N-Acetyl-L-cysteine capped quantum dots offer neuronal cell protection by inhibiting beta (1–40) amyloid fibrillation†
Olivia T. W.
Ng‡
a,
Yi
Wong‡
b,
Ho-Man
Chan
b,
Jing
Cheng
b,
Xiao
Qi
b,
Wing-Hong
Chan
b,
Ken K. L.
Yung
*a and
Hung-Wing
Li
*b
aDepartment of Biology, Hong Kong Baptist University, Kowloon Tong, Hong Kong, P. R. China. E-mail: kklyung@hkbu.edu.hk; Fax: +852-3411-5995; Tel: +852-3411-7060
bDepartment of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong, P. R. China. E-mail: hwli@hkbu.edu.hk; Fax: +852-3411-7348; Tel: +852-3411-7065
First published on 17th April 2013
Abstract
This is the first work that revealed the neuro-protective effect of functionalized quantum dots against the cytotoxicity induced by beta-amyloid peptides. This study gives insight into the future treatment of Alzheimer's disease. It opens many avenues for the development of the next generation nanotechnology for biomedical and therapeutic applications.
Alzheimer's disease (AD), an age-related neurodegenerative disorder, is the most common cause of dementia. Currently, about 35.6 million people worldwide suffer from dementia, and the number is expected to double in about 20 years.1 One of the historical hallmarks of AD is the formation of beta amyloid (Aβ) plaques in the brain. Aggregation of the Aβ peptide leads to senile plaque formation and is also accompanied by neuro-degeneration.2–4 Although the relationship between protein deposition and neuro-degeneration is not explicitly understood, it is believed that the protein aggregation process does play a significant role.5 The relative toxicity of different forms of Aβ aggregates, namely monomer, oligomer, protofibril and fibril, is still controversial. Among all of them, Aβ fibrils were the most widely reported form in the literature to cause the dysfunction and death of neural cells,2 nevertheless recent evidence suggests that oligomers may indeed, and surprisingly, have a higher toxicity than the other forms and are more crucial than fibrils in the onset of AD.6 No matter which forms of Aβ peptide is the main conformation inducing the neuro-degeneration, with the fact that abnormal expression and deposition of the Aβ peptide is assured to be a major cause of AD, strategies that could successfully reduce the Aβ monomer/oligomer/fibril-induced neurotoxicity and minimize its cytotoxic effect are desired in both the scientific and biomedical society. Such a development would be beneficial to the design of potential novel AD drugs.
The self-aggregating characteristic of Aβ peptide provides a clue to the design of an aggregation inhibitor. Several reports showed that the inhibition of Aβ1–40 fibrillation with small organic molecules, peptides and nanoparticles could effectively reduce the amyloid-induced toxicity in vitro.7–13 Our recent publications illustrated that synthetic small organic molecules and organometallic complexes with various functional end groups can control or even inhibit the growth of amyloid fibrils and demonstrate a neuro-protective effect to neuronal cells.9,13 In the studies, some of the small molecules were found to have the ability to pass through the blood–brain barrier (BBB), showing their high clinical potentials in serving as therapeutic drugs for AD. Other than that, scyllo-cyclohexanehexol was developed as an inhibitor of Aβ aggregation and tested under a phase II clinical trial.14 Cyanine dyes selectively bind to folded proteins and modulate the fibrillation process.15 Other research groups suggested that methylene blue can inhibit Aβ oligomerization by promoting fibrillation.16 Amino-modified polystyrene nanoparticles were reported to control the fibrillation kinetics by adjusting their particle surface areas.17
Quantum dots (QDs) are semiconductors with unique optical properties, such as size-dependent emission wavelengths and free of photobleaching.18 Recently, the characterization and optimization of QDs allowed the exploration of biological applications. Quantum dots are used as a tool to understand the complexity and dynamics of biological interactions among proteins or cells. Modification of QD surfaces allows QDs to interact with biological components and benefits the development of various applications, such as cellular and molecular targeting, cell tracing in vivo and in vitro, and drug delivery.18–20
Our group previously reported the inhibitory effect of N-acetyl-L-cysteine (NAC) capped quantum dots (QDs) at nanomolar concentrations on Aβ1–40 fibrillation.21 We studied the effects of the surface functionality and the size of the nanoparticles in interfering Aβ fibrillogenesis, for both promotion and inhibition, using various sizes of QDs and gold nanoparticles with different ligand functionalizations.22 With such fundamental studies, nanoparticles with optimized functionalities and sizes may be a good therapeutic agent or a nano-sized drug-carrier for AD treatment. Thus, the effect of such nanomaterials towards biological systems, especially regarding neuronal cells, should be well studied prior to clinical applications. Here, the interaction and bioactivity of quantum dots in the presence of neural cells were investigated. We studied the cell-based cytotoxicity of NAC–QDs and the effect of QDs on the beta (1–40) amyloid (Aβ1–40)-induced cytotoxicity on human neuroblastoma SH-SY5Y cells by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and ROS (reactive oxidative species) assays, respectively. We learnt that the inhibition of Aβ1–40 peptides from fibrillation by NAC–QDs had a neuroprotection effect on the neural SH-SY5Y cells. To our knowledge, this is the first work to demonstrate the neuroprotection effect of QDs on neuron cells. We hypothesized that NAC–QDs favorably interact with Aβ1–40 peptides in cell culture and inhibit Aβ1–40 aggregation, and thus are able to attenuate the toxicity induced by Aβ1–40.
The preparation of NAC–QDs is described in the experimental section and their physical characterization was reported previously23 (see the ESI, S1†). As the self-assembling process of Aβ1–40 highly depends on the experimental conditions, such as temperature, ionic strength and concentration, throughout the study a cell culture medium solution (DMEM) was used instead of PB buffer for in vitro Aβ1–40 growth in order to explore the effectiveness of NAC–QDs in the inhibition of Aβ1–40 fibrillation. Total internal reflection fluorescence microscopy (TIRFM) was employed to image the resulting fibrils grown in the presence and absence of NAC–QDs. TIRFM is a useful technique to reveal the population and sizes of the Aβ1–40 aggregates/fibrils. As shown in Fig. 1, 10−8 M NAC–QDs obviously inhibited the fibrillation of 50 μM Aβ1–40 effectively in DMEM solutions.
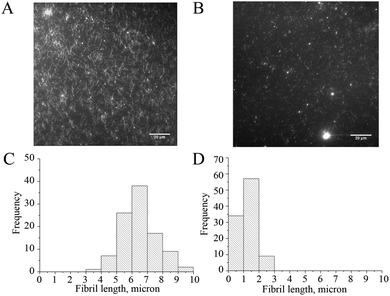 | ||
| Fig. 1 Fluorescence images and the corresponding fibril length distributions of Aβ1–40 fibrils resulting from seed-mediated growth, incubated at 37 °C in DMEM buffer without (A and C) and with (B and D) the addition of 10−8 M NAC–QDs. | ||
In the light of the inhibition of Aβ1–40 fibrillation, MTT assays were performed to determine the cytotoxicity of NAC–QDs on SH-SY5Y cells which were exposed to NAC–QDs for 2, 6 and 24 h, respectively. Fig. 2 shows the summary of the cytotoxicity data of NAC–QDs. In the short period of exposure (2 h), NAC–QDs had no significant cytotoxicity for the concentration range studied (1 nM to 1 μM). An increase in cytotoxicity of NAC–QDs was found in SH-SY5Y cells that were treated with 10 nM or above of NAC–QDs for 6 hours (n = 3, *p < 0.05). This indicated that the uptake of NAC–QDs was fast and happened within 6 h. A higher cytotoxicity (i.e. cells with more than 50% cell death) of NAC–QDs was found in SH-SY5Y cells that were treated with 10 nM or above of NAC–QDs for 24 hours (n = 3, *p < 0.05). While after a 24 h incubation of NAC–QDs there was no significant cytotoxicity reported at a concentration of 1 nM. With respect to the Aβ concentration level in AD patients’ brains, which is in the sub-nanomolar range,24 NAC–QDs at nanomolar concentrations should be more than enough for inhibiting Aβ fibrillation as confirmed by our experiments in vitro. The major hurdle of applying QDs in biomedicine is their potential cytotoxicity. Here it shown that NAC–QDs are non-toxic at a concentration of 1 nM.
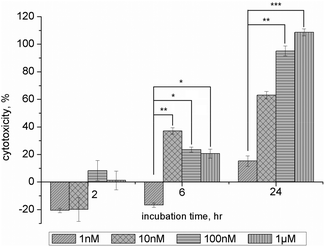 | ||
| Fig. 2 Cytotoxicity of NAC–QDs among three different time points. The histograms show the relative percentage of cytotoxicity of NAC–QDs. Low cytotoxicity was observed in NAC–QD treated SH-SY5Y cells after 2 hours, and no significant differences among the concentrations examined in the present experiment. A significant increase in cytotoxicity was found in SH-SY5Y cells that were treated with 10 nM (data = mean ± SEM, n = 3, **p < 0.01), 100 nM and 1 μM NAC–QDs after 6 hours (data = mean ± SEM, n = 3, *p < 0.05). A high cytotoxicity (i.e. cell with more than 50% cell death) was found in SH-SY5Y cells treated with higher or above 10 nM NAC–QDs concentrations after 24 hours. A significant increase in cytotoxicity was found at 100 nM (data = mean ± SEM, n = 3, **p < 0.01) and 1 μM (data = mean ± SEM, n = 3, ***p < 0.001) NAC–QDs treated SH-SY5Y cells. | ||
The MTT assay was also used to determine the effect on cell proliferation after exposure to three different micromolar Aβ1–40 solutions: monomer (Aβ1–40M), fibrils (Aβ1–40F), and monomer with fibril seeds (Aβ1–40MS) in the presence and absence of NAC–QDs at three different time points. The final concentration of Aβ1–40 and NAC–QDs were kept at 10 μM and 2 nM, respectively, in order to maintain a constant ratio between Aβ1–40 and QDs as we demonstrated in the inhibition experiments. The pre-formed fibrils were found to deposit outside the neuronal cells (see the ESI, S2†). The histogram in Fig. 3 summarizes the cytotoxicity data of different Aβ1–40 solutions (Aβ1–40M solution, Aβ1–40F solution and Aβ1–40MS solution) in the presence of NAC–QDs, relative to those without NAC–QDs. The induced toxicity of Aβ1–40 is lower with the NAC–QDs treatment, which is represented by the ratio lower than 1, while no obvious structural and morphological changes of the SH-SY5Y cells after the addition of Aβ1–40, NAC–QDs or Aβ1–40 + NAC–QDs were observed from differential interference contrast (DIC) images (see the ESI, S3†). Interestingly, a lower cytotoxicity of Aβ1–40M and Aβ1–40F was found after a 2 h incubation with NAC–QDs. Moreover, the cytotoxicity of all the Aβ1–40 solutions on SH-SY5Y cells was reduced by NAC–QDs after 6 h of incubation but then increased after 24 hours, possibly due to the toxicity of NAC–QDs themselves. These results indicated the NAC–QDs did show short-term attenuation of Aβ1–40-produced neuronal toxicity.
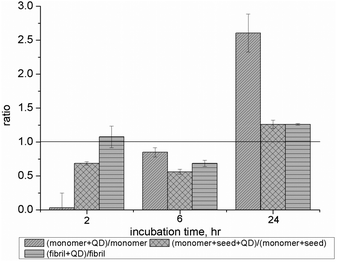 | ||
| Fig. 3 Cytotoxicity of different Aβ1–40 solutions conjugated with NAC–QDs at three different time points. The histogram shows the ratio of the relative percentage of cytotoxicity of different Aβ1–40 solutions (Aβ1–40M solution, Aβ1–40F solution and Aβ1–40MS solution) conjugated with NAC–QDs (0.2 × 10−8 M). The relative percentage of cytotoxicity was calculated and the ratio value of less than 1 indicated a lower cytotoxicity for the Aβ1–40 solutions after conjugation with NAC–QDs compared with those of SH-SY5Y cells treated with different Aβ1–40 solutions alone. | ||
ROS assay was performed to determine the oxidative stress response of cells exposed to different Aβ1–40 solutions in the presence and absence of NAC–QDs at three different time points. Fig. 4 shows the ratio of relative intracellular ROS levels of different Aβ1–40 solutions (Aβ1–40M solution, Aβ1–40F solution and Aβ1–40MS solution) with NAC–QDs (0.2 × 10−8 M). After co-incubation of Aβ1–40 and QDs for 2, 6 and 24 hours, the oxidation of dichlorofluorescein (DCF) was determined. The ratio of the relative intracellular ROS levels was calculated as (ROSβ-amyloid solution/NAC–QDs/ROSNAC–QDs) × 100%. The ratio of the relative intracellular ROS levels between different Aβ1–40 solutions and different Aβ1–40 solutions with NAC–QDs was calculated. Intracellular ROS levels in the cells were found to be lower after being treated with QDs in the presence of Aβ1–40. Lower intracellular ROS levels were found in SH-SY5Y cells treated with NAC–QDs together with all of the Aβ1–40 solutions after 2, 6 and 24 hours of incubation. Moreover, the intracellular ROS levels in SH-SY5Y cells treated with NAC–QDs and Aβ1–40 monomer solutions were consistently lower at the three time points studied compared with those treated with NAC–QDs and Aβ1–40 fibril solutions. In short, the ROS formation, which is a key toxic mechanism of neuronal cells after exposure to Aβ1–40, is successfully reduced by NAC–QDs.
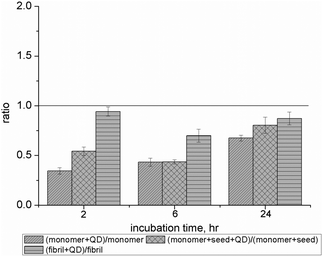 | ||
| Fig. 4 Intracellular ROS levels of different Aβ1–40 solutions conjugated with NAC–QDs at three different time points. The histogram shows the ratio of the relative intracellular ROS levels of different Aβ1–40 solutions (Aβ1–40M solution, Aβ1–40F solution and Aβ1–40MS solution) conjugated with NAC–QDs (0.2 × 10−8 M). The ratio of the relative intracellular ROS levels was calculated and the ratio value of less than 1 showed that lower intracellular ROS levels were induced by Aβ1–40 solutions after conjugation with NAC–QDs compared with those of SH-SY5Y cells treated with different Aβ1–40 solutions alone. The induction of intracellular ROS levels was found to be reduced for all Aβ1–40 solutions on SH-SY5Y cells after incubating with NAC–QDs for 2, 6 and 24 hours. | ||
The results presented here revealed the potential of NAC-capped nanoparticles as future therapeutic agents for AD. Aβ1–40 fibril formation can be inhibited by NAC–QDs in vitro.21 MTT assays demonstrated the absence of toxicity for NAC–QDs at a concentration of 1 nM. We also reported that the SH-SY5Y and NAC–QD co-incubation reduced both the ROS level and toxicity induced by Aβ1–40 aggregates of various sizes. This study provides new insight into nanomaterials as a potent tools in biomedical and biotechnological fields.
This work was fully supported by grants from the University Grants Council of the Hong Kong Special Administrative Region, China (HKBU 201309 and HKBU 262210). We thank Mr Wai-Shing Chung from the Department of Biology of HKBU for providing technical assistance.
Notes and references
- M. O. W. Grimm, J. Kuchenbecker, S. Grosgen, V. K. Burg, B. Hundsdorfer, T. L. Rothhaar, P. Friess, M. C. de Wilde, L. M. Broersen, B. Penke, M. Peter, L. Vigh, H. S. Grimm and T. Hartmann, J. Biol. Chem., 2011, 286, 14028–14039 CrossRef CAS.
- T. Okada, M. Wakabayashi, K. Ikeda and K. Matsuzaki, J. Mol. Biol., 2007, 371, 481–489 CrossRef CAS.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS.
- M. P. Mattson, Nature, 2004, 430, 631–639 CrossRef CAS.
- P. P. Bose, U. Chatterjee, L. Xie, J. Johansson, E. Gothelid and P. I. Arvidsson, ACS Chem. Neurosci., 2010, 1, 315–324 CrossRef CAS.
- M. Bartolini and V. Andrisano, ChemBioChem, 2010, 11, 1018–1035 CrossRef CAS.
- C. Cabaleiro-Lago, F. Quinlan-Pluck, I. Lynch, S. Lindman, A. M. Minogue, E. Thulin, D. M. Walsh, K. A. Dawson and S. Linse, J. Am. Chem. Soc., 2008, 130, 15437–15443 CrossRef CAS.
- H. Levine, Amyloid, 2007, 14, 185–197 CrossRef CAS.
- B. Y. W. Man, H. M. Chan, C. H. Leung, D. S. H. Chan, L. P. Bai, Z. H. Jiang, H. W. Li and D. L. Ma, Chem. Sci., 2011, 2, 917–921 RSC.
- K. Ono, K. Hasegawa, H. Naiki and M. Yamada, J. Neurosci. Res., 2004, 75, 742–750 CrossRef CAS.
- K. Ono, Y. Yoshiike, A. Takashima, K. Hasegawa, H. Naiki and M. Yamada, J. Neurochem., 2003, 87, 172–181 CrossRef CAS.
- H. Shaykhalishahi, M. Taghizadeh, R. Yazdanparast and Y. T. Chang, Chem.-Biol. Interact., 2010, 186, 16–23 CrossRef CAS.
- W. Yang, Y. Wong, O. T. W. Ng, L.-P. Bai, D. W. J. Kwong, Y. Ke, Z.-H. Jiang, H.-W. Li, K. K. L. Yung and M. S. Wong, Angew. Chem., Int. Ed., 2012, 51, 1804–1810 CrossRef.
- J. McLaurin, M. E. Kierstead, M. E. Brown, C. A. Hawkes, M. H. L. Lambermon, A. L. Phinney, A. A. Darabie, J. E. Cousins, J. E. French, M. F. Lan, F. S. Chen, S. S. N. Wong, H. T. J. Mount, P. E. Fraser, D. Westaway and P. St George-Hyslop, Nat. Med., 2006, 12, 801–808 CrossRef CAS.
- E. E. Congdon, Y. H. Figueroa, L. L. Wang, G. Toneva, E. Chang, J. Kuret, C. Conrad and K. E. Duff, J. Biol. Chem., 2009, 284, 20830–20839 CrossRef CAS.
- M. Necula, L. Breydo, S. Milton, R. Kayed, W. E. van der Veer, P. Tone and C. G. Glabe, Biochemistry, 2007, 46, 8850–8860 CrossRef CAS.
- C. Cabaleiro-Lago, F. Quinlan-Pluck, I. Lynch, K. A. Dawson and S. Linse, ACS Chem. Neurosci., 2010, 1, 279–287 CrossRef CAS.
- K. Hanaki, A. Momo, T. Oku, A. Komoto, S. Maenosono, Y. Yamaguchi and K. Yamamoto, Biochem. Biophys. Res. Commun., 2003, 302, 496–501 CrossRef CAS.
- C. Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS.
- E. B. Voura, J. K. Jaiswal, H. Mattoussi and S. M. Simon, Nat. Med., 2004, 10, 993–998 CrossRef CAS.
- L. H. Xiao, D. Zhao, W. H. Chan, M. M. F. Choi and H. W. Li, Biomaterials, 2010, 31, 91–98 CrossRef CAS.
- H.-M. Chan, L. Xiao, K.-M. Yeung, S.-L. Ho, D. Zhao, W.-H. Chan and H.-W. Li, Biomaterials, 2012, 33, 4443–4450 CrossRef CAS.
- L. Zou, Z. Y. Gu, N. Zhang, Y. L. Zhang, Z. Fang, W. H. Zhu and X. H. Zhong, J. Mater. Chem., 2008, 18, 2807–2815 RSC.
- P. Seubert, C. Vigo-Pelfrey, F. Esch, M. Lee, H. Dovey, D. Davis, S. Sinha, M. Schiossmacher, J. Whaley, C. Swindlehurst, R. McCormack, R. Wolfert, D. Selkoe, I. Lieberburg and D. Schenk, Nature, 1992, 359, 325–327 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Emission spectra and UV-vis spectra of NAC–QDs, fluorescence and DIC images on the localization of Aβ1–40 fibrils in SH-SY5Y cells, experimental procedures on the synthesis and characterization of NAC–QDs, amyloid fibrillation, cell viability assay, ROS measurements and microscopic imaging. See DOI: 10.1039/c3bm60029g |
| ‡ Authors contributed equally to the work of the manuscript. |
| This journal is © The Royal Society of Chemistry 2013 |
