A Ru(II) complex-based COX-2 targeting type I photosensitizer evokes ferroptosis and apoptosis†
Fen
Qi‡
ac,
Xiaoxue
Zheng‡
a,
Yanping
Wu
a,
Shumeng
Li
a,
Shankun
Yao
a,
Weijiang
He
 *a,
Yuncong
Chen
*a,
Yuncong
Chen
 *ab and
Zijian
Guo
*ab and
Zijian
Guo
 a
a
aState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Chemistry and Biomedicine Innovation Center (ChemBIC), ChemBioMed Interdisciplinary Research Center, Nanjing University, Nanjing 210023, Jiangsu, P. R. China. E-mail: chenyc@nju.edu.cn; heweij69@nju.edu.cn
bDepartment of Cardiothoracic Surgery, Nanjing Drum Tower Hospital, Medical School, Nanjing University, Nanjing 210008, Jiangsu, P. R. China
cKai Yuan School of Innovation and Entrepreneurship, Wuxi Institute of Technology, Wuxi 214121, China
First published on 16th October 2024
Abstract
Photodynamic therapy (PDT) often faces challenges such as oxygen dependence and limited tumour specificity. We report a tumour-targeting photosensitizer (PS), RuCXB, which enhances uptake by cancer cells by targeting overexpressed cyclooxygenase-2 enzyme in tumours. RuCXB also reduces oxygen dependence via a type I PDT mechanism and achieves a strong therapeutic effect through the synergistic induction of ferroptosis and apoptosis. This work presents a reliable strategy for developing potent PSs with enhanced PDT efficacy, tumour selectivity, and diminished oxygen dependence.
PDT is an increasingly prominent field in anticancer research due to its noninvasiveness, spatiotemporal selectivity, and minimal side effects. However, the therapeutic effect of conventional type II PDT relies on the oxygen-dependent generation of cytotoxic reactive oxygen species (ROS), which is distinctly suppressed by the hypoxic tumor microenvironment (Scheme S1, ESI†).1 Type I PDT shows great potential to overcome the malignant hypoxia of solid tumors because it requires less oxygen and relies on electron transfer or hydrogen abstraction reactions to produce superoxide radicals (O2˙−), hydroxyl radicals (˙OH), or hydrogen peroxide (H2O2).2 Noble metal complexes, such as those of ruthenium (Ru), iridium (Ir), and platinum (Pt), are considered promising candidate photosensitizers (PSs) for type I PDT.3,4 On the other hand, PDT mainly induces apoptosis for cancer cell elimination, which shows limited effect due to the overexpression of apoptosis-suppressing genes induced by the hypoxic microenvironment.5,6 Ferroptosis, a non-apoptotic regulated cell death pathway, which involves lipid peroxidation (LPO) accumulation, provides a feasible alternative for treating cancer cells.7–9
However, the potential phototoxicity to healthy tissues remains an issue in the PDT process. Recently, conjugating type I PSs with targeting moieties has emerged as an effective method for specific antitumor therapy, which could not only improve PDT efficacy but also minimize undesired side effects.10 Recently, cyclooxygenase-2 (COX-2) has become an important biomarker for early cancer diagnosis and an attractive focus in anticancer research.11,12 COX-2 is found to be highly expressed in a variety of cancers, including pancreatic, colorectal, stomach, breast, and lung carcinomas.13,14 Celecoxib (CXB), a COX-2 inhibitor, could be a promising ligand for tumor targeting due to its high affinity for COX-2.15 Therefore, developing COX-2 targeting PSs based on metal complexes shows great potential for targeted tumor inhibition with low side effects.
Herein, we report a COX-2 targeting type I photosensitizer, RuCXB, which exhibited reduced O2-dependence and improved PDT effect through the synergism of ferroptosis and apoptosis. CXB was introduced into a ruthenium(II) complex to specifically target the overexpressed COX-2 protein in cancer cells. RuCXB selectively accumulated in the Golgi apparatus (GA), causing severe GA damage and downregulation of COX-2 post-irradiation. Consequently, RuCXB activated both apoptosis and ferroptosis pathways upon PDT and exhibited higher toxicity to tumors cells than to normal cells. This work provides a feasible strategy for the design of PSs with high efficiency against hypoxic tumors.
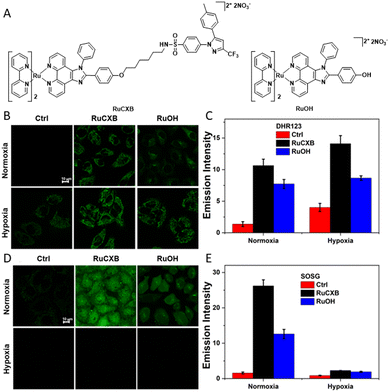
The synthesis procedures of RuCXB and RuOH are described in Scheme S2 (ESI†). All the compounds were fully characterized by 1H NMR, 13C NMR and HRMS (Fig. S1–S5, ESI†). Both RuCXB and RuOH showed a maximum absorption peak at nearly 460 nm and emission peaks ranging from 550 to 720 nm (Fig. S6 and Table S1, ESI†), which corresponded to a metal-to-ligand charge transfer (MLCT) transition of Ru(II) polypyridyl complexes.16,17 The singlet oxygen quantum yields (ΦΔ) of RuCXB and RuOH were determined to be 0.77 and 0.53, respectively (Fig. S7 and Table S1, ESI†). Meanwhile, photoinduced type II and type I ROS of the two complexes were assessed using singlet oxygen sensor green reagent (SOSG) as an 1O2 indicator and dihydrorhodamine 123 (DHR123) as an O2˙− indicator. As shown in Fig. S8 (ESI†), the emission intensity enhancement of SOSG for the two complexes was 15-fold under normoxic conditions and 1.1-fold under hypoxic conditions, which may be attributed to the fact that the production process of 1O2 relies on O2. In Fig. S9 (ESI†), the emission intensity of DHR123 for RuCXB significantly increased in a O2-independent manner and was obviously stronger than that of RuOH, which may be related to the difference in the molar extinction coefficient (ε) of the two complexes. Furthermore, a typical EPR (electron paramagnetic resonance) spectrum of O2˙− was observed after DMPO and Ru(II) complexes were added and illuminated (Fig. S10, ESI†). Collectively, these results demonstrated that RuCXB exhibited excellent photodynamic activity under hypoxia, making it a promising candidate for subsequent anti-cancer cell studies.
Next, the intracellular ROS generation ability of the two complexes in MCF-7 cells was studied using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as an indicator for total ROS detection, DHR123 for O2˙− and SOSG for 1O2, respectively (Fig. 1B–E and Fig. S11, ESI†). The luminescence intensity of RuCXB-treated cells was consistently higher than that of RuOH-treated cells, indicating that RuCXB showed superior ROS generation ability. Then, MCF-7 cells were co-incubated with different ROS inhibitors and Ru(II) complexes for 4 h, followed by photoirradiation and cell viability determination (Fig. S13, ESI†). As expected, the addition of NAC, NaN3 and MnTBAP increased the survival of cells under normoxia, while the addition of NAC and MnTBAP increased the survival of cells under hypoxia, indicating that the two complexes exhibited a self-adaptive photoinduced ROS type in cells with different O2 levels.
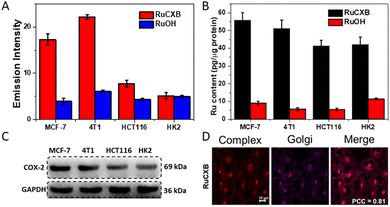
In addition, the cell imaging abilities of the two complexes were investigated in cancer cells (MCF-7, 4T1 and HCT116) and normal cells (HK2). As shown in Fig. 2A and Fig. S14 (ESI†), the phosphorescence of RuCXB-treated cells in all cell lines was significantly higher than that of RuOH. This was consistent with ICP-MS analysis, which showed higher cell uptake of RuCXB (Fig. 2B). Importantly, RuCXB exhibited stronger phosphorescence in cancer cells than in normal cells, indicating its ability to discriminate cancer cells from normal cells. To explain the differences in cellular uptake of the two complexes, the expression levels of COX-2 in various cell lines were investigated by western blot (WB). Evidently, the expression levels of COX-2 protein in HCT116 and HK2 were lower than in MCF-7 and 4T1, in agreement with cell uptake of RuCXB in different cell lines (Fig. 2C). In addition, co-localization experiments of the Ru(II) complexes were conducted. As expected, the Pearson's correlation coefficient (PCC) between RuCXB and the GA-red tracker was 0.81 in MCF-7 cells and 0.61 in HCT116 cells, illustrating that RuCXB accumulated preferentially in the GA in cells with high COX-2 expression (Fig. 2D). The results are in accordance with the fact that COX-2 is mainly distributed in the GA.18,19 As a control, RuOH failed to target the GA as indicated by low PCC values (Fig. S15 and 16, ESI†).
Subsequently, the cytotoxicity of RuCXB and RuOH was investigated. As shown in Table S2 (ESI†), RuCXB exhibited significantly higher photocytotoxicity than RuOH in four cell lines, which could be attributed to the higher levels of photoinduced ROS production and higher cellular uptake. Furthermore, RuCXB showed similar light-induced half maximal inhibitory concentration (IC50) values under both normoxia and hypoxia, demonstrating its lower O2-dependence. Moreover, RuCXB exhibited higher toxicity towards cancer cells than normal cells, which may result from its excellent COX-2 targeting effect and differential cell uptake. All of the above results confirmed that the introduction of CXB improved the ability of Ru(II) complexes to discriminate cancer cells, and further induced O2-independent photodamage by PSs.
To clarify the cell death pathway involved in this PDT process, the cell viability of MCF-7 cells treated with various cell death inhibitors and Ru(II) complexes was assessed. As shown in Fig. S17 (ESI†), both the ferroptosis and apoptosis inhibitors were effective in inhibiting cell death induced by RuCXB. However, for RuOH, only the ferroptosis inhibitor significantly inhibited cell death, indicating that a dual cell death mode was photoinduced by RuCXB, whereas RuOH primarily induced ferroptosis. The cell viability of cells was assessed by co-incubating cells with deferoxamine (DFO, an iron chelator) or holo-transferrin (HTF, an iron transporter) and Ru(II) complexes (Fig. S18, ESI†). The findings indicated that the cell viability of cells incubated with DFO and Ru(II) complexes was increased. Under hypoxic conditions, similar outcomes were noted, suggesting that the reduction in intracellular iron concentration led to the suppression of ferroptosis.20
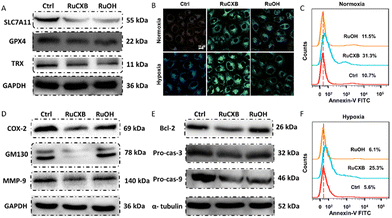
As both Ru(II) complexes induced cell ferroptosis, we investigated ferroptosis-associated proteins. In Fig. 3A, the expression of solute carrier family 7 member 11 (SLC7A11) in MCF-7 cells decreased after incubation with Ru(II) complexes upon irradiation, indicating that the disturbance of intracellular redox balance led to the inactivation of glutathione peroxidase 4 (GPX4) and decreased the expression of thioredoxin (TRX). GPX4 is a key indicator of ferroptosis; the decrease in GPX4 activity directly affects the transformation of lipid peroxides (LPOs), resulting in their accumulation in cells.21–23 Subsequently, LPOs levels in MCF-7 cells were detected by confocal laser scanning microscope (CLSM) using C11-BODIPY as a probe24 (Fig. 3B). Confocal images revealed that the fluorescence intensity of the probe increased in both normoxia and hypoxia after the addition of Ru(II) complexes and irradiation, indicating a significant accumulation of LPOs. These findings demonstrated that Ru(II) complexes could trigger ferroptosis through a GPX4-dependent mechanism in response to PDT.
Given the increased cell viability after incubation with the apoptosis inhibitor, we measured the percentage of apoptotic cells. As shown in Fig. 3C and F, under both normoxia and hypoxia, the apoptosis rate was higher in RuCXB-treated cells than in RuOH-treated cells after illumination. The percentages of apoptotic cells treated with RuCXB was 31.3% and 25.3%, respectively, under two conditions. However, little difference was observed for RuOH-treated cells compared to the control group. This may be related to the introduction of celecoxib, which can induce apoptosis as a COX-2 inhibitor. Then, the intracellular expression of COX-2 and its related proteins Golgi matrix protein 130 (GM130) and matrix metalloproteinase-9 (MMP-9) was detected by WB analysis25 (Fig. 3D). As expected, the expression of COX-2 in MCF-7 cells incubated with RuCXB after irradiation was remarkably lower than that with RuOH. GM130, a characteristic structural protein of GA,26 exhibited a similar trend to COX-2. As a downstream signal of COX-2, the expression of MMP-9 also decreased in cells treated with RuCXB. Additionally, the decreased expression of COX-2 can further inhibit the expression of the anti-apoptotic protein B-cell lymphoma-2 (Bcl-2). Subsequently, we investigated the expression of Bcl-2 and apoptosis-related proteins procaspase-3 (Pro-cas-3) and procaspase-9 (Pro-cas-9). In Fig. 3E, the levels of Bcl-2, as well as the key apoptotic proteins Pro-cas-3 and Pro-cas-9, were all reduced. These collective findings suggested that RuCXB exhibited a dual mechanism of action, capable of inducing both apoptosis and ferroptosis in cells.
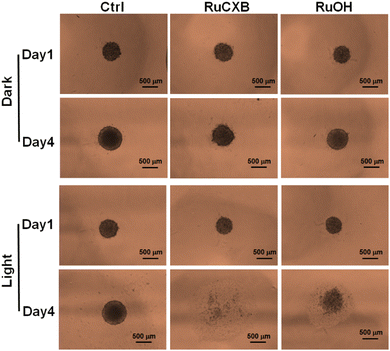
The above results indicated that RuCXB showed superior PDT efficacy compared to RuOH. 3D multicellular spheroids (MCSs) are suitable for simulating tumor tissue because they could represent the complex architecture and microenvironment of solid tumors.27,28 Thus, to validate the therapeutic potential of the Ru(II) complexes against solid tumors, we used 3D MCSs for a more physiologically relevant assessment of their effects on tumor growth and response to treatment (Fig. 4). In the control group without Ru(II) complexes, 3D MCSs grew significantly after 4 days of incubation, regardless of irradiation. The growth of 3D MCSs incubated with Ru(II) complexes was slightly inhibited in the dark; however, upon irradiation, the growth of 3D MCSs was markedly inhibited. These findings indicated that the two Ru(II) complexes are promising type I PDT agents capable of addressing tumor hypoxia and effectively inhibiting solid tumor growth. Notably, the 3D MCSs incubated with RuCXB underwent complete collapse after irradiation, indicating that the dual death modes of ferroptosis and apoptosis rendered RuCXB an effective agent for PDT.
In summary, a COX-2 targeting type I photosensitizer, RuCXB, was designed for the treatment of hypoxic tumor cells. The introduction of celecoxib enhanced the targetability of PSs to cancer cells and reduced phototoxicity to normal cells. RuCXB mainly accumulated in the GA, causing severe disruption of GA function and downregulation of COX-2 after irradiation. Importantly, RuCXB not only activated the apoptotic pathway, but also induced ferroptosis characterized by the down-regulation expression of SLC7A11, TRX and GPX4 proteins. As a result, RuCXB successfully suppressed the growth of 3D MCSs via photoirradiation. The dual death modes of ferroptosis and apoptosis provided a reliable strategy for overcoming the issues of tumor hypoxia and apoptosis-resistance.
This work was financially supported by the National Natural Science Foundation of China (22293050, 22293051, 91953201, 92153303, 22122701, 22377050, 22477054) and the Natural Science Foundation of Jiangsu Province (BK20232020).
Data availability
The data supporting this article have been included as a part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Z. H. Sun, Y. C. Chen, S. K. Yao, H. Yuan, D. F. Song, Z. J. Guo and W. J. He, CCS Chem., 2023, 5, 2078–2087 CrossRef.
- Y. P. Wu, S. M. Li, Y. C. Chen, W. J. He and Z. J. Guo, Chem. Sci., 2022, 13, 5085–5106 RSC.
- L. Wei, R. Kushwaha, T. Sadhukhan, H. R. Wu, A. Dao, Z. S. Zhang, H. T. Zhu, Q. F. Gong, J. X. Ru, C. Liang, P. Y. Zhang, S. Banerjee and H. Y. Huang, J. Med. Chem., 2024, 67, 11125–11137 CrossRef CAS PubMed.
- M. S. Zheng, X. L. Lin, K. Xiong, X. T. Zhang, Y. Chen, L. N. Ji and H. Chao, Chem. Commun., 2024, 60, 2776–2779 RSC.
- Z. Y. Wang, X. Wang, X. Y. Dai, T. M. Xu, X. Q. Qian, M. Q. Chang and Y. Chen, Adv. Mater., 2024, 36, 2312316 CrossRef CAS PubMed.
- W. R. Wilson and M. P. Hay, Nat. Rev. Cancer, 2011, 11, 393–410 CrossRef CAS PubMed.
- G. Lei, L. Zhuang and B. Y. Gan, Nat. Rev. Cancer, 2022, 22, 381–396 CrossRef CAS PubMed.
- B. R. Stockwell, Cell, 2022, 185, 2401–2421 CrossRef CAS PubMed.
- H. Yuan, Z. Han, Y. C. Chen, F. Qi, H. B. Fang, Z. J. Guo, S. R. Zhang and W. J. He, Angew. Chem., Int. Ed., 2021, 60, 8174–8181 CrossRef CAS PubMed.
- X. N. Wang, D. Luo and J. P. Basilion, Cancers, 2021, 13, 2992 CrossRef CAS PubMed.
- Y. T. Yeung, F. Aziz, A. Guerrero-Castilla and S. Argüelles, Curr. Pharm. Design, 2018, 24, 1449–1484 CrossRef CAS PubMed.
- J. S. An, K. P. Lv, C. V. Chau, J. H. Lim, R. Parida, X. Huang, S. Debnath, Y. J. Xu, S. Q. Zheng, A. C. Sedgwick, J. Y. Lee, D. X. Luo, Q. Liu, J. L. Sessler and J. S. Kim, J. Am. Chem. Soc., 2024, 146, 19434–19448 CrossRef CAS PubMed.
- M. Berbecka, A. Forma, J. Baj, M. Furtak-Niczyporuk, R. Maciejewski and R. Sitarz, J. Clin. Med., 2021, 10, 4443 CrossRef CAS PubMed.
- J. A. Glover, C. M. Hughes, M. M. Cantwell and L. J. Murray, Br. J. Cancer, 2011, 105, 13–17 CrossRef CAS PubMed.
- Z. M. Zhang, A. Ghosh, P. J. Connolly, P. King, T. Wilde, J. Y. Wang, Y. W. Dong, X. L. Li, D. H. Liao, H. Chen, G. C. Tian, J. Suarez, W. G. Bonnette, V. Pande, K. A. Diloreto, Y. F. Shi, S. Patel, B. Pietrak, L. Szewczuk, C. Sensenhauser, S. Dallas, J. P. Edwards, K. E. Bachman and D. C. Evans, J. Med. Chem., 2021, 64, 11570–11596 CrossRef CAS PubMed.
- F. Heinemann, J. Karges and G. Gasser, Acc. Chem. Res., 2017, 50, 2727–2736 CrossRef CAS PubMed.
- F. Qi, H. Yuan, Y. C. Chen, X. X. Peng, Y. P. Wu, W. J. He and Z. J. Guo, CCS Chem., 2023, 5, 1583–1591 CrossRef CAS.
- B. H. Wang, J. L. Fan, X. W. Wang, H. Zhu, J. Y. Wang, H. Y. Mu and X. J. Peng, Chem. Commun., 2015, 51, 792–795 RSC.
- H. Zhang, J. L. Fan, J. Y. Wang, S. Z. Zhang, B. R. Dou and X. J. Peng, J. Am. Chem. Soc., 2013, 135, 11663–11669 CrossRef PubMed.
- X. X. Fang, H. Ardehali, J. X. Min and F. D. Wang, Nat. Rev. Cardiol., 2023, 20, 7–23 CrossRef PubMed.
- J. P. F. Angeli, R. Shah, D. A. Pratt and M. Conrad, Trends Pharmacol. Sci., 2017, 38, 489–498 CrossRef PubMed.
- S. M. Li, H. Yuan, Y. C. Chen and Z. J. Guo, Fund. Res., 2023, 3, 525–528 Search PubMed.
- Y. Y. Zhang, B. T. Doan and G. Gasser, Chem. Rev., 2023, 123, 10135–10155 CrossRef PubMed.
- E. H. W. Pap, G. P. C. Drummen, V. J. Winter, T. W. A. Kooij, P. Rijken, K. W. A. Wirtz, J. A. F. Op den Kamp, W. J. Hage and J. A. Post, FEBS Lett., 1999, 453, 278–282 CrossRef PubMed.
- F. Finetti, L. Paradisi, C. Bernardi, M. Pannini and L. Trabalzini, Cancers, 2023, 15, 2374 CrossRef PubMed.
- M. L. Liu, Y. C. Chen, Y. Guo, H. Yuan, T. X. Cui, S. K. Yao, S. X. Jin, H. H. Fan, C. J. Wang, R. Xie, W. J. He and Z. J. Guo, Nat. Commun., 2022, 13, 2179 CrossRef PubMed.
- Y. Imamura, T. Mukohara, Y. Shimono, Y. Funakoshi, N. Chayahara, M. Toyoda, N. Kiyota, S. Takao, S. Kono, T. Nakatsura and H. Minami, Oncol. Rep., 2015, 33, 1837–1843 CrossRef PubMed.
- L. L. Sun, Y. Chen, S. Kuang, G. Y. Li, R. L. Guan, J. P. Liu, L. N. Ji and H. Chao, Chem. – Eur. J., 2016, 22, 8955–8965 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04217d |
| ‡ Fen Qi and Xiaoxue Zheng contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |