2023, 30(12), 7427–7462 CrossRef CAS.
- B. Xiang, Q. Liu, Q. Sun, J. Gong, P. Mu and J. Li, Recent advances in eco-friendly fabrics with special wettability for oil/water separation, Chem. Commun., 2022, 58(97), 13413–13438 RSC.
- S. Xia, Z. Yu, Y. Pang, Z. Chen, Y. Chen, X. Zhang and S. Guo, Advances in the application of superhydrophobic fabric surfaces for oil-water separation and extension of functionalization, J. Environ. Chem. Eng., 2024, 12(6), 114156 CrossRef CAS.
- T. S. Meiron, A. Marmur and I. S. Saguy, Contact angle measurement on rough surfaces, J. Colloid Interface Sci., 2004, 274(2), 637–644 CrossRef CAS.
- X. Zhou, J. J. Koh and C. He, Robust oil-fouling resistance of amorphous cellulose surface underwater: A wetting study and application, Langmuir, 2019, 35(4), 839–847 CrossRef CAS PubMed.
- C. D. Volpe, D. Maniglio, M. Morra and S. Siboni, The determination of a ‘stable-equilibrium’ contact angle on heterogeneous and rough surfaces, Colloids Surf., A, 2002, 206(1), 47–67 CrossRef.
- G. Whyman, E. Bormashenko and T. Stein, The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon, Chem. Phys. Lett., 2008, 450(4), 355–359 CrossRef CAS.
- R. N. Wenzel, Resistance of solid surfaces to wetting by water, Ind. Eng. Chem., 1936, 28(8), 988–994 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Wettability of porous surfaces, Trans. Faraday Soc., 1944, 40, 546–551 RSC.
- E. L. Decker, B. Frank, Y. Suo and S. Garoff, Physics of contact angle measurement, Colloids Surf., A, 1999, 156(1), 177–189 CrossRef CAS.
- G. McHale, M. I. Newton and N. J. Shirtcliffe, Water-repellent soil and its relationship to granularity, surface roughness and hydrophobicity: a materials science view, Eur. J. Soil Sci., 2005, 56(4), 445–452 CrossRef.
- N. X. Zhu, Z. W. Wei, C. X. Chen, D. Wang, C. C. Cao, Q. F. Qiu, J. J. Jiang, H. P. Wang and C. Y. Su, Self-generation of surface roughness by low-surface-energy alkyl chains for highly stable superhydrophobic/superoleophilic MOFs with multiple functionalities, Angew. Chem., Int. Ed., 2019, 58(47), 17033–17040 CrossRef CAS.
- S. Bano, U. Zulfiqar, U. Zaheer, M. Awais, I. Ahmad and T. Subhani, Durable and recyclable superhydrophobic fabric and mesh for oil–water separation, Adv. Eng. Mater., 2018, 20(1), 1700460 CrossRef.
- X. Tang and X. Yan, Dip-coating for fibrous materials: mechanism, methods and applications, J. Sol-Gel Sci. Technol., 2017, 81(2), 378–404 CrossRef CAS.
- X. Li, X. Du and J. He, Self-cleaning antireflective coatings assembled from peculiar mesoporous silica nanoparticles, Langmuir, 2010, 26(16), 13528–13534 CrossRef CAS.
- J. Lin, C. Zheng, W. Ye, H. Wang, D. Feng, Q. Li and B. Huan, A facile dip-coating approach to prepare SiO2/fluoropolymer coating for superhydrophobic and superoleophobic fabrics with self-cleaning property, J. Appl. Polym. Sci., 2015, 132(1), 41458 CrossRef.
- X. Liu, Y. Gu, T. Mi, X. Wang and X. Zhang, Dip-coating approach to fabricate durable PDMS/STA/SiO2 superhydrophobic polyester fabrics, Coatings, 2021, 11(3), 326 CrossRef CAS.
- N. Jannatun, A. Taraqqi-A-Kamal, R. Rehman, J. Kuker and S. K. Lahiri, A facile cross-linking approach to fabricate durable and self-healing superhydrophobic coatings of SiO2-PVA@PDMS on cotton textile, Eur. Polym. J., 2020, 134, 109836 CrossRef CAS.
