Raman spectroscopy study of K-birnessite single crystals†
Dong Han
Ha
 *a,
Gichang
Noh
bc,
Hakseong
Kim
d,
Dong Hwan
Kim
a,
Jeongho
Kim
b,
Suyong
Jung
a,
Chanyong
Hwang
d,
Ha Young
Lee
e,
Yong Ju
Yun
*a,
Gichang
Noh
bc,
Hakseong
Kim
d,
Dong Hwan
Kim
a,
Jeongho
Kim
b,
Suyong
Jung
a,
Chanyong
Hwang
d,
Ha Young
Lee
e,
Yong Ju
Yun
 f,
Joon Young
Kwak
f,
Joon Young
Kwak
 g,
Kibum
Kang
g,
Kibum
Kang
 b and
Sam Nyung
Yi
b and
Sam Nyung
Yi
 *e
*e
aDivision of Chemical and Material Metrology, Korea Research Institute of Standards and Science, Daejeon 34113, Korea. E-mail: dhha@kriss.re.kr
bDepartment of Materials Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon 34141, Korea
cCenter for Neuromorphic Engineering, Korea Institute of Science and Technology, Seoul 02792, Korea
dQuantum Technology Institute, Korea Research Institute of Standards and Science, Daejeon 34113, Korea
eDepartment of Nano-Semiconductor Engineering, Korea Maritime & Ocean University, Busan 49112, Korea. E-mail: snyi@kmou.ac.kr
fDepartment of Integrative Energy Engineering, Graduate School of Energy and Environment (KU-KIST Green School), College of Engineering, Korea University, Seoul 02841, Korea
gDivision of Electronic and Semiconductor Engineering, Ewha Womans University, Seoul 03760, Korea
First published on 20th November 2024
Abstract
Raman studies of manganese dioxide (MnO2), a crucial material for sustainable and innovative solutions in energy storage and environmental remediation, have predominantly been conducted on fine-grained aggregates, leaving the identification of Raman peaks open to debate. To address this, in this study the Raman spectra of potassium (K)-birnessite single crystals with varying crystal thickness, temperature, and polarization configuration are measured. An acoustic phonon mode of birnessite is identified, which is found to be sensitive to both crystal thickness and interlayer spacing, with its frequency increasing by approximately 35% when the c-axis lattice parameter is reduced from 0.70 to 0.65 nm by the removal of interlayer water. In contrast, the dependence of the optical phonon modes on crystal thickness and interlayer spacing is not particularly noticeable. It is demonstrated that the characteristic Raman peak of K-birnessite, observed at approximately 559 cm−1, originates from a two-dimensional hexagonal configuration of cations and water molecules within the interlayer space, rather than from the MnO6 octahedra. Additionally, the doubly degenerate vibrational mode of MnO6 octahedra, corresponding to the motion of oxygen atoms in the basal plane, splits into two, confirming that the MnO6 octahedra are distorted by the Jahn–Teller effect.
Introduction
One of the numerous polymorphs of manganese dioxide (MnO2) is birnessite (δ-MnO2), a material comprising layers of edge-sharing MnO6 octahedra arranged in the c-axis direction with charge-balancing cations and structural water intercalated in the interlayer space.1–3 In comparison to the tunnels of other MnO2 polymorphs, such as α-MnO2, β-MnO2, and λ-MnO2 with spinel structures,4–6 the interlayer space of birnessite has a larger volume and surface area, allowing more ions or molecules to intercalate into or deintercalate from the material. Birnessite exhibits an excellent ion exchange capacity, electrochemical activity, and catalytic performance, making it a versatile material suitable for a wide range of industries focused on sustainability and pollution control, including environmental remediation, energy storage, catalysis, and sensing.6–12In birnessite, interlayer cations and water molecules coordinate with adjacent MnO2 layers to modulate the oxidation state of manganese, thereby causing Jahn–Teller distortions of the MnO6 octahedra with Mn3+ centers and adjusting the relative positions of the MnO6 octahedra above and below.13–16 It has been reported that the symmetry of birnessite decreases from hexagonal to monoclinic or triclinic by Jahn–Teller distortions as more Mn4+ is replaced with Mn3+.17,18 The quantity and configuration of interlayer cations and water molecules are intimately related to the structural stability and functionality of layered materials, and it has been postulated that they form complex ordered or disordered hydrogen-bonded structures. Based on density functional theory (DFT) calculations, Sudare et al. proposed that in layered double hydroxides, interlayer water molecules form two-dimensional (2D) or three-dimensional (3D) irregular hydrogen-bonded structures with interlayer ions, depending on the quantity of interlayer ions and water.19 On the other hand, Matsui et al. proposed that interlayer water molecules in monoclinic Na-birnessite with C2/m symmetry are hydrated around cations bound to the MnO6 sites with Mn3+ centers, forming 2D hexamer-like structures.20
Raman spectroscopy, a powerful and non-destructive analytical technique for observing the vibrational modes of materials down to the micrometer scale without special sample preparation,21,22 has been applied to the study of MnO2 in a variety of ways. These include the identification and differentiation of different polymorphs of MnO2, the monitoring of structural changes during synthesis or electrochemical processes, and the analysis of the oxidation states of manganese ions.2,3,23–27 Since the pioneering studies conducted by Julien and colleagues,28,29 Raman spectroscopic studies of MnO2 have made significant progress over the past two decades. However, Raman studies have predominantly been conducted on aggregates of fine grains with different crystallinities, structures, and crystallographic orientations. Furthermore, the presence of impurities, interlayer cations, and water molecules poses inherent challenges to the accurate and precise analysis of Raman spectra. Consequently, inaccuracies or discrepancies persist not only in the identification of Raman peaks but also in the correlation between the crystal structure and Raman peaks, which are fundamental aspects of MnO2 Raman spectroscopy for its characterization and applications.
In order to address this limitation, in this study, the Raman spectra of potassium ion intercalated layered MnO2 (K-birnessite) single crystals were measured down to 50 cm−1 for the first time, considering variations in crystal thickness, temperature, and polarization configuration. By measuring the Raman spectra of impurity-free K-birnessite single crystals in air and a vacuum, we were able to identify both the acoustic phonon mode of birnessite and the splitting of the doubly degenerate vibrational mode of MnO6 octahedra. Moreover, the Raman peak at 559 cm−1 was verified to originate from the 2D hexagonal arrangement of K+ ions and water molecules within the interlayer space. This provides an explanation for the observation of this peak in birnessite,2,3,26,30,31 which has a relatively large and flexible 2D interlayer space. The interlayer spacing exerts a significant influence on the frequency of the acoustic phonon mode of birnessite, whereas its effect on the frequency of the optical phonon modes is negligible. The new insights into the Raman properties of K-birnessite obtained in this work are expected to play an important role in the characterization and applications of MnO2 in a variety of contexts, including determination of its crystal structure and symmetry as well as investigation of the ion storage mechanism.
Experimental
K-birnessite single crystals were grown on c-sapphire substrates; details of the crystal growth process are described in a previous study.31 The thickness of the crystals was measured in a laboratory environment using tapping mode atomic force microscopy (AFM). An X-ray diffractometer (RIGAKU, SmartLab) equipped with a CuKα source was used to obtain X-ray diffraction (XRD) data. Micro-Raman spectra were collected in a 180° backscattering configuration using a 532 nm laser line focused on the crystal surface through a 50× long working distance objective (numerical aperture = 0.8) as excitation light. The scattered light was analyzed using a Horiba Jobin Yvon LabRAM HR spectrometer, which was equipped with a grating of 1800 grooves per mm and a cooled charge-coupled device with 1024 × 256 pixels. Each Raman spectrum was obtained by conducting two measurements with an acquisition time of 60 s and averaging the two spectra. The laser power on the crystals was maintained below 0.3 mW to ensure that the Raman spectrum remained unaltered even after multiple measurements at the same location, confirming that the effect of laser heating was negligible. The temperature of the crystals was controlled by placing the sample in contact with thermal paste on a heating stage in a home-built vacuum chamber, where the vacuum was maintained below 40 mTorr. The A1g peak of c-sapphire at 417.3 cm−1 for 532 nm incident light, calibrated to the T2g peak of Si at 520.7 cm−1 in a laboratory environment, was employed as an internal reference to calibrate the frequencies of the Raman peaks of K-birnessite.Results and discussion
Shape and polarization configuration effect
Crystals grown on the c-sapphire substrate31 showed a blue, yellow, or purple color under a light microscope. They were predominantly triangular in shape, with some trapezoidal or irregular shapes; on rare occasions, a hexagonal shape was observed (ESI Fig. S1†). Blue crystals grew to over 15 μm on one side, while yellow crystals were up to 7 to 8 μm in size and purple crystals were even smaller. The thicknesses of the blue, yellow, and purple crystals employed for Raman spectral measurements were typically within the ranges of 30–50, 60–80, and 90–105 nm, respectively, indicating that as the color of the crystals changed from blue to yellow to purple, the thickness exhibited a corresponding increase (ESI Fig. S2†). In a laboratory environment (∼25 °C and air), only the (001) and (002) peaks at 2θ = 12.54° and 25.26°, respectively, were observed in XRD patterns obtained using the CuKα line, indicating that the c-axis of the crystals was oriented perpendicular to the c-sapphire substrate.31Optical images of blue and yellow crystals and their typical Raman spectra are presented in Fig. 1. It can be observed that the frequency of each Raman peak and the relative intensity of the peaks exhibit minimal variation across different crystals (ESI Fig. S4†). Furthermore, the Raman spectra of different regions within a crystal exhibit high similarity, confirming the single crystal structure (ESI Fig. S5†). All crystals display Raman features characteristic of monoclinic birnessite:3,26 (i) a ν1 peak with much lower intensity compared to the ν2 peak, (ii) two peaks, ν3 and ν4, rather than a single peak at around 500 cm−1, and (iii) the appearance of a ν6 peak. The ω1 peak is commonly observed in birnessite and varies in position and intensity depending on the type of cation, particularly in K-birnessite where it appears as a distinct peak rather than as a weak shoulder of the ν2 peak.2,31 Accordingly, the Raman spectra reveal single crystals of monoclinic K-birnessite on the c-sapphire substrate.
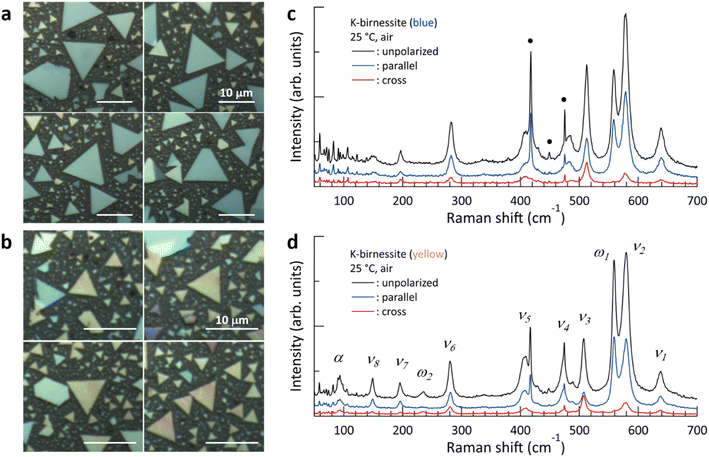 | ||
| Fig. 1 (a and b) Light microscopy images of blue and yellow crystals, respectively. (c and d) Typical Raman spectra of blue and yellow crystals obtained in a laboratory environment. The blue (parallel) and red (cross) spectra were obtained with parallel and perpendicular polarization directions, respectively, of the incident and scattered light. The black spectra (unpolarized) were obtained with no analyzer in the scattered light path. For the sake of convenience, each peak is named as in (d). The weak, narrow peaks below 150 cm−1 and those marked with black circles (●) represent the Raman peaks of the c-sapphire substrate (ESI Fig. S3†). | ||
The following differences were observed in the Raman spectra of the yellow crystals compared to those of the blue crystals: (i) a significant increase in the relative intensity of the ω1 peak compared to the other Raman peaks, accompanied by the appearance of the ω2 peak at 235 cm−1 and (ii) the appearance of the α peak at 97 cm−1. A Raman peak below 100 cm−1 was recently observed in K-birnessite by Post et al.,2 but its identity remains unknown. The Raman spectra were analyzed by fitting them with Lorentzian functions (ESI Fig. S6†), and the results are summarized in Table 1. The electrical and optical properties of layered materials are known to be influenced by interlayer interactions and interfacial effects, and these effects on material properties are observed to diminish gradually with an increase in the number of layers.22,32 Here, the frequencies of the ν3, ν4, and α peaks in the blue and yellow crystals exhibit notable differences from one another in comparison to the other peaks. Additionally, the ω2 peak emerges in the case of the thicker yellow crystals. However, there was little overall difference between the Raman spectra of the yellow and purple crystals.
| α | ν 8 | ν 7 | ω 2 | ν 6 | ν 5 | ν 4 | ν 3 | ω 1 | ν 2 | ν 1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blue crystal (cm−1) | 63.2* | 150.3 | 196.7 | — | 281.7 | 406.9 | 482.0 | 511.8 | 558.3 | 578.2 | 638.7 |
| Yellow crystal (cm−1) | 96.5 | 148.9 | 196.5 | 234.8 | 280.9 | 407.6 | 473.0 | 507.6 | 559.6 | 579.4 | 638.5 |
There are nine active Raman modes (3Ag + 6Bg) in monoclinic birnessite with point group C2h. It is known that the Raman spectrum of MnO2 consists of the vibrational modes of MnO6 octahedra in the high-frequency region above 500 cm−1 and those of the structures formed by coordination between oxygen in the MnO2 layers and interlayer cations/water in the low-frequency region.2 In particular, the ν6 peak is attributed to the stretching mode of the MO6 (M = alkali) group, while the ν5 peak is attributed to the asymmetric stretching mode of M–O tetrahedra, with some oxygen atoms from the MnO2 layer.2,3,26–28 However, these are still under debate.
The totally symmetric A1g mode of the c-sapphire with a hexagonal structure at 417 cm−1 almost disappears in the cross Raman spectra.33 The ν1 peak is the Ag mode of the MnO6 octahedra, in which the six Mn–O bonds stretch and compress in phase while Mn remains stationary in its equilibrium position.34 In the case of chalcophanite, which consists of undistorted MnO6 octahedra with all Mn in the 4+ valence state, the depolarization ratio (ρ = Icross/Iparallel) of the ν1 peak at 670 cm−1 is approximately zero, confirming a totally symmetric mode.2 However, the depolarization ratio of the ν1 peak in Fig. 1 is approximately 0.2, indicating that as some Mn4+ are replaced by Mn3+ and vacancies in K-birnessite, the symmetry of the MnO6 octahedra is broken due to Jahn–Teller distortion, and the frequency of the peak is redshifted to 639 cm−1 by the increase in the average distance of Mn–O.
Previously, the ν2 and ω1 peaks were attributed to the motion of oxygen atoms in the basal plane of MnO6. The ν2 peak was identified as the Ag mode, while the ω1 peak was attributed to the asymmetric Bg mode.2 In contrast, Rasouli et al. attributed the ω1 peak to out-of-plane vibrational modes of Mn–O bonds.31 However, Fig. 1 demonstrates that the depolarization ratio of the ω1 peak is approximately zero, indicating that the ω1 peak is a totally symmetric vibrational mode of the structure that will be revealed later. The α, ν1, ν2, and ω1 peaks will be discussed in detail in subsequent sections. The depolarization ratio of the ν3 peak at around 510 cm−1 is considerable, at times reaching more than 1 depending on the crystal as illustrated in Fig. 1d, indicating that it is the Bg mode.23,35 For the same reason, we attribute the ω2 peak to the asymmetric vibrational mode of the structure that is responsible for the ω1 peak. In the high-frequency region, where the Raman peaks are dominated by MnO6 deformations, the depolarization ratios of the ν1, ν2, and ν4 peaks are approximately 0.2 or less, with the exception of the ν3 peak. It is noteworthy that the α and ω1 peaks exhibit a markedly different behavior when compared to the other peaks. The frequencies of the α peaks observed in the blue and yellow crystals differ considerably by approximately 30 cm−1. The polarization configuration dependence of the ω1 peak, which was previously reported to be the Bg mode of the MnO6 octahedra, differs from all other peaks but is identical to that of the A1g mode of c-sapphire with sixfold symmetry (ESI Fig. S3†).
Temperature effect
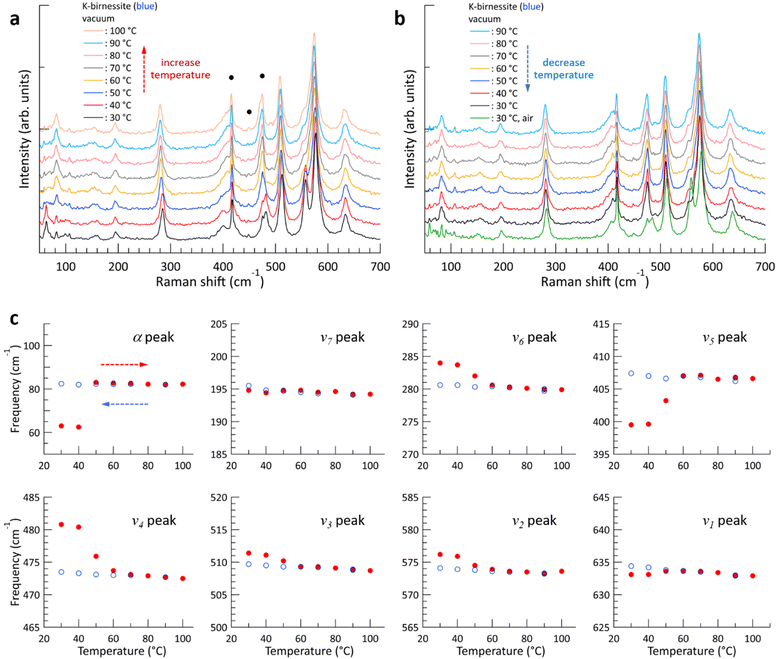
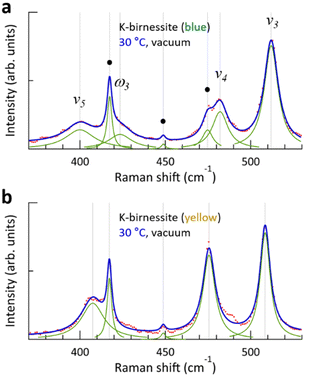
Fig. 2 shows the changes in the Raman spectrum of blue K-birnessite single crystals as the temperature increases and subsequently decreases in a vacuum. The Raman spectra demonstrate that crystals maintain a monoclinic structure at temperatures up to 100 °C. The α peak, which was undetectable in air, is clearly discernible at 63 cm−1 in a vacuum due to the elimination of the air damping effect, which refers to the attenuation of acoustic phonon amplitudes by interactions with the surrounding air. As the temperature increases, the intensity of the ω1 peak begins to decrease, becoming very weak at 50 °C and manifesting only as a faint shoulder of the ν2 peak at 60 °C. No further discernible change in the intensity of the ω1 peak is observed as the temperature continues to increase to 100 °C. However, the intensity of all the other peaks remains relatively constant throughout the temperature increase to 100 °C. As the ω1 peak diminishes, the frequency of the α peak increases from 63 to 83 cm−1, representing a considerably more pronounced change compared to any of the other peaks. Once the ω1 peak has almost disappeared, its intensity does not recover even when the temperature is decreased to 30 °C. The frequency of all peaks increases slightly due to the decrease in interatomic distances that occurs with decreasing temperature. But upon the introduction of air into the chamber at 30 °C, the intensity of the ω1 peak rapidly recovers, and likewise the frequencies of all the peaks return to their initial state at 30 °C in air. As the temperature increased and then decreased between 30 and 100 °C, the frequency of the c-sapphire exhibited a linear change (ESI Fig. S7†), confirming that the temperature of the sample reached each set temperature with precision.To elucidate the reason for the change in Raman spectra with temperature, XRD patterns were measured, where a change was indicated in the c-axis lattice parameter, namely a decrease from 0.70 to 0.65 nm with increasing temperature above 60 °C in a vacuum (ESI Fig. S8†). This suggests that interlayer water is removed from the crystal, resulting in an increase in interlayer interaction.11,28 Based on thermogravimetric measurements on Na-birnessite in a vacuum, Matsui et al. reported that strongly hydrated water molecules around Na+, which serve as structural water, begin to deintercalate at approximately 40 °C, with some remaining at 110 °C. On the other hand, weakly hydrated water can be removed even at room temperature in a vacuum,20 which is in accordance with our XRD measurements in a vacuum and air at 30 °C. It is noteworthy that the removal of interlayer water results in a more pronounced shift in the frequencies of the ν4, ν5, and ν6 peaks, which lie below 500 cm−1, in comparison to the ν1, ν2, and ν3 peaks, which are the vibrational modes of the MnO6 octahedra.
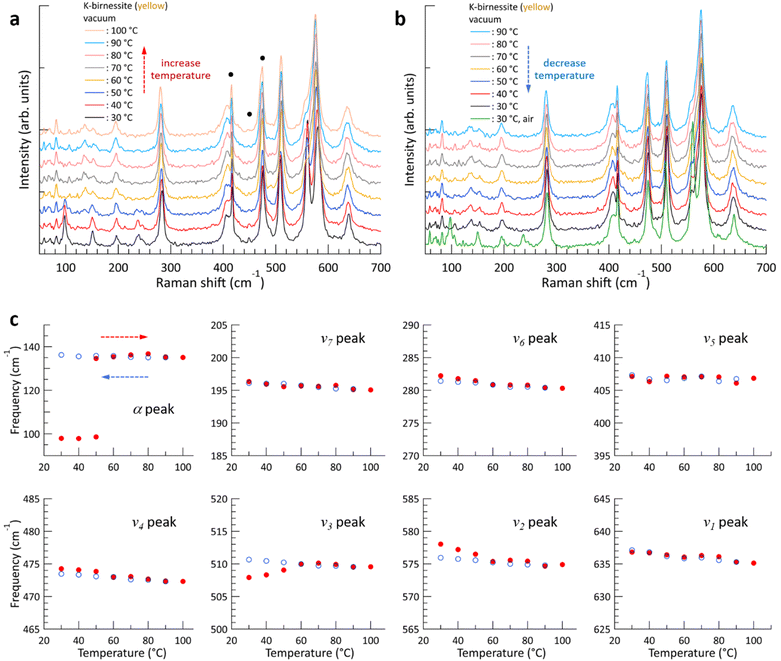
The evolution of the Raman spectrum of yellow K-birnessite single crystals with increasing and then decreasing temperature in a vacuum is depicted in Fig. 3. As the temperature increases, the intensities of the two peaks ω1 and ω2 diminish and almost disappear at 60 °C. The experimental results indicate that the ω1 and ω2 peaks do not originate from the MnO6 octahedra, but are closely related to water molecules and K+ ions in the interlayer space. This is consistent with the observation that the depolarization ratio of the ω1 peak in Fig. 1 is approximately zero, in contrast to all other Raman peaks of K-birnessite. As the quantity of interlayer water decreases from 40 °C, the intensities of the ω1 and ω2 peaks exhibit a gradual decline. Nevertheless, the ω1 peak persists as a faint shoulder on the low-frequency side of the ν2 peak even at 100 °C due to the presence of residual water molecules.20 Measurements were also conducted on crystals covered with a 30 nm thick layer of Al2O3 deposited by atomic layer deposition. In this case, the Raman spectrum remained unaltered when the temperature of the sample was elevated to 100 °C in a vacuum, as the Al2O3 layer effectively prevented interlayer water from escaping from the crystal (ESI Fig. S9†).
The overall trend of the Raman spectrum change with temperature for the yellow crystals is almost identical to that for blue crystals; however, there are some notable differences. In the case of the blue crystal, the frequencies of the ν4, ν5, and ν6 peaks undergo a significant change due to the deintercalation of interlayer water during the temperature increase to 60 °C. In contrast, the yellow crystal exhibits a minimal change in frequency. Fig. 3 illustrates that, with the exception of the α, ν2, and ν3 peaks, the frequency of each Raman peak remains largely unchanged at a given temperature before and after the interlayer water is removed. Even in the case of the ν2 and ν3 peaks, the difference in frequency before and after the change in interlayer spacing is not significant. This phenomenon is characteristic of optical phonons, which correspond to the relative motion of atoms. Consequently, the frequency of these phonons is primarily determined by the interatomic distances and local bonding environment.
The α peak shows markedly distinct behaviors compared to the other peaks, as follows. (i) It appears in a much lower frequency range than the other peaks. (ii) Its frequency is highly susceptible to alterations in crystal thickness and interlayer spacing. The frequency of the α peak of the yellow crystal increases significantly from 98 to 135 cm−1 as the ω1 peak disappears in a vacuum. (iii) The α peak of the blue crystal shows a pronounced air damping effect. However, this is observed in both air and vacuum in thicker yellow crystals, where bulk properties become more dominant, due to a reduced air damping effect. The α peak exhibits typical characteristics of acoustic phonon modes of layered materials, providing compelling evidence to assign it as an acoustic phonon mode of K-birnessite. The acoustic phonon mode is indicative of the collective motion of each MnO2 layer; accordingly, the frequency of this mode, particularly the shear mode, increases significantly as the interlayer interaction is increased by increasing the number of layers or decreasing interlayer spacing.36–38 It is observed that the frequency of the α peak for both blue and yellow crystals increases by approximately 35 ± 3% as the interlayer spacing decreases due to the removal of interlayer water. At 50 °C, the intensity of the α peak at 98 cm−1 decreases, while a weak peak appears at 135 cm−1. This suggests the coexistence of two types of interlayer spaces during the transition: those in which a substantial amount of interlayer water remains and those in which interlayer water has been largely removed.
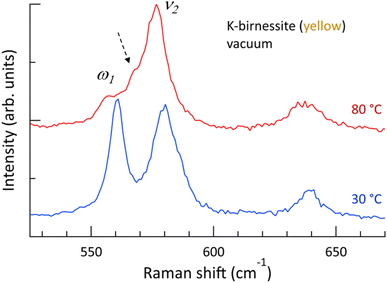
Fig. 1 shows that the depolarization ratio of the ν1 peak is approximately 0.2 due to unequal Mn–O bond lengths of MnO6 octahedra by Jahn–Teller distortion, which results in symmetry-breaking of the octahedra. The ν2 peak, which is assigned to the motion of oxygen atoms in the basal plane of MnO6, is the doubly degenerate mode (E-mode) of undistorted MnO6 octahedra.34 If the degeneracy is removed by breaking the symmetry of the MnO6 octahedra, the ν2 peak is expected to split into two peaks, as observed in transition metal dichalcogenide materials.39 The Raman spectra of yellow crystals at 30 and 80 °C in a vacuum are shown in Fig. 4, which details the region near the ν2 peak. At 30 °C, only the two strong peaks ν2 and ω1, which are in close proximity, are discernible. However, above 60 °C, the ω1 peak almost disappears and a new peak appears as a shoulder of the ν2 peak. The frequency difference between the two peaks is approximately 9 cm−1 (ESI Fig. S10†). It is postulated that the ν2 peak is also split at 30 °C but the weak shoulder is masked by the two strong peaks ω1 and ν2 located in close proximity. Although Scheitenberger et al. recently claimed that the ν1 peak originates from interlayer water,3 our results confirm that the ν1 peak is indicative of in-phase stretching and compressing of the six Mn–O bonds and that the ν2 peak is associated with the motion of oxygen atoms in the basal plane of the MnO6 octahedra.
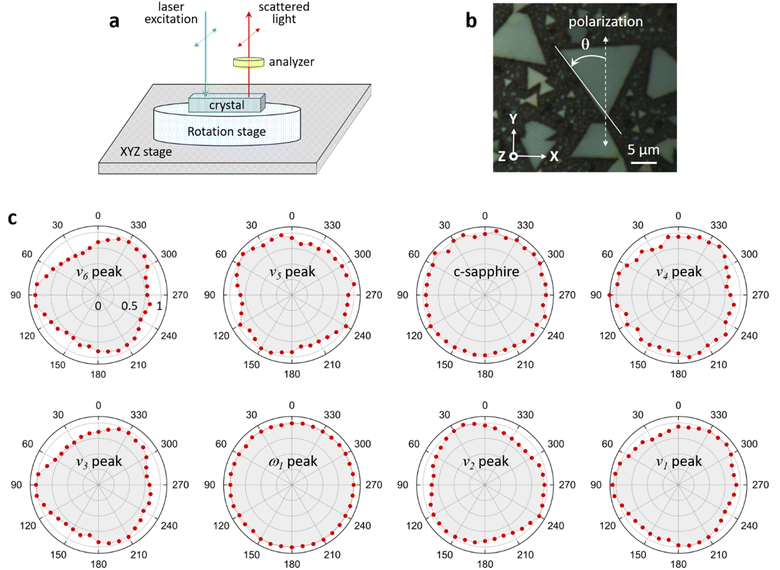
In-plane angle-resolved polarized Raman scattering
In-plane angle-resolved polarized Raman scattering (ARPRS) measurements were performed with a parallel polarization direction of the incident and scattered light (i.e., parallel polarization configuration) in order to examine the symmetry properties of each Raman mode.40,41 The angle between the polarization direction of the incident light and the crystal axis was varied in 10° steps by rotating the crystal using the measurement configuration shown in Fig. 5a. To prevent thermal damage and fluctuations, each Raman spectrum was obtained by raster scanning the incident laser over a 5 × 5 μm2 surface of the crystal, with an acquisition time of 120 s. As the crystal was rotated, the intensities of the c-sapphire and ω1 peaks remained unchanged. However, the intensities of all other Raman peaks exhibited a slight modulation with a 120° period, which differed from the 90° or 180° period observed in a previous study.42 The intensity of each Raman peak reaches a maximum when the bisection direction through the vertices of the triangle is parallel to the polarization direction of the incident light. In a recent study, Ilchenko et al. conducted ARPRS measurements on a (111) silicon plane and observed that the Raman intensity exhibited a period of 120° for off-axis (45°) measurements, which was in good agreement with theoretical calculations based on Raman tensors.43 They also observed that the Raman intensity was slightly modulated with a period of 120° for on-axis measurements. In our experiments, the c-plane of the crystal has a hexagonal structure, but the MnO6 octahedra are tilted with respect to the XY plane (ESI Fig. S11†), so it is postulated that the intensities of the Raman peaks of K-birnessite single crystals are slightly modulated by a 120° period.The Raman peak of c-sapphire at 413 cm−1 exhibits an isotropic polarization angle dependence, which is characteristic of backscattering measurements on a crystal plane with a hexagonal structure, such as the basal planes in MoS2 or highly ordered pyrolytic graphite.44,45 Post et al. postulated that K+ and water molecules form a hexagonal close-packed arrangement in the interlayer space to explain the results of Rietveld refinement and the pseudo-hexagonal arrangement observed in a selected area electron diffraction pattern corresponding to the normal direction on a K-birnessite plate.1 In addition, based on the infrared spectra of Na-birnessite and Post et al.‘s1 Rietveld refinement, Matsui et al. argued that interlayer structural water molecules hydrate Na+ ions, forming a quasi-planar hexamer-like structure.20 Our results confirm that the ω1 peak is a totally symmetric vibrational mode of the hexagonal arrangement of interlayer cations and water molecules. This result elucidates the reason for the occurrence of the ω1 peak in birnessite, which has a relatively large and highly flexible interlayer space that can accommodate various cations and water molecules to form clusters of 2D hexamer-like structures, but not in tunnel-structured MnO2 polymorphs.1,2,23
Interlayer water effect
In our experiments, increasing the temperature in a vacuum had a negligible effect on the frequency of the ν1 peak of the crystals, which are sensitive to the fraction of Mn3+. Additionally, the ω1 peak was immediately restored when air was reintroduced into the chamber at 30 °C, indicating that K+ ions are strongly bound to the MnO6 sites with Mn3+ centers and are not removed from the interlayer space even when the temperature is increased to 100 °C in a vacuum. The frequency and intensity of the ν1 peak have previously been reported to correlate positively with interlayer spacing (i.e., interlayer water content);3,24,27 however, Fig. 2 and 3 demonstrate that they have little to do with the interlayer spacing. During anodic potential sweep in aqueous solution, the quantity of cations in the interlayer space decreases while water content increases, resulting in increased frequency and intensity of the ν1 peak and interlayer spacing. It thus appeared that the interlayer spacing itself has an effect on the frequency and intensity of the ν1 peak. Our results indicate that the frequency and intensity of the ν1 peak are governed by the average Mn–O distance, which depends on the fraction of Mn3+, and have little correlation with the interlayer spacing. Furthermore, Fig. 3 demonstrates that the interlayer spacing exerts a minimal influence on the optical phonon modes below 500 cm−1 and only a limited effect on the ν2 and ν3 peaks.As illustrated in Fig. 2 and 3, the temperature-dependent alterations of the ν4 and ν5 peaks during the temperature elevation to 60 °C exhibit notable distinctions between blue and yellow crystals. As water is removed during the temperature increase, the frequencies of the two peaks undergo a significant change for the blue crystal, whereas they remain largely unaltered for the yellow crystal. A more detailed examination of the Raman spectra in this region reveals the presence of a weak peak (ω3) at 423 cm−1 for the blue crystal, which is not observed in the yellow crystal, as illustrated in Fig. 6. As the temperature increases and interlayer water is removed, the ω3 peak disappears along with the ω1 and ω2 peaks (ESI Fig. S12†), which is consistent with the results by Chen et al. that two peaks appear at around 400 cm−1 when the interlayer space is rich in water, but only a single peak is observed when the amount of water decreases.27 Through DFT calculations, Sudare et al. reported that cations and water molecules form 2D or tetrahedral 3D structures depending on the amounts of cations and water molecules in the interlayer space.19 On the other hand, Scheitenberger et al. used the peak at around 400 cm−1 as one of the indicators of the crystal structure of birnessite: as the symmetry of the crystal structure changes from monoclinic to hexagonal, the peak splits into two, accompanied by a significant enhancement of the ν1 peak.26 This is inconsistent with our results, which show that the intensity of the ν1 peak remains largely unaltered.
It is postulated that the ω3 peak originates from the tetrahedral 3D structure of cations and water molecules formed in the interlayer space. The majority of cations and water molecules form a 2D hexagonal structure in the yellow crystal, while some of them also form a 3D structure in the blue crystal. This is consistent with the observation that the relative intensity of the ω1 peak is greater in the yellow crystals compared to the blue crystals. Once the 2D and 3D structures of cations and water molecules are removed, the interlayer environments of the blue and yellow crystals become almost identical, resulting in the frequencies of all optical phonons in the two crystals being very close to each other. Further studies are required to pinpoint the reasons for the subtle differences in the structures formed by interlayer cations and water molecules in blue and yellow crystals. At present, it can be speculated that differences in interlayer interaction strength may play a role.
Conclusions
The effects of thickness, polarization configuration, and temperature on the Raman spectra of K-birnessite single crystals have been quantified. Distortion of the MnO6 octahedra was confirmed by observing that the depolarization ratio of the totally symmetric mode (ν1) is approximately 0.2, while the doubly degenerate mode (ν2) splits into two peaks. An acoustic phonon mode (α peak) of birnessite was identified for the first time. The α peak was found to be highly sensitive to changes in interlayer spacing: when the c-axis parameter was reduced from 0.70 to 0.65 nm by water evaporation, the frequency increased by approximately 35%. In contrast, the impact of interlayer spacing on the frequency of optical phonon modes is relatively modest. We conducted a comprehensive analysis of the ω1 peak at 559 cm−1, which was previously attributed to the motion of oxygen atoms in the basal plane of MnO6. The depolarization ratio of the ω1 peak was approximately zero, and in angle-resolved polarized Raman scattering measurements in parallel polarization configuration, its intensity was found to be constant for crystal rotation from 0° to 360°, which is in striking contrast to the behaviors observed for all other Raman peaks of K-birnessite single crystals. The ω1 peak was therefore assigned as a totally symmetric vibrational mode of the 2D hexagonal arrangement of interlayer cations and water molecules. This provides a clear explanation as to why the ω1 peak is observed in birnessite, which has a relatively large and highly flexible 2D interlayer space that can accommodate various cations and water molecules, allowing them to form 2D hexagonal clusters.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
D. H. Ha: conceptualization, project administration, investigation and writing – original draft. G. Noh: resources, investigation and writing – original draft. H. Kim: investigation and validation. D. H. Kim: investigation and validation. J. Kim: resources and investigation. S. Jung: investigation, funding acquisition and writing – review & editing. C. Hwang: resources and funding acquisition. H. Y. Lee: investigation and validation. Y. J. Yun: validation and writing – review & editing. J. Y. Kwak: resources and validation. K. Kang: resources and funding acquisition. S. N. Yi: conceptualization, formal analysis, validation and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. Jung was supported by a research grant for basic research funded by the Korea Research Institute of Standards and Science (no. KRISS-GP2024-0014-10). K. Kang was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (grant no. RS-2023-00030-4068).References
- J. E. Post and D. R. Veblen, Am. Mineral., 1990, 75, 477–489 CAS.
- J. E. Post, D. A. McKeown and P. J. Heaney, Am. Mineral., 2021, 106, 351–366 CrossRef.
- P. Scheitenberger, H. Euchner and M. Lindén, J. Mater. Chem. A, 2021, 9, 18466–18476 RSC.
- J. E. Post, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3447–3454 CrossRef CAS PubMed.
- T. Hatakeyama, N. L. Okamoto and T. Ichitsubo, J. Solid State Chem., 2022, 305, 122683 CrossRef CAS.
- S. Tajima, S. Fuchida and C. Tokoro, J. Environ. Sci., 2022, 121, 136–147 CrossRef CAS PubMed.
- M. Shi, Q. Li, Q. Wang, X. Yan, B. Li, L. Feng, C. Wu, R. Qiu, H. Zhang, Z. Yang, W. Yang, Q. Liao and L. Chai, J. Environ. Sci., 2024, 139, 496–515 CrossRef CAS.
- X. Zhang, Z. Wei, K. N. Dinh, N. Chen, G. Chen, F. Du and Q. Yan, Small, 2020, 16, 2002700 CrossRef CAS PubMed.
- A. Gao, M. Li, N. Guo, D. Qiu, Y. Li, S. Wang, X. Lu, F. Wang and R. Yang, Adv. Energy Mater., 2019, 9, 1802739 CrossRef.
- R. Yang, Z. Guo, L. Cai, R. Zhu, Y. Fan, Y. Zhang, P. Han, W. Zhang, X. Zhu, Q. Zhao, Z. Zhu, C. K. Chan and Z. Zeng, Small, 2021, 17, 2103052 CrossRef CAS.
- X. Tian, L. Yang, X. Qing, K. Yu and X. Wang, Sens. Actuators, B, 2015, 207, 34–42 CrossRef CAS.
- P. Wuamprakhon, A. Krittayavathananon, S. Kosasang, N. Ma, T. Maihom, J. Limtrakul, N. Chanlec, P. Kidkhunthod and M. Sawangphruk, Inorg. Chem., 2020, 59, 15595–15605 CrossRef CAS.
- K. P. Lucht and J. L. Mendoza-Cortes, J. Phys. Chem. C, 2015, 119, 22838–22846 CrossRef CAS.
- S. Zhu, Y. Wang, J. Zhang, J. Sheng, F. Yang, M. Wang, J. Ni, H. Jiang and Y. Li, Energy Environ. Mater., 2023, 6, e12382 CrossRef CAS.
- J. Liu, L. Yu, E. Hu, B. S. Guiton, X.-Q. Yang and K. Page, Inorg. Chem., 2018, 57, 6873–6882 CrossRef CAS.
- L. D. Kulish, P. Nukala, R. Scholtens, A. G. M. Uiterwijk, R. Hamming-Green and G. R. Blake, J. Mater. Chem. C, 2021, 9, 1370–1377 RSC.
- V. A. Drifts, E. Silvester, A. I. Gorshkov and A. Manceau, Am. Mineral., 1997, 82, 946–961 Search PubMed.
- F. T. Ling, J. E. Post, P. J. Heaney and E. S. Ilton, Chem. Geol., 2018, 479, 216–227 CrossRef CAS.
- T. Sudare, T. Yamaguchi, M. Ueda, H. Shiiba, H. Tanaka, M. Tipplook, F. Hayashi and K. Teshima, Nat. Commun., 2022, 13, 6448 CrossRef CAS PubMed.
- H. Matsui, J. Ju, T. Odaira and N. Toyota, J. Phys. Soc. Jpn., 2009, 78, 074801 CrossRef.
- H. -J. Kim, D. Kim, S. Jung, M. -H. Bae, Y. J. Yun, S. N. Yi, J. -S. Yu, J. -H. Kim and D. H. Ha, J. Raman Spectrosc., 2018, 49, 1938–1944 CrossRef CAS.
- D. H. Lee, N.-H. Kim, S. Jung, J. Park, B.-K. Kim, M.-H. Bae, Y. J. Yun, S. N. Yi and D. H. Ha, Adv. Mater. Interfaces, 2023, 10, 2300002 CrossRef CAS.
- J. E. Post, D. A. McKeown and P. J. Heaney, Am. Mineral., 2020, 105, 1175–1190 CrossRef.
- Y.-K. Hsu, Y.-C. Chen, Y.-G. Lin, L.-C. Chen and K.-H. Chen, Chem. Commun., 2011, 47, 1252–1254 RSC.
- H. An, Z. Chen, J. Yang, Z. Feng, X. Wang, F. Fan and C. Li, J. Catal., 2018, 367, 53–61 CrossRef CAS.
- P. Scheitenberger, S. Brimaud and M. Lindén, Mater. Adv., 2012, 2, 3940–3953 RSC.
- D. Chen, D. Ding, X. Li, G. H. Waller, X. Xiong, M. A. El-Sayed and M. Liu, Chem. Mater., 2015, 27, 6608–6619 CrossRef CAS.
- C. Julien, M. Massot, R. Baddour-Hadjean, S. Franger, S. Bach and J. P. Pereira-Ramos, Solid State Ionics, 2003, 159, 345–356 CrossRef CAS.
- C. M. Julien and M. Massot, Mater. Sci. Eng., B, 2003, 97, 217–230 CrossRef.
- M. Tu, H. Lu, S. Luo, H. Peng, S. Li, Y. Ke, S. Yuan, W. Huang, W. Jie and J. Hao, ACS Appl. Mater. Interfaces, 2020, 12, 24133 CrossRef CAS.
- H. R. Rasouli, J. Kim, N. Mehmood, A. Sheraz, M.-K. Jo, S. Song, K. Kang and T. S. Kasirga, Nano Lett., 2021, 21, 3997–4005 CrossRef CAS.
- M. Y. Chan, K. Komatsu, S.-L. Li, Y. Xu, P. Darmawan, H. Kuramochi, S. Nakaharai, A. Aparecido-Ferreira, K. Watanabe, T. Taniguchi and K. Tsukagoshi, Nanoscale, 2013, 5, 9572–9576 RSC.
- G. Pezzotti and W. Zhu, Phys. Chem. Chem. Phys., 2014, 17, 2608–2627 RSC.
- H. Meskine and S. Satpathy, J. Appl. Phys., 1999, 85, 4346–4348 CrossRef CAS.
- D. Tuschel, Spectroscopy, 2014, 29, 14–22 Search PubMed.
- A. Molina-Sánchez and L. Wirtz, Phys. Rev. B:Condens. Matter Mater. Phys., 2011, 84, 155413 CrossRef.
- H. Zeng, B. Zhu, K. Liu, J. Fan, X. Cui and Q. M. Zhang, Phys. Rev. B:Condens. Matter Mater. Phys., 2012, 86, 241301(R) CrossRef.
- P. H. Tan, W. P. Han, W. J. Zhao, Z. H. Wu, K. Chang, H. Wang, Y. F. Wang, N. Bonini, N. Marzari, N. Pugno, G. Savini, A. Lombardo and A. C. Ferrari, Nat. Mater., 2012, 11, 294–300 CrossRef CAS.
- J.-U. Lee, S. Woo, J. Park, H. C. Park, Y.-W. Son and H. Cheong, Nat. Commun., 2022, 8, 1370 CrossRef.
- D. Tuschel, Spectroscopy, 2012, 27, 2–6 Search PubMed.
- D. Rawat, A. Singh, N. K. Singh and A. Soni, Phys. Rev. B, 2023, 107, 155203 CrossRef CAS.
- Q. Song, X. Pan, H. Wang, K. Zhang, Q. Tan, P. Li, Y. Wan, Y. Wang, X. Xu, M. Lin, X. Wan, F. Song and L. Dai, Sci. Rep., 2016, 6, 29254 CrossRef.
- O. Ilchenko, Y. Pilgun, A. Kutsyk, F. Bachmann, R. Slipets, M. Todeschini, P. O. Okeyo, H. F. Poulsen and A. Boisen, Nat. Commun., 2019, 10, 5555 CrossRef CAS.
- Y. Ding, W. Zheng, M. Jin, Y. Zhu, R. Zhu, Z. Lin and F. Huang, Opt. Lett., 2020, 45, 1313–1316 CrossRef.
- X.-L. Liu, X. Zhang, M.-L. Lin and P.-H. Tan, Chin. Phys. B, 2017, 26, 067802 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06118g |
| This journal is © The Royal Society of Chemistry 2025 |