Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Wet wipes in untreated wastewater are a source of litter pollution to the arctic marine environment – a case study on the loads of litter and microplastics in wastewater effluents in Greenland†
Lis
Bach
 *a,
Jakob
Strand
a,
Hadi
Salame
bc,
Márta
Simon
a,
Janne
Fritt-Rasmussen
a and
Pernille Erland
Jensen
*a,
Jakob
Strand
a,
Hadi
Salame
bc,
Márta
Simon
a,
Janne
Fritt-Rasmussen
a and
Pernille Erland
Jensen
 bc
bc
aAarhus University, Department of Ecoscience, Frederiksborgvej 399, 4000 Roskilde, Denmark. E-mail: lb@ecos.au.dk
bDTU Sustain, Department of Environmental and Resource Engineering, Bygningstorvet, Building 115, 2800 Kgs. Lyngby, Denmark
cArctic DTU Sisimiut – Ilinniarfeqarfik Sisimiut, Siimuup Aqqutaa 32, 3911 Sisimiut, Greenland
First published on 20th September 2024
Abstract
Plastic litter is ubiquitous in the Arctic marine environment, but knowledge of the importance of specific sources is limited. This project aimed to investigate the input of plastic from untreated sewage discharged to the sea in Greenland. A method was developed to sample and quantify inputs of plastic in different size fractions from wastewater from two towns in Greenland. Plastic findings were visually characterized in terms of abundance, morphology, size, and chemically by characterizing the polymer composition using FTIR spectroscopy. The wastewater was found to be a source of both macro- and micro-sized plastic pollution. Of the total litter load, 70% of the mass was from plastic items larger than 25 mm. Wet wipes were found to be dominating and constituted 59% of the total emitted plastic by mass, but other sanitary items (sanitary pads and condoms) were also detected. A polymeric characterization of the macro-items by ATR-FTIR revealed that the wet wipes were mainly of PET (polyethylene terephthalate, a polyester) but also viscose and cellulose wet wipes were detected. In the microplastic fraction (<300 μm), the main contributor was PP (polypropylene; 65%), but also PE (polyethylene), PES (polyester), PS (polystyrene), cellulose and other polymers were detected. A characterization of the microfibers revealed a large contribution of white/transparent fibers that primarily were composed of cellulose (67%) while a smaller fraction (10%) was polyester (PES), including polyethylene terephthalate (PET). The findings of white/transparent microplastic fibers in the wastewater suggest that a fraction of these fibers is directly related to the presence of the cellulose, viscose and PET wet wipes. Our results suggest that implementing either regulatory or behavioral measures to prevent wet wipes from entering the wastewater or using technical solutions to eliminate the discharge of wet wipes into the marine environment via wastewater, could significantly reduce the emission of plastics of all sizes from wastewater to the marine environment.
Environmental significanceThe study addresses the significant issue of plastic pollution in the Arctic marine environment by identifying untreated sewage as a major source of both macro- and micro-sized plastics in Greenland. This research fills a knowledge gap, providing essential data on the types and sizes of plastics present, and highlights wet wipes as a major contribution to macro and microlitter pollution. The detailed characterization of these plastics is vital for understanding their prevalence and impact. By quantifying plastic loads from wastewater, the study informs the development of effective pollution control measures. Importantly, the research suggests actionable steps, such as regulatory or behavioural measures to prevent wet wipes from entering wastewater, which could significantly reduce marine plastic pollution. |
1 Introduction
In the Arctic marine areas, concern has been raised upon observations of high concentration of plastic litter and microplastics (MPs).1–5 Plastic litter can physically affect marine organisms through ingestion or entanglement, as well as chemically by acting as an introducer or a vector of plastic additives to the environment. Furthermore, plastic pollution contributes to biodiversity and ecosystem disturbances.1,6,7 To address this issue, the Protection of the Arctic Marine Environment (PAME), a working group of the Arctic Council, has developed a Regional Action Plan on Marine Litter.8 The plan focuses on both sea- and land-based activities, specifically targeting Arctic-specific marine litter sources and pathways. As part of this effort, the plan emphasizes improvements of onshore waste and wastewater management.8 On a national level, similar to other Arctic countries, the Greenlandic government has implemented a National Action Plan on Plastic Waste Management.9,10The presence of litter in the Arctic marine areas is closely linked to human activities such as local contribution through improper disposal of litter, fishing and leisure activities, tourism etc. But, long-distance transportation by ocean currents also introduces objects from distant areas into the Arctic marine environment.3 Recent studies highlight the presence of significant marine plastic litter pollution in Greenland.5,11 In-depth beach litter analyses in West Greenland (Sisimiut, Maniitsoq, Qaqortoq and Nuuk) found that marine litter was mostly of local origin and consisted mostly of every-day-use-products in addition to fishing and hunting equipment. This is in accordance with investigations in Arctic Canada and along the coast of the entire West Greenland, that found that litter densities were largest within 5 km of communities highlighting the role of local activities in contributing to plastic pollution.4
For microplastic, recent studies emphasized that local Arctic communities play a significant role in contributing to microplastics entering the arctic oceans.2,12 In a study where MPs were quantified (67–100 m−3 MPs in the size of 0.01–0.05 mm) in the fjord Nuup Kangerlua adjacent to Nuuk, Greenland, it was suggested that Nuuk (e.g. wastewater outlets and mismanaged waste) was the primary source.2 Similarly at Svalbard, it was found that untreated wastewater from Longyearbyen, Svalbard, with 2400 inhabitants, emits MP fibers at the level of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m−3 MPs in the size of 0.05–5 mm, which was evaluated to be at a scale similar to a modern wastewater treatment plant serving 1.3 million persons.12 Research indicates that Arctic coastal areas contain microplastics (MPs) at lower or comparable levels to those found in the southern more populated areas of the Nordic regions.2,13–19 These findings on litter and MPs have led to the identification of untreated wastewater in small and remote Arctic communities as a primary concern regarding ocean litter contamination.20 From beach litter monitoring surveys on reference beaches in Greenland there are records of various types of single use products (SUP) including sanitary waste like ear bud sticks and wet wipes.21 But, despite the observed microplastic found in the sea around communities2,15 and litter on the coastlines of Greenlandic communities,4,5,11,21 and its indicated origin from the untreated wastewater, the quantity, quality and sources of plastic in the wastewater has not yet been accurately documented.21
000 m−3 MPs in the size of 0.05–5 mm, which was evaluated to be at a scale similar to a modern wastewater treatment plant serving 1.3 million persons.12 Research indicates that Arctic coastal areas contain microplastics (MPs) at lower or comparable levels to those found in the southern more populated areas of the Nordic regions.2,13–19 These findings on litter and MPs have led to the identification of untreated wastewater in small and remote Arctic communities as a primary concern regarding ocean litter contamination.20 From beach litter monitoring surveys on reference beaches in Greenland there are records of various types of single use products (SUP) including sanitary waste like ear bud sticks and wet wipes.21 But, despite the observed microplastic found in the sea around communities2,15 and litter on the coastlines of Greenlandic communities,4,5,11,21 and its indicated origin from the untreated wastewater, the quantity, quality and sources of plastic in the wastewater has not yet been accurately documented.21
The aim of this project was to estimate the burden of plastic litter and MPs to the marine environment originating from untreated piped wastewater in Greenland by sampling and analyzing wastewater from the two biggest towns of Greenland, Nuuk and Sisimiut.
2 Methodology
2.1 Study areas
Sampling for plastics was conducted at two wastewater outlets in Greenland, one in the capital of Greenland, Nuuk, with ∼19870 inhabitants and one in the second largest town, Sisimiut, with ∼5460 inhabitants. The sampling sites were chosen based on the preference for outlets receiving wastewater primarily from households while avoiding wastewater from fish and seafood processing industry. Two outlets meeting this criterion, and with similar PEq (Person Equivalents) loads of approximately 2000 PEq (i.e., 3.6% of the Greenlandic population) and easy accessibility were selected. The outlet sampled in Sisimiut was U1 well number 08 A0001A (66°56′34′′N – 53°39′10′′W) (graphical abstract), while the outlet sampled in Nuuk was U15 well number 0620004 (64°11′47′′N – 51°42′10′′W) (Fig. 1).2.2 Sampling and processing
Four plastic size fractions were sampled: macro-plastics (>25 mm), meso-plastics (5–25 mm), large-sized microplastics (1–5 mm) and microplastics (20 μm–1 mm) according to the GESAMP recommendations for the monitoring and assessment of plastic litter in the ocean.22 The plastic fractions were classified into color, shapes, and sources according to GESAMP recommendations.22To cover differences in amounts and types of litter in the wastewater during daily/weekly routines, the sampling was conducted at different times and weekdays. The sewers in Greenland are separate to rainwater and snow melt water that runs in separate ditches. Even so, increased flow of wastewater in pipes has been observed on previous melt season/precipitation occasions. Samples diluted by rainwater were therefore avoided by not sampling for at least 48 hours after any rainfall. A detailed overview of the sampling scheme can be found in Table S1.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution, and left for 24 hours. This methodology has previously been shown to not affect commonly used plastic polymers23 and also worked as a disinfection procedure. Finally, the sieve with remaining content was rinsed with tap-water and left to air-dry before the collected macro-plastic items were retrieved, packaged and transported to the laboratory at Ecoscience, Aarhus University in Roskilde, Denmark, for further analysis.
1 solution, and left for 24 hours. This methodology has previously been shown to not affect commonly used plastic polymers23 and also worked as a disinfection procedure. Finally, the sieve with remaining content was rinsed with tap-water and left to air-dry before the collected macro-plastic items were retrieved, packaged and transported to the laboratory at Ecoscience, Aarhus University in Roskilde, Denmark, for further analysis.
The samples were weighed and thereafter purified to remove as much natural material as possible. First, the samples were filtered through a 20 μm stainless steel filter, where after the filter with the collected material was subjected to ultrasonication in acetate buffer (pH 4.8) with the addition of SDS (sodium dodecyl sulphate) as a detergent. The samples were transferred to a bottle with cellulose-degrading enzymes (cellulase and viscozyme), followed by 40 hours reaction time at 50 °C. The samples were filtered (20 μm), and the filter was ultrasonicated in the acetate buffer for 5 minutes. The samples were treated with a mixture of a strong alkaline solution (10% KOH) and hypochlorite (7% NaOCl) as an oxidizing agent for 24 hours, after which the samples were filtered (20 μm). Finally, a solution of zinc chloride with a density of 1.5 g ml−1 was used to separate heavier particles from those suspended in the liquid in a separating funnel. The upper part of the liquid fraction was filtered through a series of stainless-steel filters with mesh sizes of 1000, 100, and 20 μm. The resulting liquid fraction containing particles of <20 μm was discarded. The two size fractions 20–100 μm and 100–1000 μm were transferred to separate silicon membranes (MakroPorP12M5-350, SmartMembranes) with a diameter of 13 mm and a pore size of 5–6 μm for μFTIR spectroscopy analyses.
2.3 Sample analyses
To validate the visual classification along with polymer specific identification, representative items and particles >25 mm were identified using the ATR-FTIR (Fourier transform infrared spectroscopy-attenuated total reflectance) spectroscopy and relevant spectral libraries. Measurements were carried out using Agilent Technologies 4500a Series Portable FTIR. The spectrometer was equipped with a triple-reflection diamond ATR sample interface and an in-depth ATR polymer library. The absorbance spectra were collected using 32 background scans at a 4 cm−1 resolution, measuring a spectral range between 650 and 4000 cm−1. A background atmospheric spectrum was subtracted from all sample spectra, and 8 sample scans were performed for each sample. The library used for the polymer identification was an in-house spectral reference library of FTIR-ATR spectra of multiple synthetic and natural materials developed by the Department of Ecoscience at Aarhus University. All the litter items were dried prior to chemical analysis to reduce interference of H2O in the IR (infrared) spectrum.
For the ATR-FTIR analyses, the ‘Microlab’ software (Agilent Technologies) was used as an initial assessment as it automatically compares the collected spectrum with a spectral library and associates the best spectral match. Subsequently, the ‘Essential FTIR’ (Operant LLC.) software was applied for the data processing and interpretation of the final polymer ID. All generated spectra in this study were smoothed and baseline adjusted as such corrections are critical preprocessing techniques for improving the quality of raw FTIR spectra and obtaining a more precise analysis.
To minimize the risk of contamination, all reagents were filtered through a 0.2 μm GF filter, and all glassware and steel filters were wrapped in aluminum foil and heated at 450 °C. Rubber seals and stainless-steel filter chambers were cleaned through regular machine dishwashing, followed by ultrasonic treatment in SDS solution and then ethanol.
Field blank samples and laboratory blank samples were analyzed to assess the potential risk of external contamination during sample handling. In total, 2 field blank samples and 2 laboratory blank samples were analyzed, each divided into size fractions of 20–99 μm and 100–999 μm. Based on the blank samples, analytical detection limits (DL) were determined being equivalent to the mean value plus 3 × standard deviations (SD)29 for individual polymer types and for the total number of identified microplastics, as shown in Table S2.† For polymer types not identified in the blank samples, the detection limit is set at 1 per sample, corresponding to 2 L−1 when analyzing sample volumes of approximately 650 ml.
The quantification of fibers on the silicon membranes using visual microscopy revealed a possible internal contamination of the blank samples. With quantification of an average 35 transparent or white fibers per sample, and an average of 5 colored fibers per sample in predominantly black, blue, and red colors, this likely points to an internal fiber contamination possibly due to a contamination from GF filters while filtration of liquids used for the sample preparation. This results in a detection limit of 77 white/transparent fibers and 16 colored fibers per sample (calculated as average +3 × SD). As a result of the relatively high level of fibers in the blank samples, which for the colored fibers was at the same level as the number of colored fibers found in wastewater samples, the data on colored fibers are reported as <DL (detection limit defined as 3 × SD) for these samples.
2.4 Survey of wet wipes in trade in Greenland
A survey of the wet wipes in trade in Greenland was made. In Sisimiut, we purchased any commonly available wet wipes both for sanitary and cleaning purposes. During the 1–3 July 2023, we visited all grocery stores in Sisimiut as well as other stores that sell beauty products. All different types of wet wipes available were purchased. The wet wipes were analyzed for primary and possible secondary polymer by the same method (ATR-FTIR) as described for macro-plastics (Section 2.3.1).2.5 Beach surveys
Two rounds of beach surveys in the inner part of the Kangerluarsunnguaq Bay in Sisimiut were completed, in June and July 2023 (see white line in Fig. 1). In June, the survey for wet wipes was conducted along a larger survey following the methodology of monitoring marine litter on the beaches in the OSPAR maritime area.30 All litter items were collected in a fixed ∼100 meter section of the beach covering the whole area between the water edge to the back of the beach and the items were sorted and registered according to the guidelines. In July, the survey targeted wet wipes only.3 Results
3.1 Macro-litter (>25 mm)
All litter items with size >25 mm were easily defined and characterized due to their relatively well conserved structure. The results revealed significant amounts of items as wet wipes, sanitary pads and condoms in the wastewater in both Sisimiut and Nuuk, with total loads of 32 items in a sampling time of 445 minutes for Sisimiut, and 13 items in a sampling time of 190 minutes for Nuuk, corresponding to equal daily inputs of 104 and 99 items, respectively (Table 1). The polymeric characterization of the macro-items by ATR-FTIR analyses (Fig. 2) revealed that for Sisimiut the 23 wet wipes were of PET (polyethylene terephthalate), the single condom of rubber, the 3 sanitary pads of PP (polypropylene), one piece of foil and 3 cotton buds of cellulose. In Nuuk, the wet wipes were characterized as 6 pieces of PET, 3 of viscose and 3 of cellulose. Also, one sanitary pad was characterized as PE (polyethylene). Thus, 87% and 77% of the items were of synthetic (PET, PE, PP, rubber) or semi-synthetic (viscose) origin in Sisimiut and Nuuk, respectively (Fig. 2). Due to the limited number of samling points and times and the limited volume of sewage that could be sampled through the sieve before it clogged due to the general content of the sewage, the identified items should be comprehended as an approximation for the macroplastic content of the sewage only. Had sampling taken place at other points of time, other types of items might have been observed. The concordant observations of wet wipes, however, validates a conclusion that these are abundant and omnipresent in the sewage.| Site | Size fraction | Type of items | Abundant polymer | Number of items | Mass (g) | Total mass (g) | Yearlye marine input of plastic (g per year per capita) |
|---|---|---|---|---|---|---|---|
| a Polymer type: PE (polyethylene), PP (polypropylene), PS (polystyrene), PES (polyester), PET (polyethylene terephthalate), PVC (polyvinyl chloride), PC (polycarbonate), PMMA (polymethyl methacrylate), PA (polyamide), PUR (polyurethane), ABS (akrylonitrile butadiene styrene). b Estimated weight. c The masses of cellulose wet wipes and cotton buds are not included in the estimation of yearly marine input of plastic. d Under detection limit (the total mass and yearly marine input for these items are therefore an absolute maximum). e The yearly input of mass of plastic items through wastewater is estimated assuming a normal capita consumption of 104 L of water per day (Maréchal et al. 2022).31 | |||||||
| Sisimiut | >25 mm | Wet wipes | PET | 25 | 37.4 | 46.5 | 27.5 |
| Sanitary pads | PP | 3 | 7.5 | ||||
| Condoms | Rubber | 1 | 1.6 | ||||
| Cotton buds | Cellulose | 3 | 0.87c | ||||
| 5–25 mm | Film/foil | PE | 1 | <0.005d | 0.005 | 2.3 | |
| 1–5 mm | Fiber/thread bundle | PET | 1 | 0.01 | 0.015 | 6.9 | |
| Film/foil | PE | 1 | <0.005d | ||||
| 20–1000 μm | Microplastic particles | PE | 21 | 8.4 μg | 0.5 | ||
| PP | 86 | ||||||
| PES | 13 | ||||||
| PS | 4.5 | ||||||
| PVC | <1.4d | ||||||
| PC | <1d | ||||||
| PMMA | <1d | ||||||
| PA | <1d | ||||||
| PUR | <1d | ||||||
| ABS | <1d | ||||||
| Other polymers | 18.5 | ||||||
| Other rubbers | <3d | ||||||
| Nuuk | >25 mm | Wet wipes | PET | 6 | 10.2 | 16.9 | 23.4 |
| Viscose | 3 | 4.8b | |||||
| Cellulose | 3 | 4.8b/c | |||||
| Sanitary pads | PE | 1 | 1.9 | ||||
| 5–25 mm | N/A | — | — | 0 | 0 | 0 | |
| 1–5 mm | Film/foil/fragment | PE | 4 | 0.02b | 0.03 | 12.7 | |
| Film/foil/fragment | PUR | 2 | 0.01b | ||||
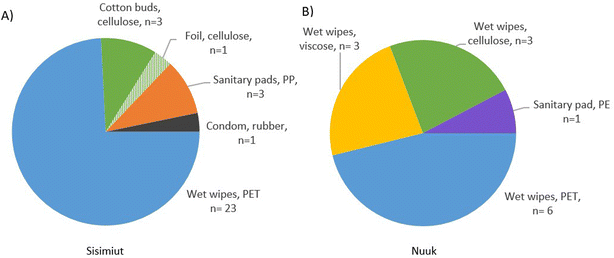 | ||
| Fig. 2 >25 mm items in raw wastewater from (A) Sisimiut, collected during 445 min, and (B) Nuuk, collected during 190 min. n = number of items. | ||
3.2 Meso-plastics (5–25 mm)
Only one meso-plastic sized particle polymeric characterized as PE was sampled in Sisimiut, whereas no particles in this size range were found in Nuuk (Table 1). This result can be attributed to the sampling method, and it is likely that objects in this size interval are underrepresented.3.3 Large-sized microplastics (1–5 mm)
Of the large-sized microplastic, 2 and 6 large-sized microplastic items were sampled in Sisimiut and Nuuk, respectively. The items from Sisimiut consisted of one PET fiber/thread and one PE film/foil (Table 1). The 6 items from Nuuk consisted of 2 items of film/foil, besides 2 pieces of PE and 2 pieces of PUR (polyurethane). The latter four items were not visually characterized as they were lost between the ATR-FTIR analysis and the visual characterization and size measurement. The items are denoted film/foil/fragments in Table 1 and for inclusion in data, the mass of the items was estimated based on size and polymer density.3.4 Microplastics (20–1000 μm)
The content of microplastics in the two wastewater samples from Sisimiut determined by μFTIR analyses showed a presence of microplastics at levels significantly higher than the detection limits (Table 1 and S2†). By employing μFTIR analyses on the sample, the average number of MPs was determined to 217 L−1 (range: 159–276 L−1). By μFTIR analyses the primary polymer types were identified as PP (65%), PE (15%), and PES (6%), but a few microplastic particles consisting of PS (polystyrene) were also identified in the samples (1%). The group of other synthetic polymers, mainly EVA (ethylene-vinylacetate), contributed with 13% (Fig. 3a).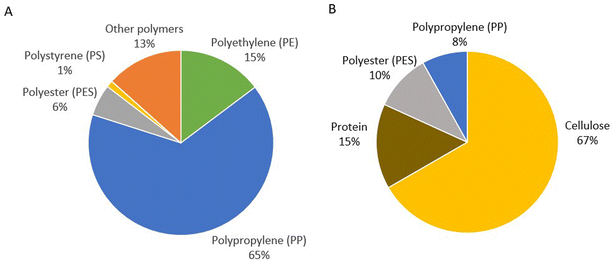 | ||
| Fig. 3 Polymer distribution of (A) all MPs (20–1000 μm) and (B) fibers only, in wastewater samples from Sisimiut identified by μFTIR analysis. | ||
Looking into the microfiber content of the wastewater, a visual inspection of the wastewater samples revealed a rather large contribution of white/transparent fibers (ranging from 102 to 352 fibers L−1) well above the detection limit (DL: 77 fibers L−1; range 17–64). The colored fibers in the wastewater (18.5 fibers L−1; range 11–31) were just above the detection limit (DL: 16 fibers L−1; range 0–8). This visual quantification was followed by μFTIR analyses. The μFTIR analyses revealed that the most part of the fibers was of organic material (cellulose or protein) and only 18% were of plastic polymers (10% PES and 8% PP) (Fig. 3b). Due to methodological limitations the cellulose fraction, or at least a portion of it, may be considered as viscose (also called rayon). This uncertainty arises from the limitations of μFTIR analysis in separating cellulose and viscose, and the inclusion of cellulose-degrading enzymes in the extraction methods, potentially leading to the degradation of true cellulose during the extraction process.
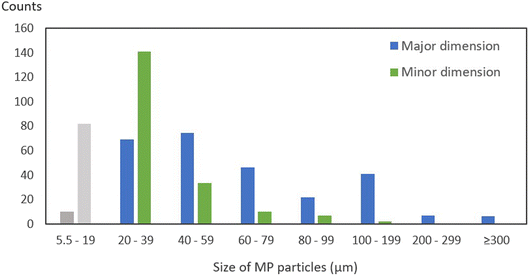
The size distribution of the identified microplastic particles revealed that 81% of the particles were in size fractions less than 100 μm, measured along the longest dimension, while only 2% were longer than 300 μm (Fig. 4). Only 4% were smaller than 20 μm, which is due to methodology limitations of the usage of a 20 μm mesh steel filter during the extraction process. Additionally, during the image analysis using siMPle software, the identification of microplastic particles was set to include only particles where at least 2 neighboring pixels, each measuring 5.5 μm, support the same identified polymer for a given particle. In addition, the μFTIR analyses are also limited by the so-called diffraction index, generally affecting the spectroscopic quality needed for identifying particles smaller than 15–20 in thickness, which also affect the inclusion of microplastic particles smaller than 20 μm. Without these methodology limitations, the size fraction of <20 μm would most likely have held significantly higher amounts.
3.5 Estimated loads of plastic litter entering the marine environment
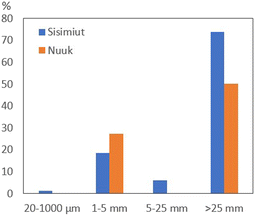
The annual input of marine plastic litter from the household wastewater to the marine environment was estimated using the loads of 2000 PEq for each of the two outlets in Sisimiut and Nuuk, the sampling time period (Table S1†) and average daily water consumption capita−1 of 104 L per day per person in Greenlandic towns.31In Sisimiut, the macro-plastic items contributed the most to the mass of litter items, which consisted primarily of wet wipes (74% of the total input) but also sanitary pads and condoms, would by estimate give a yearly input to the marine environment of 27.5 g capita−1. Taking all size fractions into consideration, an estimated yearly input will be 36.4 g per capita, thus the 5460 inhabitants in Sisimiut32 emit approximately 200 kg plastic litter per year to the marine environment via the 11 wastewater outlets.
In Nuuk, the macro-plastic items, which consisted of wet wipes and sanitary pads (23.4 g per capita per year) contributed somewhat more than the larger microplastic items (12.7 g per capita per year) to the input of litter to the marine environment. In total, the data points to a yearly input of 36.0 g per capita in Nuuk, and with a population of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 866 inhabitants,32 this approximates to a total annual input of 716 kg plastic litter to the marine environment of Nuuk Fjord via 19 wastewater outlets. It is, however, important to note that these Nuuk estimations do not include the MP size fraction of 20–1000 μm.
866 inhabitants,32 this approximates to a total annual input of 716 kg plastic litter to the marine environment of Nuuk Fjord via 19 wastewater outlets. It is, however, important to note that these Nuuk estimations do not include the MP size fraction of 20–1000 μm.
3.6 Survey of wet wipes in trade in Greenland
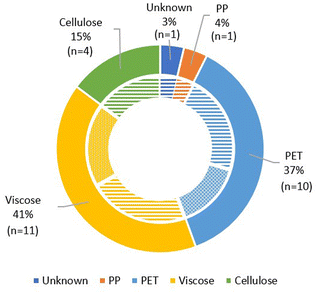
In total, 26 different wet wipes were purchased in grocery stores and health care shops in Sisimiut. The packages were grouped into 17 different wet wipes for personal care and 9 for domestic/cleaning purposes. The ATR-FTIR analysis showed that the primary polymer for wet wipes for both cleaning and sanitary purposes mainly were viscose (41%) and PET (37%), while the primary polymer was cellulose for a minor part (15%) (Fig. 5). For a number of the wet wipe products, the product declaration did not fully correspond to the ATR-FTIR analyses on polymer composition (Table S3†).3.7 Beach surveys
During the two ‘wet wipe surveys’ at an adjacent beach to the wastewater outlet in Sisimiut no wet wipes were identified.4 Discussion
In the current study we found a large input of macro- and microplastics to the sea via wastewater.4.1 Load of macro-versus microfibers from wastewater in Greenland
Estimating the total load of plastic litter by extrapolating the results from the 4000 PEq sampled in Sisimiut and Nuuk to ∼57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants in Greenland,32 approximately 2 T of plastic litter per year is discharged to the marine environment from local sources. Out of this, ∼1.2 T per year comprises plastic or semisynthetic wet wipes, approximately 1.4 T per year are items >5 mm, 0.6 T per year are items between 1 and 5 mm, and 28 kg per year are <1 mm. As a result of the sampling methods and the fact that data on meso- and larger sized microplastic items are based on very few items, these numbers must be seen as rough estimates. These approximations indicate that the largest mass proportion of litter enters the marine environment as macro plastic via wastewater (Fig. 6).
000 inhabitants in Greenland,32 approximately 2 T of plastic litter per year is discharged to the marine environment from local sources. Out of this, ∼1.2 T per year comprises plastic or semisynthetic wet wipes, approximately 1.4 T per year are items >5 mm, 0.6 T per year are items between 1 and 5 mm, and 28 kg per year are <1 mm. As a result of the sampling methods and the fact that data on meso- and larger sized microplastic items are based on very few items, these numbers must be seen as rough estimates. These approximations indicate that the largest mass proportion of litter enters the marine environment as macro plastic via wastewater (Fig. 6).
The degree of littering through wastewater may vary across different regions in Greenland e.g. due to different infrastructural conditions. For example, the extent of littering through bucket toilets (i.e. “honey buckets”) used in 20% of the households in Greenland, is unknown. In our calculation of the plastic litter load, we have assumed similar littering patterns as from sewer systems. The uncertainty on the impact of “honey buckets” should be investigated further.
4.2 Sources of macro-plastic in wastewater in Greenland
In Nuuk as well as Sisimiut, wet wipes made of plastic and semi-synthetic materials were identified as the major contributor to the macro-plastics mass in the wastewater let out to the marine environment. They constituted 82% of the macro-plastic mass, and 59% of the total identified plastic mass in the wastewater. Of the 37 wet wipes that were found in the wastewater in the present study, only 3 were made from cellulose, while the rest were made from PET (31 wet wipes) or viscose (3 wet wipes) (Fig. 3). In consistency, most wet wipes sampled from stores in Sisimiut were of PET or viscose, and even those declared to be of natural fibers, or bamboo turned out largely to of the less degradable semi-synthetic fibers as viscose than the biodegradable cellulose-based fibers (Fig. 5 and Table S3†). In accordance with our results, the presence of PET in all non-flushable wet wipes examined was demonstrated, and the presence of PET and other synthetic materials in a substantial number of flushable wet wipes.33 Based on this, it was concluded that commercially available wet wipes, even those labelled flushable or natural, can be considered as a possible source of microplastic fibers in wastewater streams. Apart from contributing to plastic contamination, the content of synthetic material as well as diverse chemical additives in wet wipes marked as natural, biodegradable or flushable may decrease their degradation rate.34 Therefore, even natural wet wipes may last for long periods in the environment,34–36 during which they can cause damages by e.g. shading, being accidentally ingested by wildlife or leaving traces of synthetic fibers and chemicals as preservatives, plasticifiers and surfactants.Even though no beach stranded wet wipes were identified during the two 'wet wipe beach surveys' in Sisimiut in June and July 2023, wet wipes were observed on the seabed next to sewer outlets in Sisimiut and another Greenlandic town, Aasiaat (personal observations – see table of contents entry). Consequently, we suggest that wet wipes deposit on the seafloor rather than being washed ashore. This is in contrast to the Marine Conservation Society's analysis of the Great British Beach Clean37 that documented the presence of wet wipes along the UK coastline equivalent to 27.5 pieces of wet wipes per 100 meters of beach cleaned. Local current patterns may also be responsible for distribution of wet wipes and the lack of wet wipes at the specific beach site investigated in Sisimiut.
4.3 Sources of MP in wastewater in Greenland
The present study found that the most abundant shape of MP particles in Greenlandic wastewater was fibers, which aligns with findings in Nuuk Fjord, where fibers <300 μm were found to be the dominant MPs at 3 marine fjord stations near Nuuk, Greenland.2 In general, fibers are the most frequently documented MP type found in the marine Arctic environment,38–40 as well as in wastewater12,15,17 and mainly source-related to domestic wash of textiles.41 As the fibers identified in our study were white/transparent and primarily of the same plastic polymer as most of the wet wipes (PET and viscose), it is reasonable to believe that a fraction of the fibers may also be linked to the presence of wet wipes. Our research thereby indicates that wet wipes may play a substantial role in microplastic (fiber) pollution, contributing through direct release of fibers from wet wipes during their passage in the sewer system and from degradation of wet wipes left in the marine environment. This aligns with the conclusions drawn by a study, where the release of microfibers from wet wipes subjected to a simulated toilet flush were studied.42 Wet wipes were immersed in water for one hour and observed a release of 1966 microplastic fibers per sheet. Thus, direct disposal of wet wipes in the sewage system will likely induce a significant release of fibers. Similarly, a study on intertidal sediment samples collected at sites of observed washed-up deposits of sewage-derived waste, found that the disposal of wet wipes and sanitary towels into toilets represents an underestimated source of white microplastic fibers in the environment.43 One of the major sources of MPs in wastewater commonly mentioned in the literature is laundry16,44 that has shown to release up to 7–800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibers from a single load of laundry.45,46 In our study, we only found limited amounts of colored fibers. Therefore, we speculate if laundry wash of textiles may not be the main contributor to the fibers detected in our samples.
000 fibers from a single load of laundry.45,46 In our study, we only found limited amounts of colored fibers. Therefore, we speculate if laundry wash of textiles may not be the main contributor to the fibers detected in our samples.
4.4 Local action plans on implementing wastewater treatment
Given that many Arctic towns and settlements face challenges related to poor wastewater treatment systems, there has been a growing awareness towards implementing improved treatment methods at the local level. These local-driven efforts are crucial for addressing the pressing environmental and public health issues associated with wastewater discharge in the Arctic region. Implementing wastewater treatment is being considered as a potential future initiative in Greenland to reduce plastic pollution in the ocean. Consequently, as part of an action plan against plastic pollution, the Greenlandic government has decided, as an initial step, to gather information on simple and effective wastewater treatment methods for removing plastics.105 Conclusion
This study documented a significant release of macro and microplastics into the environment through the discharge of untreated wastewater in two Arctic towns. Notably, large quantities of wet wipes were discovered in the wastewater and observed on the seafloor next to the outlet. Additionally, our findings of white/transparent microplastic fibers in the wastewater suggest that a fraction of these fibers is directly related to the presence of wet wipes. Further, to our knowledge on available consumer products, wet wipes would irrespectively of plastic or cellulose origin also inevitably contain chemicals as preservatives and surfactants that could potentially cause environmental harm.Our results suggest that implementing either regulatory or behavioral measures to prevent wet wipes from entering the wastewater or applying technical solutions to eliminate the discharge of wet wipes into the marine environment via wastewater, could significantly reduce the emission of plastics of all sizes from wastewater to the marine environment.
Data availability
Data obtained during the study is presented in Figures, Tables and ESI† provided in the submitted manuscript.Author contributions
Conceptualization and methodology: Lis Bach, Pernille Erland, Jakob Strand and Janne Fritt-Rasmussen. Data curation: Hadi Salame, Pernille Erland Jensen. Sampling: Pernille Erland Jensen and Hadi Salame. Formal analyses: Jakob Strand and Marta Simons. Funding acquisition: Pernille Erland Jensen and Lis Bach. Visualization and writing the original draft: Lis Bach and Pernille Erland Jensen. Writing – review & editing: Lis Bach, Pernille Erland Jensen, Janne Fritt-Rasmussen, Jakob Strand and Marta Simons.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was financed by the Swedish Environmental Protection Agency as a contribution to the Nordic Council of Ministers Project ‘Norden som en motor i arbetet mot marin nedskräpning regionalt och globalt’. The Nordic Council of Ministers has disseminated the present work as a ‘TemaNord report ‘Plastic in raw wastewater in Greenland – Load to the marine environment and mitigation. The report is available at http://dx.doi.org/10.6027/temanord2024-516. Kommunia Qeqqata and Sermersoq are thanked for the assistance during field sampling and support.References
- F. Collard and A. Ask, Plastic ingestion by Arctic fauna: A review, Sci. Total Environ., 2021, 786, 147462, DOI:10.1016/j.scitotenv.2021.147462.
- S. Rist, A. Vianello, M. H. S. Winding, T. G. Nielsen, R. Almeda and R. R. Torres, et al., Quantification of plankton-sized microplastics in a productive coastal Arctic marine ecosystem, Environ. Pollut., 2020, 266, 115248, DOI:10.1016/j.envpol.2020.115248.
- M. Bergmann, F. Collard, J. Fabres, G. W. Gabrielsen, J. F. Provencher and C. M. Rochman, et al., Plastic pollution in the Arctic, Nat. Rev. Earth Environ., 2022, 3(5), 323–337, DOI:10.1038/s43017-022-00279-8.
- M. L. Mallory, J. Baak, C. Gjerdrum, O. E. Mallory, B. Manley and C. Swan, et al., Anthropogenic litter in marine waters and coastlines of Arctic Canada and West Greenland, Sci. Total Environ., 2021, 783, 146971, DOI:10.1016/j.scitotenv.2021.146971.
- W. J. Strietman, M. J. van den Heuvel-Greve, A. M. van den Brink, E. Leemans, J. Strand and L. Bach, Beach litter in West Greenland: a source analysis, Wageningen Economic Research, Wageningen, 2021, Available at: https://edepot.wur.nl/541149 Search PubMed.
- P. Villarrubia-Gómez, S. E. Cornell and J. Fabres, Marine plastic pollution as a planetary boundary threat – The drifting piece in the sustainability puzzle, Mar. Pol., 2018, 96, 213–220, DOI:10.1016/j.marpol.2017.11.035.
- S. Werner, A. Budziak, J. van Franeker, F. Galgani, G. Hanke and T. Maes, et al., Harm Caused by Marine Litter, 2016, DOI:10.2788/690366.
- PAME, Regional Action Plan on Marine Litter in the Arctic. Arctic Council, Finland, 2021, Available at: https://pame.is/document-library/pame-reports-new/pame-ministerial-deliverables/2021-12th-arctic-council-ministerial-meeting-reykjavik-iceland/801-regional-action-plan-on-marine-litter-in-the-arctic/file Search PubMed.
- Naalakkersuisut, Selvstyrets Bekendtgørelse Om Brug Af Bæreposer Af Plast Og Engangsplastprodukter, 2022, 6 juli 2022, Available at: https://nalunaarutit.gl/groenlandsk-lovgivning/2022/bkg-23-2022?sc_lang=da Search PubMed.
- Naalakkersuisut, Mindre Plast I Naturen Naalakkersuisuts Indsatsplan for Nedbringelse Af Forbruget Af Plast (Free Translation: Less Plastic in Nature Naalakkersuisut's Action Plan for Reducing the Consumption of Plastic), 2021, Available at: https://naalakkersuisut.gl/-/media/publikationer/miljoe/2021/indsatsplan-mindre-plast-i-naturen-dk.pdf Search PubMed.
- M. L. Haarr, L. Bach, C. P. Chambers, J. Falk-Andersson, T. Juul-Pedersen and RdA. Metcalfe, et al., Beach litter sources around Nuuk, Greenland: An analysis by UArctic summer school graduate course students, Mar. Pollut. Bull., 2023, 191, 114914, DOI:10.1016/j.marpolbul.2023.114914.
- D. Herzke, P. Ghaffari, J. H. Sundet, C. A. Tranang and C. Halsband, Microplastic Fiber Emissions From Wastewater Effluents: Abundance, Transport Behavior and Exposure Risk for Biota in an Arctic Fjord, Front. Environ. Sci., 2021, 9, 662168, DOI:10.3389/fenvs.2021.662168.
- A. P. W. Barrows, S. E. Cathey and C. W. Petersen, Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins, Environ. Pollut., 2018, 237, 275–284, DOI:10.1016/j.envpol.2018.02.062.
- J.-P. W. Desforges, M. Galbraith, N. Dangerfield and P. S. Ross, Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean, Mar. Pollut. Bull., 2014, 79(1), 94–99, DOI:10.1016/j.marpolbul.2013.12.035.
- M. Granberg, L. W. von Friesen, L. Bach, F. Collard, J. Strand and G. W. Gabrielsen, Anthropogenic Microlitter in Wastewater and Marine Samples from Ny-Ålesund, Barentsburg and Signehamma, Svalbard, IVL Swedish Environmental Research Institute, 2019, Available from: https://pure.au.dk/ws/files/152355861/C373march.pdf Search PubMed.
- Y. Liu, C. Lorenz, A. Vianello, K. Syberg, A. H. Nielsen and T. G. Nielsen, et al., Exploration of occurrence and sources of microplastics (>10 μm) in Danish marine waters, Sci. Total Environ., 2023, 865, 161255, DOI:10.1016/j.scitotenv.2022.161255.
- K. Magnusson, H. Jörundsdóttir, F. Norén, H. Lloyd, J. Talvitie and O. Setälä, Microlitter in Sewage Treatment Systems : A Nordic Perspective on Waste Water Treatment Plants as Pathways for Microscopic Anthropogenic Particles to Marine Systems, Nordisk Ministerråd, Copenhagen, 2016, p. 56, DOI:10.6027/TN2016-510.
- M. Tamminga, E. Hengstmann and E. K. Fischer, Microplastic analysis in the South Funen Archipelago, Baltic Sea, implementing manta trawling and bulk sampling, Mar. Pollut. Bull., 2018, 128, 601–608, DOI:10.1016/j.marpolbul.2018.01.066.
- L. W. von Friesen, M. E. Granberg, O. Pavlova, K. Magnusson, M. Hassellöv and G. W. Gabrielsen, Summer sea ice melt and wastewater are important local sources of microlitter to Svalbard waters, Environ. Int., 2020, 139, 105511, DOI:10.1016/j.envint.2020.105511.
- PAME, Desktop Study on Marine Litter Including Microplastics in the Arctic. Arctic Council, Finland, 2019, Available from: https://oaarchive.arctic-council.org/server/api/core/bitstreams/01a163c2-fae3-4559-b17f-267360cccf31/content Search PubMed.
- J. Strand, L. Bach and T. Juul-Pedersen, Shoreline litter monitoring surveys in Greenland 2016 – 2022: Results from an ongoing monitoring activity involving a local contact network, Second International Symposium on Plastics in the Arctic and Sub-arctic Region, Reykjavik, Island, 2023, Available from: https://pure.au.dk/portal/da/publications/shoreline-litter-monitoring-surveys-in-greenland-2016-2022-result Search PubMed.
- GESAMP, Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean, 2019, Available from: https://www.GuidelinesfortheMonitoringandAssessmentofPlasticLitterintheOcean|GESAMP Search PubMed.
- J. Strand and Z. Tairova, Microplastic Particles in North Sea Sediments 2015, Aarhus University, DCE – Danish Centre for Environment and Energy, 2016, Available from: https://www.MicroplasticparticlesinNorthSeasediments2015(au.dk) Search PubMed.
- L. A. Rasmussen, L. Iordachescu, S. Tumlin and J. Vollertsen, A complete mass balance for plastics in a wastewater treatment plant - Macroplastics contributes more than microplastics, Water Res., 2021, 201, 117307, DOI:10.1016/j.watres.2021.117307.
- European Commission: Joint Research C, D. Fleet, T. Vlachogianni and G. Hanke, Joint List of Litter Categories for Marine Macro-Litter Monitoring – Manual for the Application of the Classification System, Publications Office, 2021, Available from: https://www.Jointlistoflittercategoriesformarinemacro-littermonitoring-PublicationsOfficeoftheEU(europa.eu) Search PubMed.
- K. B. Parga Martínez, V. H. da Silva, T. J. Andersen, N. R. Posth and J. Strand, Improved separation and quantification method for microplastic analysis in sediment: A fine-grained matrix from Arctic Greenland, Mar. Pollut. Bull., 2023, 196, 115574, DOI:10.1016/j.marpolbul.2023.115574.
- F. Liu, K. B. Olesen, A. R. Borregaard and J. Vollertsen, Microplastics in urban and highway stormwater retention ponds, Sci. Total Environ., 2019, 671, 992–1000, DOI:10.1016/j.scitotenv.2019.03.416.
- A. Vianello, R. L. Jensen, L. Liu and J. Vollertsen, Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin, Sci. Rep., 2019, 9(1), 8670, DOI:10.1038/s41598-019-45054-w.
- European Commission: Joint Research C, Guidance on the Monitoring of Marine Litter in European Seas – an Update to Improve the Harmonised Monitoring of Marine Litter under the Marine Strategy Framework Directive, Publications Office of the European Union, 2023, Available from: https://www.JRCPublicationsRepository-GuidanceonthemonitoringofmarinelitterinEuropeanseas(europa.eu) Search PubMed.
- Commission O, CEMP Guidelines for Marine Monitoring and Assessment of Beach Litter, London UK, OSPAR Commission, 2020, Contract No.: OSPAR Agreement 2020-02. Availble from: https://www.OSPAR_MEETING_DOCUMENT Search PubMed.
- J. Y. A. Maréchal, K. Hendriksen, L. T. Hansen, C. Gundelund and P. E. Jensen, Domestic water supply in rural Greenland - sufficiency, affordability and accessibility, Int. J. Circumpolar Health, 2022, 81(1) DOI:10.1080/22423982.2022.2138095.
- Greenland Statistics, https://bank.stat.gl, accessed 2023 Search PubMed.
- L. Pantoja Munoz, A. Gonzalez Baez, D. McKinney and H. Garelick, Characterisation of “flushable” and “non-flushable” commercial wet wipes using microRaman, FTIR spectroscopy and fluorescence microscopy: to flush or not to flush, Environ. Sci. Pollut. Res., 2018, 25(20), 20268–20279, DOI:10.1007/s11356-018-2400-9.
- T. Allison, B. D. Ward, M. Harbottle and I. Durance, Do flushed biodegradable wet wipes really degrade?, Sci. Total Environ., 2023, 894, 164912, DOI:10.1016/j.scitotenv.2023.164912.
- S. V. Afshar, A. Boldrin, T. F. Astrup, A. E. Daugaard and N. B. Hartmann, Degradation of biodegradable plastics in waste management systems and the open environment: A critical review, J. Clean. Prod., 2024, 434, 140000, DOI:10.1016/j.jclepro.2023.140000.
- M. Flury and R. Narayan, Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment, Curr. Opin. Green Sustainable Chem., 2021, 30, 100490, DOI:10.1016/j.cogsc.2021.100490.
- Society MC, Great British Beach Clean 2019 Report, Marine Conservation Society, Herefordshire, UK, 2019, Available from: https://www.774-2019GBBC2019ReportProofing.indd(solwayfirthpartnership.co.uk) Search PubMed.
- F. Amélineau, D. Bonnet, O. Heitz, V. Mortreux, A. M. A. Harding and N. Karnovsky, et al., Microplastic pollution in the Greenland Sea: Background levels and selective contamination of planktivorous diving seabirds, Environ. Pollut., 2016, 219, 1131–1139, DOI:10.1016/j.envpol.2016.09.017.
- Y. Jiang, F. Yang, Y. Zhao and J. Wang, Greenland Sea Gyre increases microplastic pollution in the surface waters of the Nordic Seas, Sci. Total Environ., 2020, 712, 136484, DOI:10.1016/j.scitotenv.2019.136484.
- A. L. Lusher, V. Tirelli, I. O'Connor and R. Officer, Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples, Sci. Rep., 2015, 5(1), 14947, DOI:10.1038/srep14947.
- P. S. Ross, S. Chastain, E. Vassilenko, A. Etemadifar, S. Zimmermann and S.-A. Quesnel, et al., Pervasive distribution of polyester fibres in the Arctic Ocean is driven by Atlantic inputs, Nat. Commun., 2021, 12(1), 106, DOI:10.1038/s41467-020-20347-1.
- J. Lee, S. Jeong and K.-J. Chae, Discharge of microplastics fibres from wet wipes in aquatic and solid environments under different release conditions, Sci. Total Environ., 2021, 784, 147144, DOI:10.1016/j.scitotenv.2021.147144.
- O. Ó Briain, A. R. Marques Mendes, S. McCarron, M. G. Healy and L. Morrison, The role of wet wipes and sanitary towels as a source of white microplastic fibres in the marine environment, Water Res., 2020, 182, 116021, DOI:10.1016/j.watres.2020.116021.
- F. Salvador Cesa, A. Turra and J. Baruque-Ramos, Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings, Sci. Total Environ., 2017, 598, 1116–1129, DOI:10.1016/j.scitotenv.2017.04.172.
- M. R. Kelly, N. J. Lant, M. Kurr and J. G. Burgess, Importance of Water-Volume on the Release of Microplastic Fibers from Laundry, Environ. Sci. Technol., 2019, 53(20), 11735–11744, DOI:10.1021/acs.est.9b03022.
- Electrolux, The Truth about Laundry (Microplastic Edition), 2022, Available from: https://www.electroluxgroup.com/wp-content/uploads/sites/2/2022/03/electrolux-electrolux-launches-microplastic-filter-to-help-tackle-rising-tide-of-plastic-pollution-220331.pdf Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00233d |
| This journal is © The Royal Society of Chemistry 2025 |