- S. Magalhães, L. Alves, B. Medronho, A. C. Fonseca, A. Romano, J. F. J. Coelho and M. Norgren, Brief overview on bio-based adhesives and sealants, Polymers, 2019, 11(10), 1685 CrossRef.
- S. H. Imam, S. H. Gordon, L. Mao and L. Chen, Environmentally friendly wood adhesive from a renewable plant polymer: characteristics and optimization, Polym. Degrad. Stab., 2001, 73(3), 529–533 CrossRef CAS.
- Q. Y. Cheng, X. P. An, Y. D. Li, C. L. Huang and J. B. Zeng, Sustainable and biodegradable superhydrophobic coating from epoxidized soybean oil and ZnO nanoparticles on cellulosic substrates for efficient oil/water separation, ACS Sustain. Chem. Eng., 2017, 5(12), 11440–11450 CrossRef CAS.
- Y. He, M. Wan, Z. Wang, X. Zhang, Y. Zhao and L. Sun, Fabrication and characterization of degradable and durable fluoride-free super-hydrophobic cotton fabrics for oil/water separation, Surf. Coat. Technol., 2019, 378, 125079 CrossRef CAS.
- K. Nakata and A. Fujishima, TiO2 photocatalysis: Design and applications, J. Photochem. Photobiol., C, 2012, 13(3), 169–189 CrossRef CAS.
- L. Feng, Y. Hou, Q. Hao, M. Chen, S. Wang, X. Hu and W. Yang, A multi-function textile with pH-induced switch wettability transition for controllable oil–water separation, Text. Res. J., 2021, 92(9–10), 1357–1368 Search PubMed.
- S. Pal, S. Mondal, P. Pal, A. Das, S. Pramanik and J. Maity, Fabrication of durable, fluorine-free superhydrophobic cotton fabric for efficient self-cleaning and heavy/light oil-water separation, Colloid Interface Sci. Commun., 2021, 44, 100469 CrossRef CAS.
- B. K. Tudu, A. Sinhamahapatra and A. Kumar, Surface modification of cotton fabric using TiO2 nanoparticles for self-cleaning, oil–water separation, antistain, anti-water absorption, and antibacterial properties, ACS Omega, 2020, 5(14), 7850–7860 CrossRef CAS PubMed.
- J. O. Ighalo, P. A. Sagboye, G. Umenweke, O. J. Ajala, F. O. Omoarukhe, C. A. Adeyanju, S. Ogunniyi and A. G. Adeniyi, CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances, Environ. Nanotechnol., Monit. Manage., 2021, 15, 100443 CAS.
- M. Ahamed, H. A. Alhadlaq, M. A. M. Khan, P. Karuppiah and N. A. Al-Dhabi, Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles, J. Nanomater., 2014, 2014, 637858 CrossRef.
- C. Cao, F. Wang and M. Lu, Superhydrophobic CuO coating fabricated on cotton fabric for oil/water separation and photocatalytic degradation, Colloids Surf., A, 2020, 601, 125033 CrossRef CAS.
- S. P. Tambe, S. D. Jagtap, R. N. Choudhari and B. P. Mallik, Influence of cross-linking agents and curing condition on the performance of epoxy coating, Pigm. Resin Technol., 2016, 45(5), 354–362 CrossRef CAS.
- N. Agrawal, P. S. Low, J. S. J. Tan, E. W. M. Fong, Y. Lai and Z. Chen, Durable easy-cleaning and antibacterial cotton fabrics using fluorine-free silane coupling agents and CuO nanoparticles, Nano Mater. Sci., 2020, 2(3), 281–291 CrossRef.
- I. De la Rosa-Gómez, M. T. Olguín and D. Alcántara, Antibacterial behavior of silver-modified clinoptilolite–heulandite rich tuff on coliform microorganisms from wastewater in a column system, J. Environ. Manage., 2008, 88(4), 853–863 CrossRef.
