Open Access Article
Open Access ArticleFacile detection of microplastics from a variety of environmental samples with conjugated polymer nanoparticles†
Angela
Awada‡
a,
Mark
Potter‡
a,
Julian
Aherne
b,
Sarah
Lavoie-Bernstein
c,
Miriam L.
Diamond
 d,
Paul A.
Helm
e,
Liisa
Jantunen
c,
Brittany
Welsh
b,
Bulent
Mutus
*a and
Simon
Rondeau-Gagné
d,
Paul A.
Helm
e,
Liisa
Jantunen
c,
Brittany
Welsh
b,
Bulent
Mutus
*a and
Simon
Rondeau-Gagné
 *a
*a
aDepartment of Chemistry & Biochemistry, University of Windsor, 401 Sunset Ave., Windsor, Ontario N9B 3P4, Canada. E-mail: mutusb@uwindsor.ca; srondeau@uwindsor.ca
bSchool of the Environment, Trent University, 1600 West Bank Drive, Peterborough, Ontario K9L 0G2, Canada
cAir Quality Processes Research Section, Environment and Climate Change Canada, Egbert, ON L0L 1N0, Canada
dDepartment of Earth Sciences, University of Toronto, 22 Ursula Franklin St., Toronto, Ontario M5S 3B1, Canada
eOntario Ministry of the Environment, Conservation and Parks, 125 Resources Road, Toronto, Ontario M9P 3V6, Canada
First published on 3rd December 2024
Abstract
Microplastic pollution constitutes a pressing global environmental issue impacting nearly every facet of human activity. This specific environmental challenge exerts profound yet still poorly understood influences on health, social dynamics, and industrial practices. A major obstacle for further investigation and mitigation of microplastics lies in their heterogeneity in size and composition. Additionally, the multitude of sources contributing to microplastic emissions further complicates their study. To enhance current detection and analytical methodologies for microplastics, this study exploits a novel approach for the easy and specific identification of microplastics within diverse environmental samples (including air, soil, lake water, rain, snow, and marine sediment) collected from various geographical locations across Canada. This method relies on fluorescent conjugated polymer nanoparticles that can be used to identify microplastics after minimal preparation. In all examined samples, originating from diverse sources and environments, microplastics were consistently present in the form of fragments and/or fibers, with polyethylene terephthalate (PET) emerging as the most abundant type, as confirmed via Raman spectroscopy either before or after labeling. This approach significantly streamlines the microplastic identification process, reducing the time needed for extraction and isolation. Our findings corroborate the efficacy of nanoparticle labeling for microplastic detection, offering promising avenues for their facile, specific, and reliable identification. Ultimately, this novel procedure holds potential to enhance remediation efforts targeting microplastics in the environment, thereby advancing our understanding of their global impact.
Environmental significanceMicroplastic (MP) pollution is a global issue that poses significant health, social, and industrial risks. More than ever, sensitive, specific, and direct analytical methods are needed to measure MP concentrations and trace their origins. Current methods are complex, costly, and time-consuming. This work demonstrates the effectiveness of new conjugated polymer nanoparticles for detecting MPs in diverse environmental samples with minimal processing. This fluorescence-based approach, tested on air, rain, snow, water, soil, and sediment across Canada, showed consistent MP detection verified by Raman spectroscopy. Our method's simplicity and sensitivity offer new opportunities for MP pollution mitigation and sensing device development, offering novel and original avenues for the evaluation and mitigation of microplastics, ultimately resulting in important impact across environmental sciences. |
Introduction
Microplastic (MP) contamination of water and air streams has become one of the major environmental concerns of the 21st century.1–3 It is estimated that cumulative production of plastics is ∼8.3 billion tons. Of this, ∼6.3 billion tons are waste, yet only 9% of the waste is recycled.4 In the environment, MPs are formed by the natural degradation of plastic waste.5 Therefore, it is worrisome that this amount of plastic waste will in time, pose an overwhelming MP accumulation problem in the environment. This constitutes a global challenge that remains to be fully understood especially with respect to the effects of MPs on living systems.6,7 The key to elucidating the effects of a potential toxin such as MPs, requires sensitive, specific, and direct analytical methods for determining their concentration (dose) in untreated environmental samples.8 These same techniques can also be utilized to effectively identify specific sources and pathways for MPs to enter and how they are temporally distributed across the environment.The current MP identification and detection methods are often time consuming due their heavy reliance on multiple, laborious, complex extraction, and concentration steps.9–11 The final MP identification is then accomplished by Fourier-transform infrared (FTIR) spectroscopy or Raman spectroscopy and, to a minor extent, gas chromatography and various mass spectrometry techniques.12–16 While often showing high resolution, these detection methods are less than ideal for detection at the source of contamination due to their complexity, lack of portability, and costs.13,17,18 Optical detection of microplastics has recently been investigated using fluorescent dyes such as Nile Red.19,20 Notably, this approach shows great promise in streamlining the detection and identification workflow for various types of microplastics. While this field is advancing rapidly, the continued development and refinement of precise detection techniques for microplastics remains critically important in our efforts to understand and combat the pervasive issue of plastic pollution in the environment. Each environmental source and condition presents a unique set of challenges and characteristics, making it essential to have specific detection methods tailored to these variables.21–23 By honing our ability to probe MPs in diverse environmental components (air, water, sediment, etc.), we can gather crucial data to guide targeted interventions and make informed decisions for mitigating the environmental impact of these persistent pollutants.
