Green solvent ionic liquids: structural directing pioneers for microwave-assisted synthesis of controlled MgO nanostructures†
Arvind H.
Jadhav
,
Alan C.
Lim
,
Gaurav M.
Thorat
,
Harsharaj S.
Jadhav
and
Jeong Gil
Seo
*
Department of Energy Science and Technology, Energy and Environment Fusion Technology Center, Myongji University, Myongji-ro 116, Cheoin-gu, Yongin-si, Gyeonggi-do 449-728, Republic of Korea. E-mail: jgseo@mju.ac.kr; Fax: +82-31-336-6336
First published on 17th March 2016
Abstract
Magnesium oxide (MgO) is one of the auspicious metal oxides which attracts much attention because of its superior performance in scientific applications. Controlled facial arrangement of MgO nanostructures with tailored properties is highly important in nanotechnology and nanoscience. Here, various MgO nanostructures were obtained via one-pot microwave (MW)-assisted synthesis in various structural directing ionic liquids (ILs). These selected ILs are based on monocationic and dicationic moieties which consist of N-methyl imidazolium and 3-methyl pyridinium cations with various halide anions. Different designer solvents with respect to their counter anions produced various nanostructures, varying from nanoflakes, interconnected nanoparticles, hexagonal nanoparticles, irregular nanoparticles and nanocapsules. In this method, green solvent ILs not only act as solvent but also act as structural directing agents. In addition, a plausible mechanism of nanomaterial formation under MW irradiation in the presence of ILs was also determined. Formation of hydrogen bonding with favorable π–π interactions by simply tailoring the IL structures by means of MW conditions is the key factor for the development of different morphology. To define the catalytic activity of the prepared nanostructures, a Claisen condensation reaction was performed. The results showed that all the nanostructures have efficient catalytic activity due to their tailored structure, basicity, and surface area. Particularly, a catalytic amount of hexagonal morphology MgO obtained from dicationic [C4(mIm)2Cl2] IL showed 100% conversion and a remarkable 95% selective yield of the respective product. The proposed approach for nanomaterial preparation does not require an additional template and harsh reaction conditions which establishes this as a simple method to reduce the cost of production using environmentally benign solvents.
1. Introduction
Nanostructured metal oxides have recently attracted considerable attention because of their widespread and interesting applications which are due to their unique shape and size dependent properties.1,2 Especially, nano MgO has a broad range of physicochemical properties and is an extremely noteworthy functional metal oxide for use in diverse scientific fields.3,4 MgO is applied as a functional material in supercapacitors, conductors, additives, toxic waste remediation, and cosmetics, and is used as a base in catalyzed reactions.3–6 In the last few decades, enormous efforts have been paid to prepare various MgO morphologies with enhanced surface area and controlled properties. Several conventional methods have been used for the synthesis of MgO morphologies such as thermal oxidation, soft template-assisted growth, hydrothermal, solvothermal, spray pyrolysis, thermal evaporation, chemical vapour deposition, and precipitation techniques.7–11 Using these methods, different morphologies have been prepared varying from particles, rods, wires, belts, tubes, nanoflakes, etc.7–11 In the most popular template-assisted methods, surfactants and polymers with suitable solvents are generally applied to stabilize the surface of nano nuclei. In this case, the kinetically regulated growth of metal oxide facets further leads to development of nanostructures with a well-defined morphology.12 However, including a template-assisted method for all these process has certain disadvantages, as they either use high temperature or high pressure. Apart from this, a template-assisted process needs expensive organic templates, surface capping agents, and toxic organic solvents, as well as crucial reaction setups. To get additional benefits from the widespread applications of MgO as a nanomaterial for various purposes, it is essential to tailor the morphology, size, and surface area as well as the surface chemistry.4,5 In the case of oxide materials, mainly engineering and catalytic phenomena take place over the surface of engineered materials.5–6 The surface property, architecture, and chemistry of MgO materials are crucial factors which lead to a selective pathway through the course of morphology development. Therefore, improving and exploring a new technique to prepare morphologically controlled MgO is still an inspiring issue in nanoscience. Synthesizing a new morphology or developing a suitable green, one-pot, simple non-conventional method using green solvent which produces outstanding architecture with enhanced properties of MgO are still desirable topics.Of the several approaches used today for construction of nanomaterials, microwave (MW) irradiation as a non-classical energy source has become increasingly popular in science fields.13–15 This approach helps to eliminate complex and time consuming non-green processes and requires minimum investment in equipment, since it can be performed in a domestic MW oven. Similarly, nowadays the use of this method in nanomaterial preparation is also increasing drastically.16 MW in-core flash heating in dramatically reducing overall processing time is the main benefit associated with this method. In addition, higher reaction rate, rapid volumetric uniform heating, and selective high yield of products are some secondary benefits of this processes.14,15 Transformation of electromagnetic radiation in to heat energy in MW heating is based on two main factors: conduction and the dipolar polarization phenomenon.14,15 Absorption of MW irradiation by a conduction mechanism is more effective and reaction media must produce a high dielectric constant and high polar nature for MW absorption.14 Indeed, polar solvents such as ILs are the best candidates for absorption of MW irradiation which leads to the production of conductive and a high polar nature for reaction media.17
ILs are generally salts accumulated with organic bulky cations and inorganic anions. Recently ILs have attracted much attention as reaction media or catalysts because of their exceptional properties. They have a wide liquid range, negligible vapor pressure, good stability at high temperature and good dissolving ability with high ionic conductivity.18 The opportunity to vary the cation and anion combination gives a number of ways to enhance IL properties.17,19,20 Additionally, being solvents ILs can also act as a reactant or catalyst in a reaction and as a template or designer agent in the synthesis of inorganic materials.21–24 In recent times, by combining the advantages of ILs and MW heating, there are very few methods report for the synthesis of inorganic materials.15,25 By using these approaches, different morphologies of inorganic nanomaterials have been produced. The results of these methods show a synergetic effect of ILs with MW irradiation, obtaining significant selective morphology, and a high yield in a short reaction time. However, there is no report which could show different controlled MgO morphologies by means of MW irradiation in different ILs as designer solvents. Therefore, it is essential to study non-conventional techniques which open up the prospect of realizing fast synthesis of nanomaterials in a green solvent system such as ILs, which make the process faster and eco-friendly, as well as cheap economically.
Herein, different perfect morphologies of MgO nanostructures were obtained in one-pot using a MW irradiation process in numerous structural directing ILs. The synthesized well accumulated designer solvents such as monocationic and dicationic ILs with counter anions controlled various MgO morphologies. Aside from a variation of IL structures, the influences of various cations and anions on morphology development were also investigated. The results revealed that ILs acted as solvent as well as a structural directing agent in the presence of MW irradiation. The obtained results of the MW-assisted synthesis were compared with those of other conventional methods. This work provides a versatile approach to synthesize various MgO crystals of different morphology by varying structural directing agents (ILs). A plausible mechanism for the formation of a different morphology of MgO in ILs is also discovered. We show here that slight structural modification of the ILs is highly responsible for achieving selective crystal growth in MW irradiation conditions. In addition, the catalytic activities of these morphologies were also determined.
2. Experimental
2.1. Materials
Magnesium acetate tetrahydrate (Mg (CH3COO)·4H2O) (99%), sodium hydroxide (NaOH) (99%) (Acros), N-methyl imidazole (99%), chlorobutane (99.0%), dichlorobutane (99%), 3-methyl pyridine (99%), acetonitrile (99%), ethyl acetate (HPLC grade), ethanol (reagent grade), and chloroform-d (CDCl3) (99%) reagents were used as received without further purification. All solvents were purchased from commercial sources. Double-distilled water was used throughout experiments.2.2. MgO nanostructure characterization methods
Crystal structures of the MgO morphology were confirmed using X-ray diffraction patterns and the results were recorded on a Rigaku Miniflex (Japan) X-ray diffractometer using Ni-filtered Cu Kα radiation. FE-TEM analyses were implemented using a TECNAI-32F30 transmission electron microscope with an acceleration voltage of 200 kV. The morphology of the nanostructures was monitored using field emission scanning electron microscopy (FE-SEM) (Carl Zeiss Sigma VP FE-SEM). Brunauer–Emmett–Teller (BET) surface areas of the nanostructures were obtained from N2 sorption isotherms acquired using a BEL Japan (Belsorp-II) instrument. Elemental composition and mapping of the nanostructures were determined using energy dispersive X-ray analysis (EDS) coupled to SEM. FT-IR spectra of the prepared nanostructures were recorded on a Varian 2000 IR spectrophotometer using the KBr disc method. Thermogravimetric analysis (TGA) was accomplished on a Scinco TGA N-100 instrument with a heating rate of 5 °C min−1 in a nitrogen atmosphere. UV-visible spectra were achieved on Varian Cary (100 Conc.) using double-distilled water as standard. Raman spectra were obtained using a LabRam HR800 UV Raman microscope (Horiba Jobin-Yvon, France) spectrometer using focal length 80 and CCD detection at room temperature. Photoluminescence analyses of the nanostructure were performed on a Princeton Instrument Co., IRY1024, USA, using a He–Cd 325 nm laser and ICCD detector at room temperature. CO2 TPD measurements were carried out using BELCAT-A (BEL Japan, Inc.).2.3. Preparation of designer solvents, monocationic and dicationic ILs
The detailed synthesis procedure and characterization data of all the ILs are described in the ESI.†2.4. Preparation of MW-assisted MgO nanostructures in ILs
For the preparation of various MgO morphologies, initially, ILs were dried in a vacuum oven at 70 °C for 12 h and utilized for further processes. 2.2 g of Mg(CH3COO)2·4H2O as a precursor was added in 5.0 g specific IL in a 50 mL Pyrex glass flask. A sufficient amount (1–2 g) of NaOH was added in the reaction mixture. The mixture was stirred in the presence of MW heating for 4–5 min. MW heating was repeated 6–7 times giving a break of 1–2 min at an appropriate temperature. The cyclic mode was preferred in order to reduce the risk of over-heating or decomposition of the ILs. The MW was operated under a power radiation of 400 using a MW oven (DAEWOO model KOC-1B3KP, Korea). The reaction temperature was kept above the melting point of the ILs by adjusting a temperature controller. After completion of MW heating cycles, the mixture slowly cooled at room temperature. Addition of 20 mL double-distilled water was done in the reaction mixture and centrifuged at 8000 rpm for 20 min to remove the aqueous layer. The obtained white slurry was washed several times with double-distilled water and centrifuged in order to remove IL from the nanostructures. Finally, the product was dried at 70 °C for 12 h in a vacuum oven and calcined at 500 °C for 5 h in the presence of N2 to obtain a white solid powder. All the different morphology development reactions were repeated 2–3 times using the same reaction conditions.3. Results and discussion
The primary aim of this study is to access the conventional tailor-made ILs as structural directing agents for nanostructure development with assistance of MW irradiation. Our key concern is whether low melting point ILs are stable enough in flash MW heating during formation of morphology, since it is reported that ILs are stable enough in conventional hydrothermal and other heating modes. In addition, the literature reveals that there was no substantial pressure created by ILs in a reaction setup in conventional mode, which indicates that they are a convenient solvent for material preparation. For this process a restricted number of ILs, such as [Bmim][BF4], [Bmim][PF6], [EmimCO2H][BF4], and [Emim][EtSO4], have been utilized in the preparation of nanostructures by means of a conventional technique.26–28 In the last decade, consideration has been paid to ILs containing BF4 or PF6, which show a hydrophobic nature and release poisonous and corrosive HF which does not follow the standards of a greener method.26 In contrast, in the present study, attention has been paid to conventional hydrophilic ILs which are highly biodegradable, easily accessible and synthesized by a greener method, which enhances the possibility to use ILs on a large scale for the development of nanostructures in both conventional and non-conventional methods.3.1. Selection of ILs as a designer agent for MgO nanostructures
Several reports confirmed that ILs can act as designer solvents or templates, and influence the physicochemical properties of nanostructures.18,29 Formation of morphology in materials can be attributed to a higher nucleation rate of the nanostructures in ILs. To determine the effect of components such as the cationic and anionic moiety of ILs on the nucleation and morphology formation, five different types of ILs were selected. These ILs have different combinations of organic bulky cations and inorganic anions. Fig. 1 shows five different ILs and their structural combinations.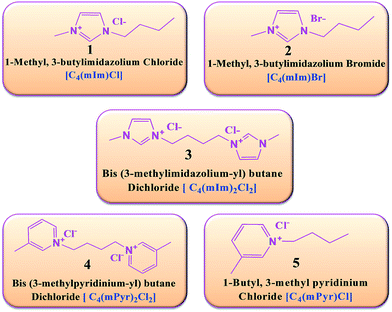 | ||
| Fig. 1 Various monocationic and dicationic ILs used for the preparation of controlled MgO nanostructures in the presence of MW irradiation. | ||
Hydrophilic ILs containing traditional anions, such as chloride (Cl−) and bromide (Br−), can be produced easily using simple methods. Previous studies report that coordination of these kinds of anions has a direct impact on particle construction as well as morphology growth with respect to the cations.29 On the basis of structural features of ILs, organization of ILs can be tailored (cations and anions) and interaction energies of ILs can be modified.30 On the other hand, MgO nanocrystals are composed of positively charged Mg layers and negatively charged O layers. Various different cationic and anionic structures of ILs possibly formed different interactions with MgO crystals which led to the development of different morphology. This factor is highly responsible for a diverse mechanism, including the formation of self-assembly, hydrogen bonds, electrostatic attractions, and π–π stacking interactions.29 The special constructed structure of ILs with respect to the fascinating properties of large cations and anions of ILs allows them to act as self-assembling templates. The pre-organized solvent structure causes nanocrystals to experience self-organization for the development of well-defined nanostructures in the presence of MW irradiation.29 Different cations such as imidazolium and pyridinium were selected by considering the following characteristics: imidazolium-based ILs are among the most frequently used species because of their outstanding physicochemical properties and biodegradability.30 Monocationic, butyl methyl imidazolium [C4(mIm)] contains a bulky organic moiety, which can contribute to the π–π interactions and hydrogen bonding.29 Attachment of a butyl chain with an aromatic ring is supposed to control the agglomeration and size distribution of the nanostructure dispersion. Meanwhile, the same counter imidazolium dication core with a short alkyl chain as a spacer between two imidazolium cations possesses two-fold π–π interactions and hydrogen bonding and enhanced other properties.19 Previous reports show that, as compared to monocationic ILs, their dicationic counter ILs possess high thermal stability, polarity, and conductivity.19,30 These enhanced properties in dicationic ILs lead to the development of different morphology and significant effects on the MgO nanostructure. Other types of ILs were selected as pyridinium-based monocationic and dicationic ILs with Cl− as counter anions. These ILs do not have any characteristic acidic protons like imidazolium-based cations have, but have an aromatic group with their effective interactions still existing which could elaborate in to π–π interactions and can produce variation in morphology.29 These five different types of ILs showed significant influence on the MgO nanostructure development. The obtained morphology of MgO in these ILs are from now on designated as MgON-1, MgON-2, MgON-3, MgON-4, and MgON-5, and they are schematically represented in Scheme 1.
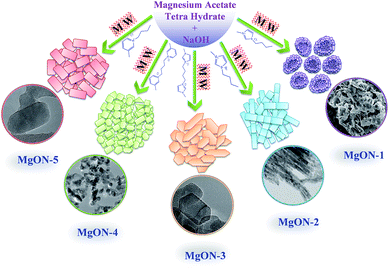 | ||
| Scheme 1 Schematic representation of various controlled MgO morphologies achieved from different ILs using MW irradiation. | ||
3.2. TGA and XRD analysis of MgO nanostructures
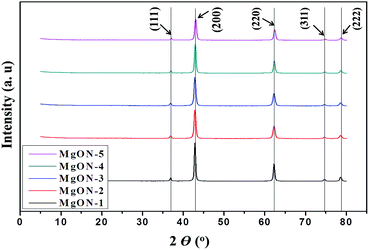
Fig. S-1 (ESI†) shows TGA of the as-synthesized MgON-3 with temperature growth from 25 to 850 °C. The TGA results show that initially the weight remained constant until 350 °C, and after that dropped rapidly. A sudden loss in weight was observed at around 350–425 °C, which correlates well with approximately 30 wt%. This weight loss corresponds to loss of hydroxyl molecules and some residual water as well as the remaining organic moieties of the ILs in the nanostructures. Above 425 °C, no weight loss is observed, showing that the obtained resultant MgO material has admirable thermal stability at high temperature. Therefore, other successive synthesized samples were calcined at 500 °C for 5 h to obtain a pure phase in the morphologies achieved from various ILs. The obtained final morphology after calcination did not show any noticeable changes, and the original morphology is preserved after calcination in the all prepared MgON samples. Few cracks/shrinkages were observed on the nanostructures, possibly due to the loss of hydroxide molecules or weight loss. However, the morphology preserved topography and crystallinity throughout the samples.XRD peaks of the calcined products of MgON acquired from different ILs are shown in Fig. 2. After annealing, all samples showed reflections corresponding to MgO as a highly pure spinal phase. Diffraction peaks at 2θ values of 36.94°, 42.80°, 62.30°, 74.67°, and 78.61° were assigned to the (111), (200), (220), (311), and (222) planes of cubic MgO nanoparticles. These obtained XRD patterns clearly confirmed the presence of a highly pure MgO cubic phase. The resulting diffraction peaks matched well with the standard XRD pattern of MgO (JCPDS 45-0946), indicating the formation of a pure MgO compound in various ILs. MgON-1 produced in IL-1 showed the highest intensity of diffraction peaks, followed by the gradually decreasing MgON-2, 3, 4, and 5, which are obtained from IL-2, 3, 4, and 5 respectively. However, the intensities of all the MgON nanostructures have comparable heights. Average particle sizes were calculated from the peak width at half height by means of the Scherrer equation for MgON-1–5.31 The calculated average sizes were obtained in the range of 10–60 nm, which closely resembles their FE-SEM and FE-TEM images.
3.3. FE-SEM and FE-TEM analysis of the prepared MgO nanostructures
All morphologies of the MgON nanostructures were analyzed by FE-SEM and FE-TEM analysis. Fig. 3 illustrates the FE-SEM and FE-TEM images and the SAED pattern of the MgON-1 sample which is obtained from monocationic IL [C4(mIm)Cl] at 90 °C. The SEM images in Fig. 3(a) and (b) show that the imidazolium-based monocationic ILs with Cl− anions developed a high quality nanoflake-like morphology throughout the sample. The imidazolium cation of [C4(mIm)Cl] used in this technique might interact with the precursor by hydrogen bonding and develop π–π interactions or electrostatic forces and hydrogen bonding. The [C4(mIm)Cl] IL has an electron-withdrawing ability by allocation of an electron pair of hydrogen and carbon at place 2 of the imidazole ring.29,32 These developed interactions pile up and stack in the presence of MW irradiation. Thus, the [C4(mIm)Cl] IL interacted with the surface of the developing MgO crystals which leads to anisotropic development of MgO crystals into nanoflakes.32 These obtained flake structures in [C4(mIm)Cl] are approximately 50–70 nm in size and have a 2–4 nm thickness. The surface of the nanostructure flakes looks rough which indicates that it is composed of many primary building blocks formed by MgO nanoparticles.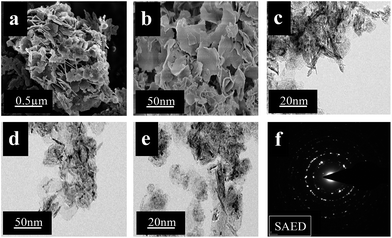 | ||
| Fig. 3 FE-SEM (a and b) and FE-TEM (c, d, and e) images, and SAED pattern (f) of MgON-1 nanoflakes obtained in [C4(mIm)Cl]. | ||
These particles are dense and well interconnected with each other. Calcination does not show any effect on the shape and size of MgON-1 but some cracks and lines were observed. In the calcination process, H2O molecules were lost between two adjacent layers which leads to periclase assembly with irregular inter-crystallite networks which produce mesopores. These results can be also confirmed by the FE-TEM images shown in Fig. 3(c)–(e). Some of these nanoflakes look to be fused with each other and transparent structures suggest that the nanoflakes are very thin. The selected area electron diffraction (SAED) pattern of MgON-1 shown in Fig. 3(f) indicates that the MgO nanoflakes are polycrystalline in nature. Single nanoflakes show three noticeable diffraction rings consistent with XRD peaks, indicating the formation of polycrystalline MgO with a cubic structure.
Fig. 4 shows FE-SEM, FE-TEM, and the SAED pattern of MgON-2 nanostructures obtained at 90 °C from monocationic IL [C4(mIm)Br] which has bromide (Br−) as an anionic moiety. From the FE-SEM images (Fig. 4(a)–(c)) it can be clearly seen that the monocationic [C4(mIm)Br] IL successfully achieved a different morphology to that produced by the IL [C4(mIm)Cl]. We believe that an approximately similar type of mechanism and interactions formed with [C4(mIm)Br] in the presence of MW irradiation, except the larger inorganic cation (Br−) with an alkyl chain is responsible for producing variation in the morphology. The [C4(mIm)Br] IL produced an interconnected capsules morphology of 20–50 nm in length and 20–25 nm in width. These capsules are formed by combination of MgO nanoparticles as a building block element. After calcination, the surface of the nanocapsules became rough and developed mesopores due to evaporation of the hydroxyl molecules. In addition, Fig. 4(d) and (e) show FE-TEM images of representative interconnected nanocapsules which further support the results obtained by the SEM images. Clear pictures of finger-like projections due to interconnected nanocapsules are seen very clearly in the TEM images. The SAED image pattern of the morphology is displayed in Fig. 4(f), which represents that the nanocapsules are well-crystallized polycrystals, which is in strong agreement with the XRD pattern of MgON-2 showing the characteristic three circular rings.
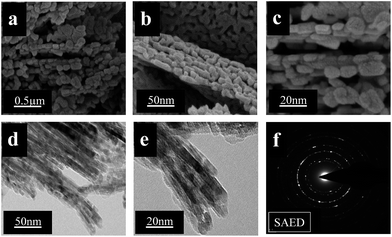 | ||
| Fig. 4 FE-SEM (a, b, and c) and FE-TEM (d and e) images, and SAED pattern (f) of MgON-2 interconnected nanocapsules obtained in [C4(mIm)Br]. | ||
Another dicationic type of IL [C4(mIm)2Cl2] was applied for the construction of a MgO nanomorphology with MW irradiation at 90 °C. In this case, organic imidazolium-based bulky dications and inorganic chloride dianions were used. These di-ions are separated by a butyl alkyl chain bridging moiety. By using [C4(mIm)2Cl2], hexagonal disc-like structures were developed. Compared with other ILs, the obtained hexagonal MgO morphology in dicationic hydrophilic [C4(mIm)2Cl2] was found to be very small in size. A high viscosity of [C4(mIm)2Cl2] is key to develop such small shaped hexagonal disc-like structures. A high nucleation rate and two-fold property as well as interactions in [C4(mIm)2Cl2] produced such a marvellous morphology. It is reported that, in dicationic ILs, the acidity as well as hydrogen bonding and interactions were two-fold as compared to monocationic ILs. In the case of the [C4(mIm)2Cl2] IL, imidazolium dications showed a major impact on the hexagonal morphology. Imidazolium cations with smaller alkyl chains between these rings associated with Cl− anions obstruct the growth of nanostructures to develop in a hexagonal shape due to steric hindrance effects. In addition, the developed two-fold acidity, hydrogen bonding, and π–π interactions are some other causes for fabrication of the hexagonal morphology.29Fig. 5(a)–(c) represent the FE-SEM images of a calcined sample of MgON-3. The results reveal a high quality hexagonal morphology developed throughout the sample. Hexagonal structures are 40–60 nm in range and 10–20 nm in thickness, which is consistent with the result calculated from the Scherrer equation. In addition, to determine the effect of increased time and reaction temperature on the hexagon morphology, the same reaction was conducted with a two-fold reaction time (10–11 MW cycles of 4–5 min duration) and increased temperature (150 °C), and showed over-growth in hexagons (Fig. S2†). Increasing the reaction temperature above 150 °C resulted in decomposition in the IL structure in MW conditions.
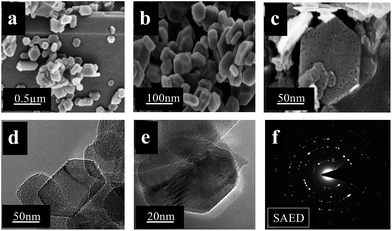 | ||
| Fig. 5 FE-SEM (a, b, and c) and FE-TEM (d and e) images, and SAED pattern (f) of MgON-3 nanohexagons obtained in [C4(mIm)2Cl2]. | ||
Moreover, hexagons were composed of MgO nanoparticles. After calcination mesopores and some cracks were produced in hexagons due to water evolution. However, the morphology was the same before and after calcination. Fig. 5(d) and (e) show the FE-TEM images of hexagons. MgON-3 shows a perfect hexagonal structure with a transparent nature. The transparent nature of the hexagons indicates that these nanostructures are very thin and porous. It can clearly be observed in TEM images that the hexagons are composed of granular MgO nanoparticles. The edges of the hexagons are approximately equal in nature. The SAED pattern shown in Fig. 5(f) indicates that nanohexagons are polycrystalline in nature and these results are in line with the XRD pattern of MgON-3. Furthermore, another group of ILs, monocationic pyridinium-based ILs [C4(mPy)2Cl2], which has chloride (Cl−) as the anion was applied for the development of morphology with MW irradiation. The results of these observations fail to give any selective morphology with the [C4(mPy)2Cl2] IL with MW irradiation.
Fig. 6(a) and (b) illustrate the FE-SEM images of the MgON-4 nanostructure, in which a specific morphology could not be found. Circular nanoparticles, capsules, a hexagonal structure, and trigonal morphology as well as nanoplates were observed throughout the sample at 120 °C. The IL [C4(mPy)2Cl2] was composed of a methyl group at a subsidiary chain of pyridinium cations with Cl− anions separated by a butyl bridging moiety. The dicationic [C4(mPy)2Cl2] IL does not have any acidic protons and it fails to do efficient hydrogen bonding with the precursor.18 The interaction between the formed MgO nuclei and ILs is too weak to serve effectively for nucleation and growth of morphology.19,29 Simple weaker π–π interactions are present due to pyridinium anions for the development of morphology. In addition, these interactions are not flexible due to the dicationic nature of ILs. Therefore, a selective morphology fails to develop under MW irradiation. The obtained morphologies are in the range of 40–50 nm in size. Calcination does not show any effect and persevered the original morphology after calcination. Some cracks and wounds were observed on these morphologies by formation of mesopores in annealing treatment. FE-TEM (Fig. 6(c)–(e)) images also supported the FE-SEM analysis. The TEM images of MgON-4 show nanodiscs, trigonal structures, particles and capsules throughout the sample. The size of the nanostructures is 40–50 nm with a 10–20 nm thickness. The bumpy surface of the nanostructure indicates that it is composed of many primary MgO nanoparticles. The SAED pattern (Fig. 6(f)) obtained by focusing an electron beam on an individual nanostructure shows a polycrystalline nature with a face-centered cubic structure.
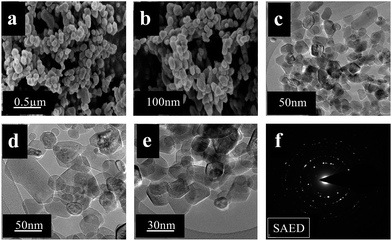 | ||
| Fig. 6 FE-SEM (a and b) and FE-TEM (c, d, and e) images, and SAED pattern (f) of MgON-4 irregular nanostructures obtained in [C4(mPy)2Cl2]. | ||
FE-SEM images of MgON-5 are shown in Fig. 7(a)–(c). These nanostructures were fabricated in pyridinium-based monocationic IL [C4(mPy)Cl], composed of pyridinium cations with Cl− anions at 90 °C. This pyridinium-based IL produced a nanocapsule morphology of MgO. The speciality of this IL is that it does not have any acidic protons but has many flexible π–π interactions between IL molecules and the precursor.29,32 The butyl alkyl chain with a pyridinium cation produces π–π interactions and allows the MgO crystal to grow in a longer fashion which leads to formation of nanocapsules. Due to the absence of acidic protons, less hydrogen bonding occurs and simple flexible π–π interactions are responsible for the morphology development.29,32 From the obtained SEM results, it is observed that after calcination, the parental morphology remained in the sample.
 | ||
| Fig. 7 FE-SEM (a, b, and c) and FE-TEM (d and e) images, and SAED pattern (f) of MgON-5 nanocapsules obtained in [C4(mPy)Cl]. | ||
However, some clefts and lines are observed compared with the as-synthesized precursor due to H2O evaporation during sintering, which leads to formation of mesopores. Nanocapsules are 50–60 nm in length and 10–20 nm in width. The FE-TEM images in Fig. 7(d) and (e) also support the SEM analysis, and it can be clearly seen that MgO nanocapsules are composed of nanoparticles. The SAED patterns of the calcined materials (Fig. 7(f)) show circled rings, an indication of the polycrystalline nature of the sample which is in good agreement with the XRD analysis of MgON-5.
3.4. Fourier transform infrared (FT-IR) and Raman spectroscopic analysis of MgO nanostructures
Fig. 8(a) demonstrates the FT-IR spectra of the MgON nanostructures obtained in this study. It is well acknowledged that MgO chemisorbs carbon dioxide and water molecules from the atmosphere due to its surface basic properties.33 However, upon calcination of MgON at 500 °C, CO2 and H2O molecules evolve and thus produce various Brønsted as well as Lewis acid and base sites on the MgO surface. The main representative peak corresponds to Mg and O (Mg–O) stretching vibrations at 442 cm−1, which is an indication of the formation of highly pure MgO.33,34 This characteristic peak was observed throughout all the MgON samples synthesized in the various IL systems. Occurrence of bands in calcined MgO at 1642, 1454, and 1029 cm−1 is assigned to carbonate species, whereas the weak absorption band at 2351 cm−1 is ascribed to stretching vibrations of CO2 due to physically adsorbed atmospheric CO2.33–35 The band at 1082 cm−1 is attributed to the H− ion.33 High intense absorption bands at 1634 and 3471 cm−1 are attributed to OH bending and stretching vibrations correspondingly of physically adsorbed H2O molecules as well as surface OH groups strongly disturbed by hydrogen bonding.33–36 All these different kinds of intense FT-IR peaks were also observed in every spectrum of MgON.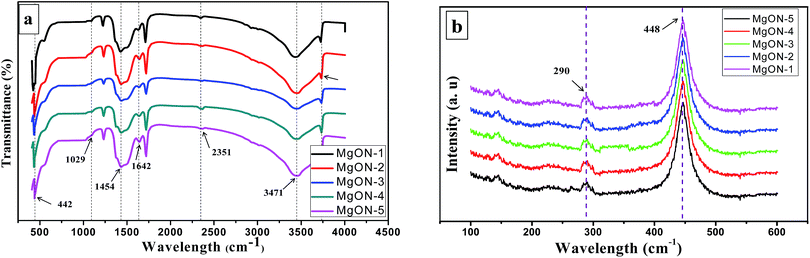 | ||
| Fig. 8 (a) FT-IR analysis, and (b) Raman spectroscopy analysis of different MgO nanostructures prepared in ILs. | ||
Furthermore, the microcrystalline nature of the morphologically controlled MgON nanostructures was also studied using Raman spectroscopy. Fig. 8(b) shows representative room temperature Raman spectra of different MgO nanostructures. The results indicate the presence of characteristic highly intense bands at 290 and 448 cm−1 in all of the prepared MgON nanostructures. A Raman intense peak at 448 cm−1 may resemble two projecting peaks in Chen’s methodology corresponding with the MgO microcrystalline structure.37,38 Consequently, it is hypothetical that the 290 cm−1 peak is accompanied by a TA (transverse acoustic) phonon at the microcrystalline nanostructure boundary, whereas the 448 cm−1 line is accompanied by a TO (transverse optic) phonon at the microcrystalline nanostructure center.38 The observed experimental Raman peaks closely resemble the previously reported results. Additionally, the observed peaks in the Raman spectrum validate the presence of the nanocrystalline phase, whereas such peaks are generally absent in bulk MgO materials.
3.5. Photoluminescence and UV-vis spectroscopy study of MgON nanostructures
Fig. S-3† shows typical photoluminescence (PL) spectra scanned at room temperature of MgON nanostructures. Oxygen vacancies or deficiencies in the metal oxide surface act as an invisible enhancer in engineering purposes or in catalytic applications which significantly alters the oxide properties.39 Therefore, determining oxygen vacancies in the nanostructures developed at the time of construction is highly significant. Being a wide bandgap material, bulk MgO normally does not demonstrate PL activity at room temperature.40 Earlier studies on the PL of MgO nanostructures showed an intense peak at around 450 nm due to the presence of oxygen vacancies on MgO.41 The presence of oxygen vacancies on the surface of nanostructures is due to the in-core MW heating preparation method or at calcination of magnesium hydroxide into MgO, resulting in an incomplete oxidation process. Due to these reasons, the nanostructure may show emission in PL.41,42 In addition, defects associated with oxygen vacancies and Mg vacancies lead to the creation of new defect levels in the bandgap section of MgO structures and these defect states freely contribute in luminescence.39–42 The obtained results illustrate the presence of a strong intense peak at nearly 450 nm in all of the prepared MgON morphologies. At 450 nm some of the MgON samples showed an increased PL emission spectra. This considerable increase in PL emission in some of the MgON samples can be explained by transfer of photo-generated carriers and optical resonant cavity development.41,42 Upon laser irradiation for PL measurement, photo-generation, transfer, and recombination of carriers occur consecutively in the core of the MgON nanostructures. Some of these photo-generated electrons and holes recombine in MgO cores to emit light and enhance the PL light.43,44 Hence, the existence of PL emission in MgON nanostructures is due to the presence of defects of oxygen vacancies on the surface of MgON. These tailored oxygen vacancies are obtained because of the ionic media used at the time of construction of the nanostructures by means of MW irradiation.Fig. S-4† shows UV-vis spectra of the MgON nanostructures. Intense peaks were found at 278 nm and 296 nm, which can be attributed to excitation of electrons present at four-coordinated anions on the edges and three-coordinated surface anions on corners, as exhibited in the crystal structure of MgO in Fig. S-5.†45 The calculated bandgap value from the UV-vis spectra was 4.3 eV and the results are displayed in Fig. S-6.† Lower bandgaps of the MgON nanostructures are due to the occurrence of anions which are four-coordinated at the surface of edges in MgON, while the bulk material holds a bandgap of 7.8 eV due to the existence of six-coordinated surface anions.45
3.6. Energy dispersive analysis (EDS) of MgO nanostructures
Fig. S-7† displays EDS spectra as well as EDS mapping of the prepared MgON. The EDS spectra revealed that the synthesized products are principally made of purely Mg and O with an atomic proportion ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼1 respectively. These EDS results are in good consistency with the XRD results and it is exposed that no other impurities were observed in the samples by EDS as well as by XRD. A slightly lower amount of oxygen indicates the presence of oxygen deficiencies in the MgON nanostructures, which might have been developed during rapid establishment of MgO crystals under MW irradiation. This oxygen deficiency occurrence has already been proven by PL spectroscopy.
∼1 respectively. These EDS results are in good consistency with the XRD results and it is exposed that no other impurities were observed in the samples by EDS as well as by XRD. A slightly lower amount of oxygen indicates the presence of oxygen deficiencies in the MgON nanostructures, which might have been developed during rapid establishment of MgO crystals under MW irradiation. This oxygen deficiency occurrence has already been proven by PL spectroscopy.
3.7. BET and pore analysis of MgO nanostructures
Additionally, to examine effect of different ILs on the texture property and effect on pores of the MgON nanostructures, BET and pore analyses were performed. The surface area, pore diameter, and pore volume, as well as the basicity of all the nanostructures prepared in various ILs are summarized in Table 1. N2 adsorption–desorption isotherms of the synthesized different MgON samples are shown in Fig. 9(a)–(e). The BET surface area of these structures is in range of 50–75 m2 g−1. These obtained isotherms show that all MgON morphologies reveal characteristic type-III isotherms with BET surface areas of 48.15, 56.23, 74.31, 49.61, and 61.27 m2 g−1 for MgON-1, 2, 3, 4, and 5, respectively. For comparison, the BET surface area of MgO prepared in different ILs by means of the MW irradiation method is larger than that obtained by conventional methods. The obtained results of surface area reveal that MgON-3 and MgON-5, which were synthesized in dicationic imidazolium and pyridinium-based ILs, have a higher surface area compared to MgON synthesized in monocationic ILs. On the other hand, chloride anions and both cationic moieties have an approximately similar surface area. The Br− anion-containing imidazolium-based monocationic ILs showed a 56.23 m2 g−1 surface area. From these obtained results, it can be concluded that the different IL media showed a strong influence on the surface area of MgON prepared by means of the MW irradiation method.| Nanostructure | Morphology | Surface area (m2 g−1) | Total pore volume (cm3 g−1) | Average pore diameter (nm) | Basicitya (mmol g−1) |
|---|---|---|---|---|---|
| a Total basicity determined by CO2 TPD. | |||||
| MgON-1 | Nanoflakes | 48.2 | 0.49 | 24.2 | 0.73 |
| MgON-2 | Interconnected nanocapsules | 61.2 | 0.91 | 31.6 | 0.86 |
| MgON-3 | Hexagonal nanostructures | 74.3 | 0.82 | 26.6 | 1.68 |
| MgON-4 | Irregular nanoparticles | 49.6 | 0.69 | 36.2 | 1.32 |
| MgON-5 | Nanocapsules | 56.2 | 0.88 | 25.5 | 0.92 |
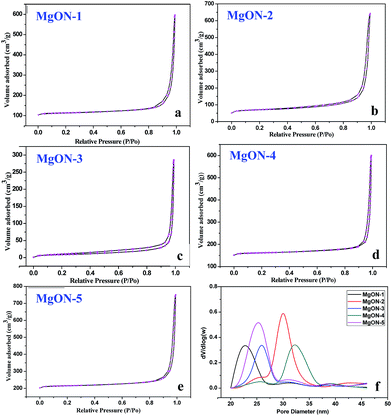 | ||
| Fig. 9 N2 adsorption–desorption isotherms of MgO nanostructures prepared in ILs (a–e), and pore diameters of the various morphologies of MgO (f). | ||
The porosity of the nanostructures is an important physical property of materials which strongly affects the adsorption properties, catalytic activity, toughness, permeability, material mechanical strength, etc. The pore size distributions of these materials are revealed in Fig. 9(f). Isotherms acquired for the MgON nanostructures indicate that the pores in all the MgON samples belong to the mesoporous category and the size range of the mesopores was found to be in between 5 and 35 nm. The pore volume of MgON did not show any trend with respect to the different ILs used in the preparation methods. The average pore diameter of MgON was in the 0.50–1.0 cm3 g−1 range. From these observations it can be concluded that the surface area and pore volume of MgON nanostructures was affected by both the cations as well as the anions present in the ILs. In addition, the basicity of MgON was also determined by the CO2 TPD method. The results reveal that the basicity was also affected tremendously with respectively different ILs. The total basicity was found to be in the range of 0.75–1.70 mmol g−1. However, detailed investigation of the effect of different ILs on basicity is in process.
3.8. Proposed mechanism
MW-assisted synthesis of nanostructures is a non-conventional method which enhances rapid homogeneous heating in contrast to the conventional heat-assisted method. To evaluate the benefit of the MW-assisted nanostructure syntheses in ILs, the results were compared with the conventional hydrothermal as well as the oil bath heating methods, which are famous conventional synthesis methods. To clarify the mechanism of the current reaction in ILs, the following control reactions were performed: Mg(CH3COO)2·4H2O with NaOH was dissolved in the respective ILs at 120 °C using the conventional heating method and the reaction was repeated under identical conditions including all the ILs used in this report. The products as MgO nanopowder without any morphology were isolated in all cases after calcination. Furthermore, Mg (CH3COO)2·4H2O was dissolved in water with NaOH and stirred at 120 °C, meanwhile the reaction was also performed under the same conditions in a MW oven. No specific morphology in the product was obtained in these reactions. Furthermore, ethylene glycol as solvent was used, because of its polarity, high viscosity and high thermal stability. This reaction was conducted at a 150 °C reaction temperature, and the same reaction was also performed in a MW oven at 150 °C. The results of these reactions also failed to give any specific morphology in the products. These same reactions were also conducted at the reflux temperature of ethylene glycol and the same conditions were maintained for up to 5 h. As a result, a MgO powder was obtained at the end as the product without any morphology. Hence, to control the morphology of the MgO nanostructures, specific ionic media as well as in-core heating are necessary to obtain selective structures, which can be provided by means of MW irradiation in IL media. Therefore, using simple non-conventional MW heating achieved highly selective nanostructures of the MgO morphology throughout the samples in the respective IL media. These observations state that a specific ionic environment with MW irradiation is highly responsible for formation of MgO morphology.In addition, a functional aspect of the reaction media (ILs) in the reaction system of the nanoparticle synthesis is to control the rate of nucleation and growth of particles, like the surfactant does in conventional methods.31 Especially, different interactions between the ILs and metal oxide structure developed at the time of morphology construction in the presence of MW irradiation, which lead to a plausible mechanism for the development of various morphologies. Fig. 10 shows the possible interactions between ILs and metal oxide particles. In continuation of the discussion, on the basis of the nature and structure of the ILs, interactions between the ILs and metal oxide can be developed and vary.29–31
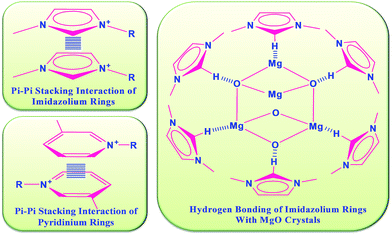 | ||
| Fig. 10 Schematic representation of interactions between ILs with MgO crystals in the presence of MW irradiation. | ||
The aromatic moiety of the ILs can contribute in π–π interactions. It serves as a highly electron-accepting section and it is also probably responsible for electrostatic attraction with the surface of metal particles with polar moieties.29 The acidic proton present in between two nitrogen atoms of the imidazolium ring structure can act as a bridging moiety at the time of formation of hydrogen bonding. It is also reported that both the electrostatic and coordination effect of the imidazolium cations contribute to the nanoparticle morphology stabilization in the presence of ILs.29,32 Depending on the different ILs with different components of cations with respect to anions, different interactions including electrostatic attraction, π–π stacking interaction and hydrogen bonding, as well as self-assembly, can be expected to occur between the ILs and precursor. These interactions of reaction components lead to MgO unit growth projecting to the development rate in a specific morphology.32
While performing the reaction in MW irradiation conditions, MW irradiation also plays a very vital role in nanostructure construction. MW irradiation supports efficient internal in-core heating and the reaction temperature was raised consistently throughout the whole IL media by direct accepting of MW energy to molecules that are present in the reaction mixture.29 MW irradiation triggers heating by two main factors, namely dipolar polarization and ionic conduction.46 Whereas, dipoles in the reaction mixture produced by the ILs are created by the dipolar polarization effect, and contribute in ionic conduction. When exposed to MW irradiation, dipoles in the reaction mixture align in the direction of the applied electric field (Fig. 11). As the electric field oscillates, molecular dipoles accordingly attempt to re-align themselves along the alternating electric field, and in such a process energy is removed in the form of heat through molecular collisions.46,47 The amount of heat released by this course is directly associated with the ability of dipoles to align with the frequency of the applied field.47 If a dipole does not have sufficient time to rearrange or re-orients too quickly with the applied field, no heating will occur at a molecular level.29,46–51 We believe that at this stage of morphology development, conventional heating methods fail to supply an applied field at a molecular level.
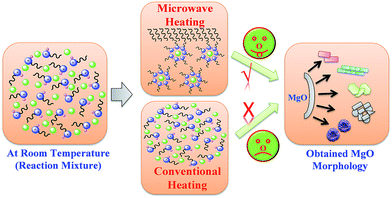 | ||
| Fig. 11 Plausible mechanism and schematic representation of the formation of MgO nanostructures in ILs using MW irradiation. | ||
Whereas in-core flash heating raised steadily throughout the whole IL media. This is the main cause for the formation of the obtained MgO nanostructures by means of MW irradiation in ILs. Moreover our experimental results also verified this hypothesis by comparison of conventional and microwave heating experimental results for MgO morphology development in various other solvents.
3.9. Catalytic activity of MgON
To determine the catalytic activity of the prepared morphologically controlled MgO nanostructures, a Claisen condensation reaction was performed. This reaction is mainly based on the basic property of the catalyst and active centers present in the catalyst. As compared to pure MgO, the synthesized MgO in this study showed a highly tailored basicity by CO2 TPD (Table 1). Therefore, this reaction gives focus on tailored basicity which alters due to the preparation method using MW irradiation in various ILs. Table 2 demonstrates the results of the synthesis of chalcones using the Claisen condensation reaction of acetophenone (1) and benzaldehyde (2) as model compounds using a catalytic amount of MgO nanostructures. Initially, a catalyst-free reaction of (1) and (2) in ethanol at 120 °C was performed which did not show any product after 72 h (entry 1). To determine the activity of the prepared MgON as catalyst, numbers of reactions were performed at the same conditions in ethanol. All the prepared different catalysts were tested for this reaction using 0.1 equiv. catalyst amount, and a remarkable catalyst was discovered among the prepared MgON samples (entries 2–6). The results show that all the performed reactions with MgON were carried out very well and provide good to excellent yields of the product (upto 95% yield) showing significant activity of the MgO nanostructures. From these results, it can be seen that the hexagonal morphology of MgON-3, which has the highest basicity as well as surface area, exhibited outstanding activity as well as selectivity compared with the other catalysts. Whereas, pure MgO showed only 21% yield of the respective product at the same reaction conditions. A high surface area as well as efficient basic sites with deficiency of oxygen created the development of morphology in MgON-3 in ILs by means of MW irradiation. Hence, these results confirmed that the MgON morphology has efficient activity which converts 100% reactant and obtained a 95% yield of the product compared to bare MgO. In continuation of this work in our laboratory, determination of the specific basicity of nano MgO and basicity oriented organic transformations is in process. In addition, other applications such as energy materials in battery applications, supercapacitors and catalyst applications are under development.| Sr. No | Catalyst | MgON (equiv.) | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a All reactions were carried out on a 1.0 mmol scale of substrate with 0.1 equiv. catalyst in 4.0 mL of ethanol. b Yield refers to the isolated product by 1H NMR. | |||||
| 1 | — | — | 120 | 72 | 0 |
| 2 | MgON-1 | 0.1 | 120 | 12 | 76 |
| 3 | MgON-2 | 0.1 | 120 | 12 | 83 |
| 4 | MgON-3 | 0.1 | 120 | 12 | 95 |
| 5 | MgON-4 | 0.1 | 120 | 12 | 72 |
| 6 | MgON-5 | 0.1 | 120 | 12 | 81 |
| 7 | Pure MgO | 0.1 | 120 | 24 | 21 |
4. Conclusions
In summary, various MgO nanostructures were successfully synthesized by applying the MW-assisted method using different kinds of structural directing monocationic and dicationic ILs. The IL itself acts as a solvent as well as a template for the formation of different morphologies. The in-core MW heating technique with ILs (ionic media) is shown to have a crucial impact on the final construction of MgO morphologies. FE-SEM and FE-TEM observations of MgO exhibit the different structures of MgO such as nanoflakes, interconnected nanoparticles, hexagonal nanoparticles, irregular nanoparticles and nanocapsules. To understand the growth mechanism of MgO with MW irradiation in the presence of ILs, various reactions were performed using different solvents by the conventional method. Formation of a controlled morphology in MgO is obtained because of the nature of the IL, such as monocationic or dicationic as well as the different anionic moiety present in the ILs. In addition, comparative study reveals that the formed hydrogen bonding with favorable π–π interactions between ILs and metal precursors effectively leads to growth of different MgO morphology under microwave conditions. Detailed characterization by different analytical and spectroscopic methods reveals that a highly crystalline and pure phase of nano MgO was obtained. There is no essential use of surfactant or template needed for this approach to construct different morphologies. The obtained materials were tested as catalysts for the Claisen condensation reaction and results showed that the nanostructures posses efficient catalytic activity in the organic transformation. Hence, it can be concluded that the ILs used in this study were proven to be effective self-directing agents and templating material for the synthesis of different MgO nanostructures by means of MW irradiation.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant number: NRF-2013R1A1A2060638 and No. 2009-0093816).Notes and references
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed.
- R. K. Joshi and J. J. Schneider, Chem. Soc. Rev., 2012, 41, 5285 RSC.
- H. Tsuji, F. Yagi, H. Hattori and H. Kita, J. Catal., 1994, 148, 759 CrossRef CAS.
- H. H. Huang, W. C. Shih and C. H. Lai, Appl. Phys. Lett., 2010, 96, 193505 CrossRef.
- B. Q. Xu, J. M. Wei, H. Y. Wang, K. Q. Sun and Q. M. Zhu, Catal. Today, 2001, 68, 217 CrossRef CAS.
- M. Verziu, B. Cojocaru, J. Hu, R. Richards, C. Ciuculescu, P. Filip and V. I. Parvulescu, Green Chem., 2008, 10, 373 RSC.
- C. M. Janet, B. Viswanathan, R. P. Viswanath and T. K. Varadarajan, J. Phys. Chem. C, 2007, 111, 10267 CAS.
- K. T. Ranjit and K. J. Klabunde, Chem. Mater., 2005, 17, 65 CrossRef CAS.
- S. Utamapanya, K. J. Klabunde and J. R. Schlup, Chem. Mater., 1991, 3, 175 CrossRef CAS.
- Y. Yin, G. Zhang and Y. Xia, Adv. Funct. Mater., 2002, 12, 293 CrossRef CAS.
- R. Richards, W. Li, S. Decker, C. Davidson, O. Koper, V. Zaikovski, A. Volodin, T. Rieker and K. J. Klabunde, J. Am. Chem. Soc., 2000, 122, 4921 CrossRef CAS.
- P. Ouraipryvan, T. Sreethawong and S. Chavadej, Mater. Lett., 2009, 63, 1862 CrossRef CAS.
- V. Polshettiwar, M. N. Nadagouda and R. S. Varma, Aust. J. Chem., 2009, 62, 16 CrossRef CAS.
- B. L. Hayes, Microwave Synthesis: Chemistry at the Speed of Light, CEM Publishing, Matthews, 2002 Search PubMed.
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164 RSC.
- I. Bilecka and M. Niederberger, Nanoscale, 2010, 2, 1358 RSC.
- R. Martnez-Palou, Mol. Diversity, 2010, 14, 3 CrossRef PubMed.
- M. B. Gawande, V. D. B. Bonifacio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC.
- A. H. Jadhav and H. Kim, Chem. Eng. J., 2012, 200, 264 CrossRef.
- A. H. Jadhav, A. Chinnappan, R. H. Patil, S. V. Kostjuk and H. Kim, Chem. Eng. J., 2014, 243, 92 CrossRef CAS.
- A. H. Jadhav and H. Kim, Tetrahedron Let.s, 2012, 53(39), 5338 CrossRef CAS.
- A. H. Jadhav, A. Chinnappan, R. H. Patil, S. V. Kostjuk and H. Kim, Chem. Eng. J., 2014, 240, 228 CrossRef CAS.
- A. H. Jadhav, K. Lee, S. Koo and J. G. Seo, RSC Adv., 2015, 5(33), 26197 RSC.
- A. H. Jadhav, H. Kim and I. T. Hwang, Catal. Commun., 2012, 21, 96 CrossRef CAS.
- Y. J. Zhu and F. Chen, Chem. Rev., 2014, 114(12), 6462 CrossRef CAS PubMed.
- A. H. Jadhav, G. M. Thorat, K. Lee, A. C. Lim, H. Kang and J. G. Seo, Catal. Today, 2016, 265, 56 CrossRef CAS.
- W. W. Wang and Y. J. Zhu, Inorg. Chem. Commu.n, 2004, 7, 1003 CrossRef CAS.
- J. M. Cao, J. Wang, B. Q. Fang, X. Chang, M. B. Zheng and H. Y. Wang, Chem. Lett., 2004, 33, 1332 CrossRef CAS.
- M. G. Ma, J. F. Zhu, Y. J. Zhu and R. C. Sun, Chem.–Asian J., 2014, 9, 2378 CrossRef CAS PubMed.
- M. Petkovic, K. R. Seddon, L. P. N. Rebello and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383 RSC.
- M. Rezaei, M. Khajenoori and B. Nematollahi, Mater. Res. Bull., 2011, 46, 1632 CrossRef CAS.
- R. R. Gandhi, S. Gowri, J. Suresh and M. Sundrarajan, J. Mater. Sci. Technol., 2013, 29(6), 533 CAS.
- N. C. S. Selvam, R. Thinesh Kumar, L. J. Kennedy and J. J. Vijaya, J. Alloys Comp.d, 2011, 509, 9809 CrossRef CAS.
- J. Zhou, S. Yang and J. Yu, Colloids Surf., A, 2011, 379, 102 CrossRef CAS.
- H. Niu, Q. Yang, K. Tang and Y. Xie, J. Nanopart. Res., 2006, 8, 881 CrossRef CAS.
- J. Xu, Y. Ao, D. Fu and C. Yuan, Appl. Surf. Sci., 2008, 255, 2365 CrossRef CAS.
- H. Ding, K. T. Yong, I. Roy, H. E. Pudavar, W. C. Law, E. J. Bergey and P. N. Prasad, J. Phys. Chem. C, 2007, 111, 12552 CAS.
- L. Bertinetti, C. Drouet, C. Combes, C. Rey, A. Tampieri, S. Coluccia and G. Martra, Langmuir, 2009, 25, 5647 CrossRef CAS PubMed.
- G. Pacchioni, ChemPhysChem, 2003, 4, 1041 CrossRef CAS PubMed.
- S. Xie, X. Han, Q. Kuang, Y. Zhao, Z. Xie and L. Zheng, J. Mater. Chem., 2011, 21, 7263 RSC.
- L. Kumari, W. Z. Li, C. H. Vannoy, R. M. Leblanc and D. Z. Wang, Ceram. Interfaces, 2009, 35, 3355 CrossRef CAS.
- B. M. Maoz, E. Tirosh, M. B. Sadan, I. Popov, Y. Rosenberg and G. Markovich, J. Mater. Chem., 2011, 21, 9532 RSC.
- H. S. Kim and H. W. Kim, Acta Phys. Pol., A, 2009, 116, 58 CrossRef CAS.
- K. Ishikawa, N. Fujima and H. Komura, J. Appl. Phys., 1985, 57, 973 CrossRef CAS.
- M. Sterrer, O. Diwald and E. Knozinger, J. Phys. Chem. B, 2000, 104, 3601 CrossRef CAS.
- C. O. Kappe and A. Stadler, Microwaves in organic and medicinal chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed.
- K. Richter, P. S. Campbell, T. Baecker, A. S. Itzek, D. Yaprak and A. V. Mudring, Phys. Status Solidi B, 2013, 250(6), 152 CrossRef.
- Z. He and P. Alexandridis, Phys. Chem. Chem. Phys., 2015, 17, 18238 RSC.
- T. Greaves and C. Drummond, Chem. Soc. Rev., 2013, 42, 1096 RSC.
- C. Vollmer and C. Janiak, Coord. Chem. Rev., 2011, 255, 2039–2057 CrossRef CAS.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2008, 37, 1709–1726 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02980a |
| This journal is © The Royal Society of Chemistry 2016 |