- X. Zhu, Z. Zhang, J. Yang, X. Xu, X. Men and X. Zhou, Facile fabrication of a superhydrophobic fabric with mechanical stability and easy-repairability, J. Colloid Interface Sci., 2012, 380(1), 182–186 CrossRef CAS PubMed.
- H. Liu, L. Yang, Y. Zhan, J. Lan, J. Shang, M. Zhou and S. Lin, A robust and antibacterial superhydrophobic cotton fabric with sunlight-driven self-cleaning performance for oil/water separation, Cellulose, 2021, 28(3), 1715–1729 CrossRef CAS.
- C. Du, J. Wang, Z. Chen and D. Chen, Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method, Appl. Surf. Sci., 2014, 313, 304–310 CrossRef CAS.
- L. Martinu and D. Poitras, Plasma deposition of optical films and coatings: A review, J. Vac. Sci. Technol., A, 2000, 18(6), 2619–2645 CrossRef CAS.
- L. Besra and M. Liu, A review on fundamentals and applications of electrophoretic deposition (EPD), Prog. Mater. Sci., 2007, 52(1), 1–61 CrossRef CAS.
- J. O. Carlsson and P. M. Martin, Chapter 7 - Chemical Vapor Deposition, in Handbook of Deposition Technologies for Films and Coatings, ed. P. M. Martin, William Andrew Publishing, Boston, 3rd edn, 2010, pp. 314–363 Search PubMed.
- N. Zhang, M. Xu and L. Cai, Improvement of mechanical, humidity resistance and thermal properties of heat-treated rubber wood by impregnation of SiO2 precursor, Sci. Rep., 2019, 9(1), 982 CrossRef.
- S. K. Lahiri, P. Zhang, C. Zhang and L. Liu, Robust fluorine-free and self-healing superhydrophobic coatings by H3BO3 incorporation with SiO2–Alkyl-Silane@PDMS on cotton fabric, ACS Appl. Mater. Interfaces, 2019, 11(10), 10262–10275 CrossRef CAS.
- T. I. Shaheen, S. S. Salem and S. Zaghloul, A new facile strategy for multifunctional textiles development through in situ deposition of SiO2/TiO2 nanosols hybrid, Ind. Eng. Chem. Res., 2019, 58(44), 20203–20212 CrossRef CAS.
- Z. Fei, B. Liu, M. Zhu, W. Wang and D. Yu, Antibacterial finishing of cotton fabrics based on thiol-maleimide click chemistry, Cellulose, 2018, 25(5), 3179–3188 CrossRef CAS.
- M. Irfan, O. Polonskyi, A. Hinz, C. Mollea, F. Bosco, T. Strunskus, C. Balagna, S. Perero, F. Faupel and M. Ferraris, Antibacterial, highly hydrophobic and semi transparent Ag/plasma polymer nanocomposite coating on cotton fabric obtained by plasma based co-deposition, Cellulose, 2019, 26(16), 8877–8894 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Mussel-inspired surface chemistry for multifunctional coatings, Science, 2007, 318(5849), 426–430 CrossRef CAS.
- Q. Huang, J. Chen, M. Liu, H. Huang, X. Zhang and Y. Wei, Polydopamine-based functional materials and their applications in energy, environmental, and catalytic fields: State-of-the-art review, Chem. Eng. J., 2020, 387, 124019 CrossRef.
- H. Zhang, J. Ou, X. Fang, S. Lei, F. Wang, C. Li, W. Li, Y. Hu, A. Amirfazli and P. Wang, Robust superhydrophobic fabric via UV-accelerated atmospheric deposition of polydopamine and silver nanoparticles for solar evaporation and water/oil separation, Chem. Eng. J., 2022, 429, 132539 CrossRef CAS.
- J. Kim, H. Lee and Y. S. Joung, Antibacterial fabric with contradictory functions of water repellency and absorbency realized by electrophoretic deposition of hydrophobic SiO2 and hydrophilic ZnO nanoparticles, Prog. Org. Coat., 2021, 161, 106455 CrossRef CAS.
- H. P. Ming, C. Y. Chan, S. Mutalik, M. W. Younas, A. Pragya and N. Noor, Sonochemical routes to superhydrophobic soft matter coatings: Comparing silica and copper oxide coatings on polyester fabric, Ind. Eng. Chem. Res., 2022, 61(51), 18729–18743 CrossRef CAS.
