Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave roasting with size grading based on the influence of carbon on vanadium extraction from stone coal via microwave roasting-acid leaching
Yi-zhong Yuan*abc,
Yi-min Zhang*abc,
Tao Liuabc and
Tie-jun Chenabc
aCollege of Resource and Environmental Engineering, Wuhan University of Science and Technology, Wuhan 430081, China. E-mail: yyz2038@163.com; zym126135@126.com; Tel: +86-159-27698430 Tel: +86-027-68862057
bHubei Provincial Engineering Technology Research Center of High Efficient Cleaning Utilization for Shale Vanadium Resource, Wuhan 430081, China
cHubei Collaborative Innovation Center for High Efficient Utilization of Vanadium Resources, China
First published on 5th January 2017
Abstract
Carbon in stone coal has a double-edged function in the microwave roasting-acid leaching of stone coal; it provides stone coal with good heating characteristics, but it hinders the oxidization of vanadium and causes the sintering phenomenon. Adjustment of the particle size and microwave input power is performed to use the advantages of carbon and avoid its disadvantages. Fine particles are not suitable for direct microwave roasting due to their low heating rate, low final temperature and serious sintering phenomenon. However, for coarse particles, a high microwave input power causes more carbon to be retained in the stone coal in the heating stage to obtain a high heating rate and final temperature. These carbon particles are removed in the temperature holding stage because of the unique cracks and pores of coarse particles under the high microwave input power condition. Comprehensively considering the heating characteristics and vanadium leaching efficiency of stone coal, a 1–3 mm particle size and 1200–1800 W microwave input power are suitable for the microwave roasting of stone coal. The vanadium leaching efficiency of the roasted sample in this condition is 14% higher than the roasted sample without size grading.
1 Introduction
Stone coal is an important vanadium resource, which is widely distributed in several provinces of China. Most of the vanadium in stone coal exists as V(III) (trivalent vanadium), which substitutes for Al3+ in the dioctahedron of mica group minerals as isomorphism.1–4 The traditional technology for vanadium extraction, which is the chloridizing roasting-water leaching technique, is gradually being replaced by blank roasting-acid/alkaline leaching or direct acid leaching because of serious environmental pollution.5,6 For stone coal that has a high ratio of vanadium in its mica lattice, the roasting process has a significant effect on the acid leaching efficiency of vanadium. In the roasting process, the reducing materials are oxidized, the mineral structures are preliminarily destroyed, and the vanadium valency changes from the trivalent state to the pentavalent state gradually.7,8 All of these changes are simulative of vanadium leaching from stone coal.The study and utilization of microwaves in the mineral and metallurgy field are becoming more and more intensive.9,10 Microwaves could be utilized for the treatment of ores in some of the unit operations, such as drying, calcining, pretreatment, prereduction roasting and smelting.11 Microwaves are a form of electromagnetic radiation with frequencies in the range of 0.3–300 GHz. Due to its different heating mechanism from conventional methods, the heating characteristics of materials in microwaves are related to their properties. Materials with high complex permittivity (ε) or complex permeability (μ) (for magnetic materials) generally have good microwave absorbing properties and heating characteristics in microwave fields. If the thermal radiation of materials and thermal effect of reactions are considered, the heating rate of materials in the microwave field can be expressed as eqn (1).12 With regard to natural ores consisting of various minerals, their heating characteristics depend on the microwave absorbing components, thermal radiation and thermal effect of reactions.
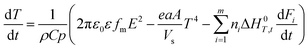 | (1) |
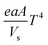 : the heat dissipation of materials.
: the heat dissipation of materials. 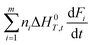 : thermal effect of reactions.
: thermal effect of reactions.
Studies on the utilization of microwaves in vanadium extraction from stone coal have been under investigation for some years. In this extraction process, microwaves are usually used in the roasting and leaching stages to improve the recovery of vanadium. Most reports on this process focused on the effect of process parameters, such as microwave input power, temperature and heating time, on vanadium recovery.13–15 Yuan et al. divided stone coal into different size grades and roasted it with microwaves. They found that the particle size of stone coal has a significant effect on the microwave roasting efficiency; however, the reason for this was not indicated.7
As mentioned above, the heating characteristics of natural ores in microwaves are related to their components. In stone coal, minerals with carbon are microwave absorbing minerals, and aluminosilicate minerals such as quartz, mica and illite are microwave transmitting minerals in general conditions. The existence of carbon in stone coal plays an important role in the microwave roasting of stone coal. Herein, the influence of carbon in stone coal on vanadium extraction by microwave roasting-acid leaching from stone coal was investigated. The relations of carbon content, particle size, microwave input power and vanadium leaching efficiency are analyzed and corresponding suggestions for the microwave roasting of stone coal are proposed.
2 Experiment
2.1 Materials
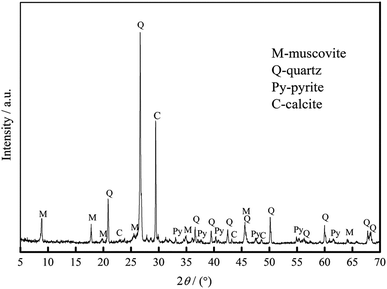
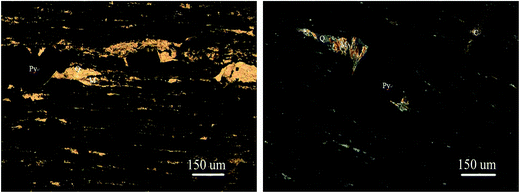
The vanadium-bearing stone coal used in this study was obtained from Teng-da Mining and Metallurgy Co. Ltd., Hubei, PR China. Its main chemical composition was analyzed via inductively coupled plasma-atomic emission spectroscopy (ICP-AES), which is listed in Table 1. Its vanadium distribution was analyzed by electron probe microanalysis (EPMA) and valence state was analyzed by potentiometric titration, and the results are shown in Table 2. The XRD analysis, microscope image and main mineral composition of the raw ore are shown in Fig. 1, 2 and Table 3, respectively.| V2O5 | SiO2 | Al2O3 | CaO | K2O | Na2O | Fe2O3 | S | C |
|---|---|---|---|---|---|---|---|---|
| 0.72 | 65.19 | 9.92 | 5.28 | 2.64 | 0.14 | 5.16 | 3.11 | 13.32 |
| Vanadium distribution | Vanadium valence state | ||||
|---|---|---|---|---|---|
| Muscovite | Organic matter | V(III) | V(IV) | V(V) | |
| Relative content | 90.03 | 9.97 | 62.97 | 37.03 | 0 |
| Content | 0.36 | 0.04 | 0.26 | 0.14 | 0 |
| Quartz | Coal | Calcite | Muscovite | Feldspar | Pyrite | Kaolinite | Other |
|---|---|---|---|---|---|---|---|
| 37 | 13 | 11 | 15 | 10 | 7 | 5 | 2 |
Tables 1 and 2 show that the raw ore is a typical low-grade mica type vanadium-bearing stone coal. The V2O5 grade is 0.72% and more than 90% of the vanadium exists in muscovite as V(III) and V(IV) without V(V). The occurrence state of vanadium in this stone coal indicates that the roasting process is necessary before acid leaching to destroy the muscovite structure and oxidize the low vanadium valence state.
As shown in Table 3, the raw ore mainly consists of 37% quartz, 13% coal, 11% calcite, 15% muscovite, 10% feldspar, 7% pyrite, 5% kaolinite and 2% other minerals.
2.2 Methods
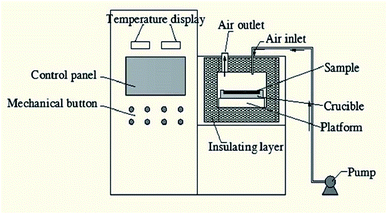
The raw ore was crushed using a Jaw crusher (XPC-60 × 100) and double-roll crusher (HLXPS-φ250 × 150) to 0–6 mm. Then, the sample was divided into three different grade sizes, 0–1, 1–3 and 3–6 mm.Roasting experiments for stone coal and pure minerals were performed in the high-temperature microwave furnace (HM-X08-16), as shown in Fig. 3, which was produced by the Kunming University of Science and Technology. The microwave input power was 0–6 kW and frequency was 2450 ± 50 MHz. The temperature measurement system consists of infrared temperature measurement (−50–2000 °C) and K type thermocouple measurement (0–1100 °C).
The temperatures of the different sample layers were measured using a portable infrared thermometer (−50–1200 °C).
All of the roasted samples were dry grinded to −0.074 mm, which accounted for more than 75%, by a vibration mill (HLXZM-100). 50 g grinded sample was put into a 1000 mL beaker and the leaching experiment was carried out in magnetic and controlling temperature stirrers (SZCL-2A). The leaching experiment was conducted under conditions where the H2SO4 concentration, liquid/solid ratio, leaching temperature, and leaching time were set as 15% (vol%), 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mL g−1), 95 °C and 6 h, respectively. The vanadium concentration of the leaching solution was measured using the ammonium ferrous sulfate method and the final vanadium leaching efficiency was the average value of three parallel experiments.16,17
1 (mL g−1), 95 °C and 6 h, respectively. The vanadium concentration of the leaching solution was measured using the ammonium ferrous sulfate method and the final vanadium leaching efficiency was the average value of three parallel experiments.16,17
3 Results and discussion
3.1 Influence of carbon on microwave roasting of stone coal
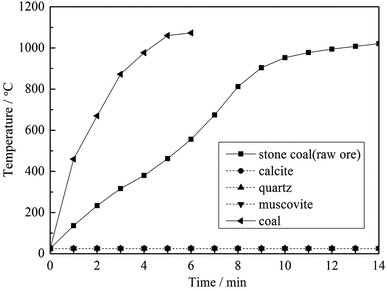
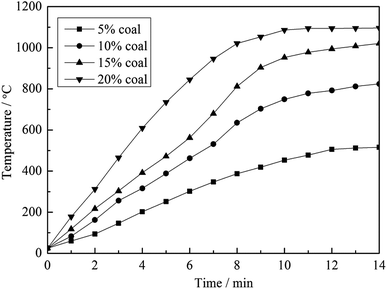
To clarify the influence of carbon content on the heating behavior of the material, pure muscovite with different coal contents was roasted in the microwave furnace at 1500 W microwave input power, and the results are shown in Fig. 5. As seen from Fig. 5, on one hand, with an increase in the carbon content in the material, the heating rate of the material in the microwave field increased. According to the Maxwell–Garnett equivalent medium theory of heterogeneous materials, the equivalent permittivity of a material increases with an increase in the content of high permittivity materials.12 This means that materials with a high carbon content have a high ε, and thus heat up quickly in a microwave field. On the other hand, the highest temperature that the materials could reach also increases along with an increase in carbon content. High temperature is an important factor in the roasting of stone coal; it has a significant effect on vanadium valency transformation and mineral structure destruction. Therefore, in terms of heating characteristics, carbon in stone coal is helpful for the microwave roasting process of stone coal; it provides stone coal with a high heating rate and high roasting temperature.
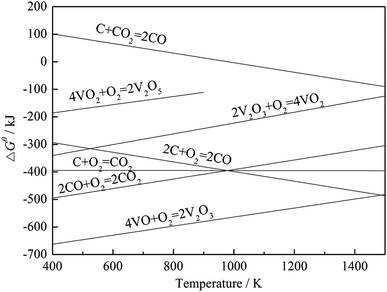
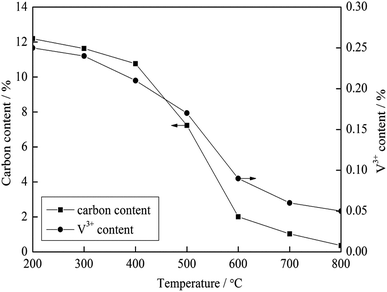
To investigate the effect of carbon on the vanadium valency, the thermodynamic calculation software, HSC Chemistry 6, was used to calculate the ΔGθ of the oxidation reactions of carbon and vanadium. The ΔGθ − T equation of each reaction (Table 4) was obtained by the least square method, and the Ellingham map is shown in Fig. 6. Based on the thermodynamic analysis, an experiment was performed for further proof. In this experiment, 100 g stone coal (0–3 mm) was roasted by 1000 W microwaves at 200 °C, 300 °C, 400 °C, 500 °C, 600 °C, 700 °C and 800 °C for 20 min, respectively. The content of carbon and trivalent vanadium in the roasted samples are presented in Fig. 7.
| Reaction | ΔGθ − T equation | Number |
|---|---|---|
| C + CO2 = 2CO | ΔGθ = 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 707 − 174.047T, J 707 − 174.047T, J |
(2) |
| 2CO + O2 = 2CO2 | ΔGθ = −564![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 + 173.64T, J 840 + 173.64T, J |
(3) |
| C + O2 = CO2 | ΔGθ = −394![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 − 0.84T, J 133 − 0.84T, J |
(4) |
| 2C + O2 = 2CO | ΔGθ = −223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426 − 175.31T, J 426 − 175.31T, J |
(5) |
| 2V + O2 = 2VO | ΔGθ = −861![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904 + 185.02T, J 904 + 185.02T, J |
(6) |
| 4/3V + O2 = 2/3V2O3 | ΔGθ = −817![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 + 178.01T, J 274 + 178.01T, J |
(7) |
| 4/5V + O2 = 2/5V2O5 | ΔGθ = −623![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 + 175.76T, J 081 + 175.76T, J |
(8) |
| 4VO + O2 = 2V2O3 | ΔGθ = −728![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016 + 164.07T, J 016 + 164.07T, J |
(9) |
| 2V2O3 + O2 = 4VO2 | ΔGθ = −418![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 + 195.81T, J 400 + 195.81T, J |
(10) |
| 4VO2 + O2 = 2V2O5 | ΔGθ = −245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 182 + 148.95T, J 182 + 148.95T, J |
(11) |
According to Fig. 6, the ΔGθ of the oxidation reactions of carbon is always less than that for the oxidation reactions of vanadium. This means that the oxidation reactions of carbon occur before vanadium, and thus more oxygen and energy are consumed by carbon before vanadium is oxidized. The result of the experiment, shown in Fig. 7, indicates that the content of V3+ decreases with a decrease in carbon content. Both the thermodynamic analysis and experiment research prove that carbon has an inhibitory effect on the oxidation of vanadium. Therefore, efficient decarburization in the roasting process could provide good oxidative reaction conditions for vanadium.
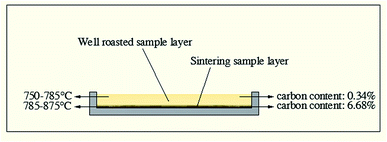 | ||
| Fig. 9 Actual temperature and carbon content of different sample layers at 750 °C in microwave roasting. | ||
Generally, carbon in stone coal has double-edged roles for microwave roasting. On one hand, carbon provides stone coal with strong microwave absorbing characteristics to obtain a high heating rate and roasting temperature. On the other hand, carbon in stone coal inhibits the oxidization of vanadium and causes the sintering phenomenon.
3.2 Microwave roasting of stone coal with size grading
According to the abovementioned analysis, carbon in stone coal plays a double-edged role in the microwave roasting process of stone coal. In order to use the advantages and avoid the disadvantages of carbon in stone coal, the microwave roasting of stone coal with size grading was conducted. The raw ore was divided into three grain sizes, and the V2O5 grades and carbon contents are shown in Table 5. The heating characteristics and carbon content of each grain size at different input powers were investigated and acid leaching of the roasted samples was performed.| Grain size | 0–1 mm | 1–3 mm | 3–6 mm |
| V2O5 grade | 0.77 | 0.63 | 0.58 |
| Carbon content | 13.69 | 12.67 | 12.48 |
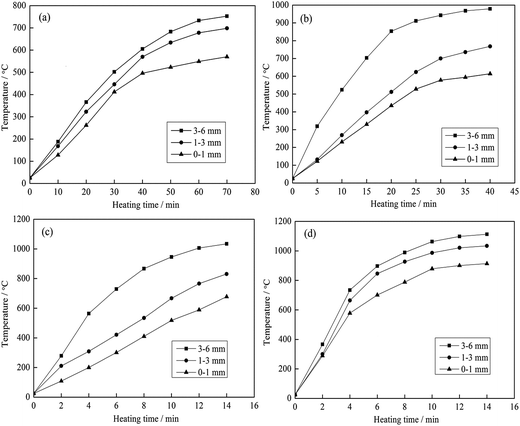 | ||
| Fig. 10 Heating characteristics of the different grain sizes at different microwave input powers ((a) 600 W; (b) 1200 W; (c) 1800 W and (d) 2400 W). | ||
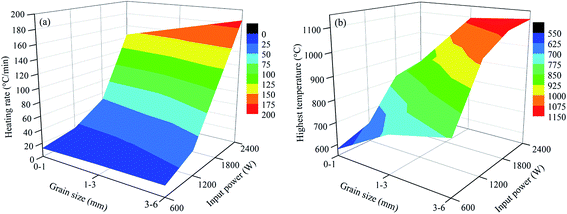 | ||
| Fig. 11 Relationship between the heating rate and highest temperature with grain sizes and input powers ((a) heating rate and (b) highest temperature). | ||
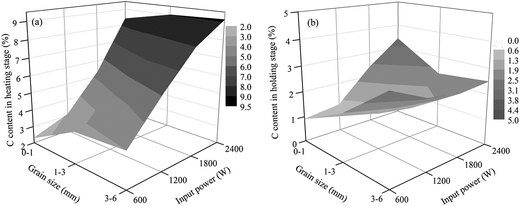 | ||
| Fig. 12 Carbon contents of the samples in different stages of the roasting process ((a) carbon content in the heating stage and (b) carbon content in the high temperature holding stage). | ||
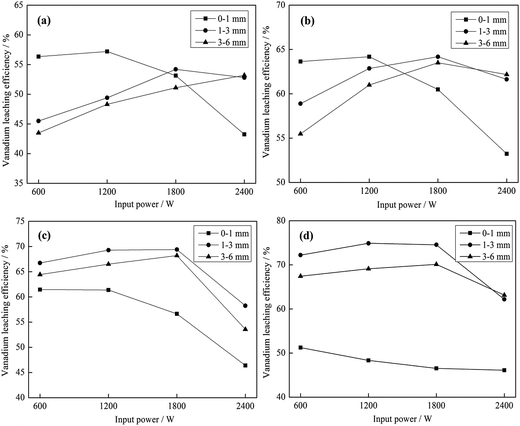
As shown in Fig. 10 and 11, the heating characteristics of the different grain sizes with different input powers are quite different. At the same input power, the heating rate and the highest roasting temperature increase with an increase in particle size. According to eqn (1), there are two reasons for this phenomenon. First, fine grained stone coal has higher heat dissipation than coarse particles due to its higher specific surface area, which decreases the heating rate of fine particles. Second, according to Fig. 12(a), the carbon contents of coarse particles are always higher than the fine particles in the heating stage because of the poor gas transfer conditions in the particles. This means that the coarse particles have higher equivalent permittivity, which causes a better microwave absorbing property. In this respect, in order to obtain a high heating rate and roasting temperature in the microwave roasting process, the size of stone coal should not be too fine.
Furthermore, for each grain size, the heating rate and highest temperature increase with an increase in input power. On one hand, a high microwave input power causes a high microwave power density. This means that more microwave energy is absorbed and transformed into heat by stone coal, consequently increasing the heating rate. On the other hand, the time to reach a certain temperature decreases with an increase in input power. This means that the reaction time of carbon with oxygen is shorter and less carbon is removed in the heating stage. There would be more carbon left to absorb microwaves and heat. In this sense, a high input power will be benefited by heating rate and highest temperature.
Generally, the carbon content of the samples in the heating stage has a significant effect on their heating characteristics, and they had similar changing tendencies when the grain size and microwave input power changed. A higher microwave input power and larger particle size ensure that more carbon is left in the samples in the heating stage and that the samples would obtain higher heating rates and roasting temperatures.
However, as mentioned above, high carbon content has synchronous disadvantages for microwave roasting. If the carbon residue in stone coal can be removed as much as possible in the high temperature holding stage, these disadvantages would be avoided. To investigate the final decarburization of each grain size at different input powers during the entire roasting stage, all the grain sizes were roasted at 800 °C for 30 min at different input powers, and the result is shown in Fig. 12(b).
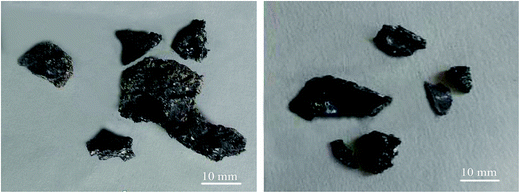
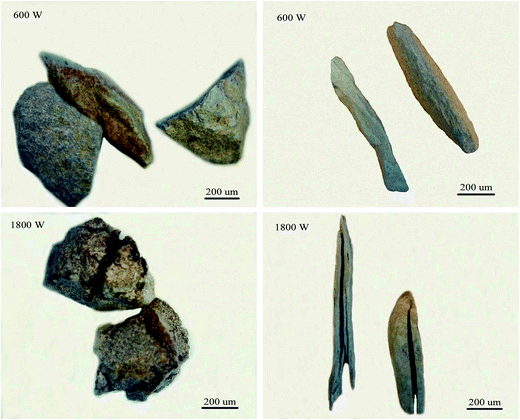
According to Fig. 12(b), for 0–1 mm stone coal, the final carbon content increased with an increase in the input power because of the severe sintering phenomenon. More carbon was wrapped up by material with a low melting point because of the sintering phenomenon. Regarding 1–3 mm and 3–6 mm, the final carbon content decreased when the input power changed from 600 W to 1800 W and then increased beyond 1800 W. As shown in Fig. 13, the roasted sample of coarse particles at 1800 W has several evident cracks and gaps; however, the roasted sample at low input power has no cracks and gaps. These cracks and gaps are mainly because of the selective heating characteristic of microwave heating.19 They provide better transfer conditions for the reaction of carbon with oxygen in the high temperature holding stage. However, too large particle size (3–6 mm in this experiment) would decrease the decarburization efficiency and there also exists the sintering phenomenon of coarse particles when the input power is too high, which leads to an increase in the final carbon content.
Generally speaking, enough cracks and gaps in the particles could ensure relatively complete decarburization in the high temperature holding stage. The synergistic action gained from a suitable particle size (1–3 mm) and microwave input power (1200–1800 W) provides the samples with a large number of cracks and gaps, which ensure the final decarburization efficiency.
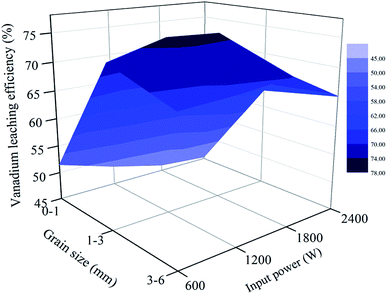 | ||
| Fig. 15 Relationship between vanadium leaching efficiency and grain size and microwave input power (850 °C for 30 min). | ||
As seen from Fig. 14, there is a downward trend of vanadium leaching efficiency for 0–1 mm when the roasting temperature is higher than 750 °C. At each temperature, the vanadium leaching efficiency of this part of stone coal decreased with an increase in input power. When the temperature was higher than 750 °C, the sintering phenomenon of these particles was prominent and a higher input power caused more severe sintering. However, this temperature level was not high enough to destroy the vanadium-bearing mineral completely and the vanadium leaching efficiencies were less than 60%. With respect to the coarse particles, the overall trend of vanadium leaching efficiency is upward with an increase in the roasting temperature, which increased before 1800 W and decreased above it. In the process of the experiment, there was almost no sintering phenomenon of coarse particles. This provided better conditions for stone coal to reach a higher temperature to destroy the structure of the vanadium-bearing mica. Furthermore, the cracks and gaps of coarse particles at high input power are also helpful for the transformation of the acid solution in particles.
From Fig. 12(b) and 15, it is not difficult to see that there is a clear correlation between vanadium leaching efficiency and final carbon content of the roasted sample. The vanadium leaching efficiencies of the 1–3 mm roasted samples with 1200–1800 W are higher than the samples roasted in other conditions. This indicates that the adjustment of grain size and microwave input power for heating characteristic could also improve the vanadium leaching efficiency. In the acid leaching condition of this experiment, the vanadium leaching efficiency of the roasted sample with 1200–1800 W input power at 850 °C for 30 min reached 76.3%, which is 14% higher than that of the roasted samples without size grading under the same roasting conditions.7
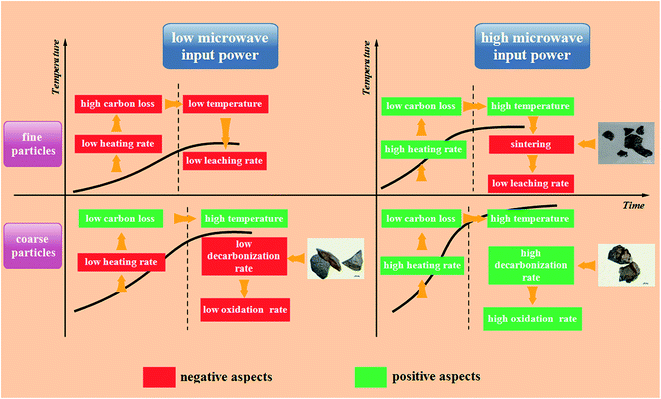
Based on the analyses mentioned above, the general rules during the microwave roasting of fine and coarse particles can be described as shown in Fig. 16.
As for the fine particles, low microwave input power causes them to heat up slowly in the heating stage and this extends the heating time, which results in high carbon loss in this stage. The microwave absorption capacity of fine particles decreases with an increase in carbon loss. Hence, the final temperature of these particles cannot reach the appropriate level to destroy the structures of vanadium-bearing minerals, which results in a low vanadium leaching efficiency. A high microwave input power causes the fine particles to have a high heating rate and a low carbon loss in the heating stage, which results in a high final temperature. However, the sintering phenomenon is incidental for the fine particles at a high temperature, which decreases the vanadium leaching rate significantly.
For the coarse particles, although a low microwave input power also causes them to heat up slowly and extend the heating time, the loss of carbon is not high in the heating stage due to the large particle size. Hence, they have a good microwave absorption capacity to obtain a high final temperature. However, the large particle size also hinders the vanadium oxidation rate, which has a negative effect on the vanadium leaching rate. A high microwave input power not only provides coarse particles with a high heating rate and a high final temperature, but also a high oxidation rate because of the abundant cracks and pores, which increase the vanadium leaching rate.
4 Conclusion
(1) Carbon is an important microwave absorbing material in this type of stone coal and it provides stone coal with good heating characteristics. However, the negative effects of carbon include the inhibition of the oxidization of vanadium and causing the sintering phenomenon.(2) For fine stone coal, because of its high decarbonization rate in the heating stage and high heat dissipation due to its high specific surface area, the heating rate and highest temperature for this part of stone coal are always low. Moreover, the vanadium leaching efficiency of these particles is not high because of their obvious sintering phenomenon.
(3) In contrast to fine stone coal, coarse particles have a higher heating rate and higher temperature during the microwave roasting process because of their low decarbonization rate and heat dissipation. A high input power in coarse particles roasting causes more carbon to be retained in stone coal in the heating stage and it is removed in the high temperature holding stage due to the cracks and gaps of the coarse particles. In this way, the advantages of carbon are used and its disadvantages are avoided. There is almost no sintering phenomenon in coarse particles. This provides better conditions for stone coal to reach a higher temperature to destroy the structure of vanadium-bearing mica.
(4) For this type of stone coal, a 1–3 mm size and 1200–1800 W input power are suitable, the vanadium leaching efficiency reaches 76.3% at 850 °C for 30 min, and it is 14% higher than the roasted samples without size grading.
Acknowledgements
This study was supported by the Project of National Natural Science Foundation of China (No. 51474162 & No. 51404174) and the National Key Technology Support Program of China (2015BAB18B01).References
- Y. M. Zhang, S. X. Bao, T. Liu, T. J. Chen and J. Huang, Hydrometallurgy, 2011, 109(1–2), 116 CrossRef CAS.
- H. L. Wu, C. Wei, G. Fan, M. Y. Li, Z. G. Deng and H. W. Ge, J. Kunming Univ. Sci. Technol., Nat. Sci. Ed., 2008, 33(6), 17 CAS.
- M. Y. Wang and X. W. Wang, Chin. J. Rare Met., 2010, 34(1), 90 CAS.
- X. B. Zhu, Y. M. Zhang and T. Liu, Chin. J. Rare Met., 2013, 37(2), 283 CAS.
- Y. H. Liu, C. Yang, P. Y. Li and S. Q. Li, Int. J. Miner., Metall. Mater., 2010, 17(4), 381 CrossRef CAS.
- D. S. He, Q. M. Feng, G. F. Zhang, L. M. Ou and Y. P. Lu, Min. Eng., 2007, 20, 1184 CrossRef CAS.
- Y. Z. Yuan, Y. M. Zhang, T. J. Chen, T. Liu, F. Wang and J. Liu, Chin. J. Rare Met., 2014, 38(3), 534 CAS.
- T. J. Chen, G. Z. Qiu and D. Q. Zhu, Min. Miner. Eng., 2008, 28(3), 64 CAS.
- W. Q. Cai, H. Q. Li and Y. Zhang, Chin. J. Process Eng., 2005, 5(2), 228 CAS.
- D. A. Jones, T. P. Lelyveld, S. D. Mavrofidis, S. W. Kingman and N. J. Miles, Resour., Conserv. Recycl., 2002, 34(2), 75 CrossRef.
- C. A. Pickles, Min. Eng., 2004, 17, 775 CrossRef CAS.
- J. H. Peng, and B. G. Liu, Microwave Calcination Technology and Application, Science Press, Beijing, 2013, p. 71 Search PubMed.
- J. Yu, Z. Z. Zhu and J. Yang, Hydrometall. China, 2011, 30(2), 111 CAS.
- H. Wang, X. Z. Lan, H. Z. Ma and Y. H. Song, Hydrometall. China, 2011, 30(4), 290 CAS.
- X. Y. Zhang, W. Q. Qin, X. D. Tian, Y. B. Chen, Y. Gu and X. G. Xi, Chin. J. of Nonferrous Met., 2011, 21(4), 908 CAS.
- China Metallurgical Information and Standardization Institute, GB/T 8704.5-2007 Ferrovanadium-Determination of Vanadium Content-the Ammonium Ferrous Sulfate Titrimetric Method and the Potentiometric Titrimetric Method, 2007 Search PubMed.
- F. Wang, Y. M. Zhang, J. Huang, T. Liu, X. Yang, Y. Wang and J. Zhao, Rare Met., 2013, 32(1), 57 CrossRef CAS.
- M. Lu, Y. M. Zhang, T. Liu and D. Yang, Chin. J. Rare Met., 2009, 33(6), 894 CAS.
- G. Q. Ou Yang, X. Y. Zhang, X. D. Tian, Y. Li and S. Xie, Chin. J. of Nonferrous Met., 2008, 18(4), 750 CAS.
| This journal is © The Royal Society of Chemistry 2017 |