Open Access Article
Open Access ArticleSilicone oil impregnated nano silica modified glass surface and influence of environmental dust particles on optical transmittance†
Bekir Sami Yilbas *ab,
Muhammad Rizwan Yousafb,
Abdullah Al-Sharafib,
Haider Alib,
Fahad Al-Sulaimanab,
Numan Abu-Dheirb,
Mazen Khaledc and
Nasser Al-Aqeelib
*ab,
Muhammad Rizwan Yousafb,
Abdullah Al-Sharafib,
Haider Alib,
Fahad Al-Sulaimanab,
Numan Abu-Dheirb,
Mazen Khaledc and
Nasser Al-Aqeelib
aCenter of Excellence in Renewable Energy, King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia. E-mail: bsyilbas@kfupm.edu.sa
bMechanical Engineering Department, King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia
cChemistry Department, King Fahd University of Petroleum & Minerals, Dhahran, Saudi Arabia
First published on 7th June 2017
Abstract
The behavior of environmental dust particles on a silicone oil impregnated glass surface is examined in relation to optical transparent surfaces for self-cleaning applications. The characteristics of environmental dust, collected in the local area, are analyzed using analytical tools. Functional silica particles are synthesized and deposited on the glass surface prior to silicone oil impregnation. Optical properties of functionalized silica particle deposited glass surfaces are examined prior to and after oil impregnation. Further tests are conducted in the open environment to assess dust settlement in silicone oil and dust particle sedimentation on the glass surface. It is found that dust particles have various sizes and shapes, and they are composed of various metallic, alkaline, and alkaline earth metallic compounds. The average size of the dust particles is of the order of 1.2 μm. Silicone oil impregnation considerably improves the optical transmittance of functionalized silica particle deposited glass. A high spreading rate gives rise to a cloaking of dust particles on the oil surface, which gradually reduces the surface tension force and modifies the vertical force balance. Consequently, dust particles immerse into the oil film and sediment on the glass surface. This, in turn, lowers the optical transmittance of the oil impregnated glass surfaces greatly.
Introduction
Self-cleaning of optically transparent wafer surfaces is critically important for solar energy harvesting devices. Recent changes in climate have given rise to an increased rate of dust storms around the globe, particularly in the Middle East. Climate change has a significant impact on the performance of energy harvesting devices, particularly for solar photovoltaic and solar concentrated power applications.1 Dust settlement on optical transparent surfaces lowers the transmittance of incident optical radiation reaching active elements of solar energy devices such as photovoltaic cells. This, in turn, lowers the device performance, in terms of efficiency and output power, and shortens the device operational life.2 Dust removal and cleaning of transparent surfaces involves a high cost as the surface area increases, such as those of solar photovoltaic farms, and increases the solar energy harvesting costs. The environmental dust has various particle shapes and sizes and, in general, they comprise of metallic oxides, alkaline (Na, K) and earth alkaline (Ca) compounds in addition to silicon and other related elements.3 The water condensates onto the dust particles in the humid ambient air and gives rise to the dissolution of alkaline and earth alkaline compounds of the dust. This forms a chemically active liquid mud solution with high pH on the surface, where the dust settles, because of liquid flow under the gravity. As ambient temperature increases, mud solution dries and forms an interfacial layer between the surface and dust particles, which in turn increases adhesion between dust particles and the surface. Furthermore, this raises the efforts required for dust removal from the surfaces and increases the cleaning costs. Consequently, investigation of dust settlement on optically transparent surfaces becomes essential.Hydrophobic surfaces demonstrate better performance in terms of effort requirements for dust removal from the surfaces. This is because of the weak adhesion of dust particles onto the surfaces.4 In general, hydrophobic characteristics of surfaces are closely related to the surface texture, comprising of micro/nano pillars, and low surface free energy of the substrate material. However, several methods are introduced and strategies are adopted to improve surface hydrophobicity of the substrate materials.5–11 These methods, in general, are related to multi-step processes in harsh conditions, require specialized reagents, and involved with a high cost. Some of these methods are phase separation,5 electrochemical deposition,6 plasma treatment,7 sol–gel processing,8 electrospinning,9 laser texturing,10 and solution immersion.11 The main focus of these studies is to reduce free energy of the surfaces by changing compositions through chemical and/or physical reactions. The cost-effective one-step process is preferable to create hydrophobic characteristics at the surface. Since surface texture and surface free energy are modified during processing, surface characteristics, such as optical reflection, scratch resistance, topology, etc. undergo changes. The textural distortion at hydrophobic surface alters the optical transmittance of the hydrophobized surface.10 Consequently, this limits the practical application of the hydrophobic surface when the transmittance of UV-visible spectrum becomes critically important.
On the other hand, considerable research studies were carried out to examine the influence of environmental dust particles on the performance of solar energy harvesting systems. The main focus was to assess the dust effect on the device efficiency and output power.12–14 The findings revealed that the photovoltaic device efficiency and output power reduced greatly because of environmental dust settlement on the optically active surface. However, the studies on prevention of dust accumulation on surfaces, in particular on solar energy devices are still in progress, despite the importance of self-cleaning on device performance as reported in the previous studies.15 Generating lotus effect on surfaces via forming hierarchical structures received considerable attention for self-cleaning applications.16–20 The rolling of water droplets on such surfaces could generate self-cleaning effect through picking dust particles from the surfaces by the droplets.21 This consideration might solve the dust accumulation problem and could ease the requirements of a large quantity of distilled water for cleaning. In some locations of the globe, water scarcity is one of the main problems yet to be met. The use of a lesser quantity of water, such as generating small size of water droplets for cleaning purposes, reduces the cleaning cost and lowers the load on the available clean water resources. Many challenges are to be met for self-cleaning applications, such as uniform cleaning of large of surfaces, cost-effective processing, the durability of treated surfaces in harsh environments, etc. Specifically, minimization of local adhesion of water droplet on surfaces and improvement of optical characteristics of treated surfaces are some of the main focuses of research.22,23 Large water droplet contact angle hysteresis could cause attachment of the water droplet and causes ceasing of droplet rolling on the treated surface. Moreover, the optical characteristics of surfaces are critical for photovoltaic applications and surface texturing towards generating lotus effect causes scattering and reflection of incident UV-visible radiation while reducing optical transmittance of the substrate material.24 The antireflection coating of the textured surface offers improvement in optical transmittance;25 however, the coating process is costly and covers over the nano-size whiskers like structures on the textured surface while lowering the lotus effect and increasing droplet adhesion at the surface. One of the solutions to overcome the transmittance problem is to introduce the correction layer of fluid with a similar refractive index of treated substrate in terms of liquid impregnation.23 Although several challenges are still waiting to be met, such as achieving high water droplet contact angle on impregnated liquid surface and hydrodynamic stability of impregnated liquid film in harsh environments, some successful results are reported for solid particle mobility on the impregnated surfaces.26 In some instances, spreading rate of oil on foreign solid particles can cause cloaking and influences particle mobility on the impregnated surface.27 In addition, a liquid ridge formed around these particles can affect the motion of particles on impregnated liquid surface. Hence, the effort required to remove these particles from impregnated liquid surface increases, which has an adverse effect on the self-cleaning process and the cleaning cost. The liquid cloaking of solid particles lowers the surface tension force because of reducing the soil–air interface; in which case, the force balance among surface tension, gravity and buoyancy forces works against the suspension of these particles on the oil surface. This can cause accelerating immersion of these particles into impregnated fluid interior. These particles further lower the optical transmittance and are difficult to clean from the impregnated fluid beds. In addition, some of these particles may dissolve in impregnated fluid. This further modifies optical characteristics of impregnated fluid and can cause an adverse effect on its optical transmittance. Consequently, investigation of the behavior of dust particles on impregnated fluid of transparent substrate becomes necessary.
Although the effect of dust particles and mud formed, from dust particles, on hydrophobic surfaces were studied previously,2,28 the main focus was to examine chemo-mechanics of dust particles2 and dry mud solution adhesion on optically transparent surfaces.28 Since oil impregnated surfaces offer improved optical transmittance to textured surfaces with high droplet mobility,23,26 they have a potential to be utilized as protective covers, with self-cleaning ability, for the photovoltaic surfaces. Consequently, the influence of environmental dust particles on oil impregnated surfaces need to be examined prior to practical applications of oil impregnated transparent surfaces in solar energy harvesting devices. In the present study, characteristics of the optically transparent hydrophobic surface are studied and properties of silicone oil impregnated hydrophobic surfaces are investigated. Chemical and physical properties of environmental dust particles are assessed using the analytical tools. Effect of environmental dust settlement on the properties of the oil impregnated transparent substrate surface is examined.
Experimental
Glass samples with dimensions of 30 mm × 40 mm × 2 mm (width × length × thickness) were used as workpieces. The chemical composition of the glass was 76.5% SiO2, 9.9% CaO, 1.2 MgO and 12.4% Na2O. The silica nanoparticles were synthesized and deposited on the glass surface. The procedure adopted for synthesizing of silica nanoparticles was similar to that reported in the previous study.29 The process is briefly described herein. Tetraethyl orthosilicate (TEOS) and isobutyltrimethoxysilane (OTES), ethanol, and ammonium hydroxide were used in the synthesizing process. In this case, 14.4 mL of ethanol, 1 mL of ultrapure water, and 25 mL of ammonium hydroxide were mixed and stirred for 12 minutes. Later, 1 mL of TEOS was diluted with 4 mL of ethanol and added to the mixture. Following 30 minutes after this process, 0.5 mL of TEOS diluted in 4 mL ethanol was added. After 5 minutes, a modified silane molecule was added in a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with respect to the second edition of TEOS. The final mixture was stirred for 20 hours at room temperature, and later centrifuged and washed with ethanol for removal of reactants. The solvent casting was applied to deposit the solution onto glass surfaces. Before coating glass surfaces were cleaned by piranha solution and later distilled water was used further cleaning the surfaces. Upon vacuum drying, until all solvent was evaporated, characterization of resulting surfaces was carried out. AFM/SPM Microscope in contact mode was used to analyze the surface texture. The tip was made of silicon nitride probes (r = 20–60 nm) with a manufacturer specified force constant, k, of 0.12 N m−1. A linear micro-scratch tester (MCTX-S/N: 01-04300, Manufacture: CMS Solutions, Switzerland) was used to measure the tangential force on the treated surface. The equipment was set at the contact load of 0.003 N and end load of 0.75 N. The scanning speed was 1 mm min−1 and loading rate was 0.75 N min−1. The total length for the scratch tests was 1 mm.
4 with respect to the second edition of TEOS. The final mixture was stirred for 20 hours at room temperature, and later centrifuged and washed with ethanol for removal of reactants. The solvent casting was applied to deposit the solution onto glass surfaces. Before coating glass surfaces were cleaned by piranha solution and later distilled water was used further cleaning the surfaces. Upon vacuum drying, until all solvent was evaporated, characterization of resulting surfaces was carried out. AFM/SPM Microscope in contact mode was used to analyze the surface texture. The tip was made of silicon nitride probes (r = 20–60 nm) with a manufacturer specified force constant, k, of 0.12 N m−1. A linear micro-scratch tester (MCTX-S/N: 01-04300, Manufacture: CMS Solutions, Switzerland) was used to measure the tangential force on the treated surface. The equipment was set at the contact load of 0.003 N and end load of 0.75 N. The scanning speed was 1 mm min−1 and loading rate was 0.75 N min−1. The total length for the scratch tests was 1 mm.
Silicone oil was used for the impregnation of the functionalized silica particles deposited glass surface. Silicone oil has dynamic viscosity 10 cst, density 935 kg m−3, and lower interfacial tension 35 mN m−1. The uniform oil film encapsulating the textured surface was assured during the oil film formation. Deposition of functionalized silica nanoparticles provided strong adhesion between the textured surface and impregnating silicone oil. Silicone oil film thickness at the surfaces was measured using an ellipsometer (Model: M-2000 Manufacture: J. A. Woolam Co., USA). The liquid film thickness measurement relies on the change in polarization state as described by two quantities: (i) amplitude ratio or Psi (Ψ), and (ii) phase difference or delta (Δ), i.e. tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΨeiΔ = Rp/Rs Rp and Rs are the Fresnel reflection coefficients for the p- and s-polarized light, respectively. A spectroscopic ellipsometer provided the measured data for psi (Ψ) and delta (Δ) value for each wavelength of incident light and generated the spectrum accordingly. The measured surface roughness and thickness of oil film was 0.42 nm and 700 ± 2 nm. Later, air jet of 6 m s−1 nozzle jet velocity and 2 mm diameter was used to remove excessive oil on the surface of functionalized silica particles deposited surface. The oil film thickness was measured. The findings revealed that the oil film thickness became 56.2 ± 2 nm while surface roughness remained same.
ΨeiΔ = Rp/Rs Rp and Rs are the Fresnel reflection coefficients for the p- and s-polarized light, respectively. A spectroscopic ellipsometer provided the measured data for psi (Ψ) and delta (Δ) value for each wavelength of incident light and generated the spectrum accordingly. The measured surface roughness and thickness of oil film was 0.42 nm and 700 ± 2 nm. Later, air jet of 6 m s−1 nozzle jet velocity and 2 mm diameter was used to remove excessive oil on the surface of functionalized silica particles deposited surface. The oil film thickness was measured. The findings revealed that the oil film thickness became 56.2 ± 2 nm while surface roughness remained same.
Water droplet contact angle for functionalized silica particles deposited and oil impregnated surfaces were measured. During the measurements, droplet volume was controlled with an automatic dispensing system having a volume step resolution of 0.1 μL. Still images were captured, and contact angle measurements were performed after one second of deposition of a water droplet on the surface. The optical transmittance of functionalized silica particles deposited and silicone oil impregnated surfaces was measured using a UV spectrometer (Model: 67 Series spectrophotometer, Manufacture: Jenway, UK), and Fourier transform infrared spectroscopy (Model: VERTEX70, Manufacture: Bruker, USA) was performed to collect the infrared absorption spectrum of the glass.
Dust particles were collected over a period of 12 months from the Solar Energy Laboratory of a Research Institute at King Fahd University of Petroleum and Minerals, which is located close to the city of Dammam in Saudi Arabia. Dust, accumulated on the surface of the protective glass of photovoltaic panels, was removed by a soft brush and stored in an airtight container. Initially, dust particles collected were analyzed in terms of weight, size, shape, and elemental composition using the analytical tools. The findings revealed that dust particles collected over one-week period within 12 months had similar characteristics in terms of elemental composition, size distribution, and shape. The amount of dust particles accumulated on the surface of the photovoltaic protective layer was 5 g m−2 within one week; however, it varied within 15% (by weight) over 12 months. This was attributed to wind speed and its direction. Although wind speed and direction changed over the time, the average wind speed remained about 4 m s−1 over the year. Dust particles were characterized using SEM and EDS (Model: 6460, Manufacture: JEOL Japan) with tungsten filament and specifications of beam voltage 0.4–40 kV, resolution 10 nm, and magnification ×10 to ×300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. EDS consisted of INCA Mics microscope image capture system including INCA X-stream X-ray acquisition and detector control unit. XRD (Model: D8 Advanced diffractometer, Manufacture: Bruker, USA). CuKα radiation was used for XRD analysis and a typical setting of XRD was 40 kV and 30 mA. Porous structures of dust particles were determined using Micro-CT scanner (Model: Skyscan 1172, Manufacture: Bruker, USA). The dust particles were mixed with desalinated water and the liquid solution that was extracted from dust was analyzed using a quadrupole inductively coupled plasma mass spectrometer (Model: XSeries 2, Manufacture: Thermo Scientific, USA). Silicone oil cloaking of dust particles was monitored and cloaking velocity was measured by using a high-speed camera (Model: SpeedSense 9040, Manufacture: Dantec Dynamic, Denmark).
000. EDS consisted of INCA Mics microscope image capture system including INCA X-stream X-ray acquisition and detector control unit. XRD (Model: D8 Advanced diffractometer, Manufacture: Bruker, USA). CuKα radiation was used for XRD analysis and a typical setting of XRD was 40 kV and 30 mA. Porous structures of dust particles were determined using Micro-CT scanner (Model: Skyscan 1172, Manufacture: Bruker, USA). The dust particles were mixed with desalinated water and the liquid solution that was extracted from dust was analyzed using a quadrupole inductively coupled plasma mass spectrometer (Model: XSeries 2, Manufacture: Thermo Scientific, USA). Silicone oil cloaking of dust particles was monitored and cloaking velocity was measured by using a high-speed camera (Model: SpeedSense 9040, Manufacture: Dantec Dynamic, Denmark).
Results and discussion
Environmental dust effect on the properties of silicone oil impregnated glass surfaces is examined. Functionalized silica particles are deposited on the glass surface to improve the spreading rate of silicone oil on the glass surface. Analytical tools are incorporated to examine the properties of the environmental dust particles and the characteristics of the oil impregnated surfaces when exposed to the outdoor environment for one week.Assessment of environmental dust particles
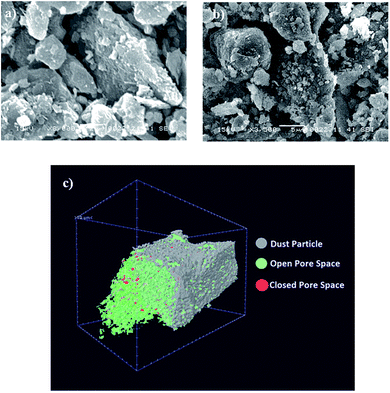
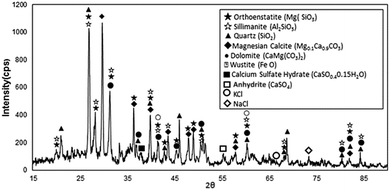
Scanning electron microscope (SEM) micrographs of environmental dust particles are shown in Fig. 1. Dust particles have various geometric features with varying sizes (Fig. 1a). Dust particles geometric configurations can be categorized by the shape factor and the aspect ratio. The shape factor can be defined by the ratio of the square of wetted perimeter over the cross-sectional area of dust particles (RShape=P2/4πA, where P is the perimeter of dust particle and A is dust particle cross-sectional area). The aspect ratio is the ratio of area corresponding to longest projection length over the cross-sectional area of dust particles (AAspect = π(Lproj)2/4A, where A is the cross-sectional area, and Lproj is the longest projection length of dust particles). The particle roundness is associated with the aspect ratio and it corresponds to the ratio of the major-to-minor axes of an ellipsoid, which can be best fit to dust particle. The dust particle circularity is inversely proportional to the shape factor and it is related to the complexity of dust particle; in which case, a perfect circle corresponds to the shape factor of unity. The particle shape is assessed and the size of dust particles with different geometries is measurement. In the case of circular dust particles, the diameter of a circle with an equivalent area of the dust particles is considered; however, the elliptic model is considered and the longest projection of dust particle is assumed to be the major axis. The relationship between the particle size and the aspect ratio or the shape factor is not linear, and it does not exactly follow the polynomial function. Between the particle size and the aspect ratio, an inverse relationship is noted; the shape factor increases with the particle size. In this case, the particle aspect ratio reduces as the shape factor increases. The cross-sectional area of a typical dust particle of size 1.75 μm is in the order of 2.4 μm2, which results in the shape factor of 1.15. However, the cross-sectional area of a typical dust particle of size 14 μm is in the order of 165 μm2 and the corresponding shape factor is about 3.11. In general, for small dust particles (≤2 μm), the shape factor approaches unity; however, for large dust particles (≥12 μm), the median shape factor approaches 3.1. The average size of dust particles is in the order of 1.2 μm and small dust particles (<0.5 μm) attach on the surface of large size dust particles (Fig. 1b). This behavior is related to electrostatic charge on small size dust particles, and they appear as bright color in the SEM micrographs due to electron charging. The presence of electrostatic charges is attributed to the long duration of residence of these particles in the atmosphere. In this case, they interact with solar radiation for longer durations than the large particles; consequently, prolonged exposure to the atmosphere in regions close to the sea, such as Arabian Gulf, causes the attachment of ionic compounds to small dust particles. The density of dust particles was measured, and it varied in between 1600 kg m−3 to 3200 kg m−3. Density variation is associated with porous structures of some dust particles, which is more pronounced for large size dust particles (≥8 μm). Fig. 1c shows image of CT scanned dust particles. Open porous structures occupy almost 20.09% of the total volume of dust particles, and the total volume of occupancy of porous structures is in the order of 21.28%. This indicates that porous structure is mainly open type in large size dust particles. Table 1 gives EDS data (wt%) for dust particles. The presence of calcium, silicon, iron, magnesium, potassium, sodium, oxygen, chlorine, and sulfur is evident. However, the elemental composition of dust particles varies slightly with the dust geometries. Oxygen and calcium contents are slightly rich for small particles while rectangular and elliptic shapes particles are rich in calcium and silicon. Chlorine concentration changes with dust particles; however, chlorine concentration does not satisfy the stoichiometric molar ratio of NaCl. Therefore, chlorine is present in the compound form rather than the crystal form. Sulfur may be attributed to monomer layer formation during the aging process in the atmosphere and/or it can be related with the calcium, such as the anhydrite or gypsum component (CaSO4) in dust particles. Iron is most likely associated to clay-aggregated hematite (Fe2O3).| Si | Ca | Na | S | Mg | K | Fe | Cl | O | |
|---|---|---|---|---|---|---|---|---|---|
| Size > 1.2 μm | 11.4 | 7.8 | 3.4 | 2.1 | 2.9 | 1.3 | 1.3 | 1.1 | Balance |
| Size < 1.2 μm | 11.6 | 7.2 | 4.8 | 1.8 | 3.6 | 2.4 | 0.8 | 2.3 | Balance |
Fig. 2 shows the X-ray diffractogram of dust particles. Calcium, iron, potassium, sodium, sulfur, and chlorine peaks are evident in the diffractogram. The peaks of silicon and iron coincide with each other. Sodium and potassium peaks are associated with the sea salt because of the regional effects, i.e. dust particles are collected from the close region of Arabian Gulf. The metal compounds in dust particles are associated with metal oxides such as Fe2O3, FeO, and MgO. To further assess the characteristics of dust particles, desalinated water are mixed with dust particles with a mass ratio of one to ten (ten being desalinated water) and, later, the mixture is placed on a mechanical shaker for one hour. pH of resulting mud solution (a mixture of water and dust particles) is measured, and the findings revealed that pH increases to 7.5 within ten hours. In this case, OH− ions in mud liquid solution give rise to the attainment of high pH level because of the dissolution of the alkaline and alkaline earth metallic compounds in water.
Characteristics of functionalized silica deposited and oil impregnated glass surface
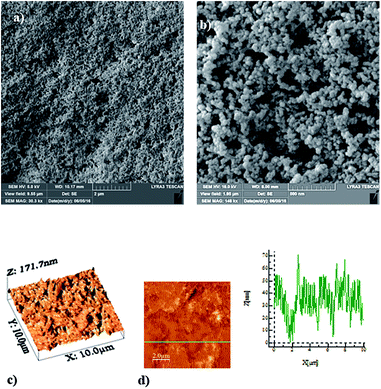
SEM micrographs of functionalized silica particles deposited onto glass surface are shown in Fig. 3. Deposited silica particles cover glass surface extensively (Fig. 3a) with some porous like textures, and they are closely spaced on the surface. Since tetraethyl orthosilicate (TEOS) is used during the synthesizing of silica particles, the functionalized shell alters the surface roughness of the particles slightly and the cell size increases slightly.30 This behavior is associated with the condensing monomer units, which are growing at a faster rate than the nucleation rate.29 Since diluted TEOS concentration with ethanol is incorporated when synthesizing the silica particles, the rate of formation of new nuclei is reduced. This gives rise to aggregation and adhesion of the particles (Fig. 3b). On the other hand, the hydroxyl groups on the functionalized silica particle surfaces have different moieties and can have different reactivity towards the modifier molecules. Therefore, the modified silane causes side reactions and condensation on the silica surface.31 Fig. 3c and d show AFM images of glass surface after deposition of functionalized silica particles. The surface texture comprises of fine size spheroid like structures, which can be observed from 3-dimensional image of the surface (Fig. 3c). The presence of small size silica particles is also observed from the line scan (Fig. 3d). Since no loose silica particles are observed from SEM micrographs (Fig. 3a and b) and AFM images (Fig. 3c and d), functionalizing silica particles adhere strongly on the glass surface.Scratch tests are carried out to assess the adhesion of functionalized silica particles on glass surfaces. Fig. 4 shows tangential force along the scratch length. Tangential force remains high indicating the strong adhesion of the functionalized silica particles on the surface. Tangential force attains high values in some regions along the scratch length. This behavior can be associated with the local aggregation and adhesion of the particles. Silicone oil impregnation is introduced on the functionalized silica particles deposited surface, and the porous like structures at the surface enable to house the impregnated oil. In this case, oil encapsulation occurs, and the oil film covers the surface with the presence of some excess oil above the textured surface. Silicon oil (Manufacture: Sigma-Aldrich, USA) with 10 cSt viscosity is used to impregnate the coating surface. However, the state of the oil impregnation depends on the energy equations; in which case, the states depend on the stable configurations in which the total interface energy remains at the lowest level.
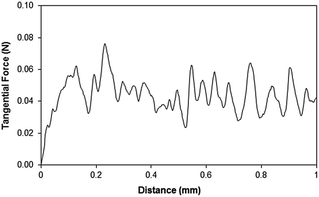 | ||
| Fig. 4 Tangential force along the scratch length of functionalized silica particles deposited surface. | ||
The state of oil impregnation is related to the interfacial energy with the texture parameters27 such that (γsa − γos)/γoa = f((1 − φ)/(r − φ)), here, φ is the fraction of the projected area of the surface that was occupied by the solid and r is the ratio of the total surface area to the projected area of the solid. Incorporating the Young's equation, cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θw = r(γsv − γsl)/γlv (where: γsv, γsl and γlv are the interfacial energies of solid–vapor, solid–liquid, and liquid–vapor phases, respectively), into the equation that relates the interfacial energy and surface texture parameters, the hemi-wicking criterion for the oil impregnation can be obtained.32,33 This yields cos
θw = r(γsv − γsl)/γlv (where: γsv, γsl and γlv are the interfacial energies of solid–vapor, solid–liquid, and liquid–vapor phases, respectively), into the equation that relates the interfacial energy and surface texture parameters, the hemi-wicking criterion for the oil impregnation can be obtained.32,33 This yields cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θos(a) = (γsa − γos)/γsa − γos, here γsa is the interfacial energy between solid and air, γos is the interfacial energy between oil and solid, and γoa is the interfacial energy between oil and air phases. The geometric features of the functionalized silica particles and line scan at the resulting surface provide the texture parameters; which yield φ = 0.01 and r = 0.68. The oil impregnated textured surface satisfies the condition of θos(a) < cos−1(1/r) and Sos(a) ≡ −γos(r − 1/r) or Sos(a) ≥ 0. The spreading rate of silicone on the textured surface is determined as Sos(a) = 7.80 mN m−1, which is greater than zero. The surface energy of silicone oil on the solid surface is measured as 16.9 mN m−1, which is consistent with that reported in the literature.27 Consequently, the silicone oil encapsulation on the textured surface is satisfied because of Sos(a) ≥ 0.
θos(a) = (γsa − γos)/γsa − γos, here γsa is the interfacial energy between solid and air, γos is the interfacial energy between oil and solid, and γoa is the interfacial energy between oil and air phases. The geometric features of the functionalized silica particles and line scan at the resulting surface provide the texture parameters; which yield φ = 0.01 and r = 0.68. The oil impregnated textured surface satisfies the condition of θos(a) < cos−1(1/r) and Sos(a) ≡ −γos(r − 1/r) or Sos(a) ≥ 0. The spreading rate of silicone on the textured surface is determined as Sos(a) = 7.80 mN m−1, which is greater than zero. The surface energy of silicone oil on the solid surface is measured as 16.9 mN m−1, which is consistent with that reported in the literature.27 Consequently, the silicone oil encapsulation on the textured surface is satisfied because of Sos(a) ≥ 0.
The water droplet contact angle is shown in Fig. 5 for functionalized silica particles deposited and silicone oil impregnated surfaces. The water droplet contact angle for thin silicone oil film due to air jet thinning is also shown for comparison. Water contact angle is in the order of 155° for the functionalized silica particles deposited glass surface, which demonstrates superhydrophobic characteristics. Some small variation of the water droplet contact angle is observed for the functionalized silica particles deposited glass surface. This is attributed the surface texture variation; in which case, porous like structures and local agglomeration of silica particles modify the texture characteristics of surface. It is in the order of 103° for silicone oil impregnated surfaces with and without thinning of oil thickness. Impregnated silicone oil film is thinned with an air jet of 2 mm diameter and 6 m s−1 jet velocity. In this case, silicone oil thickness reduces from 700 nm to 56.2 nm having a uniform thickness.
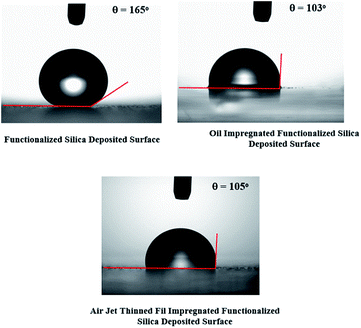 | ||
| Fig. 5 Water contact angle images of functionalized silica deposited surface prior and after silicone oil impregnation. | ||
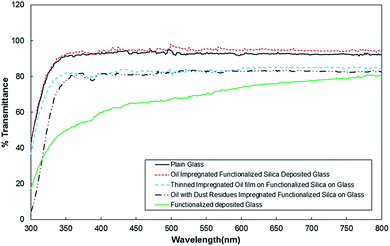
Optical transmittance of functionalized silica particles deposited glass remains low as compared to as-received glass. This situation can be seen from Fig. 6, in which UV-visible transmittance of the substrate materials is shown. The low optical transmittance of functionalized silica deposited glass surface is associated with scattering of incident optical radiation at the treated surface. This is related to the locally agglomerated functionalized silica particles and porous like structures formed at the surface. However, incorporating corrected fluid (silicone oil) having a similar refractive index of glass improves optical transmittance greatly. This is true for thick, and air jet thinned silicone oil films. Although water droplet contact angle reduces after oil impregnation at the surface, optical transmittance improves considerably. It is reported that the particle mobility remained high on the silicone oil impregnated surface; consequently, reduced water droplet contact angle does not have a notable adverse effect on the self-cleaning ability of silicone oil impregnated surface. In order to assess the optical transmittance of the silicone oil impregnated surface in open environment, oil impregnated surfaces are left in an outdoor environment for one week; in which case, the environmental dust fall onto the surface of silicone oil impregnated samples. The percentage of change of the average optical transmittance due to functionalized silica particles deposited glass surface and silicone oil impregnated and treated surface after the outdoor tests are shown in Fig. S1.† The average optical transmittance is obtained after integrating transmittance over the wavelengths and dividing it by the average wavelength. The percentage of transmittance is determined from the ratio of optical transmittance difference prior and after the outdoor tests over optical transmittance prior to start of the tests (=(TAT − To)/To, where To is the average transmittance prior to the outdoor tests and TAT is the average transmittance after the outdoor tests). The optical transmittance of the glass reduces considerably after the tests. This is associated with the sedimentation of dust particles on the impregnated glass surface. In order to assess the behavior of the immersing and sedimentation of dust particles into the silicone oil and on the glass surface, the tests for oil cloaking of dust particles are carried out.
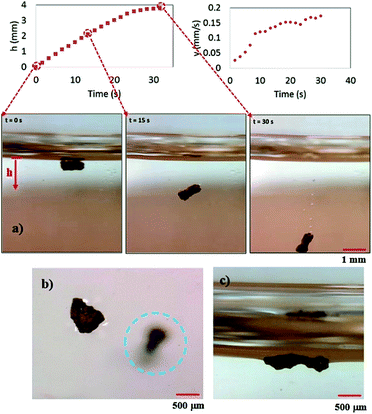
Fig. 7 shows images of two different dust particles, which are subjected to the oil cloaking, together with oil cloaking velocity and cloaking film height. Slightly large size dust particles are selected to monitor oil cloaking with high accuracy. The error estimated for cloaking height and velocity measurement is in the order of 4%, which is based on the repeatability of experiments. Cloaking velocity and height are obtained from the high-speed camera records. Cloaking film height and cloaking speed increase rapidly and the rate of increase reduces as the time progresses. Since oil film cloaking occurs opposing to the gravity, weight of the liquid film, which is cloaking around dust particle increases while lowering the net driving force opposing to gravity for cloaking. For further investigation of oil cloaking, some dust particles are functionalized to reduce surface energy to overcome the cloaking. In this case, dust particles are functionalized via covering the surface of dust particles by trichloro(1H,1H,2H,2H-perfluorooctyl) (PFOTS) via chemical vapor deposition technique. In general, silicone oil cloaking of the surface of dust particles is resulted, and the height of oil is cloaking over the dust surface increases by time. As the time progresses, cloaking covers the dust particle completely, and it causes immersion of dust particles into silicone oil (Fig. 7a). The speed of immersion of dust particle into silicone oil increases with time under the influence of gravitational force (Fig. 8a). However, silicone oil cloaking does not take place for the functionalized dust particle and functionalized dust particles float on oil surface (Fig. 8a). This is because oil cloaking on the surface of dust particles is attributed to the spreading rate of oil film at the air–dust particle interface. The spreading coefficient (Sop(a) = γpa − γpo − γoa, where γpa is the interfacial energy at dust particle–air interface, γpo is the interfacial energy at dust particle–oil interface, and γoa is the interfacial energy at oil–air interface), should remain greater than zero for possible cloaking of the outer surface of dust particle by silicone oil. Although interfacial energy between dust particle and air, and the dust particle and silicone oil are unknown, the condition γpa > (γpo + γoa) should be satisfied for oil cloaking. Since γoa = 21.2 mN m−1;27 in any case, γpa should be greater than 21.2 mN m−1. On the other hand, vertical force balance for dust particle, which is partially immersed in the silicone oil yields:
Fv = Fγ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + FB − Wp θ + FB − Wp
| (1) |
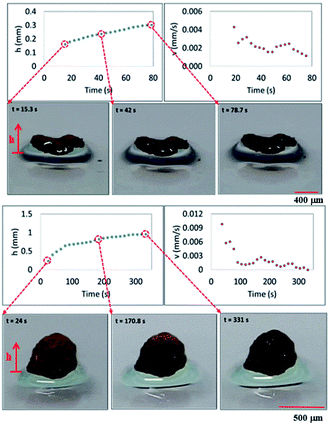 | ||
| Fig. 7 Silicone oil cloaking of dust particles: cloaking height and speed variations with time for two different dust shapes (ellipsoidal and spherical shapes). | ||
The spreading of oil on dust particle can be considered into two stages. Firstly, the balance between the surface tension gradient and the shear stress at the oil–dust interface gives rise to monolayer spreading of oil on a dust particle. Later, the location of oil spreading follows Joos law34 and spreading velocity can be related to 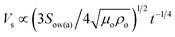 , where μo, ρo represent the dynamic viscosity and density of oil, and Sow(a) is the spreading coefficient of oil on dust particle. Later, formation of monolayer oil film is followed by plane oil film.35 The dissipating force during oil spreading around dust particle can be approximated by Ohnesorge number (
, where μo, ρo represent the dynamic viscosity and density of oil, and Sow(a) is the spreading coefficient of oil on dust particle. Later, formation of monolayer oil film is followed by plane oil film.35 The dissipating force during oil spreading around dust particle can be approximated by Ohnesorge number (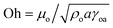 ), where a is the characteristics size of the dust particle,35 which can be considered to be an equivalent diameter.36 For average dust particle size of 1.2 μm, Oh well exits unity (Oh > 1) while implying large dissipating force for oil cloaking of dust particle. Cloaking rate is associated with cloaking time in the form of ∼kmt1/4 where km is the cloaking factor,36 and cloaking is not possible if kmt1/4 < 1. Cloaking velocity is determined from high speed camera data. The relation between cloaking velocity and cloaking time demonstrates that cloaking velocity is inversely proportional to clocking time (∼Ct−0.5, where C is a constant and varies with the shape of the dust). Temporal variation of the clocking velocity shows same trend as previous finding.34 Moreover, the net resulting force remains zero for the partially immersed and steadily floating dust particles on the oil film. The net resulting force becomes negative for continuously falling immersed dust particles in silicone oil film. However, once the oil cloaking initiates around dust particles due to spreading rate, the circumference of air–oil interface around dust particle reduces, which in turn lowers surface tension force. However, the buoyancy force increases because of dust immersion into silicone oil. Consequently, the amount of dust particle immersed into silicone oil depends on vertical force balance.
), where a is the characteristics size of the dust particle,35 which can be considered to be an equivalent diameter.36 For average dust particle size of 1.2 μm, Oh well exits unity (Oh > 1) while implying large dissipating force for oil cloaking of dust particle. Cloaking rate is associated with cloaking time in the form of ∼kmt1/4 where km is the cloaking factor,36 and cloaking is not possible if kmt1/4 < 1. Cloaking velocity is determined from high speed camera data. The relation between cloaking velocity and cloaking time demonstrates that cloaking velocity is inversely proportional to clocking time (∼Ct−0.5, where C is a constant and varies with the shape of the dust). Temporal variation of the clocking velocity shows same trend as previous finding.34 Moreover, the net resulting force remains zero for the partially immersed and steadily floating dust particles on the oil film. The net resulting force becomes negative for continuously falling immersed dust particles in silicone oil film. However, once the oil cloaking initiates around dust particles due to spreading rate, the circumference of air–oil interface around dust particle reduces, which in turn lowers surface tension force. However, the buoyancy force increases because of dust immersion into silicone oil. Consequently, the amount of dust particle immersed into silicone oil depends on vertical force balance.
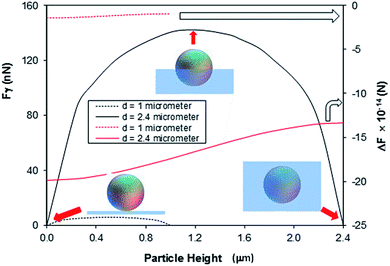
Fig. 9 shows surface tension force (Fγ) and difference between buoyancy and weight force with the height of immersed spherical dust particle in silicone oil. Surface tension force dominates over other forces when oil cloaking covers a small region of dust particle. As oil cloaking covers the surface of dust particle, size of the rim around dust particle at the interface between dust particle and oil film reduces. This in turn lowers surface tension force and causes dust particle to further immerse into silicone oil under gravitational force. The difference between buoyancy force and weight of dust particle remains negative (Fig. 9) while demonstrating that buoyancy force remains less than weight of dust particle. Consequently, environmental dust particle gradually immerses into silicone oil and settles on the treated glass surface.
Fig. S3(a–c)† show optical images of dust particles settled on glass and oil impregnated glass surface after exposing oil surface in an outdoor environment for one week. The coverage area of dust particles over the total area of the surface exposed is in the order of 20% for thick (700 nm), and air jet thinned silicone oil film (56.2 nm); however, dust particles coverage area reduces to 18% for as received glass surface. Since all samples are located close to each other in the outdoor environment and starting and ending durations of the outdoor tests are set as same for all samples, the reduction in the coverage area of dust particles could attribute to the wind effect. In this case, some of the dust particles settled, at as received glass surface, could be removed by the local wind. However, dust particles immersed in thin and thick silicone oil film remain at functionalized glass surface under the local wind conditions. The area covered by dust particles lowers optical transmittance of silicone oil impregnated glass (Fig. 6). The removal of sediment dust particles from functionalized silica particles deposited glass surface is almost not possible unless impregnated silicone oil is removed from the glass surface. Since compounds of alkaline and earth alkaline earth metals in dust particles can dissolve in a fluid, these dust compounds in silicone oil can be dissolved. This situation is examined by using inductively coupled plasma atomic emission spectroscopy. The findings are given in Table 2. The concentration of Fe, Na, Mg, K, and Cl are observed in silicone oil when dust particles are settled on the glass surface. This indicates dissolution of some dust compounds in silicone oil during the duration of dust settlement in silicone oil. To assess the effect of dissolved compounds on optical characteristics, UV-visible transmittance of silicone oil is measured after the removal of dust particles from silicone oil. Consequently, the presence of dissolved dust compounds in silicone oil reduces optical transmittance of the oil, and this reduction is in the order of 12% (Fig. 6).
| Al (ppb) | Ca (ppb) | Fe (ppb) | K (ppb) | Mg (ppb) | Na (ppb) | |
|---|---|---|---|---|---|---|
| Silicone oil | 1782 | 2191 | 243 | 2159 | 5610 | 6730 |
| Dissolved dust compounds in oil | 4687 | 4587 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
Conclusion
Properties of environmental dust particles are examined, and morphological and optical characteristics of functionalized silica particles deposited on glass surface are analyzed. In order to improve optical transmittance of functionalized silica particles deposited on glass substrate, silicone oil impregnation is introduced at the treated surface. Environmental dust particles are collected locally and characterized by using the analytical tools. Silicone oil cloaking of dust particles is analyzed, and vertical force balance is formulated for partially immersed dust particles in silicone oil film towards assessing dust particle settlement. Silicone oil impregnated surfaces are left in an outdoor environment for one week and dust particles settled on silicone oil impregnated surface are characterized. Influence of dust settlement on optical transmittance of silicone oil impregnated surface is evaluated. Silicone oil film is thinned by an air jet, and effect of dust accumulation on optical transmittance of impregnated thinned oil film is also assessed. It is found that dust particles compose of various compounds including compounds of alkaline and alkaline earth metals. Some of these compounds dissolve in water while enhancing water pH level. Average dust particle size is in the order of 1.2 μm and they have various shapes with an average shape factor of 1.15. Small dust particles (≤1.2 μm) have a tendency to attach to large size particles because of electrostatic forces, and they have the shape factor of almost unity. However, the shape factor is in the order of 3.11 for the large size particles (≥1.2 μm). Deposition of functionalized silica particles improves surface hydrophobicity of glass greatly; however, the optical transmittance of glass sample reduces considerably. Silicone oil impregnation of functionalized silica particles deposited glass surface improved optical transmittance for thick (700 nm) and thin (56.2 nm) silicone oil films. When oil impregnated surfaces are tested in an outdoor environment, optical transmittance reduces considerably because of sedimentation of dust particles in silicone oil film, which is particularly true for thick silicone oil film. Cloaking of dust particles takes place by silicone oil due to high spreading rate. This becomes the main cause for immersion of dust particles into silicone film. Dust particles immersed into silicone oil settles on the glass surface. Although surface tension force remains high prior to silicone oil spreading and cloaking of dust particles, once silicone oil cloaking covers dust particle surface, surface tension force decreases and gravity dominates over buoyancy force. Hence, dust sedimentation on silicone oil impregnated glass surfaces becomes unavoidable. Air jet thinning of silicone oil film on the surface does not prevent silicone oil cloaking of dust particles and gives rise to a negligible improvement in optical transmittance. The present study provides a fundamental understanding of dust sedimentation on silicone oil impregnated glass surface. It also includes useful information towards self-cleaning applications of oil impregnated surfaces and their optical characteristics in outdoor environments.Acknowledgements
The authors acknowledge the financial support of the Deanship of Research at King Fahd University of Petroleum and Minerals (KFUPM) and King Abdulaziz City for Science and Technology (KACST) through project #11-ADV2134-04.Notes and references
- J. A. Crook, L. A. Jones, P. M. Forstera and R. Crook, Energy Environ. Sci., 2011, 4, 3101–3109 Search PubMed.
- J. Son, S. Kundu, L. K. Verma, M. Sakhuja, A. J. Danner, C. S. Bhatia and H. Yang, Sol. Energy Mater. Sol. Cells, 2012, 98, 46–51 CrossRef CAS.
- H. Ghassan, B. S. Yilbas, S. Said, N. Al-Aqeeli and A. Matin, Sci. Rep., 2016, 6, 30253 CrossRef PubMed.
- B. S. Yilbas, H. Ali, N. Al-Aqeeli, M. Khaled, S. Said, N. Abu-Dheir, N. Merah, K. Youcef-Toumi and K. K. Varanasi, Sci. Rep., 2016, 6, 24308 CrossRef PubMed.
- J. T. Han, X. R. Xu and K. W. Cho, Langmuir, 2005, 21, 6662–6665 CrossRef CAS PubMed.
- N. J. Shirtcliffe, G. McHale, M. I. Newton, G. Chabrol and C. C. Perry, Adv. Mater., 2004, 16, 1929–1932 CrossRef CAS.
- H. Kinoshita, A. Ogasahara, Y. Fukuda and N. Ohmae, Carbon, 2010, 48, 4403–4408 CrossRef CAS.
- S. S. Latthe, H. Imai, V. Ganesan and A. V. Rao, Appl. Surf. Sci., 2009, 256, 217–222 CrossRef CAS.
- M. Ma, Y. Mao, M. Gupta, K. K. Gleason and G. C. Rutledge, Macromolecules, 2005, 38, 9742–9748 CrossRef CAS.
- B. S. Yilbas, M. Khaled, N. Abu-Dheir, N. Al-Aqeeli, S. A. M. Said, A. O. M. Ahmed, K. K. Varanasi and Y. K. Toumi, Appl. Surf. Sci., 2014, 320, 21–29 CrossRef CAS.
- X. Zhang, Y. Guo, P. Zhang, Z. Wu and Z. Zhang, Mater. Lett., 2010, 64, 1200–1203 CrossRef CAS.
- B. Abhilash and A. K. Panchal, Advances in Electrical, Electronics, Information, Communication and Bio-Informatics, 2nd International Conference (AEEICB), 2016, pp. 27–28 Search PubMed.
- K.-H. Tsui, Q. Lin, H. Chou, Q. Zhang, H. Fu, P. Qi and Z. Fan, Adv. Mater., 2014, 26, 2805–2811 CrossRef CAS PubMed.
- M. M. Tavakoli, K.-H. Tsui, Q. Zhang, J. He, Y. Yao, D. Li and Z. Fan, ACS Nano, 2015, 9, 10287–10295 CrossRef CAS PubMed.
- U. Mehmood, F. A. Al-Sulaiman and B. S. Yilbas, Sol. Energy Mater. Sol. Cells, 2016, 157, 604–623 CrossRef CAS.
- B. Bhushan and Y. C. Jung, Prog. Mater. Sci., 2011, 56, 1–108 CrossRef CAS.
- Y. Liua, A. Dasb, Z. Lina, I. B. Cooperb, A. Rohatgib and C. P. Wong, Nano Energy, 2014, 3, 127–133 CrossRef.
- A. P. Amalathas and M. M. Alkaisi, J. Phys. D: Appl. Phys., 2016, 49, 465601 CrossRef.
- D. Liu, Q. Wang, W. Shen and D. Wang, J. Mater. Chem. C, 2017, 5, 103–109 RSC.
- D. M. Giolando, Sol. Energy, 2016, 124, 76–81 CrossRef CAS.
- A. Al-Sharafi, B. S. Yilbas, A. Z. Sahin, H. Ali and H. Al-Qahtani, Appl. Therm. Eng., 2016, 108, 628–640 CrossRef.
- Y. Y. Quan, L. Z. Zhang, R. H. Qi and R. R. Cai, Sci. Rep., 2016, 6, 38239 CrossRef CAS PubMed.
- S. Anand, A. T. Paxson, R. Dhiman, J. D. Smith and K. K. Varanasi, ACS Nano, 2012, 6, 10122–10129 CrossRef CAS PubMed.
- H.-G. Park and D.-Y. Khang, Polymer, 2016, 99, 1–6 CrossRef CAS.
- H. Hanaei, M. Khalaji Assadi and R. Saidur, Renewable Sustainable Energy Rev., 2016, 59, 620–635 CrossRef CAS.
- K. S. Khalil, S. R. Mahmoudi, N. Abu-dheir and K. K. Varanasi, Appl. Phys. Lett., 2014, 105, 041604 Search PubMed.
- J. D. Smith, R. Dhiman, S. Anand, E. Reza-Garduno, R. E. Cohen, G. H. McKinley and K. K. Varanasi, Soft Matter, 2013, 9, 1772–1780 RSC.
- B. S. Yilbas, H. Ali, M. Khaled, N. Al-Aqeeli, N. Abu-Dheir and K. K. Varanasi, Sci. Rep., 2015, 5, 15833 CrossRef CAS PubMed.
- W. Y. D. Yong, Z. Zhang, G. Cristobal and W. S. Chin, Colloids Surf., A, 2014, 460, 151–157 CrossRef CAS.
- A. van Blaaderen and A. P. M. Kentgens, J. Non-Cryst. Solids, 1992, 149, 161–178 CrossRef CAS.
- J. Lin, H. Chen, Y. Ji and Y. Zhang, Colloids Surf., A, 2012, 411, 111–121 CrossRef CAS.
- A. Lafuma and D. Quere, Eur. Biophys. J., 2011, 96, 56001 Search PubMed.
- N. J. Shirtcliffe, G. McHale and M. I. Newton, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1203–1217 CrossRef CAS.
- V. V. Bergeron and D. Langevin, Phys. Rev. Lett., 1996, 76, 3152–3155 CrossRef CAS PubMed.
- A. Carlson, P. Kim, G. Amberg and H. A. Stone, Europhys. Lett., 2013, 104, 34008 CrossRef.
- S. Anand, K. Rykaczewski, S. B. Subramanyam, D. Beysens and K. K. Varanasi, Soft Matter, 2015, 11, 69–80 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03392c |
| This journal is © The Royal Society of Chemistry 2017 |