- X. Kong, C. Zhu, J. Lv, J. Zhang and J. Feng, Robust fluorine-free superhydrophobic coating on polyester fabrics by spraying commercial adhesive and hydrophobic fumed SiO2 nanoparticles, Prog. Org. Coat., 2020, 138, 105342 CrossRef CAS.
- T. He, H. Zhao, Y. Liu, C. Zhao, L. Wang, H. Wang, Y. Zhao and H. Wang, Facile fabrication of superhydrophobic Titanium dioxide-composited cotton fabrics to realize oil-water separation with efficiently photocatalytic degradation for water-soluble pollutants, Colloids Surf., A, 2020, 585, 124080 CrossRef CAS.
- D. Sun, Y. Feng, S. Sun, J. Yu, S. Jia, C. Dang, X. Hao, J. Yang, W. Ren, R. Sun, C. Shao and F. Peng, Transparent, self-adhesive, conductive organohydrogels with fast gelation from lignin-based self-catalytic system for extreme environment-resistant triboelectric nanogenerators, Adv. Funct. Mater., 2022, 32(28), 2201335 CrossRef CAS.
- Q. Wang, S. Zhu, H. He, J. Du, W. Li, Z. Kang and D. Chen, Conductive and superhydrophobic Ag/PDMS films with high stability for passive de-icing and electromagnetic shielding, Prog. Org. Coat., 2022, 169, 106919 CrossRef CAS.
- T. Song, L. Liu, F. Xu, Y. Pan, M. Qian, D. Li and R. Yang, Multi-dimensional characterizations of washing durable ZnO/phosphazene-siloxane coated fabrics via ToF-SIMS and XPS, Polym. Test., 2022, 114, 107684 CrossRef CAS.
- M. Fallahah, A. Rabiee, M. Ghashghaee and A. Ershad-Langroudi, Enhanced procedure for fabrication of an ultrahydrophobic aluminum alloy surface using fatty acid modifiers, Phys. Chem. Res., 2017, 5(2), 339–357 Search PubMed.
- M. Ghashghaee, M. Fallah and A. Rabiee, Superhydrophobic nanocomposite coatings of poly(methyl methacrylate) and stearic acid grafted CuO nanoparticles with photocatalytic activity, Prog. Org. Coat., 2019, 136, 105270 CrossRef CAS.
- X. Guo, Q. Zhang, X. Ding, Q. Shen, C. Wu, L. Zhang and H. Yang, Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: a review, J. Sol-Gel Sci. Technol., 2016, 79(2), 328–358 CrossRef CAS.
- L. F. Hao, Q. F. An, W. Xu and Q. J. Wang, Synthesis of fluoro-containing superhydrophobic cotton fabric with washing resistant property using nano-SiO2 sol-gel method, Adv. Mater. Res., 2010, 121–122, 23–26 CAS.
- M. Yang, W. Liu, C. Jiang, S. He, Y. Xie and Z. Wang, Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process, Carbohydr. Polym., 2018, 197, 75–82 CrossRef CAS PubMed.
- T. Gordon, B. Perlstein, O. Houbara, I. Felner, E. Banin and S. Margel, Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties, Colloids Surf., A, 2011, 374(1), 1–8 CrossRef CAS.
- H. Li, Y. Zhuang, H. Li, K. C. Bracamontes, D. Wang, Y. Sun, D. Sun, L. Kong, S. Gao and M. Song, Preparation, characterization, antibacterial properties and hydrophobic evaluation of SiO2/Ag nanosol coated cotton/linen fabric, J. Text. Inst., 2020, 111(1), 75–83 CrossRef CAS.
- Y. Liu, Q. Dong and H. Shi, Distribution and population structure characteristics of microorganisms in urban sewage system, Appl. Microbiol. Biotechnol., 2015, 99(18), 7723–7734 CrossRef CAS.
- M. Shaban, F. Mohamed and S. Abdallah, Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst, Sci. Rep., 2018, 8(1), 3925 CrossRef.
- A. Davies, 16 - Healthcare textiles, in Waterproof and Water Repellent Textiles and Clothing, ed. J. Williams, Woodhead Publishing, 2018, pp. 447–471 Search PubMed.
- A. Khani and N. Talebian, In vitro bactericidal effect of ultrasonically sol–gel-coated novel CuO/TiO2/PEG/cotton nanocomposite for wound care, J. Coat. Technol. Res., 2017, 14(3), 651–663 CrossRef CAS.
- D. Xiong, G. Liu and E. J. S. Duncan, Diblock-copolymer-coated water- and oil-repellent cotton fabrics, Langmuir, 2012, 28(17), 6911–6918 CrossRef CAS.
- W. Cheng, W. Liu, Q. Wang, P. Wang, M. Zhou and Y. Yu, Durable hydrophobic and antibacterial textile coating via PDA/AgNPs/ODA in situ assembly, Cellulose, 2022, 29(2), 1175–1187 CrossRef CAS.
- X. Fu, S. Wang, Z. Liu, Y. Luo, X. Du, H. Wang, M. Zhou, X. Cheng and Z. Du, Robust superhydrophobic fabric for durability, self-cleaning, and oil/water separation via thiol–acrylate polymerization, Macromol. Mater. Eng., 2021, 306(1), 2000504 CrossRef CAS.
- M. M. Abd El-Hady, S. Sharaf and A. Farouk, Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique, Cellulose, 2020, 27(2), 1099–1110 CrossRef CAS.
- C. Liu, J. Wang, J. Wan and C. Yu, MOF-on-MOF hybrids: Synthesis and applications, Coord. Chem. Rev., 2021, 432, 213743 CrossRef CAS.
- S. Yu, H. Pang, S. Huang, H. Tang, S. Wang, M. Qiu, Z. Chen, H. Yang, G. Song, D. Fu, B. Hu and X. Wang, Recent advances in metal-organic framework membranes for water treatment: A review, Sci. Total Environ., 2021, 800, 149662 CrossRef CAS PubMed.
- Z. Long, L. Yuan, J. Chen, L. Luo, C. Shi, C. Wu, H. Qiao and K. Wang, A durable fluorine-free MOF-based self-cleaning superhydrophobic cotton fabric for oil-water separation, Adv. Mater. Interfaces, 2022, 9(13), 2102427 CrossRef CAS.
- P. Ragesh, V. Anand Ganesh, S. V. Nair and A. S. Nair, A review on ‘self-cleaning and multifunctional materials’, J. Mater. Chem. A, 2014, 2(36), 14773–14797 RSC.
- R. Yue, C. An, Z. Ye, E. Owens, E. Taylor and S. Zhao, Green biomass-derived materials for oil spill response: recent advancements and future perspectives, Curr. Opin. Chem. Eng., 2022, 36, 100767 CrossRef.
- P. Yang, J. Yang, Z. Wu, X. Zhang, Y. Liu and M. Lu, Facile fabrication of superhydrophilic and underwater superoleophobic surfaces on cotton fabrics for effective oil/water separation with excellent anti-contamination ability, Colloids Surf., A, 2021, 628, 127290 CrossRef CAS.
- W. Zhang, N. Liu, Y. Cao, X. Lin, Y. Liu and L. Feng, Superwetting porous materials for wastewater treatment: from immiscible oil/water mixture to emulsion separation, Adv. Mater. Interfaces, 2017, 4(10), 1600029 CrossRef.
- W. Kang, G. Jing, H. Zhang, M. Li and Z. Wu, Influence of demulsifier on interfacial film between oil and water, Colloids Surf., A, 2006, 272(1), 27–31 CrossRef CAS.
- Y. R. Zhang, B. W. Meng, B. Hao and P. C. Ma, Aggregation-induced demulsification triggered by the hydrophilic fabric for the separation of highly emulsified oil droplets from water, Aggregate, 2022, 3(1), e131 CrossRef CAS.
- X. Li, Y. Peng, F. Zhang, Z. Yang and Z. Dong, Fast-response, no-pretreatment, and robustness air-water/oil amphibious superhydrophilic-superoleophobic surface for oil/water separation and oil-repellent fabrics, Chem. Eng. J., 2022, 427, 132043 CrossRef CAS.
- T. Takayanagi and M. Yamabe, Progress of fluoropolymers on coating applications: Development of mineral spirit soluble polymer and aqueous dispersion, Prog. Org. Coat., 2000, 40(1), 185–190 CrossRef CAS.
- S. H. Korzeniowski, R. C. Buck, R. M. Newkold, A. E. kassmi, E. Laganis, Y. Matsuoka, B. Dinelli, S. Beauchet, F. Adamsky, K. Weilandt, V. K. Soni, D. Kapoor, P. Gunasekar, M. Malvasi, G. Brinati and S. Musio, A critical review of the application of polymer of low concern regulatory criteria to fluoropolymers II: Fluoroplastics and fluoroelastomers, Integr. Environ. Assess. Manage., 2023, 19(2), 326–354 CrossRef CAS.
- H. Chi, Z. Xu, Y. Ma, T. Tang, T. Zhang and Y. Zhao, Multifunctional highly oleophobic and superhydrophilic fabric coatings prepared by facile photopolymerization, Adv. Sustainable Syst., 2020, 4(7), 2000049 CrossRef CAS.
- W. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux, Adv. Mater., 2013, 25(14), 2071–2076 CrossRef CAS PubMed.
- S. L. Loo, L. Vásquez, A. Athanassiou and D. Fragouli, Polymeric hydrogels—a promising platform in enhancing water security for a sustainable future, Adv. Mater. Interfaces, 2021, 8(24), 2100580 CrossRef.
- S. Kordjazi, K. Kamyab and N. Hemmatinejad, Super-hydrophilic/oleophobic chitosan/acrylamide hydrogel: an efficient water/oil separation filter, Adv. Compos. Hybrid Mater., 2020, 3(2), 167–176 CrossRef CAS.
- S. Kubo and J. F. Kadla, Lignin-based carbon fibers: Effect of synthetic polymer blending on fiber properties, J. Polym. Environ., 2005, 13(2), 97–105 CrossRef CAS.
- F. Sun, T. T. Li, X. Zhang, B. C. Shiu, Y. Zhang, H. T. Ren, H. K. Peng, J. H. Lin and C. W. Lou, Facile fabrication of hydrophilic-underwater superoleophobic poly(N-isopropylacrylamide) coated PP/LPET nonwoven fabrics for highly efficient oil/water separation, Prog. Org. Coat., 2020, 148, 105780 CrossRef CAS.
- J. J. Li, Y. N. Zhou and Z. H. Luo, Polymeric materials with switchable superwettability for controllable oil/water separation: A comprehensive review, Prog. Polym. Sci., 2018, 87, 1–33 CrossRef CAS.
- T. Yan, X. Chen, T. Zhang, J. Yu, X. Jiang, W. Hu and F. Jiao, A magnetic pH-induced textile fabric with switchable wettability for intelligent oil/water separation, Chem. Eng. J., 2018, 347, 52–63 CrossRef CAS.
- H. Ye, L. Zhu, W. Li, H. Liu and H. Chen, Simple spray deposition of a water-based superhydrophobic coating with high stability for flexible applications, J. Mater. Chem. A, 2017, 5(20), 9882–9890 RSC.
- H. Zhou, H. Wang, H. Niu, Y. Zhao, Z. Xu and T. Lin, A waterborne coating system for preparing robust, self-healing, superamphiphobic surfaces, Adv. Funct. Mater., 2017, 27(14), 1604261 CrossRef.
- X. Liu, Y. Wei, F. Tao, X. Zhang, L. Gai and L. Liu, All-water-based superhydrophobic coating with reversible wettability for oil-water separation and wastewater purification, Prog. Org. Coat., 2022, 165, 106726 CrossRef CAS.
- D. Rana and T. Matsuura, Surface modifications for antifouling membranes, Chem. Rev., 2010, 110(4), 2448–2471 CrossRef CAS PubMed.
- L. Dong and Y. Zhao, CO2-switchable membranes: structures, functions, and separation applications in aqueous medium, J. Mater. Chem. A, 2020, 8(33), 16738–16746 RSC.