Our team previously reported a universal high-affinity probe for the detection of MP contaminants that was based on conjugated polymer nanoparticles (CPNs).24 These MP-specific nanoparticles termed, F-HA-CPNs, were prepared from the nanoprecipitation of a semiconducting polymer and a fluorescein-tagged hyaluronic acid (F-HA), an amphiphilic polymer. The F-HA-CPNs were found to selectively bind with picomolar affinity to pristine lab-made MPs even in the presence of soil collected from outdoor surroundings. The CPNs were also tested on an environmental sample obtained through aqueous filtration/concentration, demonstrating the ability to detect MPs through fluorescence microscopy. While this work highlighted the potential of CPNs to specifically bind to MPs, the development of this approach was performed mostly on controlled samples, which can be significantly different in terms of surface and physicochemical properties from environmental samples collected directly at the source of emission.25,26
To further explore the detection of MPs through labelling with CPNs, and to expand their application to various environmental MP samples, this work investigates and provides compelling evidence that the recently developed F-HA-CPNs can maintain their specific interactions with MPs from a myriad of environmental samples, enabling the fluorescence-based detection of MP contaminants irrespective of their origins, collection techniques, and structural characteristics. Here, we tested the F-HA-CPNs on environmental samples including air, rain, snow, lake water, soil, and marine sediment samples isolated from various locations across Canada (Fig. 1). These samples underwent minimal processing to simplify analysis and were then directly mixed with F-HA-CPNs, followed by characterization by fluorescence microscopy. In all cases, the particles yielding positive fluorescent signals with F-HA-CPNs, were verified by Raman spectroscopy as being a MP polymer. Overall, this work highlights the potential for the widespread application of the F-HA-CPNs to identify a wide spectrum of MP samples, from different environmental contexts and structural features. This also opens new avenues for further exploration and adaptation of this approach in the ongoing effort to address the challenges posed by MP pollution in our communities.
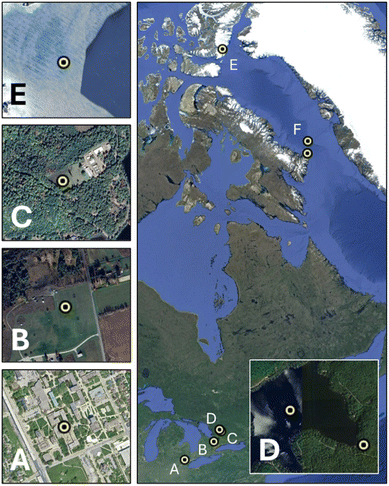 | ||
| Fig. 1 Geographical map of the samples investigated. Location of sampling sites for (A) soil, (B) air, (C and D) rainfall, snow and surface water at Harp Lake, and (E and F) sediment samples. | ||
Materials
Tetrahydrofuran (THF), sodium phosphate, and sodium dodecyl sulfate (SDS) were purchased from Millipore Sigma Canada (Oakville, Canada). Fluorescein-labeled hyaluronic acid was purchased from TdB Laboratories (Uppsala, Sweden). Poly(diketopyrrolopyrrole-co-thiophene) was synthesized via Stille cross-coupling polycondensation. Detailed experimental procedures can be found in ESI.†Instrumentation
Raman spectroscopy was performed using a WiTec Alpha 300R system (Raman, AFM, True surface Profilometer, and SNOM) using a 532 nm laser and 20× objective lens. All Raman spectral data was processed using Project FIVE (5.2) software and matched using the Wiley KnowItAll spectral database. Dynamic scattering techniques were carried out using a Panalytical Zetasizer (Malvern) and nanoparticle concentrations were obtained using a Viewsizer 3000 (Manta Instruments). Fluorescent microscope images were obtained on an epifluorescent Zeiss Axiovert 200 with a fluorescein filter set from Chroma (Bellows Falls, VT). Water-based samples were freeze dried using the Labconco Freeze Dry System, Freezone 4.5.Preparation of F-HA-CPNs
Fluorescein-labeled hyaluronic acid conjugated polymer nanoparticles were prepared following previously reported procedures.24,27,28 Nanoparticles showed a bimodal size distribution, with a zeta-average of 126 nm and polydispersity index of 0.54, as measured by dynamic light scattering techniques. Nanoparticle concentration in water was 1.86 × 1010 particles per mL, as determined by nanoparticle tracking analysis. Samples were stored at 4 °C prior to use.Environmental sample collection
The samples were collected according to previously reported procedures.29,30 Soil samples were collected using a stainless-steel trowel from the campus of the University of Windsor, Windsor, Ontario (42.305°N, −83.067°W) on October 23, 2023 (Fig. 1A). Air samples were collected using passive air sampling slides at a rural site about 1 hour north of Toronto (45.380°N, −79.135°W) on June 14, 2022 (Fig. 1B). Three water samples were collected from Muskoka, Ontario (44.233°N, −79.780°W) (Fig. 1C and D). On March 7, 2023, a snow sample was collected from the frozen surface of Harp Lake (45.380°N, −79.135°W) using a pre-cleaned shovel and 18 L bucket. On July 11, 2023, a grab sample was collected from the outflow of Harp Lake (45.376°N, −79.126°W) at the point of monitored discharge using a 500 mL PET jar that was triple rinsed with filtered Reverse Osmosis water and stream water. A two-week rainfall sample (13–27 June 2023) was collected nearby from the Dorset Environmental Science Centre (45.221°N, −78.932°W) using a bulk precipitation collector. Marine sediment samples were collected during August and September 2021, from the eastern Canadian Arctic (77.197°N, −78.593°W) (Fig. 1E and F) using a box corer from the CCGS Amundsen (depths varying from 641–1372 m).Sample treatment procedure pre-detection
