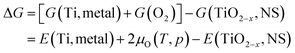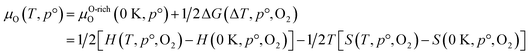Ultrafast formation of porosity and heterogeneous structures on 2D oxides via momentary photothermal effect†
Ahrom
Ryu‡
ac,
Bo-In
Park‡
b,
Hyun-Jae
Lee
g,
Jung-Won
An
a,
Jeong-Jun
Kim
e,
Sahn
Nahm
 c,
Seong H.
Kim
f,
Byungju
Lee
*d,
Ji-Won
Choi
c,
Seong H.
Kim
f,
Byungju
Lee
*d,
Ji-Won
Choi
 *ag and
Ji-Soo
Jang
*ag and
Ji-Soo
Jang
 *ah
*ah
aElectronic Materials Research Center, Korea Institute of Science and Technology, Seoul, 02792, Republic of Korea
bM.O.P. Materials, Seoul 07285, Republic of Korea
cDepartment of Materials Science and Engineering, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul, 02841, Korea
dComputational Science Research Center, Korea Institute of Science and Technology, Seongbuk-gu, Seoul 02792, Republic of Korea
eDepartment of Physics and Astronomy, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul, 08826, Korea
fDepartment of Chemical Engineering and Materials Research Institute, Pennsylvania State University, University Park, PA 16802, USA
gDivision of Nano & Information Technology, KIST School, University of Science and Technology, Seoul 02792, Republic of Korea
hDivision of Energy & Environmental Technology, KIST School, University of Science and Technology (UST), Daejeon 34113, Republic of Korea
First published on 28th November 2024
Abstract
2D metal oxides have attracted significant interest in numerous scientific research fields owing to their exceptional physicochemical properties derived from unique crystal structures and surfaces. However, unfortunately, there are still challenges to achieving fast and sensitive chemical sensing using 2D metal oxides due to their poor surface porosity. Here, a simple but powerful synthetic method for highly porous as well as reduced 2D metal oxides is suggested through a 2D oxide exfoliation approach combined with flash-thermal shock (FTS). The molecular thick-level 2D Ti0.87O2 nanosheets are simply synthesized by ion-exchange exfoliation and a subsequent ultra-fast FTS (7.5 ms) process, resulting in simultaneous formation of uniform pores and reduced Ti0.87O2−x on the 2D Ti0.87O2 surface. In this process, the heterojunctions play a crucial role in enhancing the sensitivity by facilitating charge transfer and improving the electrical properties. Density functional theory calculations and ex situ TEM analysis elucidate that the fast phase transformation of 2D Ti0.87O2 is a key driving force of porosity and reduced Ti0.87O2−x formation. Based on these features, porous 2D Ti0.87O2 exhibits an exceptional HCHO sensing characteristic including outstanding reversibility and sensitivity even at room temperature.
1. Introduction
Over the past few decades, the advancement of low-dimensional materials has opened up new opportunities to address critical challenges across various fields. In particular, gas sensing applications have benefited from the modification of surface structures and properties, leading to significant improvements in sensitivity and selectivity.1 Among them, 2D materials have gained significant interest due to their unique physical and chemical properties, attributed to quantum size effects and distinctive surface chemistry.2–4 For instance, studies have reported the improvement of electrical, optical, and chemical properties, as well as the imparting of specific characteristics, by doping, heterostructure formation, and surface treatment, utilizing the weak van der Waals bonding in 2D materials. Therefore, 2D materials hold significant potential in gas sensing systems, due to their tunable surface properties and large surface area. By enabling faster electron transport and effective catalysis on highly tunable surfaces, these applications hold the potential to not only detect hazardous gases but also to support room-temperature operation with low power consumption, thereby facilitating the integration of portable devices.5–8 However, based on such inherency, it is significantly hard to achieve sensitive chemical sensors due to their poor surface porosity and large grain size among the various material advantages of 2D oxide materials. Despite the promising advantages of 2D materials in gas sensing applications, current gas sensing devices using these materials still have simple architectures that struggle to achieve reproducibility and reliability.9 As previously reported,10 it is noted that 2D solid-state materials must be sufficiently thin (with a thickness of 30–50 nm) to achieve high sensor sensitivity. The Debye length should be small (2–5 nm) to attain a high sensor response. Only when the thickness is reduced to a few nanometers can changes in the space charge region of the surface charge due to adsorption, desorption, and catalytic processes noticeably affect the conductivity of the gas-sensitive layer. Another challenge lies in the precise positioning of nanosheets correctly on the sensor platform and in fabrication of reliable contacts.11,12 Among the various approaches for synthesizing 2D materials, chemical exfoliation is one of the most efficient methods in terms of mass productivity and reproducibility; the delamination of layered precursors into the monolayer is derived from the intercalation of organic molecules. Interestingly, exfoliated 2D materials inherently showed high single crystallinity, ultra-thin thickness (1–5 nm), and gigantic lateral size.13–18Among various oxide based sensing materials, titanium dioxide (TiO2), a high-resistance n-type semiconductor with a bandgap of approximately 3 eV, has garnered significant attention for its applications in gas sensors due to its tunable properties, catalytic capability, and environmental stability.19 Despite these advantages, conventional TiO2 material based gas sensors face limitations, such as high operating temperatures, additional sensor activators (e.g. UV irradiation), and poor reversibility when exposed to various gases. To address these performance issues, modifications such as surface porosity control and oxygen vacancy engineering have been explored. For example, optimizing 2D TiO2 nanosheets by controlling oxygen vacancies has improved ethanol detection.20 In fact, to generate pores or oxygen vacancies on 2D TiO2-based materials, high-temperature treatment is required. However, such treatments often lead to structural collapse and material degradation.21,22
In this work, we employed flash thermal shock (FTS), an ultra-fast annealing approach, to enhance reaction sites and achieve structural integrity in 2D Ti0.87O2 nanosheets, enabling efficient HCHO detection at room temperature. To achieve high-quality 2D Ti0.87O2 nanosheets, the soft chemical exfoliation method was conducted. The nanosheets were then laterally assembled on a SiO2 substrate and directly treated with FTS (7.5 ms, 5 shots), a technique that enables simultaneous porosity formation and surface reduction. Furthermore, during the FTS process, an infrared (IR) sensor system was employed to monitor millisecond-scale temperature changes, allowing for a detailed investigation into the effects of FTS power and shot number on reduction and doping mechanisms. The FTS thermal-shock not only promotes porosity formation but also achieves the reduction of Ti0.87O2−x without any structural collapse of the 2D nanosheets. The efficient combination of FTS and 2D Ti0.87O2 overcomes the typical limitations of high-temperature treatments, providing a stable, structurally intact sensor material with a high surface-to-volume ratio. Based on DFT simulations, we observed that the phase transition of 2D Ti0.87O2 NSs to the reduced Ti0.87O2 state occurs at significantly lower temperatures compared to bulk Ti0.87O2, which is essential for enhancing photothermal efficiency. As a result, our FTS-enabled approach achieved an accelerated response rate, superior sensitivity, and remarkable selectivity for HCHO gas, even at room temperature.
