Non-enzymatic low-level glucose detection electrode fabricated via single-step laser-induced forward transfer
Pong-Ping
Liu
,
Shing-Fung
Lau
and
Chien-Fang
Ding
 *
*
Department of Biomechatronics Engineering, National Taiwan University, Taipei 10617, Taiwan. E-mail: cfding@ntu.edu.tw
First published on 28th November 2024
Abstract
This study first employed single-step Laser-induced Forward Transfer (LIFT) to transfer copper metal film (1000 nm) onto Indium Tin Oxide (ITO) and Polyethylene terephthalate (PET) substrates separately, fabricating Cu2O/ITO and Cu2O/PET electrodes which were then used for non-enzymatic glucose detection. Morphology, surface elemental properties, and structural analysis were conducted using Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray Spectroscopy (EDS), and X-ray Diffraction (XRD). The Cu2O/ITO electrode demonstrated outstanding glucose detection performance, with a sensitivity of approximately 1214.33 μA mM−1 cm−2, a Limit of Detection (LOD) of 1.297 μM, and a linear relationship between current and glucose concentration of R2 = 0.989. On the other hand, the Cu2O/PET electrode showed a sensitivity of about 1188.14 μA mM−1 cm−2, an LOD of 1.824 μM, and a linear relationship between current and glucose concentration of R2 = 0.997. Both electrodes exhibited remarkable glucose sensing performance. Furthermore, both electrodes demonstrated good selectivity towards glucose in the presence of interfering substances such as Ascorbic acid (AA), Uric acid (UA), Dopamine (DA), and NaCl.
Introduction
Diabetes is a major global health issue, posing significant challenges to human health. According to the IDF Diabetes Atlas, the prevalence of diabetes among the global population aged 20–79 was estimated at 10.5% (536.6 million people) in 2021, and it is projected to rise to 12.2% (783.2 million people) by 2045.1 This indicates a worsening trend in the diabetes problem. Diabetic patients must continuously monitor their blood glucose levels to prevent complications such as blindness, kidney failure, cardiovascular diseases, and other conditions. Therefore, blood glucose monitoring is crucial not only in the medical field but also in drug development, food analysis, and industrial applications, garnering widespread attention. These glucose sensor technologies are becoming a research hotspot across multiple fields, offering possibilities for broad applications.Electrochemical glucose sensors based on glucose oxidase (GOx) or glucose dehydrogenase (GDH) are widely used due to their higher selectivity and faster testing compared to optical and Raman spectroscopy sensing techniques.2–5 Despite their dominant position in glucose sensor research and the market, the complex process of immobilizing enzymes on conductive electrodes, sensitivity to environmental changes, lack of long-term stability, and high production costs limit their further development. These drawbacks have drawn researchers' attention to the development of non-enzymatic glucose sensors.6,7 Non-enzymatic glucose sensors can directly oxidize glucose on the electrode surface without relying on enzymes, making them an attractive option for glucose analysis due to their low cost, high sensitivity, high selectivity, and stability.
To enhance catalytic performance, several methods can be employed: modifying the electrode surface to increase roughness, thereby increasing electrochemical active sites; or combining other materials such as alloys, metal–organic frameworks (MOFs), or carbon materials, to adjust the electronic structure and optimize the adsorption capacity at interface for glucose molecules.8–12 Therefore, it is evident that the modification state of the electrode material surface and interface is crucial for the electrocatalytic performance of non-enzymatic glucose sensors.
In terms of material selection, noble metals (Ag, Au, Pd, Pt),13–15 transition metals (Mn, Fe, Co, Ni),16,17 and alloys (CuAg, NiCo, PdAg)18–20 have been extensively studied due to their excellent sensing performance. Glucose sensors based on metal oxides (CuO, ZnO, NiO)have stood out among non-enzymatic glucose sensors because of their mechanical and chemical stability, as well as their excellent detection selectivity.21–23
Copper-based nanomaterials are considered one of the ideal choices for low-cost, high-electrochemical activity sensing materials.24 Among them, copper oxide (CuO) and cuprous oxide (Cu2O) are both p-type semiconductors with bandgaps of 1.2 eV and 2.1 eV, respectively.25,26 The catalytic mechanism for glucose oxidation primarily depends on the electrochemical transition of Cu2+ to Cu3+ in the presence of OH− ions under alkaline conditions. This oxidation is attributed to the formation of Cu3+ species, predominantly existing as CuOOH and Cu(OH)4−. The electrocatalytic oxidation of glucose is intrinsically linked to electron transfer associated with this transition. During the process, glucose is initially oxidized to gluconolactone (C6H10O6), followed by further oxidation to gluconic acid (C6H12O7). The electron transfer accompanying the Cu2+ to Cu3+ conversion significantly enhances the anodic current response. These electrochemical mechanisms are comprehensively described by reaction (1)–(4).27 As a result, they exhibit higher catalytic performance and greater conductivity in electroanalysis compared to unstable elementary copper.28
| Cu(OH)2 + OH− → CuOOH + H2O + e− | (1) |
| CuO + OH− → CuOOH + e− | (2) |
| CuO + H2O → Cu(OH)4− + e− | (3) |
| Cu(OH)2·2OH− → Cu(OH)4− + e− | (4) |
Various nanostructures such as nanowires,29 nanoflowers,30 nanospheres,31 nanorods,32 nanoparticles,25,33 nanocubes,34 thin films,35 nanofibers36 and nanosheet37 have been synthesized using common methods like electrodeposition, hydrothermal synthesis, and wet-chemistry routes. However, these methods, including electrodeposition, cannot be deposited on non-conductive substrates and involve time-consuming and complex chemical steps, limiting their large-scale industrial application.
Laser processing, as a simple and rapid direct and assisted fabrication method, has been widely used in the manufacture of non-enzymatic glucose sensing components.38 It effectively increases surface roughness, enhances electrochemical active sites, and is cost-effective, making it a promising alternative.39–41
Laser-induced Forward Transfer (LIFT) is a direct deposition process that allows for direct patterning without the need for masks, and it avoids the additional, time-consuming steps of lithography and etching involved in thin-film component fabrication. In this study, the thin-film process of LIFT is conducted at ambient pressure and room temperature. A pulsed laser passes through a glass substrate, focusing on the donor thin film to locally melt and eject microdroplets, transferring the copper-coated donor film onto Indium Tin Oxide (ITO) and Polyethylene Terephthalate (PET) substrates at different power levels. Morphology, surface elemental properties, structural analysis, and selectivity tests were thoroughly investigated, which helps to understand the characteristics of the working electrode and provides strong support for applications in glucose detection.