For water-based samples (Harp Lake outflow, snow, and rainfall), 40 mL of each sample was frozen and successively freeze dried using the Labconco Freeze Dry System for a duration of 48 hours. For the sediment samples, 0.1 g of sediment was subjected to an SDS solution, the mixture was agitated until bubbles formed, isolating MPs in the lipophilic meniscus. The bubbles were deposited, dried and fixed on tape on glass slides before being analyzed by Raman Spectroscopy.31 Once the samples were collected minimal processing was performed which included graph smoothing and cosmic ray removal followed by background subtraction and normalization on each of the raw spectrum. Air and soil samples were analyzed by Raman spectroscopy without any initial preparation. All samples were rinsed with a dilute SDS solution and successively labelled with 10 pM of the F-HA-CPN probe in a phosphate buffer solution (0.1 M, pH 8.5) for observation using fluorescence microscopy. A volume between 10 to 50 μL of CPNs was used for labelling depending on the sample size. Tape was used to adhere the particles from various freeze-dried water-based samples to microscope slide for both Raman and fluorescent identification. The composition of these tapes includes PET, high ethylene random copolymer, and PP, of which PP was primarily used (see ESI†). Careful consideration was taken to ensure the Raman was calibrated and was precisely aligned with the camera focal point during acquisition to avoid misidentification of MP species.MP sources and details
Environmental samples (air, rainfall, snow, lake water, soil, and marine sediment) for microplastics analysis were collected from a range of study sites across southern Ontario and the Canadian Arctic (Fig. 1); these sites have been used to assess the transport and fate of microplastics. Extracting microplastics from different environmental media is labour intensive, requiring media-specific protocols, which is further impeded by low microplastic concentrations typically observed in remote locations.32,33 Soil samples collected from the University of Windsor were representative of urban microplastic contamination sources. The air samples were collected from the Centre for Atmospheric Research Experiments, which is a regionally representative monitoring station operated by Environment and Climate Change Canada; the samples were collected using slides that were coated with an adhesive to capture atmospheric microplastics, which presents a challenge for spectrometric analysis techniques. Rainfall, snow, and surface water was collected from Harp Lake, which is a rural headwater lake, more than 200 km away from large urban and industrial centers. Harp lake has been intensively monitored by the Ministry of the Environment, Conservation, and Parks (Ontario, Canada) since the mid-1970s to better understand the impacts of multiple stressors including atmospheric deposition on water quality. The primary source of microplastics in rainwater and snow is postulated to be atmospheric transport from populated areas. In addition, the outflow from Harp Lake is influenced by the 96 shoreline residences. The sediment samples were collected from remote regions with diffuse sources in the northern Atlantic Ocean along with water that has been transported through the Canadian Archipelago. In general, sediment and soil are challenging matrices, as separating plastics from organic and inorganic matter is labour intensive and time consuming, requiring a digestion step to remove organic matter, followed by density separation to extract plastic from mineral particles.MP identification
The chemical composition of the samples was confirmed by comparing the Raman spectra with the Wiley KnowItAll spectral database. A hit quality index (HQI) above 80% was considered a very strong match, indicating high confidence in the identification. An HQI between 70–80% was regarded as a reasonably good match also indicating confidence in the identity of the material. HQI values below 70% were considered poor, often indicating noisy spectra and some uncertainty in the identity which may require further analysis due to potential factors like sample degradation or contamination. For these samples, common sense was used when confirming the identity of the species by considering peak location and intensity. For indicated samples (see ESI†), a second HQI match was selected only if it more accurately represented the experimental data based on the number and location of peaks. In addition to single-component matches, 2- and 3-component systems were considered using the Wiley KnowItAll spectral database when applicable and specified.Results and discussion
MP detection
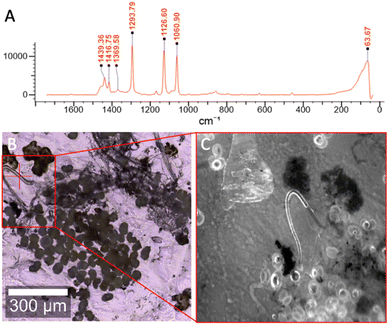
The fluorescent MP-labelling technique employed here allows for the easy detection of MPs in complex samples. The image in Fig. 2 depicts representative data showing the workflow for the identification of MPs in each sample discussed. In each case, environmental samples were surveyed for MP using Raman spectroscopy (Fig. 2A). Next, these samples were visualized under an optical microscope (Fig. 2B) and finally, via fluorescence microscopy after the application of F-HA-CPNs (Fig. 2C). Notably, non-plastic particles were not labeled as shown by the dark or gray spots (Fig. 2C), demonstrating the selectivity of the F-HA-CPNs to MPs. This Raman validation followed by fluorescent labelling approach was utilized for all samples discussed below, unless otherwise specified. It is important to note that the workflow used is the reverse of what would ultimately be employed by end users in the field to label, detect, and identify microplastics. This reverse workflow was deliberately chosen to confirm the presence of microplastics in environmental samples using established protocols prior to verify the accuracy and reliability of the detection method and its selectivity.The F-HA-CPNs specifically labeled the MPs despite the presence of large amounts of non-plastic contamination. This was confirmed by Raman spectroscopy, further demonstrating the utility and simplicity of the F-HA-CPN method for the detection of MPs.
Detection of MPs in water-based samples
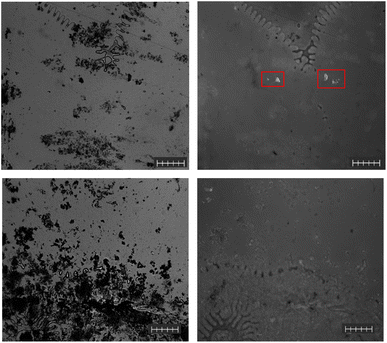
Our investigation began by focusing on water-based samples, which inherently pose a range of distinct sampling issues and important analytical challenges.17 One of the primary challenges stems from the variability in the volume of water samples across different surveys, where only a fraction of these samples can undergo analysis.34–36 Further, samples are generally limited to surface water collection, omitting the potential for MPs to settle at different water densities.35,37,38 Samples taken from non-urban areas generally require large volumes to detect the diversity of MPs. To overcome the difficulties of working with large sample volumes, the typical method involves the filtration of substantial volumes through nets, with the detection and abundance of MPs in the water samples being intricately linked to the characteristics of the sampling nets. Specifically, these factors are closely dependent on the mesh size and opening area of the net, generally resulting in the loss or under reporting of smaller MPs (those less than the net mesh size). Consequently, the development of an efficient and streamlined approach for the detection of MPs in various water-based samples becomes highly desirable.For the water samples investigated, freeze-drying was utilized to concentrate the samples with minimal risk of losing smaller MPs. The water-based samples were then resuspended in a minimal amount of F-HA-CPNs diluted in a phosphate buffer solution. The buffer solution was observed on the microscope using the F-HA-CPNs before use to ensure there were no MPs present contaminating the solution (Fig. S1†). The water-based samples analyzed in this study include rain, snow, and lake water samples. These samples contained a variety of different MP compositions and morphology. In these samples, the MPs were selected against common natural debris. This is evident in Fig. 3 which depicts the presence of organic matter, or non-plastic particles, which were not labeled. Once again, this can be observed as dark debris under visible light (Fig. 3, left) and absent of fluorescent signal when using fluorescence microscopy (Fig. 3, right), while MPs were labelled, illustrating the selectivity of the F-HA-CPNs to MPs (Fig. 3, top right).
In the Harp Lake water samples, MPs were successfully detected by the CPNs, and their identity was confirmed by Raman spectroscopy. These identified MPs took the form of both fibers, and fragments of various sizes. Fig. 4 depicts both visible and fluorescent images (left and right, respectively) of Harp Lake water, snow, and rain samples (top to bottom, respectively). In the Harp Lake water sample, there was an abundance of fragments, mostly PET or polyester (Fig. S2†) with the presence of some fibers, which were generally types of PE (Fig. 2). Additionally, a PET/p-(vinyl butyral) blend was identified (Fig. S3†) as well as low-density polyethylene (LDPE, Fig. S4†). Some contaminants were also identified including nanoscale tris(2-ethylhexyl) trimellitate, a plasticizer for polyvinyl chloride (PVC, Fig. S5†). Notably, using the F-HA-CPNs allowed for the detection of a relatively high quantity of micron-size MPs within the samples, which is more than 40 times higher than previous work focusing on headwater lakes in the same region (average lake microplastic concentration of 1.78 particles per L).29 While further validation is imperative, the high number of particles detected by the CPN can be attributed to its ability to detect smaller particles that are often missed by traditional methods such as Raman microscopy. Additionally, with the minimal preparation that is required, there is less possibility of losing MPs, unlike traditional methods.
In contrast to Harp Lake water, snow samples contained the lowest relative number of MP particles. Nevertheless, the identity of the MPs was generally PET (Fig. S6†) in the form of fragments (Fig. 4, middle) but LDPE (Fig. S7†) was also identified. In addition to the microplastics identified by the new F-HA-CPN method, Raman spectroscopy revealed additional non-labelled contaminants, including dolomite (Fig. S8†), and quartz (Fig. S9†). Finally, the rain samples contained relatively low amounts of MPs; however, PET (Fig. S10†) was still the most abundant (Fig. 4, bottom). In addition, when considering only a one component system trioctyl trimellitate was identified (Fig. S11A†); however, when considering a 3-component system, this spot was identified as a blend of PET, PP, and bis(2-ethylhexyl) adipate (Fig. S11B†). Due to the significantly higher HQI of the 3-component system, this was selected as the identity. The morphology of the MPs in these samples varied from fragments to fibers. Nevertheless, the F-HA-CPNs were capable of selectively and sensitively binding to the various MP compositions and morphologies identified regardless of their characteristics (Fig. 4). In the snow and rain samples depicted in Fig. 4, there are little if any non-plastic debris present, which was not always the case (Fig. 3); the presence of organic matter (likely plant matter) as well as various minerals were observed in the water samples using Raman spectroscopy.
Detection of MPs in sediment and soil samples
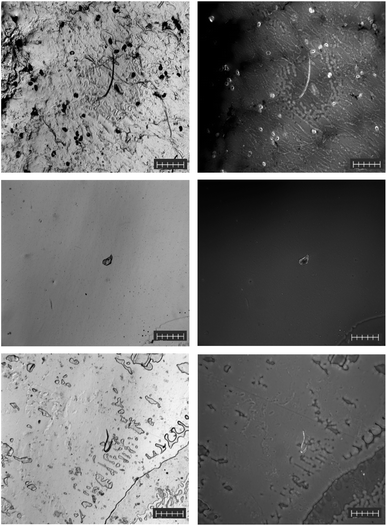
The dry soil samples did not undergo any pre-detection preparation (Fig. 5, top left) and were directly deposited onto a glass microscope slide before being labelled with the F-HA-CPNs and characterized by fluorescent microscopy. Raman spectroscopy was finally used to confirm accurate MP identification by the F-HA-CPNs. The soil sample contained PET (Fig. S12†) predominantly in the form of fragments, which was successfully labeled using the CPNs and is shown as the bright spot in Fig. 5, top right. Here, the black background is non-plastic organic debris found within the soil, which was not labelled by the CPNs, further demonstrating the selectivity of the probe to MPs. In contrast, the sediment samples (0.1 g) required some sample preparation via SDS soap isolation, which was used to reduce the amount of organic debris present and to concentrate the MPs present. These samples were found to contain PET (Fig. S13†) in the form of fragments with some fibers present as well. In addition to PET, polyester (Fig. S14†) MPs were also identified via Raman spectroscopy. Cellulose (Fig. S15A†) was also identified via Raman spectroscopy; however, when this particle was considered as a 3-component system, it had a significantly higher HQI for a blend of cellulose, PA-1121, and methylene blue (Fig. S15B†). Thus, the 3-component system was taken as the true identity. It is important to mention that our overall findings are in agreement with previous reports investigating Canadian arctic sediment samples for MPs.39 These reports confirmed concentrations of MPs in sediments ranging from 0.6 to 4.7 particles per g dry weight (dw). Microfibers comprised 82% of all these MPs, followed by fragments at 15%.40 While final concentrations were not determined, our method of detection confirmed the previous observations performed through optical microscopy/Raman spectroscopy identification procedures. The non-labelled matter, identified through Raman spectroscopy, was also observed in relatively large amounts in previous reports on Canadian artic sediment samples. This matter, primarily consisted of minerals such as quartz (Fig. S16†), and labradorite (Fig. S17†) were shown as dark spots present in the labeled sample (Fig. 5, bottom right). Notably, the fluorescence detection employed is not limited by the size of the particle as fluorescence microscopy can detect from microscale to single molecules. In all cases, the MPs detectable by the fluorescent microscope were selectively labelled with the F-HA-CPN (Fig. 5).Detection of MPs in air samples
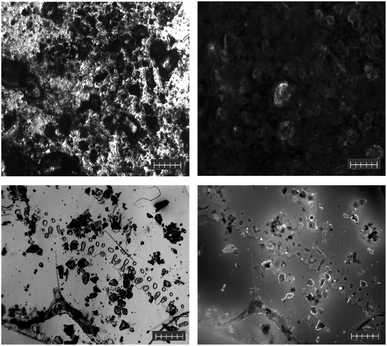
Like the soil samples, the air samples did not require any preparation before their analysis by Raman spectroscopy. This was due to the nature of the samples, which were captured in a relatively small surface area, i.e., on a microscope slide. In addition, these samples were stuck directly to the sampling adhesive, making it difficult to remove and preprocess. Due to the hydrophobic nature of the samples after collection, a small amount of SDS was applied to the microscope slides to ensure an even distribution of the water-soluble F-HA-CPNs. A pristine unused passive air sampling slide was exposed to and labelled with F-HA-CPNs in phosphate buffer to test for the possibility of MP contamination (Fig. S18†). The results reveal that the air sampling slides did not contain any MPs prior to sampling. In this sample, the predominant MP identified was PET fibers (Fig. S19†). This sample contained very little contamination with organic and inorganic debris, and due to the high concentration of PET MP fibers within the sample, the fluorescent image was well labelled and bright (Fig. 6, right). This composition was to be expected, as PET has been identified in various reports to be one of the major constituents of airborne microplastic.41–43 While concentrations were not determined in the air samples, our detection methods confirm the presence of a large quantity of PET fibers and fragments in these samples. This non-quantitative finding supports findings from the literature from various cities in Europe and Asia that reported concentrations of this airborne pollutants up to 400 particles per m2.44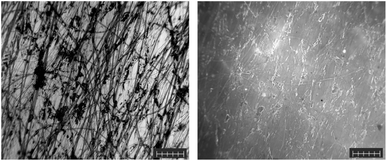 | ||
| Fig. 6 Visible (left) and fluorescent (right) images of polyethylene terephthalate microplastic fibers from an air sample. Scale bars are 100 microns. | ||
Since confirming the nature of microplastics through Raman spectroscopy on-site is challenging due to the technique's lack of portability, we investigated post-labeling identification using the CPNs. To ensure that CPNs do not interfere with Raman spectroscopy identification, we treated a native microplastic sample—composed of polycarbonate (PC), PET, PP, and polystyrene (PS)—with F-HA-CPN (10 pM) on microscope slides and dried them overnight. These samples were then subjected to Raman spectroscopy. As shown in Fig. S20–S23,† the Raman spectroscopy successfully identified the true composition of all samples, regardless of the labeling with F-HA-CPNs. This demonstrates the ability to first visualize the microplastics using CPNs and then successively characterize and identify the composition of the labeled microplastics via Raman spectroscopy.
Conclusions
This work explored the utilization of conjugated polymer nanoparticles for the selective labeling and identification of microplastic contaminants in various environmental samples obtained from different media. To verify the potential of this new labeling technique as an alternative tool that can work across various media, our investigation focused on microplastic samples obtained from soil, air, rain, lake water, snow, and marine sediment. In all samples, microplastics were present in the form of fragments and/or fibers, with PET being the most abundant type, as confirmed via Raman spectroscopy. The reasons behind this finding are not fully understood, but it may stem from an underestimation of PET abundance in environmental samples using current methods. While soap-based extraction has not been shown to selectively target PET, this possibility cannot be entirely ruled out and warrants further investigation. The novel identification and detection method explored in this study requires minimal to no sample preparation and has proven efficient for detecting particles ranging from micron to sub-micron sizes. Additionally, the use of fluorescence spectroscopy enables the identification of various types of MPs and is not limited by sample size; with appropriate photon counting techniques, fluorescence microscopy can even detect single molecules. As previously reported, our probe demonstrated a high affinity for microplastics compared to common contaminants found in samples. It is important to note that many parameters still need to be controlled and optimized to achieve rapid detection of all microplastics, regardless of their sources and size. Moreover, the nanostructure and surface chemistry of the microplastics and their impact on detection remain to be fully understood and optimized. Given their synthetic tunability and affinity modulation through design, conjugated polymer nanoparticles represent a promising tool for detecting environmental contaminants directly at the point of emission. Their use in conjunction high-throughput devices, including microfluidics, is also particularly promising for rapid labeling and isolation of MPs for further source tracing. As research into remediation practices for microplastics in the environment continues to expand, advancements in identification methods, such as the work presented here, are crucial for enhancing our collective understanding of the effects of microplastics on the environment.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
S. R.-G. and B. M. conceived and directed the project. A. A. and S. R.-G. synthesized and characterized the F-HA-CPNs and their precursors. A. A. and M. P. performed all fluorescence measurements and data analysis. A. A. and M. P. performed the complete evaluation of the microplastics in environmental samples, collected all data and performed the analysis. All coauthors wrote the manuscript and commented on the manuscript. J. A., S. L.-B., M. L. D., P. A. H., L. J. and B. W. collected microplastic samples and assisted with data analysis. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This study was financially supported by the Natural Science and Engineering Research Council of Canada (NSERC) through a Plastics Science for a Cleaner Future grant (ALLRP-558429-20). This work was also supported by NSERC through Discovery Grants (S. R.-G., RGPIN-2022-04428; B. M., RGPIN-2017-04925). S. R.-G. also acknowledges the Canada Foundation for Innovation (CFI) and Ontario Research Fund (ORF) for supporting research infrastructure, and the University of Windsor for financial support through a Vice-President Research & Innovation Research Chair. A. A. thanks the Government of Ontario for support through an Ontario Graduate Scholarship (OGS). M. P. thanks NSERC for financial support through a Canada Postgraduate Scholarship – Doctoral. This work is dedicated to Prof. Jill Crossman. ToC was created in BioRender. Awada, A. (2024) https://BioRender.com/q00m958.References
- D. Barceló and Y. Picó, Microplastics in the global aquatic environment: Analysis, effects, remediation and policy solutions, J. Environ. Chem. Eng., 2019, 7, 103421 CrossRef.
- D. Klingelhöfer, M. Braun, D. Quarcoo, D. Brüggmann and D. A. Groneberg, Research landscape of a global environmental challenge: Microplastics, Water Res., 2020, 170, 115358 CrossRef.
- A. Thacharodi, R. Meenatchi, S. Hassan, N. Hussain, M. A. Bhat, J. Arockiaraj, H. H. Ngo, Q. H. Le and A. Pugazhendhi, Microplastics in the environment: A critical overview on its fate, toxicity, implications, management, and bioremediation strategies, J. Environ. Manage., 2024, 349, 119433 CrossRef CAS PubMed.
- Global Plastics Outlook, https://www.oecd-ilibrary.org/environment/data/global-plastic-outlook_c0821f81-en, accessed 29 January 2024.
- N. Laskar and U. Kumar, Plastics and microplastics: A threat to environment, Environ. Technol. Innov., 2019, 14, 100352 CrossRef.
- E. Issaka, S. Yakubu, H. Sulemana, A. Kerkula and O. Nyame-do Aniagyei, Current status of the direct detection of microplastics in environments and implications for toxicological effects, J. Adv. Chem. Eng., 2023, 14, 100449 CrossRef CAS.
- M. Sharifinia, Z. A. Bahmanbeigloo, M. Keshavarzifard, M. H. Khanjani and B. P. Lyons, Microplastic pollution as a grand challenge in marine research: A closer look at their adverse impacts on the immune and reproductive systems, Ecotoxicol. Environ. Saf., 2020, 204, 111109 CrossRef CAS PubMed.
- Z. Akdogan and B. Guven, Microplastics in the environment: A critical review of current understanding and identification of future research needs, Environ. Pollut., 2019, 254, 113011 CrossRef CAS PubMed.
- A. B. Silva, A. S. Bastos, C. I. L. Justino, J. P. da Costa, A. C. Duarte and T. A. P. Rocha-Santos, Microplastics in the environment: Challenges in analytical chemistry - A review, Anal. Chim. Acta, 2018, 1017, 1–19 CrossRef CAS PubMed.
- F. Stock, C. Kochleus, B. Bänsch-Baltruschat, N. Brennholt and G. Reifferscheid, Sampling techniques and preparation methods for microplastic analyses in the aquatic environment – A review, Trac. Trends Anal. Chem., 2019, 113, 84–92 CrossRef CAS.
- S. Huppertsberg and T. P. Knepper, Instrumental analysis of microplastics—benefits and challenges, Anal. Bioanal. Chem., 2018, 410, 6343–6352 CrossRef CAS PubMed.
- Y. Picó and D. Barceló, Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics, Trac. Trends Anal. Chem., 2020, 130, 115964 CrossRef.
- C. F. Araujo, M. M. Nolasco, A. M. P. Ribeiro and P. J. A. Ribeiro-Claro, Identification of microplastics using Raman spectroscopy: Latest developments and future prospects, Water Res., 2018, 142, 426–440 CrossRef CAS.
- A. Cincinelli, C. Scopetani, D. Chelazzi, E. Lombardini, T. Martellini, A. Katsoyiannis, M. C. Fossi and S. Corsolini, Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR, Chemosphere, 2017, 175, 391–400 CrossRef CAS PubMed.
- W. J. Shim, S. H. Hong and S. E. Eo, Identification methods in microplastic analysis: a review, Anal. Methods, 2017, 9, 1384–1391 RSC.
- J. C. Prata, J. P. da Costa, A. C. Duarte and T. Rocha-Santos, Methods for sampling and detection of microplastics in water and sediment: A critical review, Trac. Trends Anal. Chem., 2019, 110, 150–159 CrossRef CAS.
- A. M. Elert, R. Becker, E. Duemichen, P. Eisentraut, J. Falkenhagen, H. Sturm and U. Braun, Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters?, Environ. Pollut., 2017, 231, 1256–1264 CrossRef CAS PubMed.
- A. Käppler, M. Fischer, B. M. Scholz-Böttcher, S. Oberbeckmann, M. Labrenz, D. Fischer, K.-J. Eichhorn and B. Voit, Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments, Anal. Bioanal. Chem., 2018, 410, 5313–5327 CrossRef PubMed.
- T. Maes, R. Jessop, N. Wellner, K. Haupt and A. G. Mayes, A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red, Sci. Rep., 2017, 7, 44501 CrossRef CAS PubMed.
- M. C. Konings, L. Zada, R. W. Schmidt and F. Ariese, Optimization of sample preparation, fluorescence- and Raman techniques for environmental microplastics, Spectrochim. Acta, Part A, 2024, 319, 124537 CrossRef CAS.
- L. Melymuk, M. Robson, P. A. Helm and M. L. Diamond, Application of Land Use Regression to Identify Sources and Assess Spatial Variation in Urban SVOC Concentrations, Environ. Sci. Technol., 2013, 47, 1887–1895 CrossRef CAS.
- J. Crossman, R. R. Hurley, M. Futter and L. Nizzetto, Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment, Sci. Total Environ., 2020, 724, 138334 CrossRef CAS PubMed.
- P. K. Hopke, Review of receptor modeling methods for source apportionment, J. Air Waste Manag. Assoc., 2016, 66, 237–259 CrossRef.
- A. Awada, M. Potter, D. Wijerathne, J. W. Gauld, B. Mutus and S. Rondeau-Gagné, Conjugated Polymer Nanoparticles as a Universal High-Affinity Probe for the Selective Detection of Microplastics, ACS Appl. Mater. Interfaces, 2022, 14, 46562–46568 CrossRef CAS PubMed.
- K. J. Wiggin and E. B. Holland, Validation and application of cost and time effective methods for the detection of 3–500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California, Mar. Pollut. Bull., 2019, 143, 152–162 CrossRef CAS PubMed.
- G. Vandermeersch, L. Van Cauwenberghe, C. R. Janssen, A. Marques, K. Granby, G. Fait, M. J. J. Kotterman, J. Diogène, K. Bekaert, J. Robbens and L. Devriese, A critical view on microplastic quantification in aquatic organisms, Environ. Res., 2015, 143, 46–55 CrossRef CAS PubMed.
- A. Langlois, G. T. Mason, M. H. L. Nguyen, M. Rezapour, P.-L. Karsenti, D. Marquardt and S. Rondeau-Gagné, Photophysical and Optical Properties of Semiconducting Polymer Nanoparticles Prepared from Hyaluronic Acid and Polysorbate 80, ACS Omega, 2019, 4, 22591–22600 CrossRef CAS PubMed.
- D. Lubanska, S. Alrashed, G. T. Mason, F. Nadeem, A. Awada, M. DiPasquale, A. Sorge, A. Malik, M. Kojic, M. A. R. Soliman, A. C. deCarvalho, A. Shamisa, S. Kulkarni, D. Marquardt, L. A. Porter and S. Rondeau-Gagné, Impairing proliferation of glioblastoma multiforme with CD44+ selective conjugated polymer nanoparticles, Sci. Rep., 2022, 12, 12078 CrossRef CAS.
- B. Welsh, J. Aherne, A. M. Paterson, H. Yao and C. McConnell, Spatiotemporal variability of microplastics in Muskoka-Haliburton headwater lakes, Ontario, Canada, Environ. Earth Sci., 2022, 81, 551 CrossRef CAS.
- B. Welsh, J. Aherne, A. M. Paterson, H. Yao and C. McConnell, Atmospheric deposition of anthropogenic particles and microplastics in south-central Ontario, Canada, Sci. Total Environ., 2022, 835, 155426 CrossRef CAS PubMed.
- X.-T. Bui, T.-D.-H. Vo, P.-T. Nguyen, V.-T. Nguyen, T.-S. Dao and P.-D. Nguyen, Microplastics pollution in wastewater: Characteristics, occurrence and removal technologies, Environ. Technol. Innov., 2020, 19, 101013 CrossRef.
- N. P. Ivleva, A. C. Wiesheu and R. Niessner, Microplastic in Aquatic Ecosystems, Angew. Chem., Int. Ed., 2017, 56, 1720–1739 CrossRef CAS.
- M. Rani, S. Ducoli, L. E. Depero, M. Prica, A. Tubić, Z. Ademovic, L. Morrison and S. Federici, A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices, Molecules, 2023, 28, 5710 CrossRef CAS PubMed.
- J. Bikker, J. Lawson, S. Wilson and C. M. Rochman, Microplastics and other anthropogenic particles in the surface waters of the Chesapeake Bay, Mar. Pollut. Bull., 2020, 156, 111257 CrossRef CAS PubMed.
- G. De Lucia, A. Vianello, A. Camedda, D. Vani, P. Tomassetti, S. Coppa, L. Palazzo, M. Amici, G. Romanelli, G. Zampetti, A. Cicero, S. Carpentieri, S. Di Vito and M. Matiddi, Sea Water Contamination in the Vicinity of the Italian Minor Islands Caused by Microplastic Pollution, Water, 2018, 10, 1108 CrossRef.
- H. Kye, J. Kim, S. Ju, J. Lee, C. Lim and Y. Yoon, Microplastics in water systems: A review of their impacts on the environment and their potential hazards, Heliyon, 2023, 9, e14359 CrossRef CAS PubMed.
- H. Polanco, S. Hayes, C. Roble, M. Krupitsky and B. Branco, The presence and significance of microplastics in surface water in the Lower Hudson River Estuary 2016–2019: A research note, Mar. Pollut. Bull., 2020, 161, 111702 CrossRef CAS PubMed.
- E. R. Kozak, C. Franco-Gordo, J. Mendoza-Pérez, N. Sánchez-Nuño, X. A. Martínez-Sánchez, P. Melo-Agustín, G. Pelayo-Martínez and J. Gómez-Gutiérrez, Surface layer microplastic pollution in four bays of the central Mexican Pacific, Mar. Pollut. Bull., 2021, 169, 112537 CrossRef CAS PubMed.
- S. N. Athey, J. K. Adams, L. M. Erdle, L. M. Jantunen, P. A. Helm, S. A. Finkelstein and M. L. Diamond, The Widespread Environmental Footprint of Indigo Denim Microfibers from Blue Jeans, Environ. Sci. Technol. Lett., 2020, 7, 840–847 CrossRef CAS.
- J. K. Adams, B. Y. Dean, S. N. Athey, L. M. Jantunen, S. Bernstein, G. Stern, M. L. Diamond and S. A. Finkelstein, Anthropogenic particles (including microfibers and microplastics) in marine sediments of the Canadian Arctic, Sci. Total Environ., 2021, 784, 147155 CrossRef CAS PubMed.
- G. Chen, Q. Feng and J. Wang, Mini-review of microplastics in the atmosphere and their risks to humans, Sci. Total Environ., 2020, 703, 135504 CrossRef CAS.
- D. E. Ortega and D. Cortés-Arriagada, Atmospheric microplastics and nanoplastics as vectors of primary air pollutants - A theoretical study on the polyethylene terephthalate (PET) case, Environ. Pollut., 2023, 318, 120860 CrossRef CAS PubMed.
- B. Roblin, M. Ryan, A. Vreugdenhil and J. Aherne, Ambient Atmospheric Deposition of Anthropogenic Microfibers and Microplastics on the Western Periphery of Europe (Ireland), Environ. Sci. Technol., 2020, 54, 11100–11108 CrossRef CAS PubMed.
- O. Mbachu, G. Jenkins, C. Pratt and P. Kaparaju, A New Contaminant Superhighway? A Review of Sources, Measurement Techniques and Fate of Atmospheric Microplastics, Water Air Soil Pollut., 2020, 231, 85 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed materials and instrumental section; experimental procedure for materials preparation; synthesis of the F-HA-CPNs; procedure for soap isolation and sample preparation; Raman spectra of environmental microplastics from various sources; pristine microplastics coated with F-HA-CPNs; Raman spectra controls of sample adhesives, CPNs, SDS, and glass microscope slides. See DOI: https://doi.org/10.1039/d4va00239c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |