 Open Access Article
Open Access ArticleTowards greener furfural: evaluating the technical, economic and environmental feasibility of heterogeneous catalysis in biomass conversion†
Jorge
Blanco-Cejas
 *a,
Ion
Agirre
*a,
Ion
Agirre
 *b,
Inaki
Gandarias
*b,
Inaki
Gandarias
 b,
Jovita
Moreno
ac and
Jose
Iglesias
b,
Jovita
Moreno
ac and
Jose
Iglesias
 ac
ac
aChemical & Environmental Engineering Group, Universidad Rey Juan Carlos, C/Tulipan s/n, Mostoles, 28933, Spain. E-mail: jorge.blanco@urjc.es
bDepartment of Chemical and Environmental Engineering, Bilbao School of Engineering, University of the Basque Country (UPV/EHU), Plaza Ingeniero Torres Quevedo 1, 48013 Bilbao, Spain. E-mail: ion.agirre@ehu.eus
cInstituto de Tecnologías para la Sostenibilidad, Universidad Rey Juan Carlos, C/Tulipan s/n, Mostoles, 28933, Spain
First published on 28th May 2025
Abstract
Furfural is a key biomass-derived platform chemical with a large market volume, yet its production has largely been outsourced from Europe due to the high energy demand for reactor heating and the significant environmental impact of acidic waste generation. Current industrial processes, predominantly the Chinese Batch Process (CBP), rely on sulphuric acid as a catalyst and require extensive steam stripping, contributing significant environmental constraints. This study explores the feasibility of a more sustainable furfural production by evaluating an alternative process based on a heterogeneous acid catalyst. The proposed process integrates scale-up considerations to improve reactor performance, replacing steam stripping with nitrogen stripping and sulphuric acid with Amberlyst-70® as heterogeneous catalyst. A detailed process simulation, techno-economic analysis (TEA), and life cycle assessment (LCA) were conducted to compare the material and energy balances of both processes and to assess the viability of the heterogeneous catalytic process (HCP). Results indicate that the current selectivity of Amberlyst-70® is insufficient for technical feasibility, as a 5.5-fold improvement in furfural-to-tar selectivity is required to match the steam consumption of CBP. If this target is met, both processes exhibit similar minimum selling prices (>€3000 per t), although significantly above current market levels. However, the HCP presents a potential cost reduction pathway (<€1000 per t) through the valorisation of lignin and cellulose by-products, offering a competitive advantage. Environmental analysis highlights key benefits of the HCP, including a significant reduction in freshwater ecotoxicity by eliminating sulphuric acid and improved energy efficiency through enhanced process integration. Nonetheless, energy consumption and maize cob usage remain critical environmental hotspots. Overall, the study identifies catalyst selectivity as the key bottleneck preventing the implementation of the HCP. Further development of a more selective and stable heterogeneous catalyst, alongside integrated biorefinery strategies, could enable the competitive and sustainable production of furfural.
Sustainability spotlightThe relocation of potentially polluting industrial activities to countries with lenient environmental legislation has been constant in the chemical industry since the late 20th century. However, demand for products from such processes remains, merely shifting pollution without global-scale changes. Furfural is one of the chemical products obtained through processes with significant environmental impact and serves as a case study in this contribution, which evaluates the technical, economic, and environmental feasibility of its production in Europe. This evaluation guides the development of a more sustainable furfural production process using heterogeneous catalysis, reducing environmental impact by eliminating sulphuric acid and improving energy efficiency. It also valorises by-products like lignin and cellulose, aligning with UN SDGs 9 and 13. |
1 Introduction
Climate change and resource depletion are critical environmental threats largely driven by fossil fuel dependence.1,2 The industrial sector heavily relies on fossil combustion and is the second economic sector on greenhouse gas emissions with 14 Gt CO2-eq.3 The current trend involves exploring alternative carbon sources, with biomass emerging as a key renewable option due to its abundance, low cost, and independence from international supply chains.4,5 By 2022, the number of biorefineries in Europe had reached 1250, including 500 facilities focused on chemical production.6,7Among the biomass-derived chemicals with the strongest market support, platform molecules such as lactic and succinic acids stand out as the most successful examples.8 Nevertheless, furfural is increasingly relevant, as its market volume exceeds USD 550 million and is expected to reach USD 900 million by the end of the decade,9 primarily driven by the demand for furfuryl alcohol (FOL).10 Furfural consists of a furanic ring with an aldehyde group, and it is obtained from pentoses naturally present in biomass. It finds uses as a precursor for a wide range of compounds including maleic acid, tetrahydrofurfuryl alcohol or the aforementioned succinic acid and FOL. Almost all furfural consumed in Europe is procured by Belgium and the Netherlands, and it is mainly imported from non-EU countries such as China, the Dominican Republic or Israel, with only a small percentage produced in Slovenia and Austria.11,12 The reason behind this outsourcing of production relates to the potential environmental damage associated with the operation of the production plants, as these consume large amounts of sulphuric acid.13
The industrial production of furfural dates back almost a century, with the technology patented by the Quaker Oats company.14 Despite the maturity of this process, and the associated technological developments, most of today's commercial processes follow a similar scheme based on a semi-continuous process (see Fig. S1†). The starting feedstock consists of agricultural or forestry residues, which are hydrolysed in contact with sulphuric acid. Upon hydrolysis, hemicellulosic pentose sugars – mostly xylose and arabinose – are released and subsequently dehydrated to furfural. Typically, both reactions occur in a single batch reactor, where the slurry of biomass and dilute sulphuric acid is subjected to moderate pressure and temperature conditions (about 160 °C and 7 atm) by contact with medium-pressure steam.14,15 Steam is injected into the reactor as a heating and stripping agent for the rapid separation of the evolving furfural, avoiding condensation and resinification side reactions. At the end of the reaction, the unreacted solid, bearing the sulphuric acid, is filtered and landfilled, while furfural is purified via serial double distillation. In this operation, the first stage removes the carboxylic acids (acetic and formic) present in the mixture, while the second stage separates furfural from water through an azeotropic distillation, producing 98.5–99.5% grade furfural.
Some of the most recognised technologies for furfural production include the Huaxia, SupraYield, Vedernikovs or Biofine processes.11,12 However, almost all furfural currently on the market is produced in China (Chinese batch process), the Dominican Republic (Quaker Oats) and South Africa (Rosenlew process), due to the reasons previously outlined.9 Among these technologies, the Chinese Batch Process (CBP) has the largest market share due to its production volume and the low price afforded.16–18 CBP feedstock typically comprises corn cobs from maize processing. This is a cheap and abundant material in China,19 with a high percentage of pentosans in its structure, which makes it ideal for furfural production. The process operates similarly to a Quaker-Oats-type process, although incorporating some energy improvements such as partial condensation of the reactor outlet to provide the heat at the reboiler of the first distillation column.14,15
Beyond commercial ventures, furfural production has recently received considerable attention from research community. Numerous proposals have attempted to advance homogeneous catalysis using different solvents and substrates.20–26 However, a growing trend is the use of heterogeneous catalysts that allow the minimisation of corrosion effects and hazardous waste generation, thus contributing to minimize capital expenditures through the use of simpler reaction equipment, and to minimize environmental impacts through the prevention of waste production. Many catalytic routes are based on the use commercially available of supported metal oxides27–29 and zeolites. However, some authors have reported low yields in the production of furfural through heterogeneous catalyst driven processes,30 and a significant deactivation after several reuse cycles.31,32 Gupta et al. achieved improved reusability by grafting sulfonic acid groups to H-β zeolite,33 promoting a high Brønsted acidity similar to some ion exchange resins. Notably, the use of these resins in furfural production has gained momentum in recent years, because of the wide commercial availability and low cost of these materials. Some authors have explored the use of strong cation exchange resins such as Nafion™ NR 50 (ref. 34) or Amberlyst® 15 (ref. 35) reporting moderate to high yields to furfural, but with the deactivation of the catalytic centres in consecutive reuses. Similarly, Sato et al.36 tested the use of Amberlyst® 70 (a non-commercial cation exchange resin) with a continuous flow of CO2 at 150 °C, obtaining yields similar to those provided by commercial resins. Additionally, Hu et al.37 tested the use of Amberlyst® 70 in water, although reaching very low yields due to the instability of the reaction intermediates. Despite this drawback, other authors have tested water as a solvent, mostly in homogeneous catalytic systems.38–40 Even though the use of water can be advantageous, it is mostly used in biphasic mixtures with organic compounds such as toluene41–43 or gamma-valerolactone30,44–47 to promote dehydration reactions. A comprehensive list of solvent and catalytic systems can be consulted at Edumujeze et al.48 With a few exceptions,49–51 most of the investigations focus on the laboratory scale disregarding specific scale-up effects (e.g. reactor configuration). This low technological maturity, along with the scarcity on benchmark data, hinders the analysis of constraints on an industrial level and restricts the comparison in terms of economic profitability and environmental performance.
This article aims to identify the process level keys for a sustainable furfural production, for which three critical issues must be addressed. First, the process must be technically feasible on a commercial scale, considering reactor heating limitations and purification system characteristics. Second, furfural production must be economically competitive to significantly impact the current market shares. Third, environmental impact must be notably reduced, as this is the primary reason preventing the production of furfural in countries with developed environmental protection laws. To this end, the scale-up of a furfural production process based on the use of a heterogeneous catalyst (HCP) has been rigorously simulated, in line with previous investigations.52,53 This process has been selected for its potential to reduce energy costs and to avoid acid waste due to its characteristic reaction system configuration. The process is conducted in two stages. First, pentosans are hydrolysed into sugars in the absence of external catalyst and under moderate conditions. In a second reactor, the sugars are dehydrated to furfural using Amberlyst® 70 as a heterogeneous acid catalyst instead of H2SO4. The evolving furfural is separated using a nitrogen stripping stream instead of conventional steam, potentially reducing energy consumption. Finally, the product is subjected to a double distillation as in commercial processes, yielding 99.5 wt% pure furfural. In parallel, comprehensive data on the industrial production of furfural has been generated through the rigorous simulation and optimisation of literature data.14,15,19 For this purpose, the Chinese Batch Process (CBP) has been selected as benchmark due to its current market share in the production of furfural. This inventory serves to support the comparison against the HCP and provide a reliable reference in the assessment of the economic and environmental performances. For this purpose, technoeconomic analysis (TEA) and lifecycle analysis (LCA) have been applied to both processes aiming to identify critical aspects that influence the economic competitiveness and environmental impact of the HCP compared to the current furfural production. This analysis can guide future research efforts to address the identified bottlenecks, ultimately leading to the sustainable production of furfural.
2 Methodology
2.1 Process modelling
The design of the furfural production plant was inspired by existing industrial facilities, which generally handle annual throughputs of several kilotons of furfural, with some reaching a maximum capacity of 35 kilotons per year.54,55 This study assumes an annual production scale of 15 kilotons of 99.5 wt% furfural for both conventional and heterogeneous catalysis-based plants.Process modelling was carried out using Aspen Plus© V14 software. The thermodynamic framework combined the NRTL model for liquid-phase activity coefficients with the Redlich–Kwong equation of state (EOS) for vapor-phase properties. Since Aspen Plus does not directly provide a pre-defined model for lignocellulosic biomass – e.g. corncobs, its physical properties were sourced from NREL.56
For the CBP, the kinetic data was sourced from the literature.57,58 According to these models (see Fig. S2†), xylans and arabinans are converted directly into their respective sugars (xylose and arabinose), while the acetyl groups are hydrolysed to acetic acid. Subsequently, free sugars are converted into intermediates, finally yielding furfural. Additionally, the hydrolysis of cellulose to produce glucose was also considered in the kinetic model of CBP, despite the low reaction extent at the operating conditions.
In the HCP, reaction kinetics were implemented using two CSTR reactor models. The first one, involves non-catalytic autohydrolysis reactions where xylans and arabinans are initially converted into their respective oligomers, which are subsequently broken down into sugars (see Fig. S3†). Similarly, acetyl groups are hydrolysed to acetic acid. Under the operating conditions of this stage, cellulose and lignin remain unaltered. The corresponding kinetic data were derived from the literature.59 The second reactor focuses on the conversion of the free sugars into furfural through heterogeneous catalysis. In this case the kinetic data were obtained in the laboratory.52
Both models account for the formation of tars via two mechanisms: the reaction between xylose and furfural, and the self-resinification of furfural. Moreover, the stripping effect was simulated considering the phase equilibria of the two outlet streams (waste liquid stream and the vapor stream derived from the stripping).
Distillation columns were modelled using the rigorous RadFrac model. The column configurations, including the number of stages, were optimized by minimizing total annualized costs (TAC).60 TAC, as defined in eqn (1), accounts for operational expenses by combining capital costs, amortization periods, and utility expenditures.
 | (1) |
A sensitivity analysis was conducted to identify the optimal feed stage, aiming to minimize reboiler energy consumption.
2.2 Economic analysis
The economic evaluation encompassed both capital expenditures (CapEx) and overall operational expenditures (OpEx), with the minimum selling price (MSP) of furfural serving as the benchmark for assessing economic viability.61 The MSP was determined to ensure that the annual revenue aligns with the Equivalent Annual Operating Cost (EAOC) (eqn (2)). EAOC includes both annualized capital costs and operational expenses, calculated using a discounted cash flow approach. This analysis assumed 10 year project lifespan, 20% internal rate of return, and 40% corporate tax rate.62 The annualized capital cost (eqn (3)) was distributed over the project's duration and combined with OpEx to calculate the EAOC (eqn (4)). All costs were calculated in euros, assuming a currency exchange rate of 1 USD = 0.95€.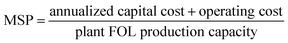 | (2) |
| EAOC = annualized capital cost + operating cost | (3) |
 | (4) |
CapEx estimations adhered to standard methodologies, utilizing equipment cost data from Aspen Process Economic Analyzer (APEA-V14), based on first-quarter 2022 figures. This tool, integrated within Aspen Plus©, accounts for equipment costs, installation, bulk plant systems (e.g., power distribution, control systems), and indirect costs. Steam ejector costs were considered negligible.63
In both the conventional CBP and the alternative HCP, carbon steel was selected as the construction material, as it is commonly employed in current furfural production plants. To withstand the corrosive effects of sulphuric acid, the reactor walls in the CBP are designed with an extraordinary thickness of 50 mm,14 and therefore this measure was considered in the CapEx estimation for the reactors in the conventional process. In contrast, for the HCP the wall thickness was adjusted according to the specific operating conditions due to the absence of acid.
Total OpEx included raw materials, utilities, catalysts, and supplementary expenses such as labour, maintenance, overheads, and administration.62 Raw material and utility costs were escalated annually by 3%.
The H2SO4 is considered an additional raw material in the CBP, with a cost of €57 per metric ton.64 In contrast, the HCP is based on heterogeneous catalysis, utilizing Amberlyst® 70 resin, priced at €47.5 per kilogram.54 The catalyst replacement time was estimated using a productivity ratio, assuming that 1 kg of catalyst produces 1000 kg of furfural before disposal.54 After optimizing the required catalyst amount (as detailed in Section 3.1) and applying this production ratio, the catalyst replacement interval was calculated to be 5.05 days. While this might seem relatively short, it aligns with experimental findings reported by Agirrezabal-Telleria et al.,52,53 who observed that catalyst particles become coated with tars formed during course of the reaction.
These prices, along with estimated process consumption rates and costs for minor reagents such as sodium carbonate used in the CBP, are summarized in Table 1. Additionally, the table provides the market prices of furfural, cellulose, and lignin.
| Raw materials | Price (€ per Mt) | Consumption CBP (Mt per year) | Consumption HCP (Mt per year) |
|---|---|---|---|
| a Figures provided by a Spanish oil refining company (2023). | |||
| Corncobs64 | 81.40 | 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.00 800.00 |
142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.00 800.00 |
| Process water | 1.61 | 164![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529.00 529.00 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797.00 797.00 |
| H2SO4 (ref. 64) | 57.00 | 2789.20 | — |
| Heterogeneous catalyst54 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
— | 8.40 |
| Sodium carbonate66 | 339.10 | 243.60 | — |
| Utilitiesa | Price |
|---|---|
| Steam @ 250 psi (€ per Mt) | 34.88 |
| Steam @ 50 psi (€ per Mt) | 34.03 |
| Electricity (€ per MW per h) | 229.23 |
| Cooling water (€ per GJ) | 1.32 |
2.3 Life cycle assessment (LCA)
The methodology used for the LCA study is described below following the ISO 14044 structure.2.4 Assumptions and limitations
Several key assumptions were considered along the assessment. These assumptions and constraints are critical to interpreting the results and provide the context necessary for evaluating the economic and technical feasibility of the process:• Although CBP is carried out in China, to ensure a fair comparison with the proposed technology, the techno-economic and environmental analyses have been carried out using European data. This avoids the price and impact differences associated with the regionalization of data. It is important to note that this may lead to deviations, such as an increase in the reference price of furfural due to the higher cost of critical raw materials, or a decrease in the impact associated with the energy mix. In any case, it ensures a fair comparison framework, which better aligns with the objective of this work.
• In CBP, two waste streams are generated: spent liquor from the reactor emptying and the bottom stream from the first distillation column. The first stream contains approximately 27 wt% of solids consisting of cellulose, lignin, other oligomers, ash and tar. These solids are filtered and sent to a controlled landfill. The liquid fraction, containing a high acid load, undergoes water treatment prior to discharge. However, some acetic and sulphuric acids are retained in the solid cake, together with other compounds such as furfural and other by-products. In the HCP, two waste streams are also produced in the autohydrolysis and stripping reactors. The first stream consists of a solid fraction with a similar composition to that of the conventional reactor, although free of mineral acids and other harmful compounds. The second reactor produces a stream containing a minor tar fraction, which is filtered and sent to a controlled landfill, while the aqueous fraction is sent to conventional water treatment.
• The CBP may produce by-products like methanol or acetic acid, as mentioned by some authors.14,19 Information on methanol production is only found in the description by Win,19 albeit it is not supported by transparent data and refers to the continuous process (Huaxia as modified by Westpro). Similarly, to the best of the authors' knowledge, there are no references to methanol production from methylated biomass structural polymer sections. Therefore, it was decided not to include this by-product in the simulation and LCA calculations. On the other hand, the bottom stream of the first distillation column contains 1.2 wt% acetic acid, which falls within the range reported in the literature,14 although is too low to justify its recovery according to consulted ref. 15. Therefore, the purification train simulated does not comprise the recovery of this compound, and it was not considered as a by-product. Finally, in the HCP, although the potential to obtain high-quality cellulose and lignin through purification—comprising filtration, washing, and precipitation—has been considered, this design was not implemented. Consequently, these by-products were assumed to undergo landfilling as a part of the waste solid cake.
• The HCP process exhibits a higher integration than the CBP due to the flexibility in its design approach. While the proprietary process was modelled to maximize performance, the CBP simulation aimed to provide a representative benchmark. Thus, only design parameters explicitly reported in the literature were used, with optimization limited to the ranges provided (e.g., stripping steam flow rate, distillation column design). However, commercial implementations may be more optimized than described in the literature, introducing a potential limitation to this comparison.
3 Results and discussion
3.1 Basic process engineering: HCP vs. CBP
As previously disclosed, HCP was initially featured with distinct characteristics as compared to CBP which were expected to strongly improve the environmental footprint of furfural production while preserving its techno-economic feasibility. Two main aspects have been investigated:(i) The use of nitrogen (N2) as a stripping agent instead of steam.
(ii) And the utilization of a heterogeneous catalyst to eliminate the need for sulphuric acid (H2SO4).
The use of N2 as a stripping agent has been published as a promising alternative due to its lower cost.52 However, process simulations about this option suggest that N2 would remove a significant portion of the water from the reaction medium since it would leave the reactor saturated in steam. This phenomenon increases the formation of tars due to the increase in the concentration of tar-precursors in the reaction medium. Moreover, it must be considered that besides acting as entrainer, steam also acts as a heating media, so that if using N2, an external heating source must be implemented. For this reason, N2 was discarded as a stripping agent in the second reactor, and steam continued to be used as in the conventional CBP.
The second potential improvement focuses on the use of commercial Amberlyst© 70 as a catalyst. Unlike H2SO4, a heterogeneous catalyst hydrolyse biomass as a solid within a liquid reaction media. Therefore, the use of this catalyst implies that the hydrolysis must be divided into two stages to favour the contact between the heterogeneous catalyst and the carbohydrate raw material (see Fig. 2). The first stage involves autothermal hydrolysis (R101), where biomass is partially hydrolysed at 190 °C (16 bar),59 aided by the presence of acetic acid evolving from biomass. This is conducted in a continuously stirred tank reactor (CSTR) with an optimized residence time of 7.5 minutes under the specified conditions. These conditions enable the depolymerization of the biomass, achieving the hydrolysis of 92% of the biomass, with the majority of xylans and arabinans (71%) converted to oligomers and 15% to free monosaccharides solubilized in the liquid water. Various temperatures (160–190 °C) and residence times were simulated in Aspen before determining the optimal conditions. Fig. S4† shows the evolution of the different species that are present in the reaction media in function of the residence time and the temperature. Higher residence times led to increased biomass hydrolysis and furfural production, but also promoted tar formation, making 7.5 minutes at 190 °C the optimal setting, keeping the solids (the sum of unhydrolyzed xylans and arabinans and tars) below 15%. Before moving to the second stage, a filter is required to separate the solid fraction, consisting of cellulose, lignin, and unhydrolyzed hemicellulose (7–8%). This stream has potential value in an integrated biorefinery concept.
 | ||
| Fig. 2 Flowsheet diagram of HCP (red lines indicate the stripping steam streams carrying furfural from the reactor to the first distillation column). | ||
The second hydrolysis step (R-102) of the HCP requires higher temperature conditions as compared to the CBP (200 °C instead of 160 °C). The main reason is the increased time required to conduct the hydrolysis of the oligomers into monosaccharides according to kinetic data.52,53 Lower operating temperatures, such as 175 °C, could be used as suggested by Agirrezabal-Telleria et al.52 However, it is important to consider that in R-102, oligomers continue depolymerizing into sugars, and at 175 °C, this process occurs significantly slower than at 200 °C (see Fig. S4†). As a result, insufficient xylose and arabinose would be available to achieve the furfural production target set in this study, given a residence time similar to that of the CBP process, which is 4 hours. Besides, since the previous hydrolysis step is conducted at 190 °C, maintaining a similar temperature seems appropriate. This is further supported by the fact that medium-pressure steam has a temperature of 205 °C, allowing the reactor to operate adiabatically at 200 °C. Bearing this in mind, multiple parallel stripping reactors were employed to reach the projected plant capacity while maintaining each reactor's volume at 14 m3, as reported for the CBP.14 However, as the bulk volume is decreased after the autohydrolysis step due to the separation of the cellulosic and lignin fractions, only 17 reactors were required instead of the 20 units estimated for the conventional process, potentially decreasing the CapEx requirements in this section of the HCP.
Initial simulations of the HCP under the mentioned conditions evidence that 325![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg h−1 of steam was required to achieve the same furfural production rate, i.e. 2550 kg h−1 (127.5 kg of vapor per kg of furfural distilled, see Fig. 3), at the stripping outlet as in the CBP, which needed only 63
000 kg h−1 of steam was required to achieve the same furfural production rate, i.e. 2550 kg h−1 (127.5 kg of vapor per kg of furfural distilled, see Fig. 3), at the stripping outlet as in the CBP, which needed only 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 kg h−1 (25.4 kg of vapor per kg of furfural distilled). These values refer to the overall values in R-102 system, i.e., the sum of the vapor required and furfural produced in each parallel reactor. This seemed counterintuitive, as the relative volatility of furfural with respect to water is slightly higher at 200 °C than at 160 °C (0.172 vs. 0.158). This discrepancy suggests that the rate of tar formation in the heterogeneous catalysts is excessively high, and therefore very large stripping vapor rates are required to limit furfural degradation.
671 kg h−1 (25.4 kg of vapor per kg of furfural distilled). These values refer to the overall values in R-102 system, i.e., the sum of the vapor required and furfural produced in each parallel reactor. This seemed counterintuitive, as the relative volatility of furfural with respect to water is slightly higher at 200 °C than at 160 °C (0.172 vs. 0.158). This discrepancy suggests that the rate of tar formation in the heterogeneous catalysts is excessively high, and therefore very large stripping vapor rates are required to limit furfural degradation.
As it is described in the techno-economic assessment results (Section 3.2, vide infra), the cost of stripping steam is very significant and accounts for almost 32.4% of the total EAOC in the CBP. Moreover, larger steam flows also increase distillation costs as more diluted furfural is obtained. Therefore, the use of Amberlyst® 70 makes the HCP economically unfeasible due to its low selectivity, which demands impractically high steam stripping flows. To enable a viable process, catalyst improvements are necessary.
Under this background, it is critically important to determine the global selectivity value required for a future heterogeneous catalytic process to enable the economically viable production of furfural on an industrial scale. This parameter can serve as a key benchmark to steer the scientific community's efforts toward the development of optimized solid acid catalysts that not only surpass the performance of Amberlyst® 70 but also compete effectively with homogeneous catalysts. As illustrated in Fig. 4, an increase in the rate of furfural formation relative to the rate of tar formation (instantaneous selectivity), while keeping the stripping vapor flow fixed at the level used in the CBP process (63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 kg h−1), gradually improves the furfural yield, bringing it closer to the furfural production achieved in CBP (2550 kg h−1). Based on these findings, the development of a catalyst exhibiting a global selectivity of 4.8 kg of furfural per kg of tar is essential. This represents a 5.5-fold improvement over Amberlyst® 70 and is necessary to match the CBP steam stripping consumption while maintaining the same furfural production rate (25 kg of vapor per kg of furfural distilled). Interestingly, the overall selectivity in the CBP is 1.5 kg of furfural per kg of tar when operating under the same conditions. Initially, it was hypothesized that the excessive steam requirement in HCP could be attributed to a high rate of tar formation on the heterogeneous catalyst. However, a closer examination of the overall selectivity (kg of furfural produced per kg of tars produced) values suggests a different explanation. In the CBP process, the overall selectivity is 1.5, whereas in HCP, when operating with 325
671 kg h−1), gradually improves the furfural yield, bringing it closer to the furfural production achieved in CBP (2550 kg h−1). Based on these findings, the development of a catalyst exhibiting a global selectivity of 4.8 kg of furfural per kg of tar is essential. This represents a 5.5-fold improvement over Amberlyst® 70 and is necessary to match the CBP steam stripping consumption while maintaining the same furfural production rate (25 kg of vapor per kg of furfural distilled). Interestingly, the overall selectivity in the CBP is 1.5 kg of furfural per kg of tar when operating under the same conditions. Initially, it was hypothesized that the excessive steam requirement in HCP could be attributed to a high rate of tar formation on the heterogeneous catalyst. However, a closer examination of the overall selectivity (kg of furfural produced per kg of tars produced) values suggests a different explanation. In the CBP process, the overall selectivity is 1.5, whereas in HCP, when operating with 325![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg h−1 of stripping steam, the selectivity increases to 3.84. This difference arises because, unlike in CBP, only approximately 80% of the hemicellulose undergoes complete hydrolysis to monomeric sugars in HCP in 4 h. Therefore, the heterogeneous catalyst must achieve a higher selectivity than sulphuric acid in CBP to ensure that the available sugars, which are fewer in HCP, are directed proportionally more towards furfural rather than tars, compared to the CBP process. These variations can be observed in Fig. 3 and 4.
000 kg h−1 of stripping steam, the selectivity increases to 3.84. This difference arises because, unlike in CBP, only approximately 80% of the hemicellulose undergoes complete hydrolysis to monomeric sugars in HCP in 4 h. Therefore, the heterogeneous catalyst must achieve a higher selectivity than sulphuric acid in CBP to ensure that the available sugars, which are fewer in HCP, are directed proportionally more towards furfural rather than tars, compared to the CBP process. These variations can be observed in Fig. 3 and 4.
A sensitivity analysis was conducted to identify the optimum amount of catalyst in each reactor (see Fig. 5). By varying the total catalyst amount (sum of the 17 parallel reactors) between 50 and 1000 kg, furfural production was found to reach its maximum at 250 kg, equivalent to 14.6 kg per reactor. Moreover, when fixing the stripping steam flow at 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 kg h−1 (25 kg steam per kg furfural), only a certain amount of furfural can be effectively removed from the reaction medium. Beyond a certain catalyst loading, while the catalyst promotes both furfural and tar formation, the additional furfural generated is not fully carried away by the stripping steam, remaining in the reactor. This leads to its resinification and therefore, increasing tar formation.
671 kg h−1 (25 kg steam per kg furfural), only a certain amount of furfural can be effectively removed from the reaction medium. Beyond a certain catalyst loading, while the catalyst promotes both furfural and tar formation, the additional furfural generated is not fully carried away by the stripping steam, remaining in the reactor. This leads to its resinification and therefore, increasing tar formation.
In addition, at least 1000 kg of furfural per kg of catalyst must be produced before disposal of the catalyst, based on the stability criteria for industrial catalyst viability established by Lange.54 Meeting this target is particularly challenging, as heterogeneous catalysts like Amberlyst® 70 are prone to deactivation due to fouling caused by tar and other heavy products deposited on the active sites. Consequently, in addition to developing a more selective catalytic material, it is essential to ensure that it can be easily regenerated. For Amberlyst® 70, regeneration through simple calcination is not feasible due to the organic nature of the material, which limits its thermal stability, and other regeneration strategies with dilute acids are required for restoring its activity.72 In this regard, inorganic materials present the advantage of allowing thermal regeneration, and therefore, they represent a more promising alternative. If a catalytic material meeting the lifetime criterion of 1000 kg of furfural per kg of catalyst is developed, the catalyst consumption is established in 15 Mt per operating year.
In summary, the intended technology substitution could be achieved by developing a more selective (with an overall selectivity of at least 4.17 kg of furfural per kg of tar, after optimizing the catalyst amount) and stable (with a lifetime of at least 1000 kg of furfural per kg) heterogeneous catalyst. It is expected that under these conditions economic parity would be accomplished while avoiding the use of sulphuric acid, which would contribute to a significant environmental benefit. In this context, and to point toward promising directions for future research, advances should focus on both functionality (selectivity increase) and catalytic support stability. Considering the first point, efficient furfural production from xylose typically benefits from catalysts combining Brønsted and Lewis acid sites (or Brønsted base sites), which promote isomerization to xylulose and subsequent dehydration.28,32 The current system uses a sulfonated ion-exchange resin providing Brønsted acidity leading to overall good results, but which may be enhanced by adding this double functionality according to literature.73 On the other hand, regarding the low thermal stability of ionic resins, alternatives like Nafion NR-50 are more thermally stable up to 240 °C but less selective.34 Zeolites, though tuneable and suitable for functionalization, often present diffusion limitations due to small pore sizes in the case of beta structures and are unstable in aqueous systems.31,33 Similarly, silica-based catalysts degrade in water, and while metal oxides are more robust and water-tolerant, their catalytic activity tends to be lower.27,29 A more promising direction may lie in acid-functionalized carbon-based catalysts, particularly those doped with nitrogen, which has demonstrated high furfural yields (>88%) from lignocellulosic feedstocks, provided gamma-valerolactone (GVL) is used as a solvent.74 However, their performance in water drops significantly due to deactivation, so this should remain a key focus.
3.2 Techno-economic assessment
Fig. 6 presents the EAOC calculated for both processes. In the HCP, the CapEx accounts for 10.2% of the EAOC, whereas in the CBP process, it represents 11.2%. The higher CapEx in the CBP process can be attributed to two primary factors:(1) Number of stripping reactors. As stated in Section 3.1, to achieve the targeted furfural production, the conventional process requires 20 stripping reactors (R-101, Fig. S1†) arranged in parallel, while the HCP requires only 17.
(2) Material and wall thickness of reactors. The conventional process operates with sulphuric acid, necessitating reactor walls with a thickness of 50 mm,14 which exceeds the thickness required by the operational pressure and temperature conditions. In HCP, despite the higher operating pressure would typically require thicker reactor walls, using a heterogeneous catalyst the wall thickness is not as thick as in CBP, thereby construction costs are cheaper.
It should also be noted that the HCP includes an additional reactor for the autothermal hydrolysis stage (R-101). However, due to the short residence time in this stage (7.5 min), the reactor volume is only 6.3 m3, approximately half the size of the stripping reactors. Consequently, the cost savings from eliminating three stripping reactors more than offsets the investment required for this initial hydrolysis reactor.
The breakdown of the operating costs for both processes is summarized in Table 2. The cost of raw materials is similar for both alternatives, amounting to €12.2 M in HCP and €12.5 M in the CBP, corresponding to 36.5% and 35.2% of their respective total OpEx. This slight difference is attributed to the water recirculation implemented in HCP. The cost of the biomass feedstock, however, is identical in both cases.
| Installed cost (€) | ||||
|---|---|---|---|---|
| CBP | HCP | |||
| a Indirect costs, plus contingency and fee, plus auxiliary facilities. b Operating labour, maintenance, overheads. c Market price. d Considering the valorisation of all biomass fractions. | ||||
| Reaction section | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 610![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
83.6% | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 937 937![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
75.8% |
| Furfural purification | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 875![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
11.5% | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 047![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
15.6% |
| Others | 797![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.9% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132 132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
8.6% |
| Total installed cost | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282 282![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
||
| Added costsa | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 512 512![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838 838![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
||
| Total capital cost | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794 794![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 956 956![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
||
| Annualized capital cost (€ per year) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 482 482![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988 988 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806 806![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 012 012 |
||
| Operating costs (€ per year) | ||||
|---|---|---|---|---|
| CBP | HCP | |||
| Raw materials cost | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 543 543![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765 765 |
35.2% | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 239 239![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 965 965 |
36.5% |
| Utilities cost | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 518![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
12.7% | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
8.0% |
| Stripping vapor cost | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 978![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 735 735 |
36.5% | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 978![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 735 735 |
38.8% |
| Catalyst cost | 158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 984 984 |
0.4% | 709![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 012 012 |
2.1% |
| Othersb | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362 362![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
15.1% | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882 882![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
14.6% |
| Operating cost | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 598 598![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492 492![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 062 062 |
||
Regarding utility costs (excluding the stripping vapor), the CBP incurs slightly higher expenses (€4.5 M vs. €2.7 M). This difference is primarily attributed to the enhanced energy integration achieved in the HCP. It is important to note that the selectivity of the catalyst in HCP was adjusted to ensure that the stripping steam consumption is equivalent in both processes.
The costs associated with the treatment of the reactor effluent, containing organic residues and spent catalysts (sulphuric acid in the CB process and a heterogeneous catalyst in the HCP), were considered negligible. Although, in CBP, neutralization of the sulphuric acid would be required, these treatment costs account for less than 1% of the total operating costs in both cases and were not considered.
As expected, the cost of the heterogeneous catalyst in the HCP is higher than the cost of sulphuric acid in the CBP (€709![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per year vs. €159
000 per year vs. €159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per year). Nevertheless, this expense represents a minor fraction of the total OpEx, contributing 2.1% and 0.4%, respectively.
000 per year). Nevertheless, this expense represents a minor fraction of the total OpEx, contributing 2.1% and 0.4%, respectively.
Once CapEx and OpEx were accounted for, the MSP calculated for the two processes was €2685 per metric ton for the CBP and €2499 per metric ton for HCP. These results indicate that the production cost of furfural would be quite similar in both cases. Given the ±30% margin of error typically associated with such calculations,62 it cannot be conclusively stated that one process is superior to the other. However, under current assumptions the proposed HCP demonstrates economic viability.
When compared to the current market price of furfural, which ranges between €1000 and €2000 per metric ton – and even lower, the estimated MSP is higher.18 This discrepancy can be attributed to several factors that were not considered in this study but could significantly reduce production costs:
(1) Energy price assumptions. This study uses the current energy prices in Europe, which are significantly higher than those in China, where the CBP is primarily implemented.
(2) Utilization of reactor residue. In the existing processes, the biomass residue exiting the stripping reactor is dried and burned to produce heat for generating the stripping steam, which reduces the associated vapor cost.14
In the HCP, the unhydrolyzed cellulose and lignin fraction forms a separate stream. While this stream could also be dried and burned as in the conventional process, the absence of mineral acids and its unhydrolyzed state open possibilities for valorisation within a bio-refinery scenario. Although the complete design of this integrated bio-refinery is beyond the scope of this study, a preliminary economic estimate was conducted to assess how the valorisation of this stream could impact the MSP of furfural. A selling price of €776.1 per Mt and €190.0 per Mt (see Table 1) was assigned to the cellulose and lignin streams, respectively. For cellulose, this estimate takes a conservative approach, reflecting the current market price of cellulose pulp. However, further valorisation of this fraction into higher-value products, such as 2,5-furandicarboxylic acid, could substantially increase its market price. In order to estimate the cost of processing this stream – including annualized capital and operating expenditures – into purified lignin and cellulose pulp, the previously described furfural production cost structure was applied, considering an annualized capital and operating costs (excluding raw materials) of 67.2% of the MSP value. Assuming this proportion remains constant, the net income from valorising lignin and cellulose streams represents the 32.8% of the selling price: €255 per Mt for cellulose and €62.3 per Mt for lignin. Using this potential net income, the recalculated MSP for furfural was determined to be €1455 per Mt. This value can be deemed competitive considering the current market price of furfural, demonstrating that a fully integrated biorefinery concept, which efficiently utilizes all biomass fractions, can lead to significant cost reductions.
Lastly, it is worth noting that in the alternative HCP, all available heat streams can be used to generate low-pressure steam. Although this was not included in the economic calculations, it could further support the valorisations of the cellulose and lignin fraction in an integrated biorefinery context.
3.3 Life cycle assessment
Life cycle assessment has been performed on both CBP and HCP following the same assumptions than for the TEA (Section 3.2). Fig. 7 summarises the impact of both processes on each environmental category. For each pair of cells, the colour scheme reflects the magnitude of the proportional difference between the values, with the highest value considered 100% (red) and the lower values graded according to their proportional difference. Values with a higher difference are shifted towards green for easier interpretation. At first glance, it is easy to see an improved environmental performance of the HCP for all measured environmental indicators. Overall, three critical inputs are identified that explain most of the impacts in both processes. These are the production of the heating steam by burning fossil fuels, the consumption of corncobs as raw material and the waste generation. Therefore, the differences in these flows between the two processes account for most of the differences in the calculated values. Thus, the lower energy requirements of the HCP and the cleaner discharge of the first reactor are the main responsible factors for the ostensible differences shown in Fig. 7. This section provides a detailed discussion for each critical input, analysing their impact on the environmental indicators and linking these effects to the configuration of both processes. | ||
| Fig. 7 Overview of the impact values of both furfural production processes over the studied environmental indicators. | ||
Despite this, the HCP reaches a better overall performance due to energy reductions in other sections of the plant. On the one hand, significant differences derive from the preheating system and the reboiler of the second distillation column, being the first one the most important. In the conventional process, 2.6 kg of steam is required per kilogram of furfural, whereas the HCP reduces this demand to 1.4 kg. This is attributed to enhanced energy integration within the process, as in the HCP, diluting water is fed at 187 °C, compared to only 25 °C in the baseline technology. This temperature increase is achieved by recirculating a portion of the water (45 wt%) from the bottom of the first column (C-101), containing 99 wt% water, at approximately 100 °C. This stream is subsequently heated by contact with the product stream exiting the stripping reactor (HX-101), reaching 187 °C prior to the introduction to the HX-102 exchanger.
On the other hand, the HCP avoids the consumption of low-pressure steam (LPS) from the second distillation column (C-102). This is achieved by forcing an identical configuration to the first tower by passing the product stream through the reboiler of this second column. In this way, the product partially condenses before entering the purification train, releasing its latent heat to allow this equipment to operate satisfactorily. This improvement saves 0.12 kg of steam per kg of furfural produced.
Finally, the HCP produces an LPS stream by contacting the product stream from the second reactor (R-102) with water at boiler B-101. This is done for total condensation before entering the C-101 column, preventing the production of steam using the conventional European mix, generating an environmental credit as shown in Fig. 2. The LPS production is particularly relevant, as for each kilogram of furfural produced, the generation of 4.3 kg of heating steam is avoided. The positive impact of this by-product has a significant impact on the comparison between the two processes, although as shown in Fig. S7.1–S7.16,† this reduction is not decisive for their comparative performance.
Corncob consumption significantly impacts indicators such as eutrophication, human toxicity, mineral resources consumption, particulate matter, and land and water use. These effects are primarily linked to the application of agrochemicals, particularly fertilizers and pesticides. Consequently, efforts to mitigate these impacts should focus on the supply chain level, as these are more extended issues.75 At the process level (foreground), solutions are more limited in terms of feedstock, as materials with a high pentosan content and at a competitive price are a current requisite. The effects of biogenic carbon are largely overshadowed by emissions associated with energy production.
Notably, the largest land use stems from both maize cultivation and steam production for heating. The latter becomes significant due to the high reactor consumption mentioned earlier, which drives intensive use of hardwood chips and coal despite their relatively low shares in the industrial mix (0.9% and 8.6%, respectively). However, around 50% of land use is due to the consumption of maize, which logically follows from cultivation activities. In addition, about 7 to 8% of the impact is related to the landfilling of the solid waste produced in both cases. An option to reduce this impact would be to avoid landfilling by recovering energy from the solid cake from the autohydrolysis reactor. This would be done at a lower energy cost than in the case of CBP, as the drying requirements are considerably lower. In addition, the combustion would be cleaner due to the absence of acids and furfural in this solid. However, this would mean a poor utilisation of this fraction as it contains cellulose and lignin with high purity. Purification and valorisation of both would lead to a much more favourable scenario. No disaggregated data are included as this purification system has not been designed—i.e. the impacts associated with its operation are not known—but a cursory analysis reveals that a net positive effect could be achieved for most indicators if the system is expanded to include these products within the envisaged functional unit.
The indicator of water consumption is dominated by the maize cultivation phase, as it accounts for 93% (HCP) and 88% (CBP) of the impact, with the remainder mainly due to steam generation equipment and extraction systems. As expected, this impact is associated in both cases with irrigation activities.
4 Conclusions
This study presents a comprehensive techno-economic and environmental comparison of two processes for furfural production: the conventional process based on homogeneous catalysis with sulphuric acid (CBP), and an alternative process employing heterogeneous catalysis (HCP). The latter was evaluated as a promising route for a more sustainable furfural production by addressing key process limitations, including reactor heating and acid usage. The HCP incorporates a two-stage reaction system, beginning with biomass auto-hydrolysis induced by temperature, followed by the conversion of released sugars into furfural using a stripping reactor with a heterogeneous catalyst. Both processes were simulated under comparable assumptions to ensure direct comparison.The study demonstrated that replacing steam with nitrogen (N2) as a stripping agent in the HCP is not viable, as it removes excessive water from the reaction medium, increasing tar formation and destabilizing the process. Furthermore, steam acts not only as a stripping agent but also as a heating fluid, making its replacement with nitrogen impractical from both a technical and economic standpoint.
For the heterogeneous catalyst used in the HCP process, Amberlyst® 70 resin was found to lack sufficient selectivity, resulting in unfeasible steam requirements for stripping. The catalyst selectivity would need to be 4.9 times higher to match the steam consumption of the conventional process. Moreover, the results indicate that the required overall selectivity must be 2.8 times higher than the one showed by H2SO4 in CBP process due to the lower sugar availability in the reaction medium. However, with a hypothetical catalyst meeting this selectivity threshold, the HCP process could become viable. The economic analysis revealed a similar minimum selling price (MSP) for furfural in both processes: €2499 per t for HCP and €2685 per t for CBP. Although the calculated MSP for the conventional process is relatively high, it should be noted that European energy prices were used in the calculations, and an important factor was not considered: the utilization of reactor residue. In existing processes, the biomass residue exiting the stripping reactor is typically dried and burned to generate heat for producing the stripping steam, which significantly reduces vapor-related costs. Furthermore, by leveraging the additional valorisation of lignin and unhydrolyzed cellulose streams in an integrated HCP biorefinery, the MSP could be reduced to €1455 per t, making it competitive with current market prices.
The environmental assessment identified energy consumption, maize cob usage, and waste emissions as key impact factors. Steam generation, particularly for reactor heating, remains the most critical aspect in both processes, and should be the focus of future developments. However, the proposed process significantly reduces steam consumption through enhanced energy integration, such as preheating via recycled streams and efficient reboiler configurations, which also generate a net environmental credit by producing low-pressure steam. Maize cob consumption is nearly identical in both processes and remains a major environmental concern due to the agrochemical inputs in maize cultivation, land use, and irrigation requirements. This highlights the need for alternative upstream solutions in the supply chain, particularly to mitigate the impacts of pesticides and fertilizers. Additionally, land use could be reduced through the recovery of solid waste, which is rich in lignin and high-purity cellulose. Since direct energy recovery would be a suboptimal use of these resources, future work should focus on developing a purification train to recover both fractions, thereby reducing environmental impacts while improving economic viability. The study also found that waste emissions, particularly acid loss in the CBP, severely impact freshwater ecotoxicity. The HCP mitigates this issue by eliminating sulphuric acid, achieving over a 99% reduction in this category using a heterogeneous catalyst.
Overall, the findings indicate that HCP process is technically and economically promising pathway for a sustainable furfural production, provided that sufficiently selective and stable heterogeneous catalyst can be developed. However, a key challenge remains: the rapid deactivation of Amberlyst® 70 due to the deposition of tar-like materials, which compromises its long-term viability. To enable an industrially feasible process, a heterogeneous catalyst must not only exhibit a selectivity at least 4.9 times higher than Amberlyst® 70 and 2.8 times higher than H2SO4 but also demonstrate long-term stability under continuous operation. Given that organic resins such as Amberlyst® 70 are thermally unstable and cannot be regenerated by calcination, alternative solid acid catalysts—particularly inorganic materials—offer a more promising route due to their higher thermal and chemical stability, allowing effective regeneration strategies. A reactor configuration that integrates the catalyst regeneration would be advisable. Addressing these limitations will be crucial for HCP to achieve technical and economic parity with conventional sulphuric acid-based processes. Additionally, integrating this process within a biorefinery concept could further enhance its environmental sustainability by maximizing resource utilization and minimizing environmental impacts, paving the way for a competitive and environmentally viable furfural industry.
Abbreviations
| AC | Acidification |
| GWP | Global warming potential |
| FWET | Freshwater ecotoxicity |
| FDP | Fossil depletion potential |
| FWEP | Freshwater eutrophication |
| MEP | Marine eutrophication |
| TEP | Terrestrial eutrophication |
| HTc | Human toxicity, cancer |
| HTnc | Human toxicity, non-cancer |
| IR | Ionising radiation |
| LU | Land use |
| MDP | Metals/minerals depletion potential |
| OD | Ozone depletion |
| PMF | Particulate matter formation |
| POF | Photochemical oxidant formation |
| WU | Water use |
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work received financial support from the Spanish Ministry of Science and Innovation through Cat4BioMon Project (PID2021-122736OB-C43 & PID2021-122736OB-C44), being funded through MCIN/AEI/10.13039/501100011033/FEDER, UE.References
- Climate Change and Health (CCH), Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s, 2024 Search PubMed.
- M. Höök and X. Tang, Energy Policy, 2013, 52, 797–809 CrossRef.
- H. Lee and J. Romero, IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Geneva, 2023 Search PubMed.
- L. Lange, K. O. Connor, S. Arason, U. Bundgård-Jørgensen, A. Canalis, D. Carrez, J. Gallagher, N. Gøtke, C. Huyghe, B. Jarry, P. Llorente, M. Marinova, L. O. Martins, P. Mengal, P. Paiano, C. Panoutsou, L. Rodrigues, D. B. Stengel, Y. van der Meer and H. Vieira, Front. Bioeng. Biotechnol., 2021, 8, 1–16 Search PubMed.
- A. Nabera, I. R. Istrate, A. J. Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, Green Chem., 2023, 25, 6603–6611 RSC.
- E. Baldoni, P. Reumerman, C. Parisi, R. Platt, H. Gonzalez Hermoso, K. Vikla, J. Vos and R. M'Barek, Chemical and Material Driven Biorefineries in the EU and beyond: Database and Dashboard Visualisation, Publications Office of the European Union, 2021 Search PubMed.
- European Commission, Chemical and Material Biorefineries in the EU|Knowledge for Policy, https://knowledge4policy.ec.europa.eu/visualisation/chemical-material-biorefineries-eu_en, accessed 27 January 2025 Search PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- Grand View Research, Furfural Market Size, Share, Growth & Trends Report, 2030, San Francisco, 2023 Search PubMed.
- D. S. S. Jorqueira, L. F. de Lima, S. F. Moya, L. Vilcocq, D. Richard, M. A. Fraga and R. S. Suppino, Appl. Catal., A, 2023, 665, 119360 CrossRef CAS.
- K. J. Yong, T. Y. Wu, C. B. T. L. Lee, Z. J. Lee, Q. Liu, J. M. Jahim, Q. Zhou and L. Zhang, Biomass Bioenergy, 2022, 161, 106458 CrossRef CAS.
- W. Adhami, A. Richel and C. Len, Mol. Catal., 2023, 545, 113178 CrossRef CAS.
- M. Dashtban, A. Gilbert and P. Fatehi, J. Sci. Technol. For. Prod. Processes, 2012, 2, 44–53 Search PubMed.
- K. J. Zeitsch, in The chemistry and technology of furfural and its many by-products, Elsevier, Sugar Series, 2000, vol. 13, pp. 36–74 Search PubMed.
- H. E. Hoydonckx, W. M. Van Rhijn, W. Van Rhijn, D. E. De Vos and P. A. Jacobs, in Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Ltd, 2007 Search PubMed.
- A. K. Mathew, A. Abraham, K. K. Mallapureddy and R. K. Sukumaran, in Waste Biorefinery: Potential and Perspectives, Elsevier, 2018, pp. 267–297 Search PubMed.
- W. Kubic Jr, X. Yang, C. Moore and A. Sutton, A Process for Converting Corn Bran to Furfural without Mineral Acids, Los Alamos, NM (United States), 2020 Search PubMed.
- M. Z. R. Mohammed, Z. W. Ng, A. Putranto, Z. Y. Kong, J. Sunarso, M. Aziz, S. H. Zein, J. Giwangkara and I. Butar, Clean Technol. Environ. Policy, 2023, 25, 1551–1567 CrossRef CAS.
- D. T. Win, AU Journal of Technology, 2005, 8, 185–190 Search PubMed.
- L. Zhang, G. Xi, J. Zhang, H. Yu and X. Wang, Bioresour. Technol., 2017, 224, 656–661 CrossRef PubMed.
- R. J. H. Grisel, J. C. Van Der Waal, E. De Jong and W. J. J. Huijgen, Catal. Today, 2014, 223, 3–10 CrossRef.
- T. L. K. Yong, N. Mohamad and N. N. M. Yusof, Procedia Eng., 2016, 148, 392–400 CrossRef.
- S. Peleteiro, V. Santos, G. Garrote and J. C. Parajó, Carbohydr. Polym., 2016, 146, 20–25 CrossRef PubMed.
- Y. Nie, Q. Hou, W. Li, C. Bai, X. Bai and M. Ju, Molecules, 2019, 24, 594 CrossRef PubMed.
- C. B. T. L. Lee, T. Y. Wu, C. H. Ting, J. K. Tan, L. F. Siow, C. K. Cheng, J. Md. Jahim and A. W. Mohammad, Bioresour. Technol., 2019, 278, 486–489 CrossRef PubMed.
- V. da Silva Lacerda, J. B. López-Sotelo, A. Correa-Guimarães, S. Hernández-Navarro, M. Sánchez-Bascones, L. M. Navas-Gracia, P. Martín-Ramos, E. Pérez-Lebeña and J. Martín-Gil, Bioresour. Technol., 2015, 180, 88–96 CrossRef.
- C. Moreno-Marrodan, P. Barbaro, S. Caporali and F. Bossola, ChemSusChem, 2018, 11, 3649–3660 CrossRef PubMed.
- P. Bhaumik and P. L. Dhepe, ChemCatChem, 2017, 9, 2709–2716 CrossRef.
- R. K. Mishra, V. B. Kumar, A. Victor, I. N. Pulidindi and A. Gedanken, Ultrason. Sonochem., 2019, 56, 55–62 CrossRef.
- S. M. Bruce, Z. Zong, A. Chatzidimitriou, L. E. Avci, J. Q. Bond, M. A. Carreon and S. G. Wettstein, J. Mol. Catal. A: Chem., 2016, 422, 18–22 CrossRef.
- Y. Wang, Y. Dai, T. Wang, M. Li, Y. Zhu and L. Zhang, Fuel Process. Technol., 2022, 237, 107472 CrossRef.
- W. Song, H. Liu, J. Zhang, Y. Sun and L. Peng, ACS Catal., 2022, 12, 12833–12844 CrossRef.
- A. Gupta, S. U. Nandanwar, P. Niphadkar, I. Simakova and V. Bokade, Biomass Bioenergy, 2020, 139, 105646 CrossRef.
- S. Le Guenic, D. Gergela, C. Ceballos, F. Delbecq and C. Len, Molecules, 2016, 21, 1102 CrossRef.
- A. Mittal, D. A. Ruddy, X. Chen and D. K. Johnson, Energy Fuels, 2023, 37, 13115–13125 CrossRef.
- O. Sato, N. Mimura, Y. Masuda, M. Shirai and A. Yamaguchi, J. Supercrit. Fluids, 2019, 144, 14–18 CrossRef.
- X. Hu, R. J. M. Westerhof, D. Dong, L. Wu and C. Z. Li, ACS Sustain. Chem. Eng., 2014, 2, 2562–2575 CrossRef.
- T. Tongtummachat, A. Jaree and N. Akkarawatkhoosith, RSC Adv., 2022, 12, 23366–23378 RSC.
- J. K. Raman and E. Gnansounou, Ind. Crops Prod., 2015, 69, 371–377 CrossRef.
- T. H. Kim, H. J. Ryu and K. K. Oh, Bioresour. Technol., 2016, 218, 367–372 CrossRef PubMed.
- C. Li, D. Ding, Q. Xia, X. Liu and Y. Wang, ChemSusChem, 2016, 9, 1712–1718 CrossRef.
- X. Wang, C. Zhang, Q. Lin, B. Cheng, F. Kong, H. Li and J. Ren, Ind. Crops Prod., 2018, 123, 118–127 CrossRef.
- S. Peleteiro, A. M. da Costa Lopes, G. Garrote, R. Bogel-Łukasik and J. C. Parajó, Ind. Crops Prod., 2015, 77, 163–166 CrossRef.
- Z. Xu, W. Li, Z. Du, H. Wu, H. Jameel, H. min Chang and L. Ma, Bioresour. Technol., 2015, 198, 764–771 CrossRef.
- P. Liu, S. Shi, L. Gao and G. Xiao, React. Kinet., Mech. Catal., 2022, 135, 795–810 CrossRef CAS.
- H. Lin, J. Chen, Y. Zhao and S. Wang, Energy Fuels, 2017, 31, 3929–3934 CrossRef CAS.
- D. M. Alonso, S. H. Hakim, S. Zhou, W. Won, O. Hosseinaei, J. Tao, V. Garcia-Negron, A. H. Motagamwala, M. A. Mellmer, K. Huang, C. J. Houtman, N. Labbé, D. P. Harper, C. T. Maravelias, T. Runge and J. A. Dumesic, Sci. Adv., 2017, 3, 1–7 Search PubMed.
- D. Edumujeze, M. C. Fournier-Salaün and S. Leveneur, Fuel, 2025, 381, 133423 CrossRef CAS.
- G. Zang, A. Shah and C. Wan, J. Cleaner Prod., 2020, 260, 120837 CrossRef CAS.
- M. S. Hossain, C. Theodoropoulos and A. Yousuf, Biochem. Eng. J., 2019, 144, 89–103 CrossRef CAS.
- S. Farzad, M. A. Mandegari, M. Guo, K. F. Haigh, N. Shah and J. F. Görgens, Biotechnol. Biofuels, 2017, 10(1), 1–24 CrossRef.
- I. Agirrezabal-Telleria, J. Requies, M. B. Güemez and P. L. Arias, Green Chem., 2012, 14, 3132–3140 RSC.
- I. Agirrezabal-Telleria, A. Larreategui, J. Requies, M. B. Güemez and P. L. Arias, Bioresour. Technol., 2011, 102, 7478–7485 CrossRef CAS PubMed.
- J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed.
- J. Slak, B. Pomeroy, A. Kostyniuk, M. Grilc and B. Likozar, Chem. Eng. J., 2022, 429, 132325 CrossRef CAS.
- R. J. Wooley and V. Putsche, Development of an ASPEN PLUS Physical Property Database for Biofuels Components, Golden (Colorado, USA), 1996 Search PubMed.
- N. Bhandari, D. G. Macdonald and N. N. Bakhshi, Biotechnol. Bioeng., 1984, 26, 320–327 CrossRef CAS PubMed.
- V. Krzelj, J. Ferreira Liberal, M. Papaioannou, J. van der Schaaf and M. F. Neira d'Angelo, Ind. Eng. Chem. Res., 2020, 59, 11991–12003 CrossRef CAS.
- D. Nabarlatz, X. Farriol and D. Montané, Ind. Eng. Chem. Res., 2004, 43, 4124–4131 CrossRef CAS.
- W. L. Luyben, Distillation Design and Control Using Aspen Simulations, John Wiley & Sons, Hoboken, New Jersey, 2nd edin, 2013 Search PubMed.
- F. K. Kazi, A. D. Patel, J. C. Serrano-Ruiz, J. A. Dumesic and R. P. Anex, Chem. Eng. J., 2011, 169, 329–338 CrossRef CAS.
- R. Turton, R. C. Bailie, W. B. Whiting, J. A. Shaeiwitz and D. Bhattacharyya, Analysis, Synthesis, and Design of Chemical Processes, Prentice Hall, Upper Saddle River, New Jersey, 4th edn, 2012 Search PubMed.
- M. S. Peters, K. D. Timmerhaus and R. E. West, Plant Design and Economics for Chemical Engineers, McGraw-Hill, New York, 5th edn, 2003 Search PubMed.
- H. Zhang, L. Hou, Y. Lin, X. Liu, S. Zhao, C. Xu and C. Chang, Bioresour. Technol., 2025, 418, 131897 CrossRef CAS PubMed.
- TNO Biobased and Circular Technologies, Phyllis2 Database Search PubMed.
- ChemAnalyst, https://www.chemanalyst.com/Pricing/Searchpricing Search PubMed.
- https://straitsresearch.com/report/cellulose-fiber-market .
- A. J. Robinson, A. Giuliano, O. Y. Abdelaziz, C. P. Hulteberg, A. Koutinas, K. S. Triantafyllidis, D. Barletta and I. De Bari, Bioresour. Technol., 2022, 364, 128004 CrossRef CAS PubMed.
- B. Steubing, D. de Koning, A. Haas and C. L. Mutel, Softw. Impacts, 2020, 3, 100012 CrossRef.
- S. Andreasi Bassi, F. Biganzoli, N. Ferrara, A. Amadei, A. Valente, S. Sala and F. Ardente, Updated Characterisation and Normalisation Factors for the Environmental Footprint 3.1 Method, Publications Office of the European Union, Luxembourg, 2023 Search PubMed.
- A. Ciroth, S. Muller, B. Weidema and P. Lesage, Int. J. Life Cycle Assess., 2016, 21, 1338–1348 CrossRef.
- G. Sampath and S. Kannan, Catal. Commun., 2013, 37, 41–44 CrossRef CAS.
- X. Teng, Z. Si, S. Li, Y. Yang, Z. Wang, G. Li, J. Zhao, D. Cai and P. Qin, Ind. Crops Prod., 2020, 151, 112481 CrossRef CAS.
- J. Gao, H. Wang, X. Cao, Z. Li, H. Guo, X. Yang, W. Wang, N. Guo and Y. Ma, Mol. Catal., 2023, 535, 112890 CrossRef CAS.
- Y. Lyu, M. Raugei, X. Zhang, S. Mellino and S. Ulgiati, Renewable Sustainable Energy Rev., 2021, 151, 111604 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5su00106d |
| This journal is © The Royal Society of Chemistry 2025 |