Results and discussion
Structural characterization
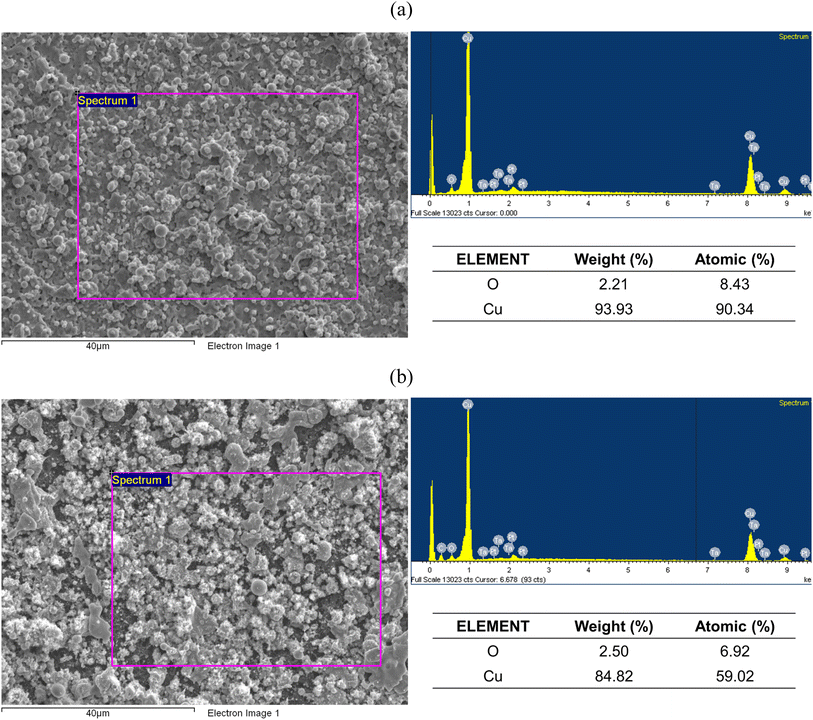
The cross-section and surface of PET and ITO after LIFT process are shown in Fig. 1 and Fig. 2, respectively. At low magnification, the surface of ITO exhibits a denser and more uniform distribution compared to the PET substrate. This difference may arise from the flexible nature of PET substrates. Previous study has indicated that conducting LIFT processes on flexible substrates may result in poor electrical performance, and in some cases, non-conductive outcomes after transfer.42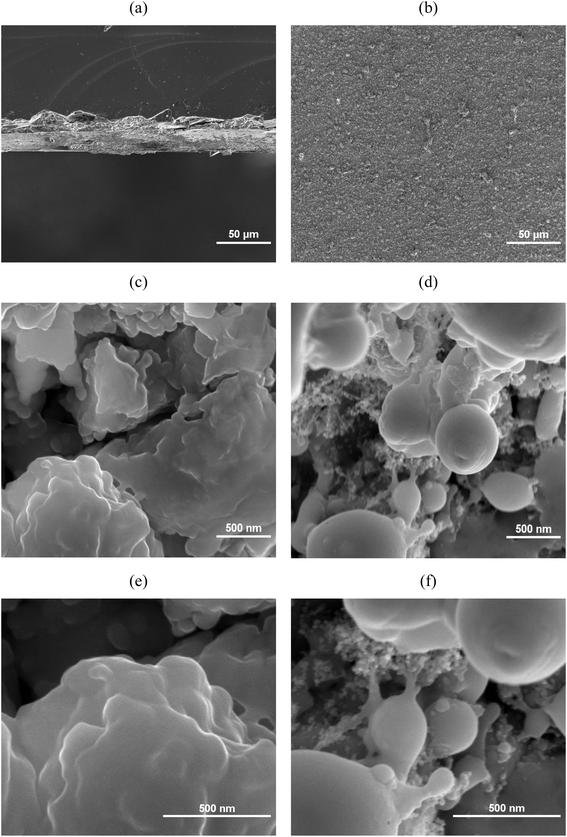 | ||
Fig. 1 SEM micrographs of Cu2O/ITO electrode: Cross-section views at (a) 500x; (c) 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00x; (e) 1 00x; (e) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× magnifications; top views at (b) 500×; (d) 500 00× magnifications; top views at (b) 500×; (d) 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00x; (f) 1 00x; (f) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× magnifications. 00× magnifications. | ||
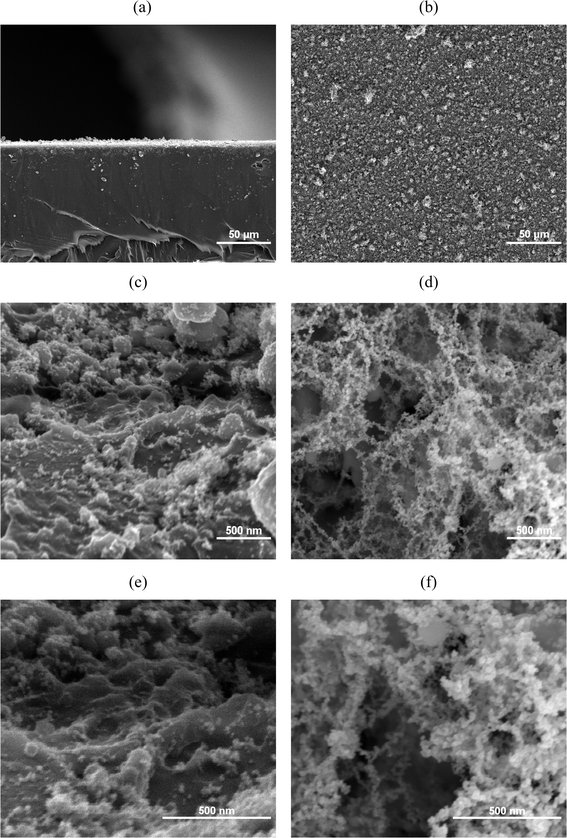 | ||
Fig. 2 SEM micrographs of Cu2O/PET electrode: Cross-section views at (a) 500x; (c) 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00x; (e) 1 00x; (e) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× magnifications; top views at (b) 500×; (d) 500 00× magnifications; top views at (b) 500×; (d) 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00x; (f) 1 00x; (f) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× magnifications. 00× magnifications. | ||
Further examination at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× and 1
00× and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× magnifications reveals that the PET surface is primarily composed of aggregated clusters of nanoparticles smaller than 100 nm in diameter, forming a distinct rough surface structure. This rough structure is believed to have a certain impact on electrical properties. In contrast, the surface of the ITO substrate is mainly composed of larger clusters with diameters exceeding 200 nm, presenting a smoother surface yet still exhibiting small spherical structures similar to those observed on the PET substrate.