- Y. Wang, S. Yang, J. Zhang, Z. Chen, B. Zhu, J. Li, S. Liang, Y. Bai, J. Xu, D. Rao, L. Dong, C. Zhang and X. Yang, Scalable and switchable CO2-responsive membranes with high wettability for separation of various oil/water systems, Nat. Commun., 2023, 14(1), 1108 CrossRef CAS PubMed.
- N. Y. Abu-Thabit, A. K. Azad, K. Mezghani, A. S. Hakeem, Q. A. Drmosh, S. Akhtar and A. Y. Adesina, Facile and green fabrication of superhydrophobic polyacrylonitrile nonwoven fabric with iron hydroxide nanoparticles for efficient oil/water separation, ACS Appl. Polym. Mater., 2022, 4(11), 8450–8460 CrossRef CAS.
- D. Lin, X. Zeng, H. Li, X. Lai and T. Wu, One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction, J. Colloid Interface Sci., 2019, 533, 198–206 CrossRef CAS PubMed.
- B. Jiang, Z. Chen, Y. Sun, H. Yang, H. Zhang, H. Dou and L. Zhang, Fabrication of superhydrophobic cotton fabrics using crosslinking polymerization method, Appl. Surf. Sci., 2018, 441, 554–563 CrossRef CAS.
- D. Cheng, Y. Zhang, X. Bai, Y. Liu, Z. Deng, J. Wu, S. Bi, J. Ran, G. Cai and X. Wang, Mussel-inspired fabrication of superhydrophobic cotton fabric for oil/water separation and visible light photocatalytic, Cellulose, 2020, 27(9), 5421–5433 CrossRef CAS.
- L. Liang, Y. Dong, W. Xu and X. Meng, Fabrication of hydrophobic/oleophilic cotton fabric based on thiol–ene click reaction for oil/water separation, J. Text. Inst., 2022, 113(9), 1838–1844 CrossRef CAS.
- L. Liang, M. Su, C. Zheng, J. Li, H. Zhan, X. Li and X. Meng, Fabrication of hydrophobic/oleophilic cotton fabric by mussel-inspired chemistry for oil/water separation, Fibers Polym., 2017, 18(12), 2307–2314 CrossRef CAS.
- X. Meng, Y. Dong, M. J. L. Arias, S. Mu and L. Liang, RAFT reaction modified cotton fabric and its application for oil/water separation, Fibers Polym., 2022, 23(2), 396–403 CrossRef CAS.
- X. Meng, C. Song, Z. Xing, M. J. Lis Arias, J. Yan, Q. Ren, Y. Xiang and L. Liang, ZIF-8/GO/PDMS modified cotton fabric to form a hierarchical-structure coating for fast oil/water separation, J. Water Process Eng., 2024, 60, 105158 CrossRef.
- L. Ghorbani, D. Caschera and B. Shokri, Effect of oxygen plasma pre-treatment on the surface properties of Si-modified cotton membranes for oil/water separations, Materials, 2022, 15(23), 8551 CrossRef CAS PubMed.
- M. E. Mohamed and B. A. Abd-El-Nabey, Fabrication of durable superhydrophobic/oleophilic cotton fabric for highly efficient oil/water separation, Water Sci. Technol., 2020, 83(1), 90–99 CrossRef PubMed.
- H. Nabipour, X. Wang, L. Song and Y. Hu, Hydrophobic and flame-retardant finishing of cotton fabrics for water–oil separation, Cellulose, 2020, 27(7), 4145–4159 CrossRef CAS.
- T. C. Lin, J. S. Chang and D. J. Lee, Hydrophobic cotton fabric with 3-mercaptopropyltriethoxysilane/polyhedral oligomeric silsesquioxane/1-octadecanethiol modification for oil/water separation, Int. J. Biol. Macromol., 2023, 253, 126748 CrossRef CAS PubMed.
- N. Li, J. Chen, J. Li, H. Wu, Z. Li, X. He and L. Cai, Facile construction of versatile cotton fabrics with robust hydrophobicity, self-cleaning and oil–water separation, Fibers Polym., 2024, 25(2), 565–575 CrossRef CAS.
- X. Liu, X. Chen, H. Bian, S. Ni, Z. Li, N. Liu, M. Qin and F. Zhang, Highly hydrophobic cotton fabric by in situ co-deposition of lignin/metal particles for oil/water separation, Ind. Crops Prod., 2023, 204, 117393 CrossRef CAS.
- Y. R. Zhang, B. W. Meng, B. Hao and P. C. Ma, Aggregation-induced demulsification triggered by the hydrophilic fabric for the separation of highly emulsified oil droplets from water, Aggregate, 2021, 3, e131 CrossRef.
- B. Yu, K. Hou, Z. Fan, K. Jin and Z. Cai, Design fiber-based membrane with interfacial wettability rapidly regulated behavior by pH for oily wastewater high-efficient treatment, Prog. Org. Coat., 2024, 189, 108326 CrossRef CAS.
- J. Li, L. Yang, H. Liu, G. Li, R. Li, Y. Cao and H. Zeng, Simple preparation method for hydrophilic/oleophobic coatings, ACS Appl. Mater. Interfaces, 2020, 12(40), 45266–45273 CrossRef CAS.
- Y. Wang, C. You, C. Kowall and L. Li, A nanometer-thick, mechanically robust, and easy-to-fabricate simultaneously oleophobic/hydrophilic polymer coating for oil–water separation, Ind. Eng. Chem. Res., 2018, 57(45), 15395–15399 CAS.
- M. Xiao, Y. Huang, A. Xu, T. Zhang, C. Zhan and L. Hong, On-demand oil–water separation by environmentally responsive cotton fabrics, ACS Omega, 2019, 4(7), 12333–12341 CrossRef CAS PubMed.
- J. Fu, F. Yang and Z. Guo, Fabrication of switchable surface wettability with UV-triggered on cotton fabric, Mater. Lett., 2021, 283, 128767 CrossRef CAS.
- J. Chen, C. Shen, S. Yang, M. Rana and P. C. Ma, Acid and temperature dual-responsive cotton fabrics with polymer coating, Compos. Commun., 2017, 4, 10–15 CrossRef.
- Y. Wang, Y. Xiao, X. Fu, L. Jiang, A. Yuan, H. Xu, Z. Wei, Y. Lei and J. Lei, Facile preparation of cotton fabric with superhydrophilicity–oleophobicity in air and superoleophobicity under water by using branched polyethyleneimine/perfluorooctanoic acid composites, New J. Chem., 2021, 45(34), 15321–15327 RSC.
- G. Zhang, Y. Liu, C. Chen, L. Long, J. He, D. Tian, L. Luo, G. Yang, X. Zhang and Y. Zhang, MOF-based cotton fabrics with switchable superwettability for oil–water separation, Chem. Eng. Sci., 2022, 256, 117695 CrossRef CAS.
- Y. Qiuyu, L. Xinyue, R. Qing, T. Jiang, W. Peng, L. Ming and X. Hang, Anionic ammonium polyphosphate coated underwater superoleophobic cotton fabric for effective oil/water separation, Ind. Crops Prod., 2023, 202, 117080 CrossRef.
- Y. R. Zhang, J. T. Chen, B. Hao, R. Wang and P. C. Ma, Preparation of cellulose-coated cotton fabric and its application for the separation of emulsified oil in water, Carbohydr. Polym., 2020, 240, 116318 CrossRef CAS PubMed.
- M. Wang, M. Peng, J. Zhu, Y. D. Li and J. B. Zeng, Mussel-inspired chitosan modified superhydrophilic and underwater superoleophobic cotton fabric for efficient oil/water separation, Carbohydr. Polym., 2020, 244, 116449 CrossRef CAS.
- M. Qu, Y. Pang, J. Li, R. Wang, D. He, Z. Luo, F. Shi, L. Peng and J. He, Eco-friendly superwettable functionalized-fabric with pH-bidirectional responsiveness for controllable oil-water and multi-organic components separation, Colloids Surf., A, 2021, 624, 126817 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |