Gold(I) “click” 1,2,3-triazolylidenes: synthesis, self-assembly and catalysis†‡
Kelly J.
Kilpin
a,
Ursula S. D.
Paul
b,
Ai-Lan
Lee
*b and
James D.
Crowley
*a
aDepartment of Chemistry, University of Otago, PO Box 56, Dunedin, New Zealand. E-mail: jcrowley@chemistry.otago.ac.nz; Fax: +64 3 479 7906; Tel: +64 3 479 7731
bSchool of Engineering and Physical Sciences, Chemistry – William H. Perkin Building, Heriot-Watt University, Edinburgh EH14 4AS, UK. E-mail: A.Lee@hw.ac.uk; Tel: +44 (0)131-4518030
First published on 23rd August 2010
Abstract
Novel gold(I) “click” carbene(1,2,3-triazolylidene) complexes have been synthesised, characterised and exploited for the self-assembly of a metallomacrocycle and as precatalysts for gold(I)-catalysed reactions.
In the past 20 years N-heterocyclic carbenes (NHCs) have evolved from chemical curiosities to standard organometallic ligands. The strong covalent metal–carbene bond within these complexes makes them very stable and this has led to applications in a number of areas. Indeed, late metal–carbene complexes, including those of gold(I), have been exploited to generate new catalysts, medicines and materials.1 Arduengo-type imidazolylidene ligands still dominate the area, because this class of carbenes are relatively easily synthesised and handled.2 However, despite the availability of excellent synthetic methods for the generation of imidazolylidenes the majority of these ligands are symmetrically aryl- or alkyl-substituted.2 As such, new modular synthetic methods that allow ready incorporation of functionality and the controlled modification of steric and electronic properties of NHCs could enable the rapid generation of new catalysts, medicines and materials. One potential method is the Cu(I)-catalyzed 1,3-cycloaddition of organic azides with terminal alkynes (the CuAAC reaction).3 The mild reaction conditions and wide substrate scope of this “click”4 reaction has led to its extensive use in the synthesis of functional molecules.3 Albrecht and co-workers have recently shown that 1,2,3-triazolylidenes, synthesised using the CuAAC reaction, form metal–carbene complexes with Pd(II), Ag(I), Ru(II), Rh(I) and Ir(I).5 Inspired by Albrecht's discovery and building on our own interest in the generation of functionalised ligand architectures using CuAAC “click” chemistry,6 we report the synthesis of novel gold(I) “click” carbene(1,2,3-triazolylidene) complexes.7 Furthermore, we demonstrate for the first time that these gold(I)–1,2,3-triazolylidene complexes can be exploited to self-assemble a metallomacrocycle and are active catalytic precursors for a variety of gold(I)-catalysed organic transformations.
1-Benzyl-4-phenyl-1H-1,2,3-triazole, 1,6 formed readily through a one-pot “click” reaction, was quantitatively methylated in dichloromethane using Meerwein's salt,8 providing the triazolium salt 2 (X = BF4−) (Scheme 1). Conversion of 2 (X = BF4−) into 2′ (X = PF6−) allowed the molecular structure to be determined unambiguously by X-ray crystallography (see ESI‡) and showed that the triazole was methylated regioselectively at the N3 nitrogen atom. 2 or 2′ was readily metalated with Ag2O providing the silver(I) carbene.5 The Ag(I) complex was immediately treated with Au(SMe2)Cl and the resulting transmetallation provided the neutral 1,2,3-triazolylidene gold(I) chloride complex, 3, in 82% yield (Scheme 1). The chloride anion of 3 can be readily replaced by other ligands (Scheme 1). The neutral phenylacetylide complex 4 was obtained by reacting 3 with the potassium salt of phenylacetylide in methanol. Likewise the cationic complex, 5, was synthesised by treating 3 with AgSbF6 and N,N′-dimethylaminopyridine (DMAP) in an acetone solution at RT. The gold(I)–1,2,3-triazolylidene complexes, 3, 4 and 5, have been fully characterised by elemental analysis, HR-ESMS, IR, 1H and 13C NMR (see ESI‡). X-Ray structural analyses of 3, 4 and 5 unambiguously confirmed the connectivity patterns (Scheme 1 and ESI‡). As expected the gold(I) ions are coordinated in a linear fashion (L–Au–C bond angles range from 173.6°–177.2°) to the carbon of the 1,2,3-triazolylidene and the other ligand. The Au–C and Au–L bond lengths are similar to those observed in other gold(I) N-heterocyclic carbene complexes (Scheme 1 and ESI‡).9,10
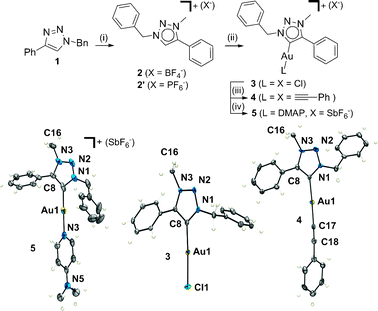 | ||
Scheme 1 (i) Me3OBF4, CH2Cl2, RT, 3 d; (ii) (a) Ag2O, NMe4Cl, CH3CN–CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RT, 18 h, (b) Au(SMe2)Cl, CH2Cl2, RT, 2 h; (iii) KOtBu, phenylacetylene, MeOH, RT, 18 h; (iv) DMAP, AgSbF6, acetone, RT, 1 h. ORTEP diagrams of 3 (centre), 4 (left) and 5 (right) with thermal ellipsoids shown at the 50% probability level. Counterions are omitted for clarity. Selected bond lengths (Å) and angles (°): for 3, Au1–C8 1.982(4), Au1–Cl1 2.2940(10), Cl1–Au1–C8 177.2(1); for 4, Au1–C8 2.030(6), Au1–C17 2.024(6), C17–Au1–C8 177.8(5); for 5, Au1–C8 1.962(8), Au1–N4 2.055(6), N5–Au1–C8 173.6(2). 1), RT, 18 h, (b) Au(SMe2)Cl, CH2Cl2, RT, 2 h; (iii) KOtBu, phenylacetylene, MeOH, RT, 18 h; (iv) DMAP, AgSbF6, acetone, RT, 1 h. ORTEP diagrams of 3 (centre), 4 (left) and 5 (right) with thermal ellipsoids shown at the 50% probability level. Counterions are omitted for clarity. Selected bond lengths (Å) and angles (°): for 3, Au1–C8 1.982(4), Au1–Cl1 2.2940(10), Cl1–Au1–C8 177.2(1); for 4, Au1–C8 2.030(6), Au1–C17 2.024(6), C17–Au1–C8 177.8(5); for 5, Au1–C8 1.962(8), Au1–N4 2.055(6), N5–Au1–C8 173.6(2). | ||
The chemical shift of the carbene carbon of 3, 4 and 5 appears between δC 174–155 ppm in the 13C-NMR spectra, values which are similar to those observed for other reported 1,2,3-triazolylidene metal–carbenes,5 but significantly lower in value than those observed for previously reported gold(I)–carbene complexes.9
Recently the use of NHCs as building blocks in the assembly of metallosupramolecular architectures has been explored.11 As such, we next attempted to use the di-1,2,3-triazolylidene gold(I) chloride complex, 8, to self-assemble a metallomacrocycle. 8 was synthesised from the known ditriazole, 66 (Scheme 2) using the same methodologies that provided the parent compound 3. Treatment of 8 with 1 eq. of the in situ generated (from 7) disilver(I) triazolylidene complex provided the digold(I) metallomacrocycle 9 (see ESI‡ for full characterisation). 1H and diffusion-ordered NMR spectroscopy (DOSY) along with positive ion electrospray mass spectrometry (ESMS) experiments provide conclusive evidence for the formation of metallomacrocycle, 9. The ESMS spectrum of 9 shows a series of isotopically resolved peaks consistent with the formulation [M–n(BF4)]n+ (n = 1–2). The 1H NMR spectrum (CD3CN, 298 K) of 9 shows a complicated pattern of signals, however, the protons Ha–c of the phenyl spacer units of the ligands are upfield shifted, characteristic of a face to face π–π stacking interaction. 1H DOSY12spectra provided further support for the selective formation of the metallomacrocycle in CD3CN solution. All proton signals of 9 showed the same diffusion coefficient of 4.8 × 10−10 m2 s−1, whereas a diffusion coefficient of 6.1 × 10−10 m2 s−1 was obtained for 8 alone in CD3CN solution, which is consistent with the formation of the larger metallomacrocycle in solution (ESI‡).
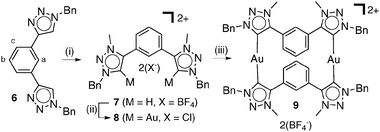 | ||
Scheme 2 (i) Me3OBF4, CH2Cl2, RT, 3 d; (ii) (a) Ag2O, NMe4Cl, CH3CN–CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RT, 18 h, (b) Au(SMe2)Cl, CH2Cl2, RT, 2 h; (iii) 7, Ag2O, NMe4Cl, CH3CN–CH2Cl2 (1 1), RT, 18 h, (b) Au(SMe2)Cl, CH2Cl2, RT, 2 h; (iii) 7, Ag2O, NMe4Cl, CH3CN–CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), RT, 2 h. 1), RT, 2 h. | ||
Another obvious potential application of the gold(I) “click” carbene complexes such as 3 is in catalysis. There has recently been much interest in gold(I)-catalysed synthetic organic chemistry as gold(I)-complexes can act as efficient carbophilic Lewis acids to activate π-systems such as alkynes, allenes, dienes and alkenes.13 A combination of phosphine or NHC ligated AuCl complexes in the presence of a silver salt (such as AgOTf and AgSbF6) has been widely employed as highly active cationic Au(I) species.1,13,14 It has also been shown that the nature of the ancillary ligand can greatly affect the reactivity and selectivity of the gold(I)-catalysts.13 We were thus keen to investigate whether gold(I)–1,2,3-triazolylidene complexes such as 3 are viable catalytic precursors for gold(I)-catalysed organic transformations.
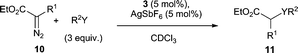
We were delighted to find that the air and moisture stable complex 3 in the presence of AgSbF6 successfully catalyses the carbene-transfer reaction from ethyl diazoacetate (EDA) 10 (R1 = H) (Table 1).15 The insertion of the :CHCOOEt unit into O–H bonds of primary, secondary and tertiary alcohols occurs readily (entries 1–4) and the insertion into the O–H bond of phenol also proceeds albeit in a moderate yield (50%, entry 5). Insertion into the N–H bond of aniline is also successfully catalysed by complex 3/AgSbF6 (entry 6). As reports on gold(I)-catalysed carbene-transfer reactions with alcohols and anilines tend to focus only on EDA 10 (R1 = H),15,16 we were keen to investigate whether more substituted diazo compounds such as 10 (R1 = Ph) are also viable substrates. In the presence of 3/AgSbF6, methyl 2-diazo-2-phenylacetate 10 (R1 = Ph), does indeed react with ethanol to give 11g, but more sluggishly when compared to EDA (entry 7). Finally, formal insertion of the carbene moiety into the aldehyde C–H and C–C of benzaldehyde is also catalysed by 3/AgSbF6 (entry 8).17
| Entry | R1 | R2Y | Product | Yielda (%) |
|---|---|---|---|---|
| a Determined by 1H-NMR analysis using trimethoxybenzene as the internal standard. No diethyl fumarate or maleate was detected in any experiment. b 20 °C, 3.5 h. c Reaction was allowed to stir for 18 h. d 40 °C, 70 h. e 20 °C, 6 d. f 20 °C, 1 h. g 11i = ethyl 3-oxo-2-phenylpropanoate, 11j = (Z)-ethyl 3-hydroxy-2-phenylacrylate (see ESI2). | ||||
| 1b | H | MeOH | 11a | 88 |
| 2b | H | EtOH | 11b | >95 |
| 3b | H | i-PrOH | 11c | 82 |
| 4c | H | t-BuOH | 11d | 81 |
| 5b | H | PhOH | 11e | 50 |
| 6d | H | PhNH2 | 11f | 62 |
| 7e | Ph | EtOH | 11g | 50 |
| 8f | H | Ph(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)H O)H |
11h | 40% + 22% 11i + 27% 11jg |
Additionally, complex 3/AgSbF6 successfully catalyses the hydroalkoxylation of allene 12 to furnish 13 in 80% isolated yield (Scheme 3).18 Cycloisomerisation of enyne 14 also proceeds smoothly in the presence of 3/AgSbF6 to produce isomers 15 and 16 in 42% yield (Scheme 4). In the presence of MeOH as the co-solvent, methoxycyclisation occurs to give an excellent yield of methoxylated product 17 (98%, Scheme 4).
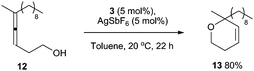 | ||
| Scheme 3 Hydroalkoxylation of allene 12, catalysed by complex 3/AgSbF6. | ||
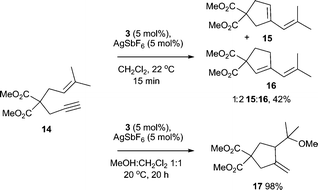 | ||
| Scheme 4 Cycloisomerisation and methoxycyclisation of enyne 15, catalysed by complex 3/AgSbF6. | ||
Following our successful preliminary results with the unmodified parent gold(I)–1,2,3-triazolylidene complex 3, we envisage that the easy access to analogues of 3via the CuAAC “click” reaction should now open the door to ready and rapid tuning of the steric and electronic properties of gold(I)-precatalysts via modification of the substituents. Since the nature of the ancillary ligand can greatly affect the reactivity and selectivity of the gold(I)-catalysts, analogues of complex 3 with different electronic and steric properties could be beneficial to the development and optimisation of gold(I)-catalysed reactions. Work is currently underway in this area and will be reported in due course.
In conclusion, we have reported the synthesis and characterisation of novel gold(I) “click” carbene complexes. These complexes have been used for the self-assembly of a metallomacrocycle and as precatalysts for gold(I)-catalysed reactions. Exploitation of the CuAAC methodology used to generate the carbene ligands should provide the ability to readily and rapidly tune the steric, electronic and functional properties of the resulting gold(I)–1,2,3-triazolylidene complexes potentially enabling the generation of new catalysts, medicines and materials.
We would like to thank Erasmus (USDP), EPSRC, The Royal Society, Heriot-Watt University and the University of Otago for providing financial support for this work. Bevan Jarman (University of Waikato) and the EPSRC Mass Spectrometry services at Swansea are thanked for collecting MS data.
Notes and references
- For recent reviews see: L. Mercs and M. Albrecht, Chem. Soc. Rev., 2010, 39, 1903 Search PubMed; S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 RSC; J. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Hwang and I. J. B. Lin, Chem. Rev., 2009, 109, 3561 CrossRef CAS.
- F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122 CrossRef CAS; O. Kuehl, Chem. Soc. Rev., 2007, 36, 592 RSC.
- M. Meldal and C. W. Tornoe, Chem. Rev., 2008, 108, 2952 CrossRef CAS; P. Wu and V. V. Fokin, Aldrichimica Acta, 2007, 40, 7 CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- P. Mathew, A. Neels and M. Albrecht, J. Am. Chem. Soc., 2008, 130, 13534 CrossRef; T. Karthikeyan and S. Sankararaman, Tetrahedron Lett., 2009, 50, 5834 CrossRef CAS.
- J. D. Crowley and P. H. Bandeen, Dalton Trans., 2010, 39, 612 RSC; J. D. Crowley, P. H. Bandeen and L. R. Hanton, Polyhedron, 2010, 29, 70 CrossRef CAS; J. D. Crowley and E. L. Gavey, Dalton Trans., 2010, 39, 4035 RSC; M. L. Gower and J. D. Crowley, Dalton Trans., 2010, 39, 2371 RSC.
- For a review of “abnormal carbenes” see: O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445 Search PubMed . Recently, 1,2,3-triazolylidenes have also been termed “mesoionic carbenes”, see: G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 4759 CrossRef CAS.
- B. Schulze, C. Friebe, M. D. Hager, W. Guenther, U. Koehn, B. O. Jahn, H. Goerls and U. S. Schubert, Org. Lett., 2010, 12, 2710 CrossRef CAS; K. M. Mullen, J. Mercurio, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2009, 48, 4781 CrossRef CAS.
- C. Dash, M. M. Shaikh, R. J. Butcher and P. Ghosh, Inorg. Chem., 2010, 49, 4972 CrossRef CAS; P. De Fremont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 2411 CrossRef CAS.
- P. de Fremont, N. Marion and S. P. Nolan, J. Organomet. Chem., 2009, 694, 551 CrossRef CAS; J. Y. Z. Chiou, S. C. Luo, W. C. You, A. Bhattacharyya, C. S. Vasam, C. H. Huang and I. J. B. Lin, Eur. J. Inorg. Chem., 2009, 1950 CrossRef CAS.
- A. Rit, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2010, 132, 4572 CrossRef CAS; F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Chem.–Eur. J., 2008, 14, 10900–10904 CrossRef CAS; F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Organometallics, 2008, 27, 6408–6410 CrossRef CAS; C. Radloff, J. J. Weigand and F. E. Hahn, Dalton Trans., 2009, 9392–9394 RSC.
- A. Pastor and E. Martinez-Viviente, Coord. Chem. Rev., 2008, 252, 2314 CrossRef CAS.
- Z. G. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS; D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS; H. C. Shen, Tetrahedron, 2008, 64, 3885 CrossRef CAS; H. C. Shen, Tetrahedron, 2008, 64, 7847 CrossRef CAS; A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef.
- N. Marion and S. P. Nolan, Chem. Soc. Rev., 2008, 37, 1776 RSC; H. G. Raubenheimer and S. Cronje, Chem. Soc. Rev., 2008, 37, 1998 RSC; M. S. Hadfield and A.-L. Lee, Org. Lett., 2010, 12, 484 CrossRef CAS; J. T. Bauer, M. S. Hadfield and A.-L. Lee, Chem. Commun., 2008, 6405 RSC.
- M. R. Fructos, T. R. Belderrain, P. de Fremont, N. M. Scott, S. P. Nolan, M. M. Diaz-Requejo and P. J. Perez, Angew. Chem., Int. Ed., 2005, 44, 5284 CrossRef CAS; L. Ricard and F. Gagozs, Organometallics, 2007, 26, 4704 CrossRef CAS; P. de Fremont, E. D. Stevens, M. R. Fructos, M. Mar Diaz-Requejo, P. J. Perez and S. P. Nolan, Chem. Commun., 2006, 2045 RSC.
- A. Prieto, M. R. Fructos, M. M. Diaz-Requejo, P. J. Perez, P. Perez-Galan, N. Delpont and A. M. Echavarren, Tetrahedron, 2009, 65, 1790 CrossRef CAS.
- The selectivity is comparable with reports using [IPrAu(NCMe)]BF4: M. R. Fructos, M. M. Diaz-Requejo and P. J. Perez, Chem. Commun., 2009, 5153 Search PubMed.
- C. Bartolome, Z. Ramiro, D. Garcia-Cuadrado, P. Perez-Galan, M. Raducan, C. Bour, A. M. Echavarren and P. Espinet, Organometallics, 2010, 29, 951 CrossRef CAS; C. Nieto-Oberhuber, M. R. Munoz, S. Lopez, E. Jemenez-Nunez, C. Nevado, E. Herrero-Gomez, M. Raducan and A. M. Echavarren, Chem.–Eur. J., 2008, 14, 5096 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, 1H and 13C NMR and ESMS spectra, 1H NMR stacked plots, and crystallographic data. CCDC reference numbers 782313–782316. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc02185g |
| This journal is © The Royal Society of Chemistry 2011 |