00× magnifications reveals that the PET surface is primarily composed of aggregated clusters of nanoparticles smaller than 100 nm in diameter, forming a distinct rough surface structure. This rough structure is believed to have a certain impact on electrical properties. In contrast, the surface of the ITO substrate is mainly composed of larger clusters with diameters exceeding 200 nm, presenting a smoother surface yet still exhibiting small spherical structures similar to those observed on the PET substrate.
The EDS spectrum, shown in Fig. 3, results show strong copper signals detected on both ITO and PET surfaces, indicating successful transfer of the copper thin film by the LIFT process onto the substrates.
Structural analysis of PET, ITO, and Cu donor after LIFT was performed using XRD (Fig. 4). All samples exhibited peaks corresponding to planes (111), (200), and (220) at 2θ = 43.29°, 50.41°, and 74.12°, respectively, according to PDF 04-0836 and PDF 05-0667.43,44 After LIFT process, a decrease in the peak intensity of Cu (111) was observed, indicating potential formation of rougher surface grains affecting crystal orientation. Additionally, Cu2O (111) phases were detected post-transfer on both PET and ITO electrode surfaces, with an additional observation of Cu2O (110) on ITO. Furthermore, a prominent PET (100) phase was observed at 2θ = 25.8° on the PET substrate.
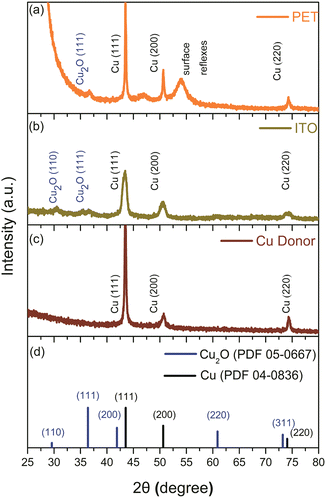 | ||
| Fig. 4 X-ray diffractogram of (a) Cu2O/PET electrode; (b) Cu2O/ITO electrode; (c) copper donor; (d) standard PDF card of Cu2O and Cu. | ||
Electrochemical performance
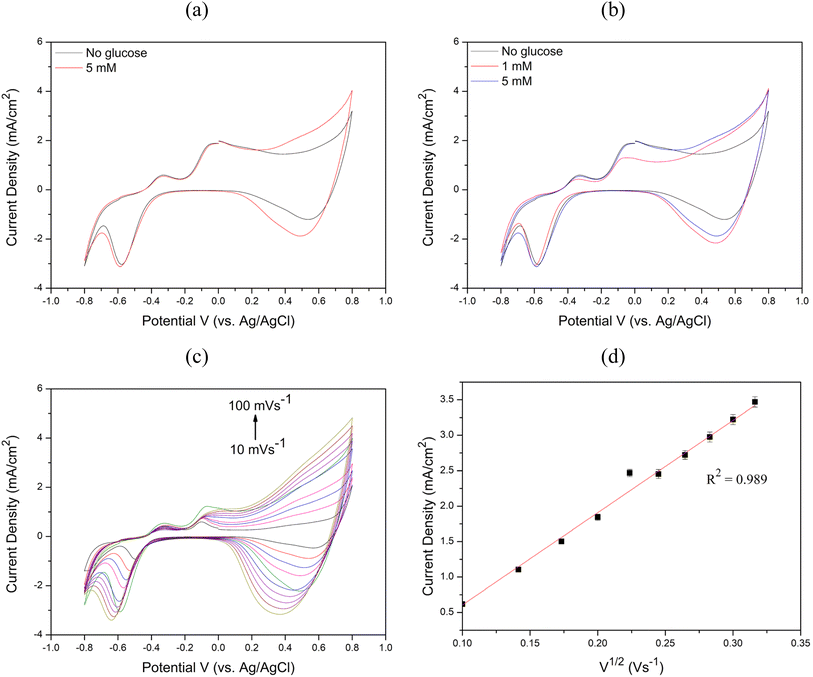
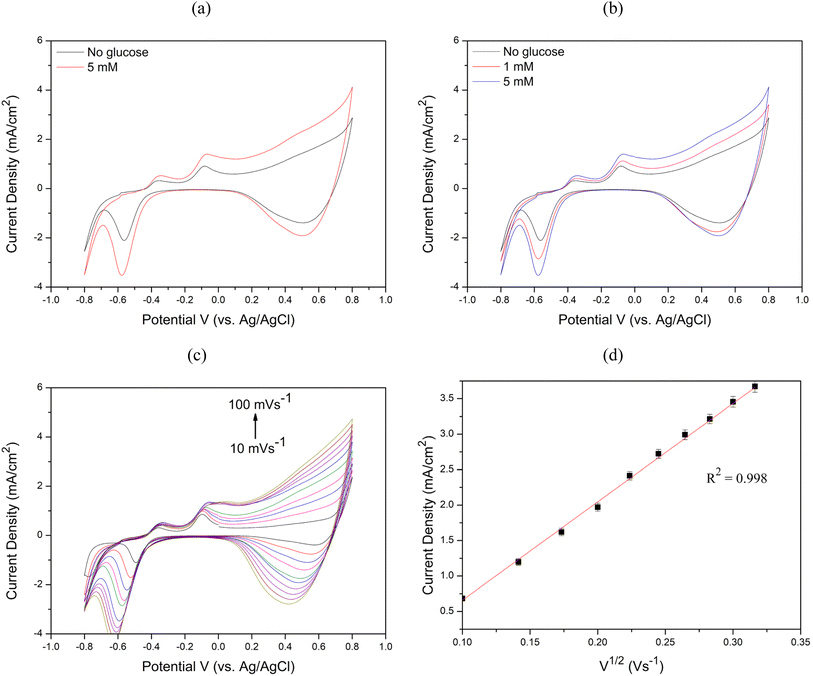
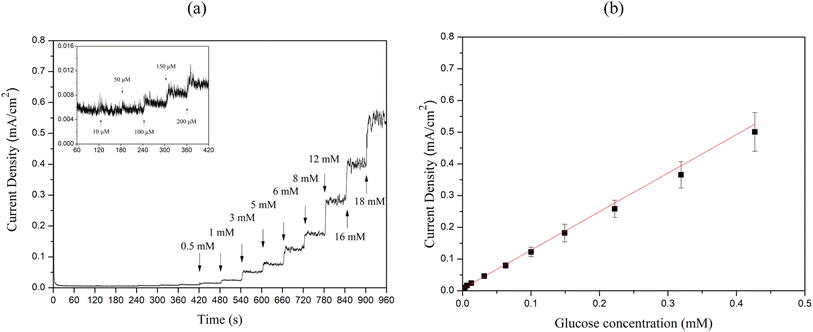
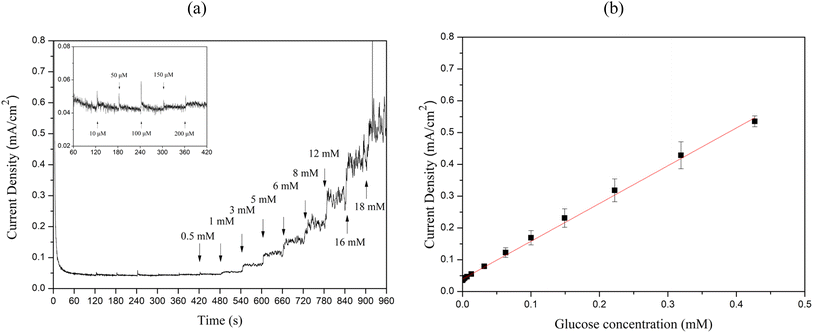
To evaluate the sensitivity, limit of detection (LOD), and linear range of the sensors, chronoamperometry measurements were performed by successively adding 10 μM to 18 mM glucose to 0.1 M NaOH at 60 second intervals, as illustrated in Fig. 9(a) and Fig. 10(a). The calibration curves between glucose concentration and peak oxidation current, shown in Fig. 9(b) and Fig. 10(b), demonstrate a linear relationship. The calculated sensitivities were approximately 1214.33 μA × mM−1 × cm−2 (R2 = 0.989) and 1188.14 μA × mM−1 × cm−2 (R2 = 0.997), respectively. The LOD was determined using the equation LOD = 3S m−1, where S is the standard deviation of the blank solution and m is the slope of the calibration curve. Based on the standard deviation of the blank solution for Cu2O/ITO (0.264 μA) and the sensitivity (0.607 μA × μM−1), the calculated LOD for Cu2O/ITO was 1.297 μM. Similarly, for Cu2O/PET, the LOD was calculated to be 1.824 μM based on the standard deviation of the blank solution (0.361 μA) and the sensitivity (0.594 μA × μM−1).
Therefore, summarizing the sensitivity, LOD, and linear range of Cu2O/ITO and Cu2O/PET electrodes based on LIFT in this study, along with performance comparisons with other relevant literature on Cu-based non-enzymatic glucose sensors, are presented in Table 1. The glucose concentration in human sweat typically ranges from 0.02 to 0.6 mM.45 Consequently, the method developed in this study for the rapid, single-step fabrication of glucose sensors exhibits significant potential for advancement into a practical sweat sensor. Although the Cu2O/ITO and Cu2O/PET electrodes may not exhibit the highest performance compared to other reported studies, they still demonstrate promising results. The LIFT process provides a rapid and efficient alternative to conventional fabrication techniques, bypassing the need for time-intensive chemical preparation. Furthermore, LIFT facilitates the deposition of metal thin films onto non-conductive substrates, such as PET, without the use of adhesives, thereby preventing the obstruction of active catalytic sites and mitigating any potential reduction in electrocatalytic activity. This capability to transfer electrodes onto flexible substrates presents a significant advantage for the development of wearable glucose sensors. Future research could investigate the use of alternative metals or bimetallic materials, as well as the integration of microfluidic systems for non-invasive glucose monitoring applications.