2. Methods
2.1 Fabrication of 2D Ti0.87O2 nanosheets
First, Ti0.87O2 colloidal nanosheets were prepared from the exfoliation of layered titanate crystals, K0.8Ti1.73Li0.27O4 (KTLO). The layered titanate crystal of this KTLO was synthesized by the flux method. TiO2, K2CO3, and Li2CO3 powders (all from Sigma-Aldrich, USA) were mixed using a zirconia mortar in a stoichiometric ratio for 15 min. Additionally, K2MoO4 was mixed at the molar ratio of flux. Subsequently, the ground mixture was maintained at 1200 °C for 10 hours, then slowly cooled to 950 °C for 50 hours, and finally cooled to room temperature for 5 hours. The KTLO single crystals were recovered by dissolving the K2MoO4 flux melt with water. The KTLO powder (4 g) was then immersed in a 0.5 M HCl solution (200 mL) at room temperature and protonated to H1.07Ti1.73O4 (HTO) via an acid exchange reaction. The acid solution was replaced daily with a fresh one by decantation. During this process, the interlayer potassium cation (K+) was exchanged for a hydronium ion (H3O+). After treatment for 5 days, HTO·H2O was collected by vacuum filtration and washed with distilled water. A drying process was followed in a convection oven at 60 °C for 24 hours. After drying, the HTO powder was introduced into a tetrabutylammonium hydroxide (TBA+OH−) aqueous solution. The molar ratio of TBA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H+ was maintained at 1
H+ was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This mixture was placed on a shaker. After shaking for 14 days, an exfoliated titania nanosheet colloidal solution was obtained, which is a silky-textured suspension23–27
1. This mixture was placed on a shaker. After shaking for 14 days, an exfoliated titania nanosheet colloidal solution was obtained, which is a silky-textured suspension23–27
2.2 Fabrication of 2D Ti0.87O2 nanosheet films
The well-exfoliated Ti0.87O2 nanosheet colloids were coated by a drop-coating method onto a Pt/Ti inter-digitated electrode (IDE) pattern (25 μm wide and 350 μm long lengths) as an active sensing layer (Fig. S1†). A micropipette was used to drop 20 μL of colloid and dry for 24 hours in ambient air.2.3 Flash-thermal shock (FTS) irradiation
A xenon flash lamp (ILC technology, L6755) was utilized as the light source to form sub-nm-scale pores on Ti0.87O2 nanosheets. The light source emitted a spectrum ranging from 400 to 1100 nm. The pulse light irradiated the films through a quartz crystal. The FTS process was performed with an applied voltage of 250 to 500 V, a light pulse irradiation time of 7.5 ms, an off time between light pulses of 7.5 ms, and a frequency of 1 Hz. The pulse interval was optimized to 7.5 ms to accommodate the equipment conditions, as adequate charging time for the energy bank was necessary between pulses. The films were exposed to a series of single light pulses (number of shots) for a total of 5 times.2.4 IR sensor system
An IR thermometer (CTlaser 3MH2, Optris) measures IR information at a wavelength of 2.3 μm from samples. The IR sensor can measure temperatures ranging from 200 to 1500 °C. It should be noted that the base temperature is displayed as 200 °C due to the detectable temperature range, and the emissivity is set to unity when temperature is measured. After applying emissivity correction, the upper-temperature limit of the sensor system is elevated to approximately 1630 °C. This temperature corresponds to the saturation peaks observed in the transient temperature profile. To get reliable measurements, samples should be placed 15 cm apart from the IR sensor. A data acquisition board (National Instruments USB-6341 X series Multifunction DAQ) corrects and converts measured analog signals to digital signals. Finally, measured data are presented in the form of temperature.2.5 Gas sensing measurements
The gas sensing properties of pristine Ti0.87O2 nanosheets and those after FTS treatment were measured within a quartz tube under ambient conditions. To ensure precise gas flow control, a mixture of dry air and water vapor was used, and a mass-flow controller (MFC) was employed to maintain a consistent flow rate of 1000 sccm. The sensor resistance was measured using a Keithley 2401 source/meter with a DC bias voltage of 0.5 V applied. All experimental data were recorded on a computer through a general-purpose interface bus (GPIB) utilizing LabVIEW software.2.6 Measurement and characterization
To investigate the structural properties, pristine Ti0.87O2 nanosheets were investigated by Raman (LabRAM ARAMIS IR2, HORIBA JOBIN YVON) and X-ray diffraction (XRD, DMAX 2200, Rigaku, Japan) at a scan rate of 4° min−1 at 5°–50° with Cu Kα radiation (λ = 1.54 Å). The surface morphology of the nanosheets was examined by SEM (FE-SEM, Inspect50, FEI, USA) and AFM (Park XE-15, Park Systems, Korea). The morphology of the samples was observed by high-resolution transmission electron microscopy (HRTEM, Hitachi H-9000, Japan) operating at 200 kV. A droplet of the diluted colloidal nanosheet suspension was placed on a holey carbon grid, allowing the suspension to penetrate through. After drying, the specimen was prepared for TEM observations. The chemical bonding of before and after FTS irradiation samples was confirmed by X-ray photoelectron spectroscopy (XPS, PHI 5000 VersaProbe, ULVAC-PHI). The binding energy was calculated using a monochromated Al Ka X-ray source (1486.6 eV). The optical properties were measured using a UV-vis spectrometer (Lambda 35, PerkinElmer) in the 200–900 nm wavelength range.2.7 DFT calculations
We conducted first-principles calculations based on density-functional theory (DFT) using the VASP(5.4.4) code.28–31 We adopted a meta-generalized gradient approximation (GGA) for describing the exchange correlation energy functional and pseudopotentials generated under the projector-augmented planewave (PAW)32 scheme using r2SCAN.33 Energy cutoff for the plane wave basis was set to 500 eV and force tolerance for the structure optimization was 0.015 eV Å−1. For anatase TiO2, a Γ-centered 6 × 6 × 6 k-point grid was chosen for sampling integrations over the Brillouin zone. In the unit cell of four formula units, we fully optimized the anatase TiO2 structure which has the lattice constants a = 3.806(0) Å, b = 3.806(0) Å and c = 9.669(4) Å, consistent with earlier reported theoretical and experimental values.34,35 For TiO2 nanosheets, the cell under slab conditions containing eight formula units has lattice constants a = 6.049(1) Å and b = 7.498(6) Å, and an 8 × 8 × 1 k-point grid was used. The 4 × 4 × 1 k-point grid was used for the 2 × 2 × 1 supercell containing 32 formula units in Ti vacancy-induced calculations. All slab calculations included a vacuum region with a thickness exceeding 15 Å and lattice constants were fixed during structure optimization. All the figures of atomic structures in this manuscript were drawn by using the VESTA code.362.8 Calculation of formation energy
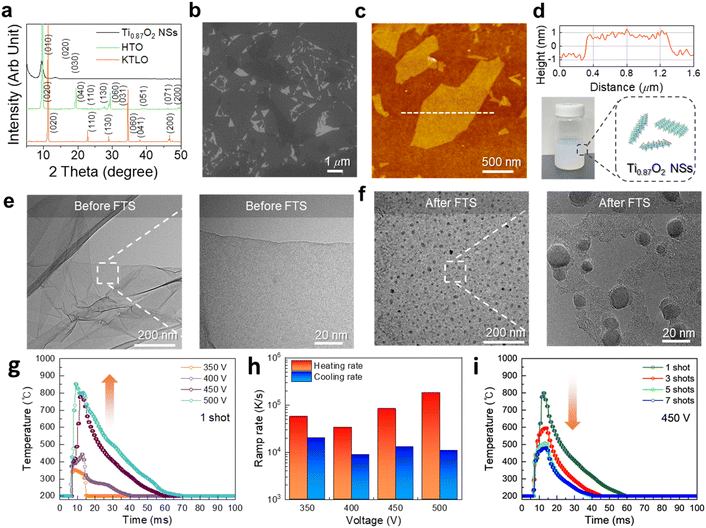
Assuming Ti and TiO2−x NSs as rigid solids with minimal sensitivity to thermal effects (entropy) compared to O2, we simplified the calculation of the formation energy for the decomposition of TiO2−x NSs into Ti and oxygen as follows:where G is Gibbs free energy and E(Ti, metal) and E(TiO2−x, NS) are calculated total energy of Ti metal and TiO2−x from DFT calculations, respectively. μO(T,p) is the oxygen chemical potential calculated using the following formalism:
| μO(T,p) = μO(T,p°) + 1/2 kTln(p/p°), |
where T, p, p° and k are temperature, pressure, standard state pressure (1 atm) and Boltzmann constant, respectively. Enthalpy (H) and entropy (S) contributions in finite temperature and pressure are determined from the NIST-JANAF thermochemical tables.37 As a reference, we compared the calculated formation energy of anatase TiO2 with experimental measurement. The calculated formation energy of anatase TiO2 was −3.44 eV per atom, slightly larger than −3.23 eV per atom in the experimental measurement.35 Thus, the correction −0.64 eV per atom was added to the total energy of the oxygen molecule to match the formation energy of anatase TiO2.
3. Results and discussion
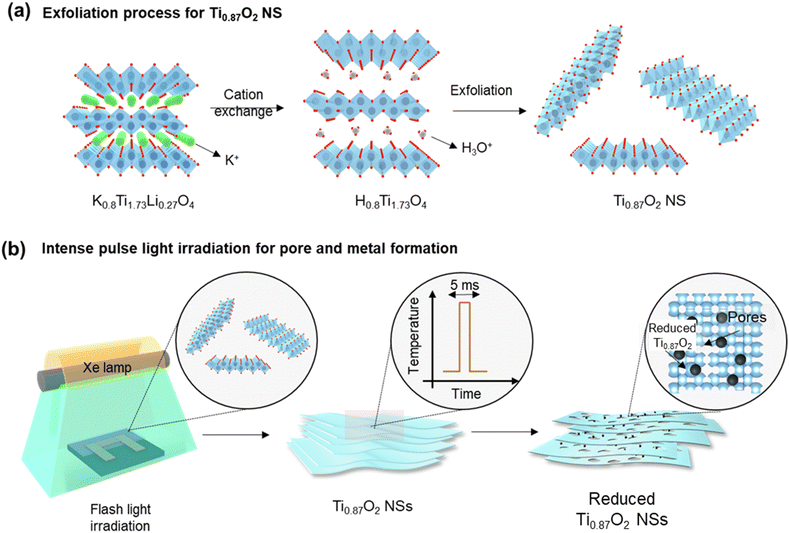
The exfoliation process of 2D Ti0.87O2 nanosheets (Ti0.87O2 NSs) is shown in Fig. 1a. The Ti0.87O2 NSs were exfoliated by a solution-based soft-chemical method (see the Methods section). During the cation exchange process, the interlayer spacing in K0.8Ti1.73Li0.27O4 (KTLO) gradually increases as the intercalation compounds are replaced, eventually leading to the exfoliation of mono-layer Ti0.87O2 NSs. This liquid exfoliation method facilitates the synthesis of various 2D oxides. Layered oxide materials are derived from precursors that consist of host compounds featuring corner- or edge-sharing MO6 octahedra (where M = Ti, Mn, Nb, etc.) and interlayer alkali metal cations (K+, Rb+, CS+, etc.). Typical examples include metal oxides (K0.8Ti1.73Li0.27O4, KTiNbO5, KNb3O8, K0.45MnO2, etc.) and perovskite oxides (KSr2Nb3O10, KCa2Ta3O10, KCa2Nb3O10etc.).38–41 To fabricate the chemiresistive sensing device, the exfoliated 2D colloidal nanosheet solution was drop-cast directly onto the SiO2/Si sensing substrate as shown in Fig. S1.† To generate surface pores on 2D Ti0.87O2 NSs, the 2D Ti0.87O2 NSs were exposed to millisecond-scale intense pulse light (IPL) generated using the Xe flash lamp (Fig. 1b). Hereafter, IPL irradiation is called flash-thermal shock (FTS). During the FTS (7.5 ms), 2D Ti0.87O2 NSs absorbed light, resulting in a sudden temperature increase of Ti0.87O2 NSs due to photothermal effects.42,43 To elucidate the details of the millisecond-scale process, the temperature changes were monitored as a function of the number of FTS pulses using an IR sensor system. The sample was placed at a distance of 4.5 cm from the FTS quartz plate to ensure that the IR light could pass through the sensor system. Such dramatic temperature increases of 2D Ti0.87O2 NSs facilitated the self-reduction of reduced Ti0.87O2−x on 2D Ti0.87O2 NSs as well as the formation of numerous pores (hereafter, FTS-Ti0.87O2).To investigate the microstructure and crystallographic structure of Ti0.87O2 NSs, X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis were carried out. As shown in Fig. 2a, the X-ray diffraction (XRD) patterns were used to identify the crystal structure of KTLO, HTO, and Ti0.87O2, respectively. The typical layered structure of KTLO and HTO powders can be seen at the edge of the powders, as shown in Fig. S2.† The XRD peaks in the pattern of KTLO were well-matched with those of the lepidocrocite-type titanate. Then, diffraction peaks of HTO were consistent with those of bulk KTLO, indicating that the single phase is well formed. Furthermore, based on this analysis, the calculated d-spacing of the (020) plane was found to be ≈7.79 Å for KTLO and ≈9.22 Å for HTO, respectively. The increase in d-spacing is attributed to interlayer swelling, as the molecular size of the intercalated hydronium ions (H3O+) is larger than that of the replacing K+. And the d-spacing of the (020) plane was calculated to be ≈17.5 Å for Ti0.87O2 nanosheets. As shown in Fig. 2b, it shows that the nanosheets formed a parallel layer structure and the lateral size was in the range of several micrometers.
To analyse the morphological characteristics of Ti0.87O2 nanosheets (NSs), atomic force microscopy (AFM) analysis was conducted. As shown in Fig. 2c, the Ti0.87O2 NS exhibited lateral dimensions ranging from 0.5 to 2 μm. The cross-sectional profile measured along the dashed line in Fig. 2c is presented in the top row of Fig. 2d, where the thickness of a single Ti0.87O2 NS is estimated to be approximately 2 nm.
In addition to assessing the dimensions of individual nanosheets, AFM images were obtained after spin coating to evaluate the size and thickness distribution of multiple nanosheets. Nanosheets were uniformly dispersed on the SiO2/Si substrate, indicating that the nanosheets remained distinct within the colloidal solution. The surface of the nanosheets appeared clean and extremely flat, as confirmed in Fig. S3.† The thickness and lateral distribution data confirm the uniformity of the nanosheets with an average thickness of 1.02 ± 2.1 nm and 3.56 ± 2.8 nm of lateral size. Fig. 2d (bottom row) shows the digital image of Ti0.87O2 NSs dispersed in deionized (DI) water.
As shown in Fig. S4,† FTS treatment is a key process that induces porosity and heterogeneous structure formation in Ti0.87O2 nanosheets (NSs). This process is significantly influenced by the intense thermal energy generated during FTS treatment. The thermal energy facilitates structural rearrangement at the nanoscale, promoting pore formation, which is associated with phase changes in the material and the removal of volatile components.44 These complex processes demonstrate that FTS treatment effectively modifies the structural properties of the nanosheet surface, contributing to the formation of porosity and heterogeneous structures. The transformation of the nanosheet morphology highlights the effectiveness of the FTS treatment in enhancing the surface properties of Ti0.87O2, thereby optimizing its potential for gas sensing applications.
The surface morphology of nanosheets was further observed using TEM, before and after FTS 5 shots at a fixed applied voltage of 450 V. Fig. 2e clearly shows the single monolayer nanosheet confirmed thin-layered 2D structures. After FTS, interestingly, numerous pores and reduced Ti0.87O2−x NSs were formed on the surface of FTS-Ti0.87O2 as shown in Fig. 2f. These spherical particles were analysed as reduced Ti0.87O2−x NSs by energy dispersive spectroscopy (TEM-EDS) analysis as shown in Fig. S5.† In Fig. S6b and f,† the fast Fourier transform (FFT) pattern was collected from the red box in Fig. S6a and e.† The red box in Fig S6e† contains the selected portion of the circular particle observed in the FTS-Ti0.87O2 sample, which was confirmed to be reduced Ti0.87O2−x by TEM-EDS analysis shown in Fig. S5.† Then, the fringes in the HRTEM images are with calculated d-spacing values of 0.21 nm and 0.23 nm, indicating the Ti0.87O2 lepidocrocite (130) planes and Ti HCP (002) planes. Therefore, these results indicate that there is no noticeable variation in the crystallite size and thickness of Ti0.87O2 NS films after FTS irradiation.
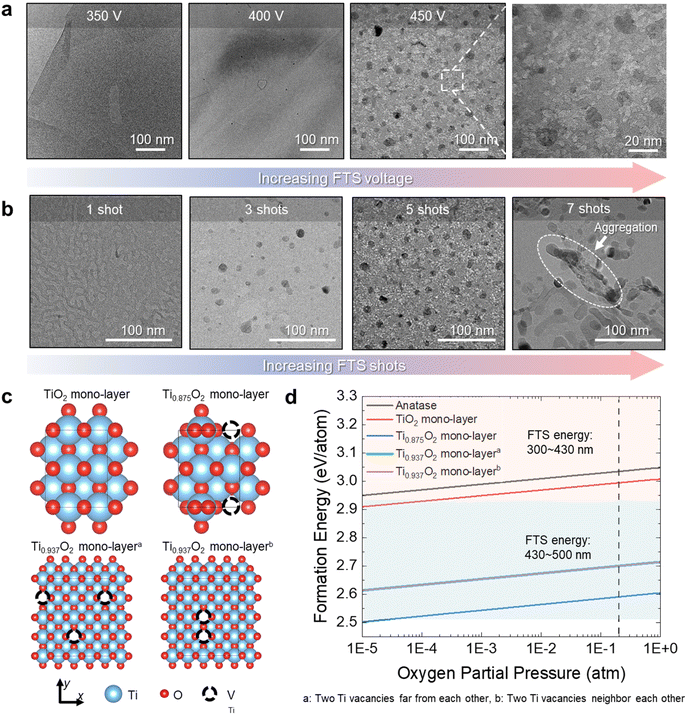
To investigate the optical reduction behavior induced by FTS, the effects of FTS voltage and the number of shots on the temperature profile were examined (Fig. 2g–i). The thermal accumulation effect occurs only when the next pulse arrives within milliseconds, so the interval between pulses was set to more than 1 second. As shown in Fig. 2g, the temperature changes during FTS treatment at various voltages ranging from 350 V to 500 V with a 7.5 ms pulse interval were measured using an IR system. The heating and cooling rates during this process are depicted in Fig. 2h. As shown in Fig. 2g, the maximum temperatures increased from 355 °C at 350 V to 443 °C, 800 °C, and 853 °C at 400 V, 450 V, and 500 V, respectively. These behaviors are attributed to the partial reduction of Ti0.87O2, where heating due to the photothermal effect occurs when exposed to photons with energies exceeding the bandgap.45 Compared to 350 V, the temperature at 400 V increased by 88 °C, while the difference in maximum temperature between 450 V and 500 V was only 54 °C. However, between 400 V and 450 V, a much larger temperature increase of 356 °C was observed. As shown in Fig. 2h, while the heating rate increased with the increase of voltage, the cooling rate remained within the range of 8743–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 884 K s−1. Such rapid thermal processing using FTS is advantageous, as traditional methods such as box-furnaces require prolonged exposure to high temperatures, potentially leading to structural instability or thermal deformation of the nanosheets.46 In this study, we applied the FTS process, which is capable of ultra-high-speed optical sintering. Due to the short sintering time of several milliseconds (5–20 ms), the FTS process minimizes sample damage. This enables rapid sintering within the required temperature range without shape deformation commonly observed in conventional high-temperature sintering processes, such as furnaces or rapid thermal annealing (RTA). Therefore, it is applicable to temperature-sensitive substrates as well. In a previously reported study, SEM images of ZnO nanosheets subjected to vacuum annealing at various heat treatment temperatures showed that they could not withstand a temperature of 700 °C, exhibiting structural breakage and partial collapse. The thickness of these nanosheets was generally between 20 and 100 nm. However, the FTS process we employed offers a significant advantage: Ti0.87O2 nanosheets, which are even thinner, were able to withstand the higher FTS process temperature of 800 °C while maintaining their structure and forming pores and reduced particles.47,48 As shown in Fig. 2i, peak temperatures recorded after the 1st, 3rd, 5th, and 7th shots at 450 V were 800 °C, 594 °C, 507 °C, and 481 °C, respectively. These trends suggest that after the first FTS shot, changes in the microstructure of the sample surface, such as pore formation and the reduction of Ti0.87O2−x, partially blocked direct light absorption, thereby reducing the photothermal effect. Consequently, as the number of shots increases under the same voltage conditions, the energy absorbed during FTS photothermal processing decreases, leading to a gradual reduction in maximum temperature. This behavior indicates that 2D Ti0.87O2 nanosheets exfoliated to atomic thickness, and pore formation and phase transitions can occur sufficiently with only a few shots under FTS voltage and millisecond pulse conditions.
884 K s−1. Such rapid thermal processing using FTS is advantageous, as traditional methods such as box-furnaces require prolonged exposure to high temperatures, potentially leading to structural instability or thermal deformation of the nanosheets.46 In this study, we applied the FTS process, which is capable of ultra-high-speed optical sintering. Due to the short sintering time of several milliseconds (5–20 ms), the FTS process minimizes sample damage. This enables rapid sintering within the required temperature range without shape deformation commonly observed in conventional high-temperature sintering processes, such as furnaces or rapid thermal annealing (RTA). Therefore, it is applicable to temperature-sensitive substrates as well. In a previously reported study, SEM images of ZnO nanosheets subjected to vacuum annealing at various heat treatment temperatures showed that they could not withstand a temperature of 700 °C, exhibiting structural breakage and partial collapse. The thickness of these nanosheets was generally between 20 and 100 nm. However, the FTS process we employed offers a significant advantage: Ti0.87O2 nanosheets, which are even thinner, were able to withstand the higher FTS process temperature of 800 °C while maintaining their structure and forming pores and reduced particles.47,48 As shown in Fig. 2i, peak temperatures recorded after the 1st, 3rd, 5th, and 7th shots at 450 V were 800 °C, 594 °C, 507 °C, and 481 °C, respectively. These trends suggest that after the first FTS shot, changes in the microstructure of the sample surface, such as pore formation and the reduction of Ti0.87O2−x, partially blocked direct light absorption, thereby reducing the photothermal effect. Consequently, as the number of shots increases under the same voltage conditions, the energy absorbed during FTS photothermal processing decreases, leading to a gradual reduction in maximum temperature. This behavior indicates that 2D Ti0.87O2 nanosheets exfoliated to atomic thickness, and pore formation and phase transitions can occur sufficiently with only a few shots under FTS voltage and millisecond pulse conditions.
To confirm the presence of oxygen vacancies on the 2D oxide surface, we conducted electron paramagnetic resonance (EPR) analysis and X-ray Photoelectron Spectroscopy (XPS). As shown in Fig S7,† the peak of the oxygen vacancy around g = 2.02 is observed in the EPR spectrum, which is too subtle to detect definitively. Instead of the EPR analysis, we carried out XPS analysis with before and after FTS treatment samples. The XPS data in Fig. S8† provide the survey analysis, which identifies the elemental composition of the sample. The XPS presented in Fig. S9† shows the O 1s XPS spectra for Ti0.87O2 and FTS-Ti0.87O2. Each spectrum was deconvoluted into three peaks using Gaussian fitting to represent different oxygen chemical states. The peaks at 529.1 eV, 531 eV, and 532 eV correspond to lattice oxygen within the structure, highly oxidative oxygen species from oxygen-deficient areas such as oxygen vacancies, and chemisorbed oxygen on the film surface such as O2 and H2O, respectively. Based on the relative peak areas, the surface oxygen vacancy concentrations for the Ti0.87O2 and FTS-Ti0.87O2 samples were calculated to be 6% and 32%, indicating that surface oxygen vacancies increase following the FTS treatment.
The peaks at 456.82 eV and 461.74 eV correspond to Ti3+ species. Under reductive conditions, Ti3+ species are generated from the reduction of Ti3+, resulting in oxygen vacancies as Ti4+ is replaced by Ti3+ in the lattice. The presence of these Ti3+ species and oxygen vacancies contributes to enhanced electrical conductivity. These Ti3+ ions, predominantly formed due to oxygen deficiencies, are primarily located on the surface or subsurface of the crystal. Consequently, the introduction of these Ti3+ species significantly improves electrochemical conductivity and reduces electron transfer resistance, which is crucial for enhancing the electrochemical performance of the Ti0.87O2 material.49 Thus, it seems that the phase transition is observed on the surface of the nanosheets due to the instantaneous FTS. That is, the condition of instantaneous photothermal effect during the FTS process caused the phase separation of Ti and O. For XRD, the Ti0.87O2-based NS samples were fabricated on a Si wafer using the drop coating method. The films showed 0k0 basal reflections up to the seventh order line, indicating high structural order as shown in Fig S13.†50 Based on these results, it has been confirmed that there was no peak change observed before and after FTS. This indicates that the formation of reduced Ti0.87O2−x predominantly occurs on the surface, rendering it challenging to detect any alterations in the Ti0.87O2 lepidocrocite structure, which serves as the backbone, via XRD analysis. To further confirm the effects of FTS through optical properties, the UV-vis analysis was carried out before and after FTS. The nanosheet film for UV-vis spectroscopy and Raman spectroscopy was prepared using the drop coating process, with a glass substrate. Fig. S10a† shows the UV-vis absorbance as a function of the wavelength and Fig. S10b† shows the Tauc plot, which was established using the UV-vis absorption spectrum. The absorption coefficient obtained from UV-vis was converted to a band gap using the Tauc plot method (1)
| (α·hv)1/γ = B (hv − Eg) | (1) |
To clearly understand the FTS effects on 2D Ti0.87O2, we further carried out ex situ TEM analysis and DFT calculations. As shown in Fig. 3a, with an increase in power of FTS, the morphology of FTS-Ti0.87O2 has evolved toward highly porous surface structures with reduced Ti0.87O2−x. Compared to 400 V FTS-2D Ti0.87O2 NSs, 450 V FTS-2D Ti0.87O2 NSs showed distinctive porosity and numerous reduced Ti0.87O2−x on 2D Ti0.87O2 NSs. In this sense, we realized that higher power FTS can induce the higher temperature increase of 2D Ti0.87O2 NSs, thus resulting in the self-reduction of 2D Ti0.87O2 NSs. To further investigate the FTS effect on 2D oxides, we additionally conducted the ex situ TEM analysis with various FTS-Ti0.87O2 samples which were treated by a different number of FTS shots with fixed FTS power. As shown in Fig. 3b with an increase in the number of FTS shots (1–7 shots of FTS), the pores as well as reduced Ti0.87O2−x are gradually increased. Following the 5-shot process at FTS 450 V, TEM images (Fig. 3b) of the nanosheet surface were recorded.
In Fig. 3b, it was observed that the deformed and aggregated nanosheets were at 7 shots. This can be explained by applying the Gibbs–Thomson equation. The Gibbs–Thomson equation provides an estimate of the size dependence of particle stability, typically expressed as a shift in particle chemical potential:
In addition to ex situ TEM analysis, we further investigated the process wherein TiO2−x NSs undergo decomposition into Ti and oxygen during ultra-fast thermal annealing by FTS, leading to the formation of reduced Ti0.87O2−x on Ti0.87O2 NSs by employing DFT calculations as shown in Fig. 3c. In particular, we conducted an analysis of various TiO2−x structures (Fig. 3c) and calculated the formation energies for the decomposition of each configuration into Ti and oxygen (Fig. 3d). As depicted in Fig. 3c, a structure of stoichiometric TiO2−x NS was optimized by incorporating the structural information acquired from experimental measurements (top and left). For the stoichiometric structure, the formation energy for decomposition into Ti and oxygen (see the Methods section) was also calculated, represented as the red line in Fig. 3d. Under the experimental condition (oxygen partial pressure = 0.2 atm), the formation energy is calculated to be 2.99 eV per atom within the range of FTS energy. Moreover, to simulate a composition similar to the experimental conditions, one of the eight Ti atoms was removed (denoted as a dotted circle), leading to the Ti0.875O2 structure (top and right). In this configuration, oxygen atoms adjacent to the Ti vacancy shift closer to neighboring Ti atoms, forming a local Ti-rich region. This structure, where Ti and oxygen are already separated locally, might lower formation energy for the decomposition. The blue line in Fig. 3d shows that, in practice, the formation energy is 2.59 eV per atom (oxygen partial pressure of 0.2 atm), which is less than that of the stoichiometric structure. It is important to note, however, that this calculation could be considered unrealistic due to the relatively small cell size and periodic boundary conditions, which tend to arrange vacancies in a regular pattern. To address this, more realistic calculations were conducted using larger supercell configurations. We compared two extreme cases: one with distant vacancies (bottom and left, denoted as case *) and another with closely positioned vacancies (bottom and right, denoted as case **), although the composition slightly deviates from the experimental one. Surprisingly, despite the significant disparity in the distribution of Ti vacancies, both cases exhibited nearly identical energy states. The formation energies for decomposition into Ti and oxygen were also remarkably similar for both cases, 2.70 eV per atom under an oxygen partial pressure of 0.2 atm, indicating a consistent trend (represented by green and purple lines in Fig. 3d). Analyzing these four cases reveals that as the number of Ti vacancies increases, the formation energy decreases, suggesting that less energy is needed for decomposition. This confirms that nearly all cases of TiO2−x NSs can decompose into Ti and oxygen when exposed to FTS energy within the range of 300 to 430 nm, even enough to decompose the anatase phase (as shown by the black line in Fig. 3c). In the case of Ti0.87O2 NSs, corresponding to the experimental composition, this suggests the potential for forming porous Ti0.87O2 NSs with reduced Ti0.87O2−x by local decomposition into Ti and oxygen, at FTS energy ranging from 430 to 500 nm.
To investigate the surface activity of porous Ti0.87O2 NSs after FTS, as a case study, we conducted chemical sensing measurements with five gas species of VOC gases including formaldehyde (HCHO), toluene (C6H5CH3), benzene (C6H6), o-xylene (C6H4(CH3)2) and ammonia (NH3). The prepared Ti0.87O2 NSs and FTS-Ti0.87O2 NSs were coated on sensing substrates that consisted of interdigitated Pt electrodes and all the measurements were carried out by applying a constant voltage of 0.5 V. Fig. S16† shows the gas sensing system under working conditions. To reveal the effect of formation of porosity and reduced Ti0.87O2−x, we carried out dynamic response measurements on Ti0.87O2 and FTS-Ti0.87O2. The respective sensor devices were exposed to various concentrations of HCHO in air for a period of 10 min, followed by a 10 min purge with clean air. For most concentrations, we found that 10 minutes was sufficient to return back to approximately the base resistance values even at room temperature. The gas response to HCHO is defined by the following equation:
| Response (%) = [Rg − Ra/Ra] × 100 | (2) |
| Y = 7.89x + 2.62 and Y = 40.57x + 8.57 (Y, sensor response; x, gas concentration) | (3) |
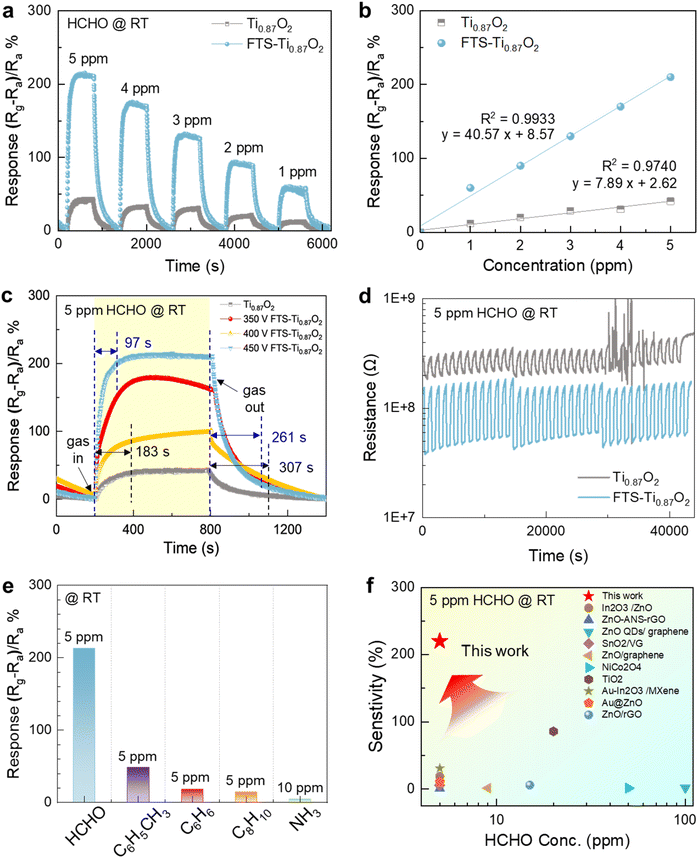
The correlation coefficient (R2) is 0.9740 and 0.9933, respectively. These results provided high reliability and accuracy of our sensors. Furthermore, we assessed the sensor's detection limit (LOD) by analyzing the root-mean-square deviation (rmsd) of the baseline signal.55 According to the equation LOD (ppm) = 3 × RMSnoise/slope, we determined a theoretical LOD of approximately 99.13 ppb (Fig. 4b). Therefore, the FTS-Ti0.87O2 sensor can be a candidate for detecting HCHO in indoor settings and human breath samples.
As shown in Fig. 4c, in terms of the sensing speed, response time refers to the time from the initial sensitivity to reach a value corresponding to 90% of the maximum sensitivity and recovery time refers to the time to return to a value corresponding to 10% of the maximum response after reaching the maximum response. Pristine Ti0.87O2 and FTS-Ti0.87O2 exhibited significantly rapid sensing responses (183 s and 97 s) at the 5 ppm level for HCHO and room temperature. In the case of the FTS treatment sample, FTS-Ti0.87O2 includes pores and reduced nanoparticles on the surface, which enhance permeability thus enabling fast HCHO response. Furthermore, our 2D metal oxide based sensors showed great recovery sensing behavior with competitive recovery speed (307 s for pristine Ti0.87O2 and 261 s for FTS-Ti0.87O2).
In addition, we measured the sensitivity properties of HCHO gas under various FTS voltage conditions, as shown in Fig. 4c. Note that the different FTS voltages induce different porosities on the 2D oxides. Interestingly, the results show that sensitivity gradually increases with the degree of pore formation observed in the TEM analysis. This suggests that the increase in reaction rates on the porous and reduced surfaces contributes to the overall sensitivity of the gas sensor, leading to the determination of 450 V as the optimal voltage condition for the FTS process.
As shown in Fig. S12,† the NO2 gas sensing properties were investigated at various FTS voltages (250, 300, 350, 400, 450, and 500 V). After applying five shots of FTS treatment at each voltage, the gas sensing performance was evaluated. The results demonstrated that at 450 V, the sensor exhibited the most stable and fastest response and recovery times. This enhanced performance is attributed to the formation of reduced nanoparticles and a porous surface, as confirmed by the temperature profiles measured during the FTS process and the TEM images. Considering the previously reported room temperature operated HCHO sensors, our 2D oxide materials with high porosity showed exceptional sensing reversibility even at room temperature. Fig. 4d shows the typical results of a sensing response cycling test of the Ti0.87O2 based gas sensors toward HCHO gas (5 ppm) at room temperature. As shown in Fig. 4d, no matter whether exposed to HCHO vapor or air, the response value of the after FTS-Ti0.87O2 sensor was relatively stable even after 35 cycles, indicating that our sensor had outstanding reliability and reversibility. To confirm the stability of the nanosheets, we conducted both XRD and microstructural SEM analyses before and after the cycle test as shown in Fig. S13 and S14,† respectively. The results confirm that the phase and morphology of the 2D Ti0.87O2 nanosheets remained unchanged, indicating excellent structural stability. These findings suggest that the nanosheets are capable of repeatable performance, maintaining their structure through continuous cycles. To investigate the selectivity of FTS-Ti0.87O2, the cross-selectivity of the FTS-Ti0.87O2 sensors, under the same conditions, to several typical gases, including HCHO, toluene, benzene, o-xylene and NH3 was tested as shown in Fig. 4e. Apparently, it could be seen that the FTS-Ti0.87O2 sensor showed remarkably higher response (213%) to HCHO than to the other gases (45.8%). These results indicated the excellent selectivity of the FTS-Ti0.87O2 sensor to HCHO gas. Additionally, the resistance changes were confirmed for 3 types of mixed gases (formaldehyde, toluene, and ammonia, each at 5 ppm) as shown in Fig. S15.† It was observed that the sensor's response is maintained, allowing for its detection even among mixed gases. To demonstrate the superiority of our sensors, we compared the gas-sensing performances of several state-of-the-art gas sensors to detect HCHO at room temperature. The results are briefly summarized in Fig. 4f and Table S1.†58–69 In the previously reported paper,59 HCHO gas was excellently detected at room temperature. In these studies, the synthesis method for the composite materials can be complex making reproducibility challenging and costly. In contrast, nanosheets offer advantages as they can be mass-produced with a uniform atomic layer thickness (∼2 nm) through liquid exfoliation. Furthermore, thinner thicknesses of 2D materials provide better sensitivity in gas sensors, and using small amounts of material makes it economical to use them as sensors. In consideration of the gas-sensing properties, FTS-Ti0.87O2 exhibited a relatively better HCHO detection performance compared with the other previously reported sensors in two-dimensional materials (Fig. 4f). Therefore, we demonstrated the photothermal treatment effect by formation of porosity and reduced Ti0.87O2−x on the Ti0.87O2 nanosheets, which facilitates electron transport. Additionally, the effect includes an increase in the number of active sites. Based on the aforementioned FTS effects, the simultaneous formation of pores and reduced Ti0.87O2−x on thin-layered Ti0.87O2 by FTS results in rapid HCHO gas diffusion to sensing layers and dramatic electrical resistivity modulation even at room temperature. Although multiple 2D oxide layers on sensing substrates conventionally blocked smooth gas diffusion to the whole 2D oxide surface due to narrow channel pores (d-spacing of 2D materials), the FTS inspired surface pores on 2D oxides successfully break through such limitations thus resulting in exceptional HCHO sensing behavior at room temperature. We expected that the atomic level thin layered structures of FTS-Ti0.87O2 with high porosity play a critical role in the enhancement of the sensing response and sensing reversibility even at room temperature. In terms of the sensing mechanism of room temperature operated sensors, the electrical resistivity of 2D oxide sensing layers can be modulated by adsorption of charged molecules and charge transfer.70 For example, charged HCHO molecules on 2D oxides can attract electrons and/or holes, which induces the resistivity modulation of 2D oxides. Therefore, the atomic level thickness and single crystalline 2D oxides can induce rapid gas adsorption/desorption behavior and porosity of 2D oxides may possibly result in fast gas diffusion.71
4. Conclusion
In this work, we investigated the simultaneous formation of pores as well as reduced Ti0.87O2−x effects on 2D Ti0.87O2 nanosheets through simple and rapid flash-thermal shock by the FTS technique. Since 2D Ti0.87O2 nanosheets were rapidly annealed by flash-thermal shock (7.5 ms), desired pores and reduced Ti0.87O2−x were achieved on Ti0.87O2 nanosheets, while maintaining the atomically thin 2D structures and crystal structures. Furthermore, the DFT calculations and ex situ TEM analysis depending on the power of flash-thermal shock were conducted to deeply understand the mechanism of pores and reduced Ti0.87O2−x formation on 2D Ti0.87O2. To investigate the surface activity of surface porosity as well as reduced Ti0.87O2−x on 2D Ti0.87O2, we conducted chemical sensing measurements by using samples before and after FTS treatment. The flash-thermal shock treated 2D Ti0.87O2 having numerous pores and reduced Ti0.87O2−x showed exceptional HCHO detection capability with excellent reversibility even at room temperature. Compared to state-of-the-art room temperature operated HCHO sensors, our flash-thermal shock treated 2D Ti0.87O2 based sensors showed superior sensitivity and selectivity to ppm level HCHO. We believe that flash-thermal shock treated 2D oxide structures will open new avenues for rapidly producing porous 2D oxides with reduced Ti0.87O2−x, especially optimized for applications in sensors, catalysis, and energy generation systems.Data availability
The data supporting this article have been included as part of the ESI.† Data for this article are available at KIST [Korea Institute of Science & Technology].Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Korea Institute of Science and Technology Future Resource Program (2E32491) and National Research of Korea (NRF) grant funded by the Korean government (2021R1A2C201069513). This research was also supported by KIST [2V10301 & 2V10120]. This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (RS-2024-00407291). This research was also supported by the Technology Innovation Program (00144157, Development of Heterogeneous Multi-Sensor Micro-System Platform) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).References
- V. Kamysbayev, A. S. Filatov, H. Hu, X. Rui, F. Lagunas, D. Wang, R. F. Klie and D. V. Talapin, Science, 2020, 369, 979–983 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D.-e. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. Chhowalla, Z. Liu and H. Zhang, Chem. Soc. Rev., 2015, 44, 2584–2586 RSC.
- J. Shen, Y. Zhu, H. Jiang and C. Li, Nano Today, 2016, 11, 483–520 CrossRef CAS.
- G. Qian, J. Chen, T. Yu, J. Liu, L. Luo and S. Yin, Nano-Micro Lett., 2022, 14, 1–15 CrossRef PubMed.
- H.-P. Li, J. Wen, S.-M. Ding, J.-B. Ding, Z.-H. Song, C. Zhang, Z. Ge, X. Liu, R.-Z. Zhao and F.-C. Li, Nano Mater. Sci., 2023, 5, 421–428 CrossRef CAS.
- H.-F. Zhang, J.-Y. Xuan, Q. Zhang, M.-L. Sun, F.-C. Jia, X.-M. Wang, G.-C. Yin and S.-Y. Lu, Rare Met., 2022, 41, 3976–3999 CrossRef CAS.
- S. A. Zahra and S. Rizwan, RSC Adv., 2022, 12, 8405–8413 RSC.
- B. Wang, Y. Gu, L. Chen, L. Ji, H. Zhu and Q. Sun, Nat. Nanotechnol., 2022, 33, 252001 CrossRef CAS PubMed.
- G. Korotcenkov, Mater. Sci. Eng., 2008, 61, 1–39 CrossRef.
- H. Yim, S. Y. Yoo, Y. H. Kim, K. H. Chae, Y.-H. Kim, S. K. Kim, S.-H. Baek, C.-H. Lee and J.-W. Choi, Chem. Mater., 2021, 33, 8685–8692 CrossRef CAS.
- Z. Zhang, D. Jiang, D. Li, M. He and M. Chen, Appl. Catal., B, 2016, 183, 113–123 CrossRef CAS.
- H. Zhang, ACS Nano, 2015, 9, 9451–9469 CrossRef CAS PubMed.
- M. Im, W.-H. Lee, S.-H. Kweon, C.-Y. Kang and S. Nahm, J. Eur. Ceram. Soc., 2019, 39, 1149–1155 CrossRef CAS.
- W.-H. Lee, J.-U. Woo, H.-G. Hwang, S. Nahm, G. Lee and J.-W. Choi, Appl. Surf. Sci., 2020, 525, 146640 CrossRef CAS.
- J.-H. Kim, S. H. Kweon and S. Nahm, Nano Res., 2019, 12, 2559–2567 CrossRef CAS.
- S.-Y. Yoo, H. Yim, A. Ryu, C. Yoon, B. H. Park, S. Nahm and J.-W. Choi, npj 2D Mater. Appl., 2023, 7, 53 CrossRef CAS.
- S.-H. Kweon, M. Im, W.-H. Lee, S. Nahm, J.-W. Choi and S.-J. Hwang, J. Mater. Chem. C, 2016, 4, 178–184 RSC.
- B. Bhowmik and P. Bhattacharyya, IEEE Trans. Electron Devices, 2017, 64, 2357–2363 CAS.
- B. Bhowmik and P. Bhattacharyya, IEEE Trans. Electron Devices, 2017, 64, 2357–2363 CAS.
- Y. Wang, Y. Zhang, Y. Liu and Z. Wu, Appl. Catal., B, 2022, 316, 121610 CrossRef CAS.
- X. Wang, S. Wang, G. Qiao, W. Liu, Y. Xiong, J. Tian, N. Wu and X. Wang, J. Alloys Compd., 2023, 930, 167468 CrossRef CAS.
- K. Saito, K. Inaguma, M. Ogawa, P. T. Ha, H. Akiyama, S. Yamaguchi, H. Minokoshi, M. Ogasawara and S. Kato, ACS Appl. Nano Mater., 2022, 5, 9053–9062 CAS.
- M. Ogawa, M. Morita, S. Igarashi and S. Sato, J. Solid State Chem., 2013, 206, 9–13 CAS.
- T. Sasaki, F. Kooli, M. Iida, Y. Michiue, S. Takenouchi, Y. Yajima, F. Izumi, B. C. Chakoumakos and M. Watanabe, Chem. Mater., 1998, 10, 4123–4128 CrossRef CAS.
- H. J. Park, S. E. Lee and J. Y. Park, Thin Solid Films, 2017, 636, 99–106 CAS.
- J. Y. Hwang, Y. Lee, G. H. Lee, S. Y. Lee, H.-S. Kim, S.-i. Kim, H. J. Park, S.-J. Kim, B. Z. Lee and M. S. Choi, Discover Nano, 2023, 18, 47 CrossRef PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse, J. Non-Cryst. Solids, 1995, 192–193, 222–229 CrossRef.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- J. W. Furness, A. D. Kaplan, J. Ning, J. P. Perdew and J. Sun, J. Phys. Chem. Lett., 2020, 11, 8208–8215 CrossRef CAS PubMed.
- E. Araujo-Lopez, L. A. Varilla, N. Seriani and J. A. Montoya, Surf. Sci., 2016, 653, 187–196 CrossRef CAS.
- D. D. Wagman, Selected Values of Chemical Thermodynamic Properties, Institute for Materials Research, National Bureau of Standards, 1966 Search PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- E. W. Lemmon, Thermophysical properties of fluid systems, NIST Chemistry WebBook, 2010 Search PubMed.
- W. H. Lee, M. Im, S. H. Kweon, J. U. Woo, S. Nahm, J. W. Choi and S. J. Hwang, J. Am. Ceram. Soc., 2017, 100, 1098–1107 CrossRef CAS.
- R. Ma and T. Sasaki, Acc. Chem. Res., 2015, 48, 136–143 CrossRef CAS PubMed.
- H. Yim, S.-Y. Yoo, S. Nahm, S.-J. Hwang, S.-J. Yoon and J.-W. Choi, Ceram. Int., 2013, 39, S611–S614 CrossRef CAS.
- M. Im, S.-H. Kweon, J.-S. Kim, S. Nahm, J.-W. Choi and S.-J. Hwang, Ceram. Int., 2014, 40, 5861–5867 CrossRef CAS.
- D.-H. Kim, J.-H. Cha, G. Shim, Y. H. Kim, J.-S. Jang, H. Shin, J. Ahn, S.-Y. Choi and I.-D. Kim, Chem, 2022, 8, 1014–1033 CAS.
- J. H. Bang, M. S. Choi, H. G. Na, W. Oum, S.-W. Choi, S. S. Kim, H. W. Kim and C. Jin, Sci. Rep., 2019, 9, 8129 Search PubMed.
- S. J. Choi, J. S. Jang, H. J. Park and I. D. Kim, Adv. Funct. Mater., 2017, 27, 1606026 Search PubMed.
- H. Lu, J. Wang, H. Li, W. Zhou, Q. Yuan and S. Liu, Mater. Chem. Front., 2023, 7, 4372–4399 CAS.
- Y.-S. Lee, H. Yim, S.-Y. Yoo, B.-K. Ju and J.-W. Choi, J. Alloys Compd., 2017, 711, 51–57 CAS.
- S. Prucnal, J. Sun, A. Muecklich and W. Skorupa, Electrochem. Solid-State Lett., 2006, 10, H50 CrossRef.
- C. J. Barnett, N. A. Smith, D. R. Jones, T. G. Maffeis and R. J. Cobley, Nanoscale Res. Lett., 2015, 10, 1–6 CAS.
- D.-N. Pei, L. Gong, A.-Y. Zhang, X. Zhang, J.-J. Chen, Y. Mu and H.-Q. Yu, Nat. Commun., 2015, 6, 8696 CAS.
- K. Akatsuka, M.-a. Haga, Y. Ebina, M. Osada, K. Fukuda and T. Sasaki, ACS Nano, 2009, 3, 1097–1106 CAS.
- P. Makuła, M. Pacia and W. Macyk, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef PubMed.
- D. Zhu, H. Zhao, B. Wang and S. Yang, CrystEngComm, 2022, 24, 1319–1333 RSC.
- V. P. Indrakanti, J. D. Kubicki and H. H. Schobert, Energy Environ. Sci., 2009, 2, 745–758 RSC.
- O. K. Varghese, D. Gong, M. Paulose, K. G. Ong and C. A. Grimes, Sens. Actuators, B, 2003, 93, 338–344 CrossRef CAS.
- C. A. Grimes, J. Mater. Chem., 2007, 17, 1451–1457 RSC.
- S. Yang, G. Lei, H. Xu, Z. Lan, Z. Wang and H. Gu, Nanomaterials, 2021, 11(4), 1026 CrossRef CAS PubMed.
- S. Park, S. Kim, G.-J. Sun, S. Choi, S. Lee and C. Lee, Ceram. Int., 2015, 41, 9823–9827 CrossRef CAS.
- Z. Chen, B. Zhou, M. Xiao, T. Bhowmick, P. Karthick Kannan, L. G. Occhipinti, J. W. Gardner and T. Hasan, Sci. Adv., 2024, 10, eadk6856 CrossRef CAS PubMed.
- Y. K. Jo, S.-Y. Jeong, Y. K. Moon, Y.-M. Jo, J.-W. Yoon and J.-H. Lee, Nat. Commun., 2021, 12, 4955 CrossRef CAS PubMed.
- D. Rajkumar, H. Umamahesvari and P. Nagaraju, Results Chem., 2023, 5, 100946 CrossRef CAS.
- M. Liu, R. Sun, Z. Sima, P. Song, Y. Ding and Q. Wang, Appl. Surf. Sci., 2022, 605, 154839 CrossRef CAS.
- S. Karmakar, A. Sett, P. C. Maity, R. Sha, T. K. Bhattacharyya and I. Lahiri, Mater. Lett., 2023, 350, 134927 CrossRef CAS.
- J. Fan, H. Li, H. Hu, Y. Niu, R. Hao, A. Umar, M. Al-Assiri, M. A. Alsaiari and Y. Wang, Microchem. J., 2021, 160, 105607 CrossRef CAS.
- Z. Bo, M. Yuan, S. Mao, X. Chen, J. Yan and K. Cen, Sens. Actuators, B, 2018, 256, 1011–1020 CrossRef CAS.
- W. Guo, J. Electrochem. Soc., 2016, 163, B517 CrossRef CAS.
- X. Li, J. Wang, D. Xie, J. Xu, R. Dai, L. Xiang, H. Zhu and Y. Jiang, Sens. Actuators, B, 2015, 221, 1290–1298 CrossRef CAS.
- H. Mu, Z. Zhang, X. Zhao, F. Liu, K. Wang and H. Xie, Appl. Phys. Lett., 2014, 105(3), 033107 CrossRef.
- Q. Huang, D. Zeng, H. Li and C. Xie, Nanoscale, 2012, 4, 5651–5658 RSC.
- L. Han, D. Wang, J. Cui, L. Chen, T. Jiang and Y. Lin, J. Mater. Chem., 2012, 22, 12915–12920 RSC.
- J. Zhang, X. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795–831 CrossRef CAS PubMed.
- N. Yamazoe, Sens. Actuators, B, 1991, 5, 7–19 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06114d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |