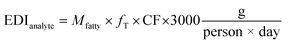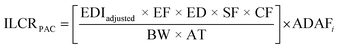Open Access Article
Open Access ArticleEstimating dietary exposure to polycyclic aromatic compounds from food grade plastics†
Kara B.
Loudon‡
*,
Thane M. Z.
Tomy‡
,
Erin C.
Liebzeit
,
Thor
Halldorson
,
Zhe
Xia
 ,
Sara
Sambanthan
,
Duc Luong
Hoang
,
Nipuni
Vitharana
and
Gregg T.
Tomy
,
Sara
Sambanthan
,
Duc Luong
Hoang
,
Nipuni
Vitharana
and
Gregg T.
Tomy
 *
*
Department of Chemistry, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada. E-mail: loudonk@myumanitoba.ca; gregg.tomy@umanitoba.ca
First published on 9th December 2024
Abstract
Plastics are extensively involved in our everyday lives, including use as food storage containers. Greater than 95% of plastics produced are derived from petrochemicals. Numerous studies have shown that chemical additives (e.g., phthalates) can migrate out of food grade plastics into foods. Based on this we hypothesize that petrochemicals used in the manufacturer of plastics also migrate into foods. To test this hypothesis, we simulated chemical migration from petrochemical-based plastics under refrigeration and microwave conditions using the United States Food and Drug Administration testing guidelines. Specifically, we measured the amounts of polycyclic aromatic compounds (PACs) migrating from four plastics used heavily in the food industry namely polypropylene, polyethylene, polycarbonate and polyethylene terephthalate glycol. Our results showed that several alkylated and non-alkylated PACs could be detected in the food simulant used with relatively greater amounts of the alkylated PACs compared to their non-alkylated analogs. Data from our studies were used to estimate daily intake where it was shown that the greatest risk of exposure to humans stems from migration of PACs from PE into foods with total EDIs of 1794.4 ± 163.5 and 169.4 ± 23.5 ng per person per day under refrigeration and microwave conditions, respectively. Finally, an assessment of human health risk resulting from dietary exposure to PACs migrating from the four plastics studied under the two usage scenarios, suggests that at current exposure levels, PACs pose negligible cancer risk to humans.
Environmental significanceThe paper presents results of empirical studies to elucidate compounds that migrate from petrochemical-based food-grade plastics into foods. Our focus was on polycyclic aromatic compounds including polycyclic aromatic hydrocarbons (PAHs), alkylated-PAHs and some heterocyclic-based heterocyclic aromatic compounds. We tested migration from single use of four popular food-grade plastics including polypropylene, polyethylene, polycarbonate and polyethylene terephthalate glycol. To our knowledge, this work represents the first comprehensive study to measure the migration of these compounds from plastics into foods. Further work is warranted to understand if repeated plastic use constitutes a greater risk of exposure to PACs and what these risks mean for human health. |
Introduction
Plastics are high-molecular weight organic polymers composed of various elements like carbon, hydrogen, oxygen, nitrogen, sulphur and chlorine. Most of the plastics in use today are synthetic based (i.e., derived from petrochemicals) with biobased plastics occupying a much smaller market share of the global plastic economy (∼1%).1 With environmental concerns surrounding the fossil-fuel industry, it is interesting that the production of synthetic-based plastics continues to grow. In 2019, production estimates were 460 MT which was more than double the production amounts in 2000 (234 MT).2,3 Furthermore, it is estimated that plastic production will triple from 2019 to 2060 with a predicted growth-rate of 2.5–4.6% per year.4,5There are numerous types of synthetic plastics produced from petrochemicals. Of those used in the packaging industry, polyethylene (PE), polypropylene (PP) and polyethylene terephthalate glycol (PETG) occupy the largest market share with respective contributions of 27%, 19% and 6%.5 While these plastics are used in the construction and transportation industries as well as textiles and consumer products, they are used mostly in the packaging industry (∼36%).5 Their resistance to other chemicals (e.g., oils, acids/bases), malleability, durability and cost-effectiveness to produce are some of the characteristics that make them desirable for use in packaging.
The scientific literature is replete with examples of the deleterious impacts of plastics in the environment (see reviews by Zhang et al. (2021),6 Wayman and Niemann (2021),7 Li et al. (2020)8 references therein). Much of the focus to date has been on micro- and nano-plastics and more recently on the detection of chemical additives that migrate from plastics into the environment.3,4,9 Knowing that synthetic-based plastics are derived from petrochemicals, it is surprising that relatively little is known about the migration of these chemicals, which are likely to be present in residual amounts, from plastics.10–12 Due to many of these plastics being used in the food packaging industry, if petrochemicals can migrate from plastics dietary intake is a likely route of exposure of these chemicals to humans.
Food contact chemicals (FCCs) are chemicals present in food contact articles that are intentionally or non-intentionally added or associated with the manufacture of food contact materials (FCMs). Bisphenol-A (BPA) is an example of an FCC which has received considerable scientific attention.13,14 In Canada BPA is identified in the priority substances list under the Canadian Environmental Protection Act, 1999 and was readily detected in polycarbonate-based plastics used in the food packaging industry especially in infant baby bottles.15 Groh et al. (2021) recently compiled an inventory of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 FCCs and this list is likely to continue to increase as non-targeted analytical testing capabilities increase.16 New research also shows that many per- and polyfluoralkyl substances, plasticizers and antioxidants are measurable in renewable biobased food contact materials.17
285 FCCs and this list is likely to continue to increase as non-targeted analytical testing capabilities increase.16 New research also shows that many per- and polyfluoralkyl substances, plasticizers and antioxidants are measurable in renewable biobased food contact materials.17
The US Food and Drug Administration (US-FDA) and the European Union both have similar protocols for empirically measuring the migration of FCCs from plastic into food.18,19 Because of the analytical challenges of food as a matrix, chemical simulants are used as a surrogate of foods. Depending on the nature of the foods and the chemical properties of the FCC studied different simulants can be employed. The mass transfer of FCC into the simulant is diffusion controlled and, in most cases, thought to obey Fick's law.18
Here we hypothesize that petrochemicals used as raw materials in the manufacturer of synthetic plastics can migrate from the FCM into foods. To test this hypothesis, we used the US-FDA migration testing protocol to guide the design of our experiments.18 We selected four plastic-types used heavily in the food packaging industry, PP, PE, PETG and polycarbonate (PC) for testing. Our analytes of interest were polycyclic aromatic compounds (PACs) and were measured in an ISO-17025 accredited laboratory using high resolution gas chromatography tandem mass spectrometry (HRGC-MS/MS). Our experiments were conducted to simulate migration under refrigerated and microwave conditions which enabled us to estimate the probable daily dietary intake of PACs by humans under these two realistic usage scenarios. The probable daily dietary intake was then used to calculate the potential risk of cancer from exposure to PACs resulting from their mass-transfer from plastics into foods.
Material and methods
Chemicals
All organic solvents used were high purity (Optima grade) and purchased from Fisher Chemicals (Ottawa, Ontario, Canada). Alkylated polycyclic aromatic hydrocarbons (APAHs) were purchased from Accustandard Inc. (New Haven, Cincinnati, USA) and Caledon Laboratory Chemicals (Georgetown, Ontario, Canada). These include thirty-seven (36) individual (APAHs): 1,7-dimethylphenanthrene, 1,8-dimethylphenanthrene, 1-methylnaphthalene, 1-methylphenanthrene, 2,6-dimethylphenanthrene, 2-methylnaphthalene, 2-methylphenanthrene, 3,6-dimethylphenanthrene, 3-methylphenanthrene, 9-methylphenanthrene, 5-methylchrysene, 6-ethylchrysene, 1,4-dimethylnaphthalene, 1,3-dimethylphenanthrene, 6-n-propylchrysene, 2,3,5-trimethylnaphthalene, 1,2,6-trimethylphenanthrene, 6-n-butylchrysene, 1,4,6,7-tetramethylnaphthalene, 1,2,6,9-tetramethylphenanthrene, retene, 6-methylbenzo[a]pyrene, 2-methyldibenzothiophene, 1-methylfluorene, 1-methylpyrene, 7,10-dimethylbenzo[a]pyrene, 1,2-dimethyldibenzothiophene, 9-ethylfluorene, 1-ethylpyrene, 4-n-propyldibenzothiophene, 9-n-propylfluorene, 1-n-propylpyrene, 4,6-diethyldibenzothiophene, 9-n-butylfluorene, 1-n-butylpyrene, dibenzothiophene. Sixteen (16) unsubstituted PAHs (naphthalene, acenaphthylene, anthracene, acenaphthene, fluorene, phenanthrene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-c,d)pyrene, fluoranthene, dibenzo(a,h)anthracene, benzo(g,h,i)perylene) as a native mix and deuterium mass labeled d10-anthracene were purchased from Accustandard Inc., while PAH deuterated analogs were from Cambridge Isotope Laboratories Inc. (Tewksbury, Massachusetts, USA). All standards were >98% purity. The deuterium mass labeled PAHs used as the recovery internal standard (RIS) were d8-naphthalene, d8-acenaphthylene, d10-acenaphthene, d10-fluorene, d10-phenanthrene, d10-pyrene, d12-benzo(a)anthracene, d12-chrysene, d12-benzo(b)fluoranthene, d12-benzo(k)fluoranthene, d12-benzo(a)pyrene, d12-indeno(1,2,3-c,d)pyrene, d10-fluoranthene, d14-dibenzo(a,h)anthracene, d14-benzo(g,h,i)perylene. Labeled anthracene, d10-anthracene, was used as the instrument performance internal standard (IPIS). The size-exclusion S-X3 Biobeads were purchased from Bio-Rad Laboratories (Mississauga, Ontario, Canada). Splendido cold pressed extra virgin olive oil stored in a metal container was purchased from a local grocery in the city. Straight-sided glass jars (30 mL) were purchased from Uline (Milton, Ontario, Canada).Plastic samples
Sheets (121.9 × 121.9 cm) of food grade plastic were purchased from McMaster Carr (Illinois, USA). Polyethylene and PP were both 1.59 mm in thickness while PC and PETG were 3.17 and 1.02 mm thick, respectively. Plastic sheets were first rough cut using a band saw into strips (∼15 cm). Strips were then precision cut on a milling machine so 2 sides were parallel. These machined strips were further rough cut using a band saw into ∼2.54 cm lengths. Squares (2 sides milled) were then loaded into a fixture to mill the saw cut edges to finish size ensuring squareness. The final dimensions of each plastic were 3.10 × 3.10 cm (1.22 × 1.22′′). All sheets came with a protective removable plastic adhesive which was only removed prior to the start of our migration experiments thereby minimizing potential contamination during milling. Finally, plastic surfaces were rinsed with HPLC water and a mild detergent to remove any residual adhesive left after removing the protective coating.Experimental design
Sample processing
After spiking 1 g of each sample with RIS the volume was adjusted to 5 mL using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dichloromethane (DCM) and hexane. Samples were then applied to the head of a gel permeation column (60 g of S-X3 Biobeads, 29.5 mm × i.d. 400 mm) using a J2 AccuPrepMPS Scientific gel permeation chromatographic system (Pennsylvania, USA). The mobile phase consisted of a 1
1 mixture of dichloromethane (DCM) and hexane. Samples were then applied to the head of a gel permeation column (60 g of S-X3 Biobeads, 29.5 mm × i.d. 400 mm) using a J2 AccuPrepMPS Scientific gel permeation chromatographic system (Pennsylvania, USA). The mobile phase consisted of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DCM and hexane and at flow rate of 5 mL min−1 our target analytes eluted from 140 to 360 mL. The collected extracts were reduced to 2 mL using centrifugal roto-evaporation (Genevac, Thermo Scientific) and another cleanup step was performed using a dispersive solid phase adsorption chromatography method developed by Xia et al.20 In brief, extracts were transferred to a 125 mL round bottom flask containing a mixture of silica gel (4 g), sodium sulfate (0.5 g) and 5% deactivated alumina (1 g). Flasks were then allowed to sit for 30 min with periodic swirling every 10 min. The dispersed extracts were transferred into 60 mL tubes through glass wool to remove any dispersant using a 70
1 mixture of DCM and hexane and at flow rate of 5 mL min−1 our target analytes eluted from 140 to 360 mL. The collected extracts were reduced to 2 mL using centrifugal roto-evaporation (Genevac, Thermo Scientific) and another cleanup step was performed using a dispersive solid phase adsorption chromatography method developed by Xia et al.20 In brief, extracts were transferred to a 125 mL round bottom flask containing a mixture of silica gel (4 g), sodium sulfate (0.5 g) and 5% deactivated alumina (1 g). Flasks were then allowed to sit for 30 min with periodic swirling every 10 min. The dispersed extracts were transferred into 60 mL tubes through glass wool to remove any dispersant using a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mixture of DCM
30 mixture of DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane. Extracts were carefully reduced to 200 μL using a gentle stream of UHP nitrogen and transferred to GC vials. Prior to detection and quantitation using HRGC-MS/MS each extract was spiked with 10 ng (5 μL of 2 ng μL−1) of IPIS.
hexane. Extracts were carefully reduced to 200 μL using a gentle stream of UHP nitrogen and transferred to GC vials. Prior to detection and quantitation using HRGC-MS/MS each extract was spiked with 10 ng (5 μL of 2 ng μL−1) of IPIS.
Quality control
Balances and syringes were calibrated daily prior to use. All glass jars were rinsed with water and acetone and baked at 250 °C for 24 hours prior to use. All 1 g samples were fortified with a known amount of RIS to account for any losses incurred during sample processing. Mass labeled d10-anthracene was added to sample extracts prior to HRGC-MS/MS injection to account for any fluctuations in the performance of our system. For the refrigeration migration studies, five procedural blanks per time point consisting of 30 mL glass jars filled with oil were sampled and processed in an identical manner to our samples (n = 30). Similarly, five procedural blanks were used in the microwave migration studies. Glass jars filled with oil were also purposely fortified with 16 native PAHs (20 μL of 15 ng μL−1) and sampled on prescribed time-points to assess the potential for loss of our analytes: (1) to the headspace and (2) from chemical/physical transformations (n = 30). Finally, glass jars with oil (30 mL) and plastic (2 pieces of each type, n = 3 in each case) were purposely fortified with 15 mass labeled PAHs (75 μL of 1200 μg μL−1) and sampled on day 0 and day 10. This allowed us to assess the potential for re-adsorption of PACs onto the surface of the plastics.Detection and quantitation of polycyclic aromatic compounds
An Agilent 7890 GC coupled with a 7000C triple quadrupole mass spectrometer fitted with an electron ionization (EI) source was used for the MS/MS acquisition. An Agilent J&W HP-5ms ultra inert column (30 m × 0.25 mm × 0.25 μm) was used with helium as the carrier gas at a 1.2 mL min−1 constant flow rate. Splitless injections of 1 μL into a glass tapered liner with wool at its base were achieved using a PAL RSI 85 autosampler into our injector port maintained at 300 °C. Bot the GC transfer line and MS ion source were at 300 °C. The details of the oven temperature ramp and MS/MS analysis including multiple reaction monitoring ion transitions and our approach to detection and quantitation of our analytes are described in detailed in Idowu et al.21Results and discussion
Sources of experimental uncertainty
Although our experimental approach subscribed to the US-FDA testing guidelines there are opportunities for analyte losses because of the experimental design. Here we discuss them and our approach to mitigating them.There are three potential sources of loss of analytes. First, analytes can partition from the oil into the headspace. Second, chemical or physical induced transformations of native PACs can occur while in the oil. Third, once PACs migrate from the plastic, they can re-adsorb onto the surface of the plastic. To assess the first two scenarios, oil (30 mL, n = 5) was added to our glass jars which did not contain any plastic and spiked with sixteen (16) native PAHs. The amount of each PAH was determined at each of our sampling time points and the measured amounts of the 16 PAHs recovered on each of the sampling days are presented in Fig. ESI-1.† Our results showed that there was no statistical change (ANOVA, p < 0.01) in the amount of any PAH at any point of our exposure and implies that analytes losses from chemical/physical transformations and losses to the headspace throughout the duration of our experiment are both negligible.
The potential for chemicals that have partitioned into the oil to re-adsorb onto each plastic type was also assessed. The percent recovery of each mass labeled PAHs measured in oil on day 0 and 10 are shown in Table ESI-1.† While there are a few instances in which recoveries for the mass labeled PAHs were statistically different between time points (Student's t-test, p < 0.05), these differences were small and implies that re-adsorption of our analytes onto plastic is not a major loss mechanism.
There are also sources of experimental variability that we made every effort to control. Naturally, there can be analyte variability arising from the analytical protocol. To monitor this, once 1 g of oil was drawn from the glass jar it was purposely spiked with 15 mass labeled internal standards, and then taken through our analytical method. The mass labeled internal standards allowed us to correct for any losses of our target analytes. For our migration studies at 20 °C, the bias of our IS ranged from 64.4 to 98.0% (n = 120) and our uncertainty, expressed as standard deviation, ranged from 13.6 to 26.9 (see Table 1). Similar values were obtained for our IS spiked into our microwave samples (see Table ESI-2).† This implies that while some variability does occur, the magnitude of the variability is certainly within acceptable limits.22
| Compound | pg | Mean + 3 × SDa | Recovery |
|---|---|---|---|
| a If our analytes in samples were smaller than their respective mean +3 × SD amounts then it was considered a non-detect. b Mass labeled anthracene was purposely not added as a recovery internal standard because it was used as an instrument performance standard. | |||
| Acenaphthene | 10.9 ± 1.5 | 15.5 | 80.0 ± 17.3 |
| Acenaphthylene | 23.9 ± 3.8 | 35.3 | 64.4 ± 13.6 |
| Anthracene | 9.1 ± 2.1 | 15.4 | —b |
| Benz[a]anthracene | 2.8 ± 0.5 | 4.2 | 89.2 ± 19.1 |
| Benzo[a]pyrene | 0.9 ± 0.2 | 1.5 | 79.6 ± 20.6 |
| Benzo[b]fluoranthene | 1.8 ± 0.5 | 3.3 | 87.3 ± 20.4 |
| Benzo[g,h,i]perylene | 1.0 ± 0.2 | 1.6 | 84.5 ± 22.2 |
| Benzo[k]fluoranthene | 1.3 ± 0.2 | 2.0 | 86.2 ± 20.3 |
| Chrysene | 13.1 ± 2.2 | 19.5 | 87.7 ± 19.0 |
| Dibenzo[a,h]anthracene | 0.2 ± 0.1 | 0.4 | 97.4 ± 26.2 |
| Fluoranthene | 37.0 ± 9.1 | 64.2 | 98.0 ± 18.9 |
| Fluorene | 23.7 ± 4.7 | 37.9 | 89.2 ± 18.6 |
| Indeno[1,2,3-c,d]pyrene | 0.7 ± 0.2 | 1.2 | 93.6 ± 26.9 |
| Naphthalene | 110.2 ± 15.5 | 156.6 | 66.9 ± 16.7 |
| Phenanthrene | 160.7 ± 38.3 | 275.8 | 94.6 ± 18.8 |
| Pyrene | 34.3 ± 8.4 | 59.5 | 96.8 ± 18.0 |
There can also be experimental variability because of small fluctuations in the instrument performance between injections. To monitor for this, mass labeled anthracene was added to extracts prior to injecting on our GC-MS/MS. The variability of our IS was less than 10% (n = 150). Indeed, while this variability contributes to our overall experimental uncertainty this can be assumed to be small.
The final source of variability can arise from the non-homogenous distribution of our analytes on the plastic itself. The plastics used in our studies were cut from a sheet of plastic and it is unreasonable to assume that there is uniform distribution of PACs in each plastic used in our experiment. Unfortunately, there was no way for us to account for this variability. This likely resulted in variable frequencies of detection (fD) in some analyte replicate measurements and high relative standard deviations.
Treatment of procedural blanks
Blanks were used for both migration studies. In total, there were 30 and 5 blanks for our refrigeration and microwave migration studies, respectively. Since there were no statistical differences (ANOVA, p < 0.05) in the amounts of our analytes in procedural blanks collected at any time-point of our refrigeration studies we decided to combine them. Similarly, there were no statistical differences in analytes amounts in the 5 blanks used in our microwave studies. Analytes in our samples were blank corrected using their respective mean blank value from our procedural blank. It was important to blank correct our samples as some PACs were routinely detected in the oil. Table 1, ESI-2 and 3† shows the average amounts of PACs (pg) and their measurement uncertainty in both types of our procedural blanks along with respective method detection limits (MDLs). Method detection limits for our refrigeration and microwave studies ranged from 2.2 pg (1,8-dimethylphenanthrene) to 198.3 pg (C2-dibenzothiophene) and 4.0 pg (1,8-dimethylphenanthrene) to 165.3 pg (C2-dibenzothiophene), respectively. It is worth acknowledging that there was good agreement between the blank values of our analytes irrespective of the migration study. Positive detection of our analytes in samples was assigned if the blank corrected value of analytes in our samples exceeded the mean plus 3 standard deviations of each PAC in the procedural blank.Detection of analytes in oil
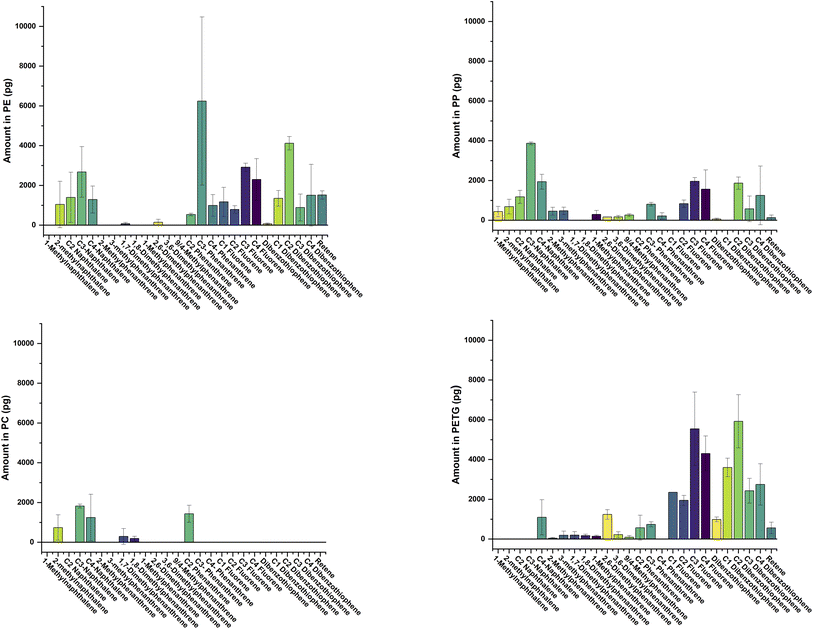
Experimental results for studies under refrigeration are presented in Tables 2–5 while results under microwave conditions are presented in Fig. 1, 2 and Table ESI-4.† For our refrigeration studies, there is more than one instance where a compound is detectable in oil at one time-point and is then undetectable at a subsequent time-point. For example, C3-naphthalene is measurable in oil incubated with PP at day 4 (758.8 ± 125.2 pg), undetectable at days 6 and 8, and detectable again at day 10 (801.7 ± 458.8 pg). Because of the stringent quality controls and procedures taken to reduce or account for both systematic errors and experimental uncertainty, it is felt that this phenomenon is not an analytical artifact but rather related to the nature of PAC distribution in the plastic. This is further evidenced by the variable fD as stated earlier. Furthermore, because the pieces of plastic used in our incubation experiment were randomly chosen, it is very possible that instances arose whereby the plastic pieces selected contained negligible amounts of PACs. This claim is supported by the work of Kuzmicz and Ciemniak (2017) who noted that PAHs in plastic packaging materials can vary between manufacturers and between production batches from the same manufacturer.23 Naturally, there was no way to control for this. If PACs were evenly distributed throughout the plastic used, we would expect the amounts of PACs to increase continuously with time according to Fisk's law of diffusion until reaching equilibrium. Yet this clearly was not observed. However, by imposing the stringent analyte detection criteria described earlier we feel confident about the claims made throughout the paper.| Compound | Time (days) | |||||
|---|---|---|---|---|---|---|
| 0.083 | 1 | 4 | 6 | 8 | 10 | |
| a Total surface area of plastic used in each incubation was 19.36 mm2 (2 × 9.68 mm2). b Arithmetic mean and standard deviation (shown in brackets). c Median and median absolute error (shown in brackets). d The frequency of detection. | ||||||
| C3-Naphthalene | n.d. | n.d. | 994.8(438.2)b | n.d. | n.d. | n.d. |
| 912.6(309.0)c | ||||||
| 80d | ||||||
| C4-Naphthalene | n.d. | 4874.4(1902.1) | 10126.6(808.6) | n.d. | n.d. | n.d. |
| 4634.9(1685.2) | 10255.8(589.5) | |||||
| 100 | 100 | |||||
| 3-Methyl-phenanthrene | n.d. | n.d. | n.d. | 410.2(432.5) | n.d. | n.d. |
| 410.2(305.8) | ||||||
| 40 | ||||||
| C4-Pheanthrene | n.d. | n.d. | 452.6(176.2) | n.d. | n.d. | n.d. |
| 452.6(124.6) | ||||||
| 40 | ||||||
| C2-Fluorene | n.d. | n.d. | 3284.3(4194.4) | n.d. | n.d. | n.d. |
| 744.6(534.1) | ||||||
| 100 | ||||||
| C3-Fluorene | n.d. | n.d. | 5592.4(6261.4) | n.d. | n.d. | n.d. |
| 3398.0(2674.6) | ||||||
| 80 | ||||||
| C4-Fluorene | n.d. | 1992.0(1307.6) | 5899.0(4713.5) | n.d. | n.d. | n.d. |
| 2596.0(845.9) | 5529.3(3781.7) | |||||
| 100 | 100 | |||||
| C3-Chrysene | n.d. | n.d. | n.d. | n.d. | 675.0(1067.7) | n.d. |
| 103.5(88.9) | ||||||
| 60 | ||||||
| C4-Chrysene | n.d. | n.d. | n.d. | n.d. | 958.5(1520.6) | n.d. |
| 94.4(27.6) | ||||||
| 60 | ||||||
| C3-Pyrene | n.d. | 1924.0(1320.0) | 1580.0(2100.4) | n.d. | n.d. | n.d. |
| 2131.0(997.2) | 707.7(363.7) | |||||
| 60 | 80 | |||||
| C4-Pyrene | n.d. | 328.5(52.7) | 2071.6(1788.0) | n.d. | n.d. | n.d. |
| 328.5(37.2) | 1521.5(655.2) | |||||
| 40 | 100 | |||||
| C2-Dibenzothiophene | n.d. | n.d. | 4675.4(1141.8) | n.d. | n.d. | n.d. |
| 4711.2(1041.6) | ||||||
| 100 | ||||||
| C4-Dibenzothiophene | n.d. | 575.0(180.6) | 1128.2(528.4) | n.d. | n.d. | n.d. |
| 590.3(157.1) | 1281.2(3781.7) | |||||
| 60 | 100 | |||||
| Compound | Time (days) | |||||
|---|---|---|---|---|---|---|
| 0.083 | 1 | 4 | 6 | 8 | 10 | |
| a Total surface area of plastic used in each incubation was 19.36 mm2 (2 × 9.68 mm2). b Arithmetic mean and standard deviation (shown in brackets). c Median and median absolute error (shown in brackets). d The frequency of detection. | ||||||
| C3-Naphthalene | n.d. | n.d. | 758.8(125.2)b | n.d. | n.d. | 801.7(458.8) |
| 758.8(88.6)c | 801.7(324.4) | |||||
| 40d | 40 | |||||
| C4-Naphthalene | n.d. | n.d. | 9532.4(2192.2) | 270.9(309.5) | 5481.2(1213.1) | 7270.5(1923.7) |
| 9532.2(1550.1) | 270.9(218.8) | 5591.2(462.9) | 6611.7(608.9) | |||
| 40 | 40 | 100 | 80 | |||
| 2,6-Dimethyl-phenanthrene | n.d. | n.d. | 266.7(90.6) | n.d. | n.d. | n.d. |
| 222.4(14.9) | ||||||
| 100 | ||||||
| C4-Phenanthrene | n.d. | n.d. | 941.8(980.3) | n.d. | n.d. | 1846.6(1723.0) |
| 941.8(693.2) | 1846.6(1218) | |||||
| 40 | 40 | |||||
| C2-Fluorene | n.d. | n.d. | 3695.3(3785.3) | n.d. | n.d. | n.d. |
| 3695.3(2676.6) | ||||||
| 40 | ||||||
| C3-Fluorene | n.d. | n.d. | n.d. | n.d. | n.d. | 2674.3(3842.8) |
| 661.3(404.7) | ||||||
| 60 | ||||||
| C4-Fluorene | n.d. | n.d. | 6308.8(5566.9) | n.d. | 2607.5(1569.7) | 4032.3(2135.8) |
| 6308.8(3936.4) | 2475.2(935.9) | 4904.7(296.1) | ||||
| 40 | 100 | 80 | ||||
| C2-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | 486.9(660.1) |
| 486.9(466.8) | ||||||
| 40 | ||||||
| C3-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | 77.8(47.9) |
| 77.8(33.8) | ||||||
| 40 | ||||||
| C4-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C2-Pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 215.6(157.7) |
| 215.6(111.5) | ||||||
| 40 | ||||||
| C4-Pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 619.9(624.1) |
| 619.9(441.3) | ||||||
| 40 | ||||||
| C1-Benzo(a)pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 118.2(15.3) |
| 118.2(10.8) | ||||||
| 40 | ||||||
| C2-Benzo(a)pyrene | n.d. | n.d. | 8224.5(8928.4) | n.d. | n.d. | 1125.8(331.8) |
| 8224.5(6313/3) | 1125.8(234.6) | |||||
| 40 | 40 | |||||
| Retene | n.d. | n.d. | 438.0(316.4) | n.d. | n.d. | 431.0(120.5) |
| 438.0(223.7) | 431.0(85.2) | |||||
| 40 | 40 | |||||
| C2-Dibenzothiophene | n.d. | n.d. | 10874.5(7040.9) | n.d. | 10612.9(4589.4) | n.d. |
| 10874.5(4979.7) | 10612.9(3245.2) | |||||
| 40 | 40 | |||||
| C4-Dibenzothiophene | n.d. | n.d. | 3464.3(3764.1) | n.d. | n.d. | 260.6(13.4) |
| 3464.3(2661.6) | 253.4(1.1) | |||||
| 40 | 60 | |||||
| Compound | Time (days) | |||||
|---|---|---|---|---|---|---|
| 0.083 | 1 | 4 | 6 | 8 | 10 | |
| a Total surface area of plastic used in each incubation was 19.36 mm2 (2 × 9.68 mm2). b Arithmetic mean and standard deviation (shown in brackets). c Median and median absolute error (shown in brackets). d The frequency of detection. | ||||||
| C3-Naphthalene | n.d. | n.d. | 618.1(78.2)b | n.d. | n.d. | 445.9(391.9) |
| 597.0(42.7)c | 445.9(277.2) | |||||
| 60d | 60 | |||||
| C4-Naphthalene | n.d. | 5540.4(1226.0) | 11338.1(1558.4) | n.d. | n.d. | 7949.8(1965.9) |
| 5499.2(935.4) | 12174.5(472.1) | 8574.7(1166.8) | ||||
| 100 | 100 | 100 | ||||
| 3-Methyl-phenanthrene | n.d. | n.d. | n.d. | n.d. | n.d. | 166.1(187.3) |
| 166.1(132.4) | ||||||
| 40 | ||||||
| 2,6-Dimethyl-phenanthrene | n.d. | n.d. | n.d. | — | — | — |
| C4-Phenanthrene | n.d. | n.d. | 341.3(253.6) | n.d. | 5979.8(1543.9) | n.d. |
| 293.8(158.7) | 6317.9(1010.9) | |||||
| 80 | 100 | |||||
| C2-Fluorene | n.d. | n.d. | 7382.6(11841.0) | n.d. | n.d. | 1458.0(2123.2) |
| 2203.8(143.4) | 596.0(363.3) | |||||
| 100 | 80 | |||||
| C3-Fluorene | n.d. | n.d. | 15364.2(20501.9) | n.d. | n.d. | 6137.5(3698.8) |
| 15364.2(14497.1) | 4322.2(497.6) | |||||
| 40 | 60 | |||||
| C4-Fluorene | n.d. | 5333.9(1129.5) | 6456.8(7742.2) | n.d. | 2227.1(1514.9) | 3830.5(2496.3) |
| 5178.2(672.6) | 3633.6(1227.3) | 2002.5(1165.6) | 3184.9(1078.9) | |||
| 100 | 100 | 60 | 80 | |||
| C2-Chrysene | n.d. | n.d. | Nd | n.d. | n.d. | 1219.8(487.3) |
| 1075.8(254.9) | ||||||
| 60 | ||||||
| C3-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | 211.4(69.0) |
| 227.9(42.7) | ||||||
| 60 | ||||||
| C4-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C3-Pyrene | n.d. | 3821.0(1023.8) | 7844.9(9876.5) | n.d. | n.d. | 563.2(703.7) |
| 3467.1(318.2) | 7844.9(6983.8) | 563.2(497.6) | ||||
| 80 | 40 | 40 | ||||
| C4-Pyrene | n.d. | 2421.1(1467.9) | 6394.0(8656.6) | n.d. | n.d. | 877.4(373.9) |
| 2422.4(1188.8) | 2304.7(1765.0) | 866.8(373.8) | ||||
| 80 | 60 | 60 | ||||
| C2-Benzo(a)pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 418.0(360.5) |
| 126.9(63.7) | ||||||
| 80 | ||||||
| C2-Dibenzothiophene | n.d. | n.d. | 4576.3(5473.4) | n.d. | 6030.0(627.6) | 8382.8(2645.5) |
| 2366.3(1673.3) | 5882.9(393.9) | 7666.8(1497.7) | ||||
| 100 | 60 | 80 | ||||
| C4-Dibenzothiophene | n.d. | n.d. | 1584.9(1874.5) | n.d. | n.d. | n.d. |
| 816.3(542.3) | ||||||
| 100 | ||||||
| Compound | Time (days) | |||||
|---|---|---|---|---|---|---|
| 0.083 | 1 | 4 | 6 | 8 | 10 | |
| a Total surface area of plastic used in each incubation was 19.36 mm2 (2 × 9.68 mm2). b Arithmetic mean and standard deviation (shown in brackets). c Median and median absolute error (shown in brackets). d The frequency of detection. | ||||||
| C3-Naphthalene | n.d. | n.d. | n.d. | n.d. | n.d. | 1005.7(39.6) |
| 1005.7(28.0) | ||||||
| 40 | ||||||
| C4-Naphthalene | n.d. | 6112.1(820.5)b | n.d. | n.d. | n.d. | 7038.7(1713.8) |
| 6081.2(773.8)c | 7038.7(1211.8) | |||||
| 80d | 40 | |||||
| C4-Phenanthrene | n.d. | n.d. | n.d. | n.d. | n.d. | 643.4(41.9) |
| 643.4(29.6) | ||||||
| 40 | ||||||
| C2-Fluorene | n.d. | 511.3(549.6) | n.d. | n.d. | n.d. | 843.6(1.7) |
| 284.0(172.2) | 843.7(1.2) | |||||
| 60 | 40 | |||||
| C3-Fluorene | n.d. | n.d. | n.d. | n.d. | n.d. | 3107.3(367.0) |
| 3107.3(259.5) | ||||||
| 40 | ||||||
| C4-Fluorene | n.d. | 2879.2(1511.6) | n.d. | n.d. | n.d. | 1863.2(956.3) |
| 3034.5(1272.7) | 1863.2(676.2) | |||||
| 40 | 40 | |||||
| C3-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | 241.5(142.0) |
| 241.5(100.4) | ||||||
| 40 | ||||||
| C4-Chrysene | n.d. | n.d. | n.d. | n.d. | n.d. | 204.3(148.6) |
| 204.3(105.0) | ||||||
| 40 | ||||||
| C1-Pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 388.0(254.6) |
| 388.0(180.0) | ||||||
| 40 | ||||||
| C2-Pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 441.2(588.6) |
| 441.2(416.2) | ||||||
| 40 | ||||||
| C2-Benzo(a)pyrene | n.d. | n.d. | n.d. | n.d. | n.d. | 2428.9(47.3) |
| 2428.9(33.4) | ||||||
| 40 | ||||||
| C2-Dibenzothiophene | n.d. | n.d. | n.d. | n.d. | n.d. | 11107.7(2357.5) |
| 11107.7(1667.0) | ||||||
| 40 | ||||||
| C4-Dibenzothiophene | n.d. | 588.9(164.5) | n.d. | n.d. | n.d. | n.d. |
| 588.9(166.3) | ||||||
| 40 | ||||||
Polycyclic aromatic hydrocarbons in oil
It was not until day 10 that we were able to detect PAHs in our PETG, PC and PE samples incubated at 20 °C. Polycyclic aromatic hydrocarbons were undetectable in the PP type plastic. In PE samples, acenaphthylene was detected in all five samples with a range from 895.4 to 1763.1 pg and arithmetic mean ± standard deviation of 1278.0 ± 437.1 pg. Anthracene was also detected in all 5 PE samples, with a value of 334.6 ± 150.3 pg. In PETG, acenaphthylene was detected in 2 of the 5 samples at amounts of 465.3 and 514.8 pg. Finally, both acenaphthylene and anthracene were detected in PC at amounts of 537.0 ± 100.9 and 87.3 ± 41.5 pg, respectively (fD = 100% in both instances).Under microwave incubation conditions, PAHs were readily detectable in PETGs and PC (see Table ESI-4).† For example, there were 8 PAHs detected in oil containing PETG with amounts ranging 82.1 ± 63.0 pg for dibenzo[a,h]anthracene (fD = 100%) to 5183.1 ± 806.2 pg for naphthalene (fD = 75%). Nine PAHs were detectable in oil containing PC with the total (Σ) amount of PAH measured in oil of 3129.4 ± 1228.4 pg. Only acenaphthene was detected in oil containing PE (58.0 ± 67.3 pg, fD = 75%) and PAHs in oil incubated with PP were all below our MDLs.
In general, there were more alkylated PACs than PAHs detected in our samples and the amounts of many alkylated PACs were significantly greater than PAHs. For ease of reading, we will discuss the amounts of alkylated PACs in each of the plastic types separately.
Alkylated PACs in oil incubated with PE
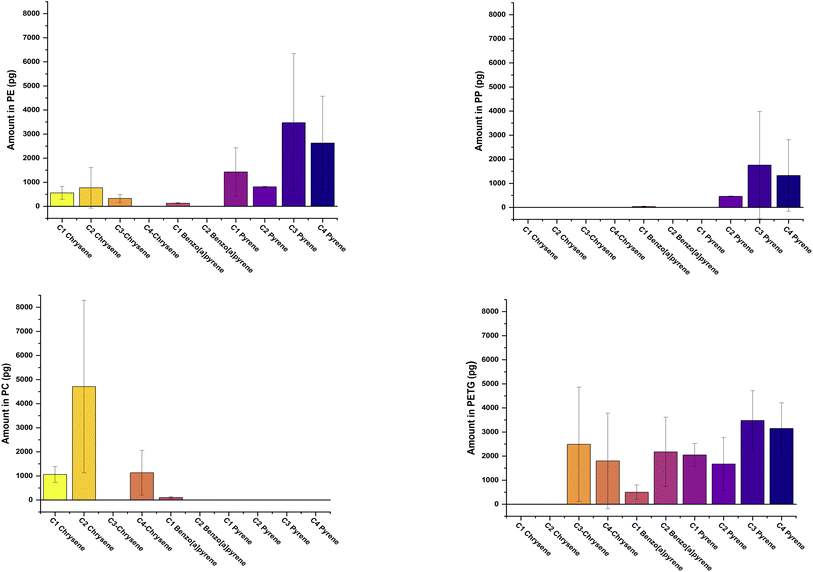
All alkylated PACs that we investigated were undetected in oil at 2 h and day 10 of our refrigeration migration experiment (see Table 2). Five alkylated PACs were detected in oil at day 1 with amounts ranging from 328.5 ± 52.7 for C4-pyrene (fD = 40%) to 4874.4 ± 1902.1 pg for C4-naphthalene (fD = 100%). Similar amounts of C3-pyrene (1924.0 ± 1320.0 pg, fD = 60%) and C4-fluorene (1992.9 ± 1307.6 pg, fD = 100) were measured in oil both of which were ca. 3.5 times greater than that of C4-dibenzothiophene (575.0 ± 180.6 pg, fD = 60%). The greatest amount of alkylated PACs migrating from PE into oil were measured on day 4 of the exposure. The Σ mass of alkylated PACs measured on day 4 in oil was ca. 36.2 ng with C4-naphthalene accounting for almost 30% of the total. Six compounds were detected in all our replicate samples while three compounds were detected in 4 of our 5 replicates and one compound detected in 40% of our replicates. 3-Methylphenanthrene was detected in oil only on day 6 in 40% of our replicate samples while on day 8, both C3 and C4-chrysenes were detected in oil at relative amounts of 675.0 ± 1067.7 pg (fD = 60%) and 958.5 ± 1520.6 (fD = 60%) pg, respectively.The Σ mass of alkylated PACs measured in oil incubated at 120 °C and the distribution patterns of our analytes are shown in Fig. 1 and 2. For visual clarity, PACs are divided into low- (2–3 rings) and high- (4–5 rings) molecular weight compounds (LMW and HMW). The Σ mass of LMW-PACs (30.1 ± 5.3 ng) were ca. 3× greater than ΣHMW-PACs (10.1 ± 3.7 ng) with C3-phenanthrene accounting for ∼20% of total. For the HMW-PACs, C3- and C4-pyrene account for 34 and 26% of the Σ mass of HMW-PACs measured in oil.
Alkylated PACs in oil incubated with PP
None of the alkylated PACs investigated were measurable in oil at 2 h and 1 day of the refrigeration migration experiment (see Table 3). Most of the alkylated PACs were detected in oil on days 4 and 10. The rank order of the mass of alkylated PACs measured in oil at day 4 was C2-dibenzothiophene ≅ C4-naphthalene > C2-benzo(a)pyrene > C4-fluorene > C2-fluorene ≅ C4-dibenzothiophene > C4-phenanthrene > C3-naphthalene > retene > 2,6-dimethylphenanthrene. All the compounds except 2,6-dimethylphenanthrene (fD = 100%) measured in oil on day 4 had a fD of 40%. On day 6, only C4-naphthalene (fD = 40%) was detected in oil while at day 8 amounts of C2-dibenzothiophene (10612.9 ± 4589.4 pg, fD = 40%) were significantly greater than both C4-fluorene (2607.6 ± 1569.7 pg, fD = 100%) and C4-naphthalene (5481.2 ± 1213.1 pg, fD = 100%).Under elevated heating conditions, the Σ mass of the 21 LWM-PACs detected (19.2 ± 2.1 ng) was ca. 5× greater than Σ mass of the 4 HWM-PACs (3.6 ± 2.7 ng) measured in oil (see Fig. 1 and 2). For the LMW-PACs, C3-naphthalene accounted for 20% of the Σ mass. The rank order of HMW-PACs in oil was C3-pyrene > C4-pyrene > C2-pyrene > C1-benzo[a]pyrene.
Alkylated PACs in oil incubated with PETG
Like the other plastic types, none of the alkylated PACs were detectable in oil at our first sampling time-point (see Table 4). However, at day 1, significant amounts of C4-fluorene (5333.9 ± 1129.5 pg, fD = 100%), C4-naphthalene (5540.4 ± 1226.0 pg, fD = 100%) and C3/C4-pyrene (3821.0 ± 1023.8 and 2421.1 ± 1467.9 pg), respectively could be measured in oil with high fD (80%). Day 4 showed maximum amounts of alkylated PACs with amounts ranging from 341.2 ± 253.5 pg (C4-phenanthrene, fD = 80%) to 15364.2 ± 20501.9 pg (C3-fluorene, fD = 40%) and Σ mass of alkylated PACs of ∼62 ng. Because of the large variability and the low fD the amounts of C3-fluorene should be taken with some caution. Conversely, there was a small relative standard deviation (14%), high fD (100%) and significant amounts of C4-naphthalene (11338.1 ± 1558.4 pg) in samples at day 4. None of the alkylated PACs investigated were measurable in oil at day 6 and only 3 alkylated PACs could be measured in oil on day 8. Amounts and number of alkylated PACs measurable on day 10 increased relative to days 6 and 8. C4-Naphthalene and C2-dibenzothiophene were the dominant alkylated PACs measured in oil on day 10 with respective amounts of 7949.8 ± 1965.9 (fD = 100%) and 8382.8 ± 2645.5 pg (fD = 80%).There were more alkylated PACs detected in oil incubated at 120 °C with PETG than for any of the other plastics. The Σ mass of LWM-PACs (33.1 ± 29.7 ng) detected in oil was ca. 2× greater than Σ HWM-PACs (17.3 ± 4.0 ng) and the fD for all LMW-PACs were 75% or greater. Except for C3-chrysene, the fD for all the other HMW-PAC detected were 100% and only C1-pyrene had a relative standard deviation of less than 30% in the replicates.
Alkylated PACs in oil incubated with PC
Four and twelve of the investigated alkylated PACs were measurable in oil on days 1 and 10, respectively (see Table 5). All other days contained undetectable amounts of alkylated PACs. Only C4-naphthalene measured in oil on day 1 (6112.1 ± 820.5 pg) had a fD > than 60%. Amounts of C2-fluorene in oil on day 1 (511.3 ± 549.6 pg, fD = 60%) were ∼1.6× smaller than day 10 (843.6 ± 1.7 pg, fD = 40). Conversely, the amounts of C4-fluorene measured in oil on day 10 (1863.2 ± 956.3, fD = 40%) were ∼1.5× smaller than that measured on day 1 (2879.2 ± 1511.6 pg, fD = 40%). The total alkylated PACs measured in oil on day 10 was ∼29 ng with C2-dibenzothiophene and C4-naphthalene accounting for 38 and 24% of the total, respectively.Compared to the other plastics, oil incubated with PC at elevated temperature contained the smallest amounts of LMW- and HMW-PACs. There were 6 LMW-PACs that contributed to the Σ mass of LWM-PACs (5.7 ± 1.5 ng) with 4 compounds detected at a fD of 100% and the other 2 at a fD of 50%. Only C3-naphthalene and C2-phenanthrene had relative standard deviations of less than 30% in replicate measurements. All the HMW-PACs (Σ 7.0 ± 2.7 ng) were detected at a fD of 75% or less and the relative standard deviation all exceeded 30%.
Reported migration studies on PACs
There are a few studies that have examined the migration of PACs from plastics. Ciemniak and Kuzmicz (2021) studied PAH absorption and desorption of PAHs from LDPE, HDPE and PETG and showed that PAHs can migrate from fortified edible oil to plastics24. It was shown that migration of PAHs from oil to LDPE was more effective than any other plastic type and that most absorption occurred within 24 h. Others have shown similar mass-transfer of PAHs from media onto plastic.23,25–28 Finally, desorption of PAHs from plastics containing PAHs into non-contaminated oil was also shown to readily occur.24 Earlier work of Simko et al. (1995) also showed effective mass-transfer of PAHs from LDPE plastic packages into oil.29These previously reported migration studies of PAHs from plastic into oil corroborates the results of our study. Without knowledge of accurate amounts of PACs embedded in the plastics used in our study, it is difficult to speculate which plastic type led to the greatest mass-transfer into oil. Conversely, unlike other studies, our control study indicated that at 20 °C negligible migration of PAHs from oil to plastic occurs. Temperature is a known driver for diffusion, and it is possible that limited migration of PAHs from oil to plastic occurred because of the temperature used in our incubations.
Estimated daily intake
Two criteria were chosen for our estimated daily intake (EDI) calculations. The % relative standard deviation of our analyte must be less than 30% in our replicate measurements and, the fD of that analyte had to be 75% or greater. These criteria will result in more conservative EDI values, but we felt that these criteria were necessary as they are intuitively satisfying. It should be acknowledged that EDI are based on the highest level of migration to food as stated by the US-FDA.18Some of the important criteria for estimating migration of food contact substances from plastics as described by the US-FDA are (1) the simulant volume to exposed surface area must be at a ratio of 10 mL per square inch, (2) sample thickness should be stated so as to determine if migration is one- or two-sided, (3) for refrigerated applications a test temperature should be 20 °C and an incubation time of 10 days and (4) for microwave studies a testing temperature of 120 °C for 2 hours must be used.18 Our experimental protocols strictly adhered to the US-FDA testing protocol with the addition of sampling at prescribed time-points for the refrigeration studies to determine kinetics of migration of analytes.18 Because of the slow rate of migration and lack of detectability of many analytes, kinetic studies were not feasible.
Assuming that our plastics are intended for single-use, the approach to calculating an EDI uses two constants. The first is the consumption factor (CF) which describes the fraction of the daily diet expected to contact specific packaging materials.18 The nature of food drives the partitioning of chemicals out of the plastic, therefore, the food-type distribution factor (fT) accounts for the different nature of foods.18 The overall EDI can then be estimated using the equation below:
As an example using PE, we can calculate the EDI for acenaphthene under refrigeration conditions. For PE, the maximum amount of acenaphthene we measured was 1278.1 pg (in 1 g of oil simulant). The US-FDA ascribed values for fT and CF are 0.31 and 0.12, respectively. Using these values the maximum EDI can be calculated as follows:
In a similar manner we can calculate maximum EDI's for the other PACs that fulfill the criteria noted above. Tables 6 and 7 shows the EDI of PACs investigated in this study under refrigeration and microwave migration conditions, respectively. Under refrigeration conditions, the greatest exposure to PACs stems from PE with a ΣEDI of 1794.4 ± 163.5 ng of PACs per person per day with C4-naphthalene accounting for 63% of the total. C4-Naphthalene also accounted for all the daily exposure to PACs in PP with an EDI value of 270.5 ± 71.5 ng per person per day. Four alkylated PACs made up the ΣEDI for PETG with C4-naphthalene, C4-phenanthrene, C4-fluorene and C4-pyrene accounting for 43, 22, 20 and 14% of the total (127.0 ± 12.8 ng per person per day). Respective EDI values for acenaphthylene and C4-naphthalene migrating from PC were 0.8 ± 0.1 and 9.2 ± 1.2 ng per person per day.
| Compound | PE | PC | PETG | PP |
|---|---|---|---|---|
| a C F and fT used were taken from the US-EPA Guidance Document for Migration Testing.16 Respective CF for PE, PC, PETG and PP were 0.12, 0.05, 0.16 and 0.04, and fT for PE, PC, PETG and PP were 0.31, 0.01, 0.01 and 0.31, respectively. | ||||
| Acenaphthene | 142.6 ± 48.8 | |||
| Acenaphthylene | 0.8 ± 0.1 | |||
| C4-Naphthalene | 1130.1 ± 90.2 | 9.2 ± 1.2 | 54.4 ± 7.5 | 270.5 ± 71.5 |
| C4-Phenanthrene | 28.7 ± 7.4 | |||
| C4-Fluorene | 25.6 ± 5.4 | |||
| C4-Pyrene | 18.3 ± 4.9 | |||
| C2-Dibenzothiophene | 521.7 ± 127.4 | |||
| Total | 1794.4 ± 163.5 | 10.0 ± 1.2 | 127.0 ± 12.8 | 270.5 ± 71.5 |
| Compound | PE | PC | PETG | PP |
|---|---|---|---|---|
| a C F and fT used were taken from the US-EPA Guidance Document for Migration Testing.16 Respective CF for PE, PC, PETG and PP were 0.12, 0.05, 0.16 and 0.04, and fT for PE, PC, PETG and PP were 0.31, 0.01, 0.01 and 0.31, respectively. | ||||
| Naphthalene | 24.9 ± 3.9 | |||
| Phenanthrene | 23.8 ± 3.6 | |||
| Fluorene | 3.3 ± 0.1 | |||
| Dibenzoanthracene | 4.7 ± 0.6 | |||
| Dibenzothiophene | 4.7 ± 0.6 | |||
| 2,6-Dimethylphenanthrene | 5.9 ± 1.2 | |||
| C1-Pyrene | 9.8 ± 2.2 | |||
| C2-Fluorene | 9.3 ± 1.2 | |||
| C2-Dibenzothiophene | 28.4 ± 6.4 | |||
| C4-Fluorene | 20.7 ± 4.2 | |||
| C2-Phenanthrene | 2.1 ± 0.6 | |||
| Retene | 169.4 ± 23.5 | |||
| Total | 169.4 ± 23.5 | 2.1 ± 0.6 | 135.6 ± 9.8 | |
Under microwave conditions and based on our imposed criteria, 10 PACs were found to contribute to our EDI calculations for PETG while only one compound contributed to the EDI for PC and PE. No compound measured in oil incubated with PP was found to fulfill our criteria. Total EDI from PETG was 135.6 ± 24.0 ng per person per day which is similar to that observed under refrigeration conditions. The major contributors to the ΣEDI for PETG were from naphthalene (18%), phenanthrene (17%), C2-dibenzothiophene (21%) and C4-fluorene (15%). Like our observations for PE, the large ΣEDI for retene migrating from PE is likely driven, in part, by the relatively high CF (0.12) and fT (0.31) constant values.
Exposure risk assessment
The mean incremental lifetime cancer risk (ILCR) of exposure to PACs migrating from plastic into foods were estimated using the maximum EDI values in Tables 6 and 7 along with their respective toxic equivalence factors (TEFs). Toxic equivalence factors express the relative toxicity of a PAC to that of benzo[a]pyrene.30 For example, the TEF of phenanthrene is 0.001 relative to that of benzo[a]pyrene. To estimate the overall carcinogenic potency of PACs, daily exposure values are first multiplied by their respective TEF value. Where literature TEF values for an individual PAC were unavailable, Samburova et al. (2017) suggests substituting reported TEFs values for compounds similar in molecular structure i.e., isomeric in nature or containing the same number of rings.31 This approach was adopted in our ILCR estimations. For example, the reported TEF value for phenanthrene is 0.001 which was also used to estimate the ILCR for 2,6-dimethylphenanthrene.31There are additional elements required to estimate the dimensionless ILCR's for a given PAC and the overall expression developed by the US-EPA is given by:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 days) and ADAFi is the age dependent adjustment factor for the ith age group (10 for ages 0 and 1, 3 for ages 2–15 and 1 for ages 16 and greater).32–36 For simplicity, we calculated ILCR's for adults only i.e., ADAFi = 1.
500 days) and ADAFi is the age dependent adjustment factor for the ith age group (10 for ages 0 and 1, 3 for ages 2–15 and 1 for ages 16 and greater).32–36 For simplicity, we calculated ILCR's for adults only i.e., ADAFi = 1.
Calculated ILCR values for adults of individual PACs and the combined ILCR are provided in Table ESI-5.† The US-EPA places ILCR values into 3 cancer risk categories: ILCR > 10−4 high potential health risk, 10−6 to 10−4 potential health risk and <10−6 insignificant or negligible health risk.36 Unsurprisingly, with a TEF 3 orders of magnitude greater than any other PAC, dietary exposure to dibenzoanthracene by migration from PETG under microwave conditions had the greatest ILCR value (2.9 × 10−7). Nevertheless, this value is still an order of magnitude smaller than the range which would place dibenzoanthracene in the category of being a potential health risk. Overall, and based on our calculated values shown in Table ESI-5,† it can be concluded that human exposure to these compounds (either individually or summed) stemming from the mass-transfer of PACs from plastics into foods poses negligible cancer risks to humans.
It was acknowledged earlier that PACs distribution in plastic materials varies between manufacturers and within batches from the same manufacturer. Naturally, this would suggest that there are likely systematic biases in our ILCR values resulting from the plastic samples used in our incubations. Considering that our ILCR values reported here are based on the random selection of plastics, some caution must be exercised when interpretating the ILCR values reported here.
To put our findings into perspective, there are many reports in the literature highlighting the potential risk of cancer from dietary exposure to PAHs from other sources.37–40 For example, Ma et al. (2023) assessed the ILCR risk from exposure to a variety of teas from sources in China and showed that chronic exposure to PAHs by consuming different types of tea can result in a potential carcinogenic risk.37 A study by Aamir et al. (2021), demonstrated that PAH levels in some food groups viz. wheat and eggs consumed by the Pakistani population posed a potential cancer risk to both adults and children.38 High-PAH containing foods like barbequed, smoked or deep-fried meats were shown to pose a potential cancer risk to residents in urban China.39 Finally, Naghashan et al. (2023) showed that PAHs can migrate from PETG plastic packaging into fruit juice media.40 In this study, while the ∑16PAHs concentrations reported ranged from 2.7 to 10.6 μg L−1, ILCR values were smaller than 10−6 indicating no potential health risk to the population.
Taken together, while there are reports of migration of PAHs into food media with accompanying health risk assessments in some instances, studies examining human exposure to PACs beyond the 16 PAHs are limited. Our study provides experimental evidence that a broader suite of PACs are present in food simulants and that measurements beyond the 16 PAHs are warranted.
Conclusions
Our results clearly indicate that PACs can partition from food-grade plastics studied into foods. Irrespective of the temperature, both PE and PETG showed the greatest mass-transfer of PACs into the food simulant. Conversely, PC showed the smallest amounts of PACs in the food simulant. Under both usage scenarios, the migration of PACs from PE showed the greatest risks to humans with ΣEDI of 1794.4 ± 163.5 and 169.4 ± 23.5 ng per person per day at 20 °C and 120 °C, respectively. The lack of homogeneity in the distribution of PACs in our plastics led to low analyte detection frequencies in some instances and precluded kinetic data analyses. To our knowledge, this is the first comprehensive study that highlights the partitioning of PACs from food-grade plastics into foods. Our ILCR estimates suggests that at the current exposure level, and based on the four plastic types studied, dietary exposure to PACs resulting from their migration to foods under refrigeration or microwave conditions poses negligible human cancer risks.Data availability
Data will be made available on request.Author contributions
Kara Loudon: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, riting – review & editing, visualization. Thane Tomy: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review & editing, visualization. Erin Liebzeit: investigation. Thor Halldorson: validation, investigation. Zhe Xia: validation, formal analysis, writing – review & editing. Sara Sambanthan: validation, investigation. Duc Luong Hoang: investigation. Gregg T. Tomy: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to GTT (grant number: RGPIN-05354-2019), an NSERC Undergraduate Student Research Award to KBL, a University of Manitoba Undergraduate Research Award to TMZT and an NSERC Canada Graduate Scholarship – Master's Award to ECL.References
- M. Wagner, L. Monciús, H. P. h. Arp, K. J. Groh, M. E. Løseth, J. Muncke, Z. Wang, R. Wolf and L. Zimmermann, State-of-the-Art Review on Hazardous Substances in Plastics, PlastChem, project number 341954 2024, https://plastchem-project.org Search PubMed.
- R. Geyer, Production, Use, and Fate of Synthetic Polymers. Plastic Waste and Recycling, 2020, pp. 13–32, DOI:10.1016/B978-0-12-817880-5.00002-5.
- United Nations Environment Programme and Secretariat of the Basel, Rotterdam and Stockholm Conventions, Chemicals in Plastics: a Technical Report, Geneva, 2023, https://www.unep.org/resources/report/chemicals-plastics-technical-report Search PubMed.
- OECD, Global Plastics Outlook: Policy Scenarios to 2060: Organization for Economic Co-operation and Development, Paris, 2022, DOI:10.1787/aa1edf33-en.
- J.-P. Lange, Towards circular carbo-chemicals-the metamorphosis of petrochemicals, Energy Environ. Sci., 2021, 14, 4358–4376, 10.1039/d1ee00532d.
- K. Zhang, A. H. Hamidian, A. Tubic, Y. Zhang, J. K. H. Fang, C. X. Wu and P. K. S. Lam, Understanding plastic degradation and microplastic formation in the environment: a review, Environ. Pollut., 2021, 274, 116554, DOI:10.1016/j.envpol.2021.116554.
- C. Wayman and H. Niemann, The fate of plastic in the ocean environment: a minireview, Environ. Sci.: Processes Impacts, 2021, 23(2), 198–212, 10.1039/d0em00446d.
- C. R. Li, R. Busquets and L. C. Campos, Assessment of microplastics in freshwater systems: a review, Sci. Total Environ., 2020, 707, 135578, DOI:10.1016/j.scitotenv.2019.135578.
- C. Campanale, C. Massarelli, I. Savino, V. Locaputo and V. F. Uricchio, A detailed review study on potential effects of microplastics and additives of concern on human health, Int. J. Environ. Res. Public Health, 2020, 17(4), 1212, DOI:10.3390/ijerph17041212.
- C. M. Rochman, C. Manzano, B. T. Hentschel, S. L. M. Simonich and E. Hoh, Polystyrene plastic: a source and sink for polycyclic aromatic hydrocarbons in the marine environment, Environ. Sci. Technol., 2013, 4794, 13976–13984, DOI:10.1021/es403605f.
- B. G. Yeo, K. Mizukawa, H. Takada, H. Tait and J. Frechou, Polycyclic aromatic hydrocarbons (PAHs) in new unexposed and beached expanded polystyrene foams, Environ. Monit. Contam. Res., 2022, 2, 14–21, DOI:10.5985/emcr.20210012.
- S.-Q. Li, H.-G. Ni and H. Zeng, PAHs in polystyrene food contact materials: an unintended consequence, Sci. Total Environ., 2017, 609, 1126–1131, DOI:10.1016/j-scitotenv.2017.07.262.
- A. Abraham and P. Chakraborty, A review on sources and health impacts of bisphenol-A, Rev. Environ. Health, 2020, 35(2), 201–210, DOI:10.1515/reveh-2019-0034.
- J. N. Hahadakis, E. Iacovidou and S. Gerassimidou, An overview of the occurrence, fate, and human risks of bisphenol-A present in plastic materials, components, and products, Integr. Environ. Assess. Manage., 2023, 19(1), 45–62, DOI:10.1002/ieam.4611.
- Order Amending Schedule I to the Hazardous Products Act (Bisphenol A), Government of Canada, Canada Gazette Part II, 2010 Search PubMed.
- K. J. Groh, B. Geueke, O. Martin, M. Maffini and J. Muncke, Overview of intentionally used food contact chemicals and their hazards, Environ. Int., 2021, 150, 106225, DOI:10.1016/j.envint.2020.106225.
- K. Bouma, D. K.-V. Wijk, L. Steendam, D. T. H. M. Sijm, T. de Rijk, R. Kause, R. Hoogenboom and S. van Leeuwen, Plant-based food contact materials: presence of hazardous substances, Food Addit. Contam.,:Part A, 2024, 1–10, DOI:10.1080/19440049.2024.2357350.
- United States Food and Drug Administration, Guidance for Industry: Preparation of premarket submissions for food contact substance (chemistry recommendations), 2007, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-premarket-submissions-food-contact-substances-chemistry Search PubMed.
- Commission regulation (EU), No. 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food, Official Journal of the European Union, 2011, p. 89, http://data.europa.eu/eli/reg/2011/10/oj Search PubMed.
- Z. Xia, E. Kerr, N. Klaassen, H. Assi, H. Bray, C. Marvin, P. J. Thomas, J. Stetefeld and G. T. Tomy, New approaches to reduce sample processing times for the determination of polycyclic aromatic compounds in environmental samples, Chemosphere, 2021, 274, 129738, DOI:10.1016/j.chemosphere.2021.129738.
- I. Idowu, O. Francisco, P. J. Thomas, W. Johnson, C. Marvin, J. Stetefeld and G. T. Tomy, Validation of a simultaneous method for determining polycyclic aromatic compounds and alkylated isomers in biota, Rapid Commun. Mass Spectrom., 2018, 32, 277–287, DOI:10.1002/rcm.8035.
- Association of Official Analytical Chemist, Appendix F: Guidelines for Standard Method Performance Requirements, 2016 Search PubMed.
- K. Kuzmicz and A. Ciemniak, Assessing contamination of smoke sprats (Sprattus sprattus) with polycyclic aromatic hydrocarbons (PAHs) and changes in its levels during storage in various types of packaging, J. Environ. Sci. Health, Part B, 2017, 53, 1–11, DOI:10.1080/03601234.2017.1369306.
- A. Ciemniak and K. Kuzmicz, Effect of a packaging material type on PAHs contents in oils and water, J. Stored Prod. Res., 2021, 92, 101810, DOI:10.1016/j.jspr.2021.101810.
- P. Simko and B. Brunckova, Lowering polycyclic aromatic hydrocarbons in liquid smoke flavor by sorption into polyethylene packaging, Food Addit. Contam.,:Part A, 1993, 2, 257–263, DOI:10.1080/02652039309374147.
- P. Simko, B. Sklarsova, P. Simon and E. Belajova, Decreased benzo(a)pyrene in rapeseed oil packed in polyethylene terephthalate, Eur. J. Lipid Sci. Technol., 2005, 107, 187–192, DOI:10.1002/ejlt.200401108.
- B. Sklarsova, P. Simko, P. Simon and E. Belajova, Diffusion and adsorption of BaP from vegetable oils into polyethylene terephthalate and low density polyethylene package, J. Food Nutr. Res., 2006, 45, 12–16 CAS.
- J. Semanova, B. Sklarsova, P. Simon and P. Simko, Elimination of polycyclic aromatic hydrocarbons from smoked sausages by migration into polyethylene package, Food Chem., 2016, 201, 1–6, DOI:10.1016/j.foodchem.2016.01.057.
- P. Simko, V. Khunova, P. Simon and M. Hruba, Kinetics of sunflower oil contamination with polycyclic aromatic hydrocarbons from contaminated recycled low density polyethylene terephthalate, Int. J. Food Sci. Technol., 1995, 30, 807–812, DOI:10.1111/j.1365-2621.1995.tb01428.x.
- I. C. T. Nisbet and P. K. LaGoy, Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs), Regul. Toxicol. Pharmacol., 1992, 16, 290–300, DOI:10.1016/0273-2300(92)90009-X.
- V. Samburova, B. Zielinska and A. Khlystov, Do 16 polycyclic aromatic hydrocarbons represent PAH air toxicity, Toxics, 2017, 5, 1–16, DOI:10.3390/toxics5030017.
- USEPA, Dose–Response Analysis of Ingested Benzo[a]Pyrene (CAS No. 50-32-8). Human Health Assessment Group, Office of Health and Environmental Assessment, EPA/600/R-92/045, Washington, DC, 1991, available online at, http://refhub.elsevier.com/S0048-9697(20)37359-9/rf0185 Search PubMed.
- USEPA, Drinking Water Criteria Document for PAH. Prepared by the Office of Health and Environmental Assessment, Environmental Criteria and Assessment off Ice, Cincinnati, OH for the Office of Water Regulations and Standards, Washington, DC, 1991, available online at, http://refhub.elsevier.com/S0048-9697(20)37359-9/rf0190 Search PubMed.
- U.S. EPA, Exposure Factors Handbook (1997, Final Report), U.S. Environmental Protection Agency, Washington, DC, 1997, EPA/600/P-95/002F a-c Search PubMed.
- USEPA, Toxicological Review of Benzo[a]Pyrene Executive Summary [CASRN50-32-8]. Integrated Risk Information System, National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC, 2017, available online at https://iris.epa.gov/static/pdfs/0136tr.pdf Search PubMed.
- U.S. Environmental Protection Agency, Guidelines for carcinogen risk assessment, Federal Register, 2005, vol. 70, pp. 17765–17817, available online at https://www3.epa.gov/airtoxics/%20cancer_guidelines_final_3-25-05.pdf Search PubMed.
- J. Ma, Z. Zhu, S. Du, D. Zhang, X. Li, Q. Zheng, J. Shen, L. Xiao, X. Wu, Y. Chen, J. Ji and S. Lu, Polycyclic aromatic hydrocarbons in commercial tea from China and implications for human exposure, J. Food Compos. Anal., 2023, 116, 105075, DOI:10.1016/j.jfca.2022.105075.
- M. Aamir, S. Yin, Y. Liu, H. Ullah, S. Khan and W. Liu, Dietary exposure and cancer risk assessment of the Pakistani population exposed to polycyclic aromatic hydrocarbons, Sci. Total Environ., 2021, 757, 143828, DOI:10.1016/j.scitotenv.2020.143838.
- X. Duan, G. Shen, H. Yang, J. Tian, F. Wei, J. Gong and J. Zhang, Dietary intake polycyclic aromatic hydrocarbons (PAHs) and associated cancer risk in a cohort of Chinese urban adults: Inter- and intra-indiviudal variability, Chemosphere, 2016, 144, 2469–2475, DOI:10.1016/j.chemosphere.2015.11.019.
- M. Naghashan, P. Kargarghomsheh, R. R. Nazari, A. Mehrale, F. Tooryan and N. Shariatfar, Health risk assessment of PAHs in fruit juice samples marketed in city of Tehran, Iran, Environ. Sci. Pollut. Res., 2023, 30, 20077–20088 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00195h |
| ‡ Kara B. Loudon and Thane M. Z. Tomy contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2025 |