| Sensor material | Manufacturing process | Linear range (up to mM) | Detection limit (μM) | Sensitivity (μA × mM−1 × cm−2) | Ref. |
|---|---|---|---|---|---|
| CuO | Solution deposition | 0–2.2 | 1.19 | 1207 | Adijat Inyang et al. (2020)46 |
| CuxO/Cu | Thermal oxidation | 0–1.6 | 10 | 1212.06 | Suocheng Wang et al. (2020)47 |
| Cu2O/ITO | Electrochemical deposition | 0.04–0.4 | 4 | 2322.5 | Samiha Laidoudi et al. (2021)48 |
| CuO/Cu2O | Laser induced oxidation | 0–5 | 2.81 | 6950 | Sedahat Sedaghat et al. (2021)40 |
| Cu2O/FTO | Physical vapor deposition | 0.01–2 | 0.052 | 1394 | Amir R. Amirsoleimani et al. (2023)49 |
| Cu nanofibril | Chemical reduction | 0.005–3.88 | 1.41 | 4131.57 | Muchharla et al. (2023)50 |
| Cu2O/ITO | LIFT-fabricated | 0.003–0.4 | 1.2968 | 1214.33 | This work |
| Cu2O/PET | 0.003–0.4 | 1.824 | 1188.14 |
Selectivity test
Selectivity tests were conducted on Cu2O/ITO and Cu2O/PET electrodes to evaluate their response to glucose and common interferents, as shown in Fig. 11. According to previous study, glucose concentrations in both blood and urine are typically 20–30 times higher than those of interferents.51 The selectivity test involved the sequential addition of 1 mM glucose and common interferents, including ascorbic acid (AA), dopamine (DA), uric acid (UA), and sodium chloride (NaCl), at 100 second intervals, with their current responses being recorded.52 It can be observed that the current response from interferents is negligible compared to that generated after glucose addition. This indicates that both Cu2O/ITO and Cu2O/PET electrodes fabricated in this study exhibit adequate selectivity.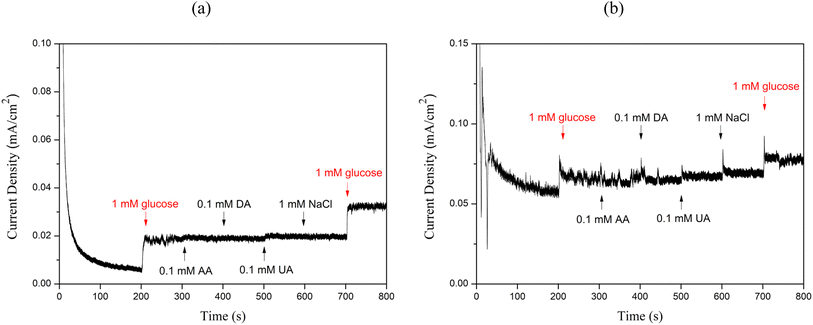 | ||
| Fig. 11 Selectivity test of the (a)Cu2O/ITO electrode, and (b) Cu2O/PET electrode in the presence of glucose and other interfering agents such as AA, DA, UA, NaCl. | ||
Experimental
Materials
Copper films with a purity of 99.9%, dimensions of 20 mm × 20 mm, and a thickness of 1000 nm were deposited using electron beam evaporation onto Corning glass (Ruilong Optoelectronics, Taiwan). The donor and two types of receiver substrates were Indium Tin Oxide (ITO) with a sheet resistance of approximately 20 Ω/sq and Polyethylene Terephthalate (PET), both with dimensions of 20 mm × 20 mm. D-(+)-Glucose (C6H12O6), Dopamine (C8H11NO2), and Sodium chloride (NaCl) were purchased from Sigma-Aldrich. Uric acid (C5H4N4O3) was obtained from Alfa Aesar, and Ascorbic acid (C6H8O6) was sourced from Honeywell.Process and material characterization
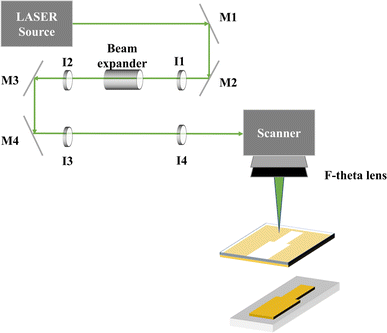
The LIFT process utilized a nanosecond green diode-pumped solid-state laser (DX-532-10, Photonics Industries, USA) with a central wavelength of 532 nm, a pulse repetition rate of 50 kHz, and a maximum pulse energy of 300 μJ. The LIFT process in this study used a pulse width of 15 ns, as shown in Fig. 12. The laser was deflected using an XY galvanometer scanner (MINISCAN-14, Raylase, Germany) and then focused with an f-theta lens (focal length = 250 mm) to a spot size of approximately 50 μm on the donor substrate. The laser average power, pulse repetition frequency, and scanning speed were controlled via commercial software. Field emission scanning electron microscopy (FESEM, JEOL, JSM-7800F Prime, Japen) equipped with energy-dispersive X-ray spectroscopy (EDS) was used for structural characterization of the deposited thin film patterns. X-ray diffraction (XRD, Bruker D2 PHASER XRD, USA) was employed to analyze the crystalline structure. Finally, a four-point probe instrument (EMP4, Sadhu Design Corporation, Taiwan) was used to measure the film resistance after material transfer and induced oxidation (probe spacing of 8.45 mm).Electrode preparation
The distance between the donor and receiver substrates was approximately 400 μm (3M tape, Minnesota, USA). Prior to the LIFT process, these substrates underwent ultrasonic cleaning for 10 minutes each using acetone, ethanol, isopropanol (IPA), and deionized (DI) water. All processes followed the optimized parameters by our previous study, starting with the transfer of the first layer of 5 mm × 10 mm film at 2 W power and a fixed scanning speed of 1300 mm s−1, followed by the transfer of the second layer at 1.5 W power, as shown in Fig. 13.42 Subsequently, the ITO electrodes were subjected to laser-induced oxidation (LIO) treatment at a defocus working distance of 362 mm using 2 W power. After completing the process, the substrates were cleaned using ultrasonic treatment with ethanol and deionized water to remove any remaining particles or debris from the substrate surface.Electrochemical characterization
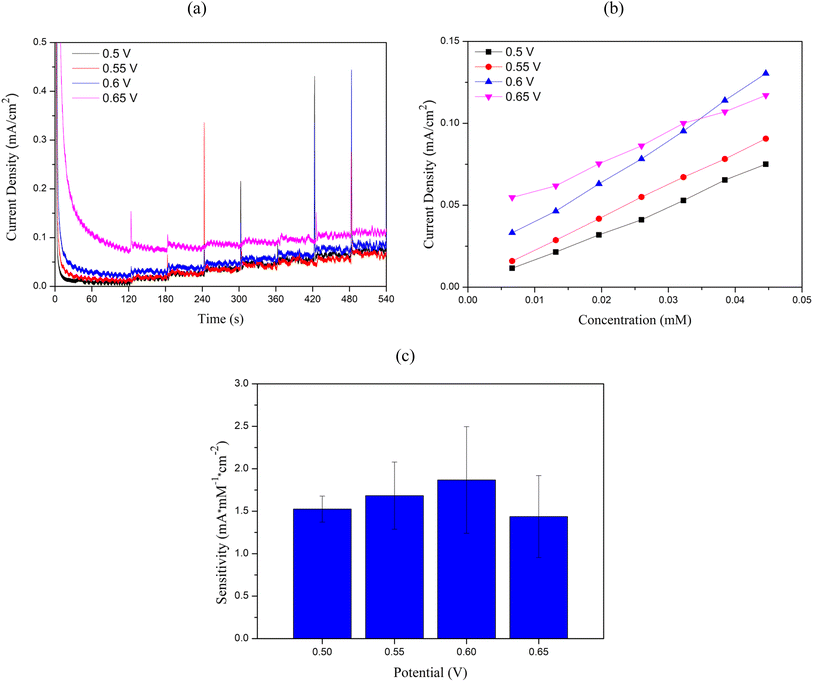
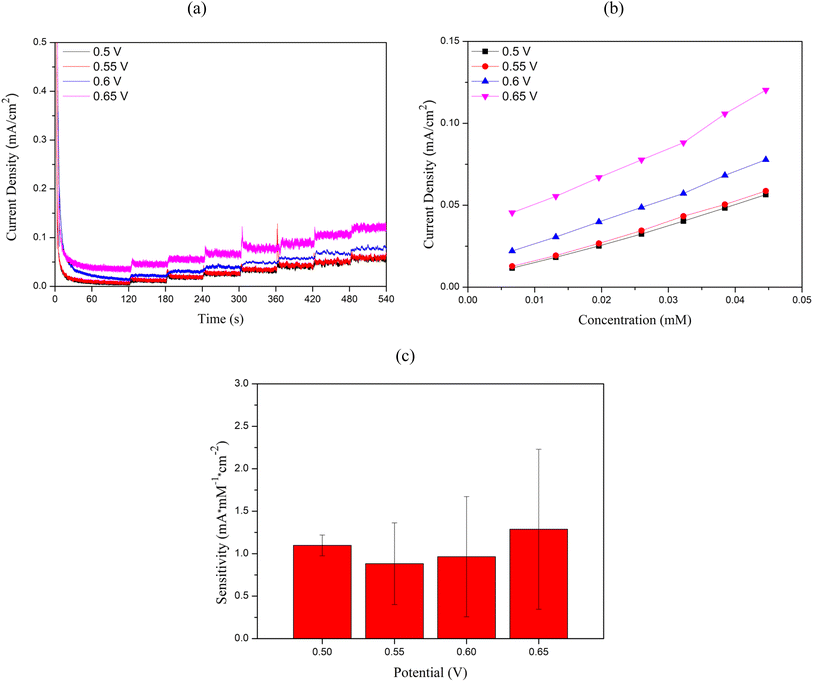
Electrochemical measurements were performed using the Nova operating software to control the potentiostat (Metrohm Autolab Type III). Cu2O/ITO and Cu2O/PET electrodes with an active area of 0.5 cm2 were employed as working electrodes. ITO functions as a transparent conductive electrode, enabling efficient electron collection and transfer to the external circuit due to its excellent electrical conductivity, while its optical transparency facilitates potential integration with optical sensing techniques. Meanwhile, PET serves as a flexible substrate, providing mechanical stability, flexibility, and biocompatibility, which are important for the potential development of wearable or portable sensor applications. Ag/AgCl served as the reference electrode, and a Pt coil was used as the auxiliary electrode. Cyclic voltammetry experiments were conducted using a solution of 0.1 M NaOH + 5 mM glucose to investigate its effect on glucose oxidation performance. The potential scan range was set from −0.8 V to +0.8 V, with a scan rate of 50 mV s−1. Chronoamperometric curves were recorded relative to the Ag/AgCl reference electrode at applied potentials of +0.5 V, +0.55 V, +0.6 V, and +0.65 V. The results showed that the Cu2O/ITO electrode exhibited the highest current response at +0.6 V, while the Cu2O/PET electrode showed the highest response at +0.65 V. Therefore, working potentials of +0.6 V and +0.65 V were chosen to evaluate the sensor sensitivity by adding glucose solutions with concentrations ranging from 10 μM to 18 mM into 0.1 M NaOH, which was continuously stirred at 500 rpm using a magnetic stirrer.Conclusion
This study employed the simple, rapid, and cost-effective LIFT technique to directly deposit films on PET and ITO, creating Cu2O/ITO and Cu2O/PET electrodes. Both samples demonstrated significantly enhanced electrocatalytic activity when used as non-enzymatic glucose sensing elements and exhibited satisfactory sensitivity. Future plans involve integrating multilayer structures, such as microfluidic channels, to fabricate non-invasive sensors. This advancement holds potential to improve patient comfort and facilitate the development of new clinical applications. The following conclusions can be drawn from the present work:1. FESEM observations revealed that the Cu2O/ITO electrode surface exhibited a denser and more uniform distribution with larger spherical aggregates (>200 nm), while the Cu2O/PET electrode showed a rougher structure with smaller aggregates (<100 nm). The EDS analysis reveals prominent copper signals on both the ITO and PET surfaces, suggesting the successful transfer of the copper thin film onto the substrates via the LIFT process.
2. XRD analysis confirmed Cu2O phase formation on both substrates. The Cu2O/ITO electrode showed an additional Cu2O (110) phase, while the Cu2O/PET electrode exhibited a strong PET (100) peak at 2θ = 25.8°. The decrease in Cu (111) peak intensity for both electrodes may be attributed to increased surface roughness.
3. Cyclic voltammetry demonstrated increasing oxidation current with higher glucose concentrations for both electrodes. The anodic current increased with scan rates (10–100 mV s−1), indicating diffusion-controlled electrochemical kinetics. Chronoamperometry revealed optimal working potentials of 0.6 V for Cu2O/ITO and 0.65 V for Cu2O/PET, with Cu2O/ITO showing higher overall sensitivity.
4. In the glucose concentration range of 3 μM to 0.4 mM, Cu2O/ITO electrodes exhibited a sensitivity of 1214.33 μA × mM−1 × cm−2 (R2 = 0.989) with an LOD of 1.297 μM, while Cu2O/PET electrodes showed 1188.14 μA × mM−1 × cm−2 (R2 = 0.997) with an LOD of 1.824 μM.
5. Both electrodes demonstrated significant current responses to 1 mM glucose, with negligible interference from substances like AA, DA, UA, and NaCl, indicating good glucose selectivity.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.Author contributions
P.-P. L., S.-F. L.: conceptualization, data curation, formal analysis, writing – original draft; C.-F. D.: resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Science and Technology Council, Taiwan for financially supporting this research under contract nos. NSTC 112-2221-E-002-218 and NSTC 113-2221-E-002-111-MY2.References
- H. Sun, P. Saeedi, S. Karuranga, M. Pinkepank, K. Ogurtsova, B. B. Duncan, C. Stein, A. Basit, J. C. N. Chan, J. C. Mbanya, M. E. Pavkov, A. Ramachandaran, S. H. Wild, S. James, W. H. Herman, P. Zhang, C. Bommer, S. Kuo, E. J. Boyko and D. J. Magliano, IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045, Diabetes Res. Clin. Pract., 2022, 183, 109119 CrossRef PubMed.
- J. Liu, M. Agarwal and K. Varahramyan, Glucose sensor based on organic thin film transistor using glucose oxidase and conducting polymer, Sens. Actuators, B, 2008, 135, 195–199 CrossRef CAS.
- J. Okuda-Shimazaki, H. Yoshida and K. Sode, FAD dependent glucose dehydrogenases – Discovery and engineering of representative glucose sensing enzymes, Bioelectrochemistry, 2020, 132, 107414 CrossRef CAS PubMed.
- T. Ramon-Marquez, A. M. Sesay, P. Panjan, A. L. Medina-Castillo, A. Fernandez-Gutierrez and J. F. Fernandez-Sanchez, A microfluidic device with integrated coaxial nanofibre membranes for optical determination of glucose, Sens. Actuators, B, 2017, 250, 156–161 CrossRef CAS.
- Y. Hu, H. Cheng, X. Zhao, J. Wu, F. Muhammad, S. Lin, J. He, L. Zhou, C. Zhang, Y. Deng, P. Wang, Z. Zhou, S. Nie and H. Wei, Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues, ACS Nano, 2017, 11, 5558–5566 CrossRef CAS PubMed.
- M. Govindaraj, A. Srivastava, M. K. Muthukumaran, P. C. Tsai, Y. C. Lin, B. K. Raja, J. Rejendran, V. K. Pounnusamy and J. A. Selvi, Current advancements and prospects of enzymatic and non-enzymatic electrochemical glucose sensors, Int. J. Biol. Macromol., 2023, 253, 126680 CrossRef CAS PubMed.
- D. W. Hwang, S. Lee, M. Seo and T. D. Chung, Recent advances in electrochemical non-enzymatic glucose sensors – A review, Anal. Chim. Acta, 2018, 1033, 1–34 CrossRef CAS PubMed.
- Y. Hu, X. Wang, W. Li, Y. Lai, Y. Chen, Z. Wei and H. Yang, Metal-organic frameworks and related materials for nonenzymatic electrochemical glucose sensors, Int. J. Electrochem. Sci., 2024, 19, 100466 CrossRef CAS.
- S. Gupta and N. H. Tai, Carbon nanomaterials and their composites for electrochemical glucose biosensors: A review on fabrication and sensing properties, J. Taiwan Inst. Chem. Eng., 2023, 154, 104957 CrossRef.
- M. Wei, Y. Qiao, H. Zhao, J. Liang, T. Li, Y. Luo, S. Lu, X. Shi, W. Lu and X. Sun, Electrochemical non-enzymatic glucose sensors: recent progress and perspectives, Chem. Commun., 2020, 56, 14553–14569 RSC.
- C. He, M. Asif, Q. Liu, F. Xiao, H. Liu and B. Y. Xia, Noble Metal Construction for Electrochemical Nonenzymatic Glucose Detection, Adv. Mater. Technol., 2023, 8, 2200272 CrossRef CAS.
- S. Radhakrishnan, S. Lakshmy, S. Santhosh, N. Kalarikkal, B. Chakraborty and C. S. Rout, Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials, Biosens, 2022, 12, 467 CrossRef CAS PubMed.
- L. Han, S. Zhang, L. Han, D. P. Yang, C. Hou and A. Liu, Porous gold cluster film prepared from Au@BSA microspheres for electrochemical nonenzymatic glucose sensor, Electrochim. Acta, 2014, 138, 109–114 CrossRef CAS.
- S. H. Kim, J. B. Choi, Q. N. Nguyen, J. M. Lee, S. Park, T. D. Chung and J. Y. Byun, Nanoporous platinum thin films synthesized by electrochemical dealloying for nonenzymatic glucose detection, Phys. Chem. Chem. Phys., 2013, 15, 5782–5787 RSC.
- X. Wang, X. Xia, X. Zhang, W. Meng, C. Yuan and M. Guo, Nonenzymatic glucose sensor based on Ag&Pt hollow nanoparticles supported on TiO2 nanotubes, Mater. Sci. Eng., C, 2017, 80, 174–179 CrossRef CAS PubMed.
- X. Niu, X. Li, J. Pan, Y. He, F. Qiu and Y. Yan, Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: opportunities and challenges, RSC Adv., 2016, 6, 84893–84905 RSC.
- R. Akter, P. Saha, S. S. Shah, M. N. Shaikh, M. A. Aziz and A. S. Ahammad, Nanostructured Nickel-based Non-enzymatic Electrochemical Glucose Sensors, Chem.–Asian J., 2022, 17, 202200897 CrossRef PubMed.
- H. Li, C. Y. Guo and C. L. Xu, A highly sensitive non-enzymatic glucose sensor based on bimetallic Cu–Ag superstructures, Biosens. Bioelectron., 2015, 63, 339–346 CrossRef CAS PubMed.
- K. Ramachandran, T. Raj kumar, K. J. Babu and G. Gnana Kumar, Ni-Co bimetal nanowires filled multiwalled carbon nanotubes for the highly sensitive and selective non-enzymatic glucose sensor applications, Sci. Rep., 2016, 6, 36583 CrossRef CAS PubMed.
- S. Liu, C. Zhang, L. Yuan, J. Bao, W. Tu, M. Han and Z. Dai, Component-Controlled Synthesis of Small-Sized Pd-Ag Bimetallic Alloy Nanocrystals and Their Application in a Non-Enzymatic Glucose Biosensor, Part. Part. Syst. Charact., 2013, 30, 549–556 CrossRef CAS.
- Q. Dong, H. Ryu and Y. Lei, Metal oxide based non-enzymatic electrochemical sensors for glucose detection, Electrochim. Acta, 2021, 370, 137744 CrossRef CAS.
- H. Zhu, L. Li, W. Zhou, Z. Shao and X. Chen, Advances in non-enzymatic glucose sensors based on metal oxides, J. Mater. Chem. B, 2016, 4, 7333–7349 RSC.
- G. A. Naikoo, H. Salim, I. U. Hassan, T. Awan, F. Arshad, M. Z. Perdram, W. Ahmed and A. Qurashi, Recent Advances in Non-Enzymatic Glucose Sensors Based on Metal and Metal Oxide Nanostructures for Diabetes Management- A Review, Front. Chem., 2021, 9, 748957 CrossRef CAS PubMed.
- G. A. Naikoo, T. Awan, H. Salim, F. Arshad, I. U. Hassan, M. Z. Pedram, W. Ahmed, H. L. Faruck, A. A. A. Aljabali, V. Mishra, A. S. Aroca, R. Goyal, P. Negi, M. Birkett, M. M. Nasef, N. B. Charbe, H. A. Bakshi and M. M. Tambuwala, Fourth-generation glucose sensors composed of copper nanostructures for diabetes management: A critical review, Bioeng. Transl. Med., 2022, 7, e10248 CrossRef CAS PubMed.
- A. Ashok, A. Kumar and F. Tarlochan, Highly efficient nonenzymatic glucose sensors based on CuO nanoparticles, Appl. Surf. Sci., 2019, 481, 712–722 CrossRef CAS.
- B. Meyer, A. Polity, D. Reppin, M. Becker, P. Hering, P. J. Klar, Th. Sander, C. Reindl, J. Benz, M. Eickhoff, C. Heiliger, M. Heinemann, J. Blasing, A. Krost, S. Shokovets, C. Muller and C. Ronning, Binary copper oxide semiconductors: From materials towards devices, Phys. Status Solidi B, 2012, 249, 1487–1509 CrossRef CAS.
- M. M. Alam and M. M. Howlader, Nonenzymatic electrochemical sensors via Cu native oxides (CuNOx) for sweat glucose monitoring, Sensing and Bio-Sensing Research, 2021, 34, 100453 CrossRef.
- C. Li, Y. Su, S. Zhang, X. Lv, H. Xia and Y. Wang, An improved sensitivity nonenzymatic glucose biosensor based on a CuxO modified electrode, Biosens. Bioelectron., 2010, 26, 903–907 CrossRef CAS PubMed.
- Y. Zhang, L. Su, D. Manuzzi, H. Monteros, W. Jia, D. Huo, C. Hou and Y. Lei, Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires, Biosens. Bioelectron., 2012, 31, 426–432 CrossRef CAS PubMed.
- S. Sun, X. Zhang, Y. Sun, S. Yang, X. Song and Z. Yang, Hierarchical CuO nanoflowers: water-required synthesis and their application in a nonenzymatic glucose biosensor, Phys. Chem. Chem. Phys., 2013, 15, 10904–10913 RSC.
- H. Cao, A. Yang, H. Li, L. Wang, S. Li, J. Kong, X. Bao and R. Yang, A non-enzymatic glucose sensing based on hollow cuprous oxide nanospheres in a Nafion matrix, Sens. Actuators, B, 2015, 214, 169–173 CrossRef CAS.
- X. Liu, W. Yang, L. Chen and J. Jia, Synthesis of copper nanorods for non-enzymatic amperometric sensing of glucose, Mikrochim. Acta, 2016, 183, 2369–2375 CrossRef CAS.
- B. Wu, L. Xiao, M. Zhang, C. Yang, Q. Li, G. Li, Q. He and J. Liu, Facile synthesis of dendritic-like CeO2/rGO composite and application for detection of uric acid and tryptophan simultaneously, Solid State Chem.:Compd., 2021, 296, 122023 CrossRef CAS.
- W. Liu, G. Chai, J. Zhang, M. Wang, Y. Dai and Q. Yang, Preparation of Cu2O nanocubes with different sizes and rough surfaces by a seed-mediated self-assembly process and their application as a non-enzymatic glucose sensor, New J. Chem., 2020, 44, 15662–15670 RSC.
- S. Laidoudi, M. R. Khelladi, L. Lamiri, O. Belgherbi, S. Boudour, C. Dehchar and R. Boufnik, Non-enzymatic glucose detection based on cuprous oxide thin film synthesized via electrochemical deposition, Appl. Phys. A, 2021, 127, 160 CrossRef CAS.
- B. Muchharla, B. Barbee, M. Darby, W. Cao, H. Sadasivuni, A. Adedeji, K. Kumar, A. Karoui, P. Panwar, G. Slaughter and B. Kumar, Oxide derived Cu nanofibril assembly for enhanced nonenzymatic glucose sensing, Mater. Today Commun., 2023, 35, 106286 CrossRef CAS.
- S. Zhang, P. Ling, Y. Chen, J. Liu and C. Yang, 2D/2D porous Co3O4/rGO nanosheets act as an electrochemical sensor for voltammetric tryptophan detection, Diamond Relat. Mater., 2023, 135, 109811 CrossRef CAS.
- I. I. Tumkin, E. M. Khairullina, M. S. Panov, K. Yoshidomi and M. Mizoshiri, Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection, Materials, 2021, 14, 2493 CrossRef CAS PubMed.
- S. Wang, L. Jiang, J. Hu, Q. Wang, S. Zhan and Y. Lu, Dual-functional CuxO/Cu electrodes for supercapacitors and non-enzymatic glucose sensors fabricated by femtosecond laser enhanced thermal oxidation, J. Alloys Compd., 2020, 815, 152105 CrossRef CAS.
- S. Sedaghat, S. Nejati, L. H. Bermejo, Z. He, A. M. Alcaraz, A. Roth, Z. Li, V. G. Pol, H. Wang and R. Rahimi, Laser-induced atmospheric CuxO formation on copper surface with enhanced electrochemical performance for non-enzymatic glucose sensing, J. Mater. Chem. C, 2021, 9, 14997–15010 RSC.
- X. Zhou, W. Guo, Y. Yao, R. Peng and P. Peng, Flexible Nonenzymatic Glucose Sensing with One-Step Laser-Fabricated Cu2O/Cu Porous Structure, Adv. Eng. Mater., 2021, 23, 2100192 CrossRef CAS.
- A. Das and C.-F. Ding, Innovative Fabrication of Metal Alloy Structures via Laser-Induced Forward Transfer on Flexible Substrates, Small Methods, 2023, 2301429 Search PubMed.
- E. Alp, The Facile Synthesis of Cu2O-Cu hybrid cubes as efficient visible-light-driven photocatalysts for water remediation processes, Powder Technol., 2021, 394, 1111–1120 CrossRef CAS.
- G. Martinez-Saucedo, F. M. Cuevas-Muñiz, R. Sanchez-Fraga, I. Mejia, J. J. Alcantar-Peña and I. R. Chavez-Urbiola, Cellulose microfluidic pH boosting on copper oxide non-enzymatic glucose sensor strip for neutral pH samples, Talanta, 2023, 253, 123926 CrossRef CAS PubMed.
- L. Johnston, G. Wang, K. Hu, C. Qian and G. Liu, Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables, Front Bioeng. Biotechnol., 2021, 9, 733810 CrossRef PubMed.
- A. Inyang, G. Kibamboa, M. Palmera, F. Cummingsb, M. Masikinia, C. Sundaya and M. Chowdhurya, One step copper oxide (CuO) thin film deposition for non-enzymatic electrochemical glucose detection, Thin Solid Films, 2021, 709, 138244 CrossRef.
- S. Wang, S. Dong, X. Liu and S. Yan, Multifunctional surface of titanium alloy with dual-scale hierarchical micro/nanostructures fabricated by femtosecond laser processing, Opt. Laser Technol., 2023, 164, 109423 CrossRef CAS.
- S. Laidoudi, M. R. Khelladi, L. Lamiri, O. Belgherbi, S. Boudour, C. Dehchar and R. Boufnik, Non-enzymatic glucose detection based on cuprous oxide thin film synthesized via electrochemical deposition, Appl. Phys. A: Mater. Sci. Process., 2021, 127, 160 CrossRef CAS.
- A. R. Amirsoleimani, H. Siampour, S. Abbasian, G. B. Rad, A. Moshaii and Z. Zaradshan, Copper oxide nanocolumns for high-sensitive non-enzymatic glucose sensing, Sens. Bio-Sens. Res., 2023, 42, 100589 CrossRef.
- B. Muchharla, B. Barbee, M. Darby, W. Cao, H. Elsayed–Ali, K. K. Sadasivuni, A. Adedeji, K. Kumar, A. Karoui, P. Panwar, G. Slaughter and B. Kumar, Oxide derived Cu nanofibril assembly for enhanced nonenzymatic glucose sensing, Mater. Today Commun., 2023, 35, 106286 CrossRef CAS.
- F. Cao, S. Guo, H. Ma, G. Yang, S. Yang and J. Gong, Highly sensitive nonenzymatic glucose sensor based on electrospun copper oxide-doped nickel oxide composite microfibers, Talanta, 2011, 86, 214–220 CrossRef CAS PubMed.
- H.-H. Fan, W.-L. Weng, C.-Y. Lee and C.-N. Liao, Electrochemical cycling-induced spiky Cu x O/Cu nanowire array for glucose sensing, ACS omega, 2019, 4, 12222–12229 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |