Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of a mixed-valence Cu(I)/Cu(II) metal–organic framework with the full light spectrum and high selectivity of CO2 photoreduction into CH4†
Yajun
Gao‡
 a,
Lei
Zhang‡
d,
Yuming
Gu‡
c,
Wenwei
Zhang
a,
Lei
Zhang‡
d,
Yuming
Gu‡
c,
Wenwei
Zhang
 a,
Yi
Pan
a,
Weihai
Fang
c,
Jing
Ma
a,
Yi
Pan
a,
Weihai
Fang
c,
Jing
Ma
 *c,
Ya-Qian
Lan
*c,
Ya-Qian
Lan
 *d and
Junfeng
Bai
*d and
Junfeng
Bai
 *ab
*ab
aState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
bSchool of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China. E-mail: bjunfeng@nju.edu.cn; bjunfeng@snnu.edu.cn
cKey Laboratory of Mesoscopic Chemistry of Ministry of Education, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China. E-mail: majing@nju.edu.cn
dJiangsu Collaborative Innovation Centre of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China. E-mail: yqlan@njnu.edu.cn
First published on 2nd September 2020
Abstract
Based upon the hetero-N,O ligand of pyrimidine-5-carboxylic acid (Hpmc), a new semiconductive Cu(I)/Cu(II) mixed-valence MOF with the full light spectrum and a novel topology of {43·612·86}2{43·63}2{63}6{64·82}3, {(Cu4I4)2.5[Cu3(μ4-O) (μ3-I) (pmc)3(Dabco)3]·2.5DMF·2MeCN}∞ (NJU-Bai61, NJU-Bai for Nanjing University Bai group; Dabco = 1,4-diazabicyclo [2.2.2] octane), was synthesized stepwise. NJU-Bai61 exhibits good water/pH stabilities and a relatively large CO2 adsorption capacity (29.82 cm3 g−1 at 1 atm, 273 K) and could photocatalyze the reduction of CO2 into CH4 without additional photosensitizers and cocatalysts and with a high CH4 production rate (15.75 μmol g−1 h−1) and a CH4 selectivity of 72.8%. The CH4 selectivity is the highest among the reported MOFs in aqueous solution. Experimental data and theoretical calculations further revealed that the Cu4I4 cluster may adsorb light to generate photoelectrons and transfer them to its Cu3OI(CO2)3 cluster, and the Cu3OI(CO2)3 cluster could provide active sites to adsorb and reduce CO2 and deliver sufficient electrons for CO2 to produce CH4. This is the first time that the old Cu(I)xXyLz coordination polymers' application has been extended for the photoreduction of CO2 to CH4 and this opens up a new platform for the effective photoreduction of CO2 to CH4.
Introduction
Due to climate change, CO2 capture and conversion has recently, become one of the greatest concerns.1 In particular, the photoreduction of CO2 into value-added chemicals (such as CO, HCOOH, CH4, and so on) has attracted great attention, because it can be considered as a promising approach for solar-to-chemical energy conversion by mimicking the natural photosynthetic process to achieve a carbon neutral economy.2 In the past few decades, diverse photocatalysts have been extensively employed for the photocatalytic CO2 reduction reaction (CO2RR).3 Homogeneous/molecular catalysts exhibit high selectivity and efficiency, but low activity due to catalyst deactivation,4 whereas heterogeneous/inorganic catalysts show high activity and efficiency, but low selectivity.5 Very recently, due to their high surface area, inorganic–organic hybrid nature, structural and functional diversity and tunability, metal–organic frameworks (MOFs) may combine the advantages of the traditional homogeneous/heterogeneous catalysts and are emerging as promising platforms for the photocatalytic CO2RR.6Since 2011,7 many MOFs have been designed for the photocatalytic CO2RR targeting to improve their efficiency, activity and selectivity by functionalizing organic ligands, optimizing metal ions/clusters, and making MOF-based composites.8 Although, some achievements have been made, research on MOF-based photocatalysts to date is still in its early stages. In terms of the reductive products, most reported MOFs predominantly produce the 2e−/2H+ products of CO/HCOOH.8a,9 Due to the fact that the photocatalytic reduction of CO2 into CH4 is more difficult than with other C1 fuels, because it involves a complex 8e−/8H+ reduction process, i.e., multiple steps of hydrogenation and deoxygenation reactions, and requiring the highest kinetic barrier of up to 818.3 kJ mol−1,10 the reported MOF catalysts capable of producing even low or moderate yields of CH4 are still rare. Thus, design of MOFs with high selectivity for the reduction of CO2 into CH4 is a great challenge.11
The Cu(I)xXyLz (where X = Cl, Br or I; L = N, P or S containing organic ligands) are almost the oldest coordination polymers with diversified structures and interesting properties, such as luminescence and semiconductivity, and so on.12 Very recently, their use has been demonstrated for photocatalytic H2 evolution.13 Herein the exploration of these polymers as promising platforms for CO2 capture and conversion is reported. From a simple hetero-N,O ligand pyrimidine-5-carboxylic acid, a Cu4I4 and Cu3OI(CO2)3 cluster based and semiconductive Cu(I)/Cu(II) mixed-valence MOF (NJU-Bai61) with a full light spectrum, which exhibits good water and pH stabilities and the relatively large CO2 adsorption capacity (29.82 cm3 g−1 at 1 atm, 273 K) was successfully constructed. In addition, NJU-Bai61 could photocatalyze the reduction of CO2 into CH4 without additional photosensitizers and cocatalysts and with a high CH4 production (15.75 μmol g−1 h−1) and CH4 selectivity of 72.8%. As far as is known, the CH4 selectivity is the highest among the reported MOFs in the aqueous solution. Upon light irradiation, its Cu4I4 clusters as photoelectron generators could transfer photoelectrons to the Cu3OI(CO2)3 clusters, whereas the Cu3OI(CO2)3 clusters could provide active sites for adsorbing and reducing CO2 and act as photoelectron collectors for delivering enough electrons to CO2 for CH4 evolution.
Results and discussion
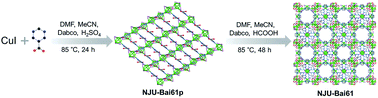
From CuI and the Hpmc ligand and using Dabco as the structural directing agent, like many Cu(I)xXyLz, a Cu4I4 cluster-based copper(I) coordination polymer, {(Cu4I4) (Hpmc)2}∞ (NJU-Bai61p) was initially obtained. NJU-Bai61p is a 2D layered and 4-connected network with sql topology (Fig. S3, ESI†), in which each Hpmc ligand uses its N-donor center to link to a 4-coordinated Cu(I) in a tetrahedral coordination geometry resulting in a [Cu4I4N4] moiety, leaving its COOH functional group uncoordinated (Fig. S4, ESI†).Later on, by changing the acid and extending the time, NJU-Bai61p was further transformed into NJU-Bai61 (Scheme 1). Compared with NJU-Bai61p, the Hpmc ligands in NJU-Bai61 were deprotonated, coordinated with Cu(II) ions in a bridging bidentate mode, facilitating the formation of the Cu3OI(CO2)3 cluster. The Cu3OI(CO2)3 cluster is 7-connected and surrounded by one Cu4I4 cluster, three pmc and three Dabco auxiliary ligands. All the Cu(II) ions in this new cluster adopt 5-coordinated geometry with two O atoms from two independent pmc linkers, one N atom from the Dabco linker, one μ3-I− ion shared by three Cu(II) ions, and one μ4-O2− ion shared by three Cu(II) ions and one Cu(I) ion from the Cu4I4 cluster (Fig. S6, ESI†). Remarkably, the Cu4I4 clusters in NJU-Bai61 exist in two different coordination environments. One is the same as that of NJU-Bai61p and can form a 4-connected [Cu4I4N4] moiety, whereas the other is the Cu4I4 cluster which is linked by three N atoms from three Dabco ligands and one μ4-O2− ion to form a 4-connected [Cu4I4N3O] moiety (Fig. S5, ESI†).
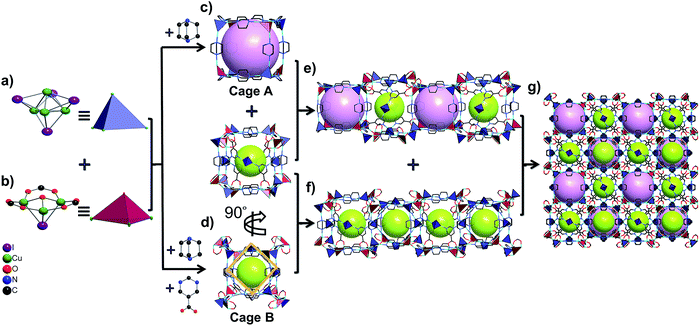
Furthermore, these Cu4I4 and Cu3OI(CO2)3 clusters are bridged by pmc and Dabco ligands to form two types of cubic cages. The larger one (cage A) is composed of four Cu4I4 clusters and four Cu3OI(CO2)3 clusters arranged alternately as vertices and 12 linear Dabco ligands as edges with a diameter of about 8.0 Å (Fig. 1c). The smaller one (cage B) is composed of eight pairs of [Cu4I4–Cu3OI(CO2)3] linkage clusters as vertices and 12 Dabco ligands as edges, in which there exists a square with a diameter of about 6.4 Å based on four pmc linkers and Cu4I4 clusters located at the center of the four facets of this cage (Fig. 1d and S7, ESI†). The cages A and B connect alternately with each other to form a 1D channel by sharing quadrilateral windows, whereas the B cages connect with each other to form a 1D cage-stacked chain by sharing the facets including a quadrilateral window and a Cu4I4 cluster (Fig. 1e, f, and S8, ESI†). Therefore, these 1D channels and chains are arranged in an alternating fashion to form a 3D porous framework based on the cages A and B ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in which each cage A shares facets with six cage Bs and each cage B shares facets with two cage As and four cage Bs (Fig. 1g and S9, ESI†). From the viewpoint of structural topology, pmc ligands, Cu4I4 and Cu3OI(CO2)3 clusters could be regarded as 3-connected triangular nodes, 4-connected tetrahedral nodes, and 7-connected single cap octahedron nodes, respectively. Consequently, NJU-Bai61 is a new (3,4,4,7)-connected network with the point symbol {43·612·86}2{43·63}2{63}6{64·82}3 (Fig. S10, ESI†).
3, in which each cage A shares facets with six cage Bs and each cage B shares facets with two cage As and four cage Bs (Fig. 1g and S9, ESI†). From the viewpoint of structural topology, pmc ligands, Cu4I4 and Cu3OI(CO2)3 clusters could be regarded as 3-connected triangular nodes, 4-connected tetrahedral nodes, and 7-connected single cap octahedron nodes, respectively. Consequently, NJU-Bai61 is a new (3,4,4,7)-connected network with the point symbol {43·612·86}2{43·63}2{63}6{64·82}3 (Fig. S10, ESI†).
The phase purities and thermal stabilities of NJU-Bai61p and NJU-Bai61 were confirmed using PXRD and TG analyses (Fig. S13 and S14, ESI†). As shown in Fig. S15–S17 (ESI†), they are quite stable under water and other organic solvents. Furthermore, they are also stable under the broad variation of the pH values.
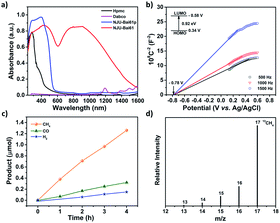
NJU-Bai61p exhibits a visible light adsorption up to 550 nm due to the Cu4I4 cluster to linker charge transfer (CLCT) transition (Fig. 2a and Table S2, ESI†). Very interestingly, NJU-Bai61 shows the widest absorption band among the reported MOFs with the edge up to 1400 nm, which are mainly dominated by intra metal cluster transfer (ICT), CLCT, and metal cluster-to-metal cluster charge transfer (CCCT) transitions (Fig. 2a and Table S3, ESI†). The bandgaps of semiconductive NJU-Bai61p and NJU-Bai61 were estimated to be 2.33 eV and 0.92 eV, respectively, (Fig. S18, ESI†), which could be correlated with the calculated HOMO–LUMO gaps of 2.16 eV and 1.25 eV for the corresponding cluster models, respectively, (Tables S4 and S5, ESI†). The solid state of NJU-Bai61 with a periodic boundary condition (PBC) model for the band gap was further calculated, showing a narrow band gap of 0.65 eV (Fig. S19, ESI†). The Mott–Schottky measurements further revealed that they were typical n-type semiconductors and their conduction bands (CB) were −0.55 V and −0.58 V, which were more negative than the reduction potentials for the conversion of CO2 to CO and CH4 (Fig. 2b and S20, ESI†).8a Thus, they are very promising for the CO2 photoreduction applications.
The photocatalytic reduction of CO2 over the activated NJU-Bai61 was further investigated. The amount of CH4 was 1.26 μmol (i.e., 15.75 μmol g−1 h−1) after 4 h. Except for the small amounts of CO (0.32 μmol, i.e., 4 μmol g−1 h−1) and H2 (0.15 μmol, i.e., 1.87 μmol g−1 h−1), no other products, such as HCOOH, CH3OH and HCHO, were detected (Fig. 2c, S22 and S23, ESI†). The NJU-Bai61 exhibited a CH4 selectivity of 72.8% in aqueous solution, which was the highest among the reported MOFs (Table S8, ESI†). No obvious change of the CH4 activity occurred during the four continuous runs (Fig. S24, ESI†). The XRD patterns obtained before and after its photocatalytic experiments revealed the structural robustness of the catalyst (Fig. S27, ESI†). The isotopic 13CO2 tracing experiment was also performed to confirm that the carbon source of CH4 did indeed come from the used CO2 rather than the degradation of organics in the reaction (Fig. 2d).11b For comparison, the use of NJU-Bai61p as the photocatalyst was also investigated under the same conditions and only CO (1.37 μmol, i.e., 17.13 μmol g−1 h−1) and H2 (1.34 μmol, i.e., 16.75 μmol g−1 h−1) were detected after 4 h (Fig. S25, ESI†). This result may reveal that Cu3OI(CO2)3 clusters in NJU-Bai61 could provide active sites for CH4 evolution.
Then in-depth research was carried out to discover the reason underlying the high efficiency of CH4 evolution. As for NJU-Bai61, the BET surface area was 248.1 m2 g−1 and the CO2 uptakes at 273 K and 298 K were 29.82 and 19.69 cm3 g−1, respectively, which was helpful for the subsequent CO2 conversion (Fig. S28–S30, ESI†). The electrostatic potential analysis may further reveal that the Cu(II) centers in Cu3OI(CO2)3 clusters are the most favorable sites for the nucleophilic attack of CO2 (Fig. S31, ESI†). The local interactions between Cu(II) sites and CO2 molecules were investigated using the in situ FTIR technology. The adsorption of CO2 onto the Cu(II) sites in NJU-Bai61 was a 16 cm−1 red shift of the asymmetric stretching mode of CO2 (ν = 2359 cm−1), indicating the stronger binding between the CO2 and Cu(II) sites (Fig. S33, ESI†).11b However, for NJU-Bai61p, no shift existed after CO2 adsorption (Fig. S32, ESI†). Moreover, this experimental phenomenon was explained by the DFT calculations in which the peaks were also red-shifted and the adsorbed CO2 molecule takes a slightly bent geometry to facilitate the CO2 activation (Fig. S34 and Table S9, ESI†).14 Furthermore, its fluorescence was quenched in comparison to NJU-Bai61p, indicating that the photo-excited electrons of the Cu4I4 clusters were transferred to the Cu3OI(CO2)3 clusters, making it act as a photoelectron collector to provide electrons for the adsorbed CO2 (Fig. S35, ESI†).
An energetically feasible reaction pathway was calculated using DFT with the relative free energy, ΔG, for each step shown in Fig. 3 and S38 (ESI).† Upon light irradiation, the Cu4I4 clusters in NJU-Bai61 may adsorb light to generate the photoelectrons and transfer them to the Cu3OI(CO2)3 clusters, whereas the Cu3OI(CO2)3 clusters could supply electrons to the adsorbed CO2 for CH4 evolution. In the first step, the adsorbed CO2 molecule accepted an electron and a proton to generate the COOH*. Then the COOH* combines with the second electron–proton pair to generate CO*. The CO* was reduced to the CHO* by accepting two electrons and a proton, and further combined with a total of four electrons and five protons to generate CH4. In the photocatalytic process, the Cu4I4 cluster could serve as a photosensitizer and donated the energy of 2.16 eV to the conversion process of CO* to CHO* at the Cu3OI(CO2)3 cluster which was an endothermic process with the ΔG of 1.2 eV. Moreover, the stronger CO binding affinity on NJU-Bai61 (Eb = −20.13 eV) in comparison with that on only Cu(I)-contained NJU-Bai61p (Eb = −8.05 eV) may further stabilize the CO@Cu3IO(CO2)3 complex to complete the CO2-to-CH4 conversion (Fig. S39, ESI†).
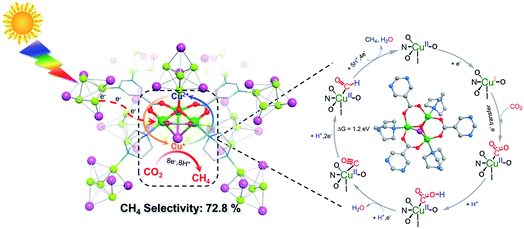 | ||
| Fig. 3 A proposed reaction pathway together with free energy difference (ΔG) for the photocatalytic CO2-to-CH4 conversion over NJU-Bai61. | ||
Conclusions
In summary, a novel Cu4I4 and Cu3OI(CO2)3 cluster based and semiconductive Cu(I)/Cu(II) mixed-valence MOF with the full light spectrum, NJU-Bai61, was successfully produced, which exhibits good water stability, pH stability and a relatively large CO2 adsorption capacity. NJU-Bai61 could photocatalyze the reduction of CO2 into CH4, without additional photosensitizers and cocatalysts, but with a high CH4 production and significantly high CH4 selectivity of 72.8% (the highest among the reported MOFs in aqueous solution). It was revealed that the Cu4I4 and Cu3OI(CO2)3 clusters may play the role of photoelectron generators and collectors, respectively. This work firstly expands the old Cu(I)xXyLz coordination polymers' application into the reduction of CO2 to CH4 and may open up a new system of MOFs for the reduction of CO2 to CH4 with high selectivity.Experimental section
Synthesis of NJU-Bai61p
A mixture of Hpmc (11 mg, 0.09 mmol), CuI (30 mg, 0.16 mmol), Dabco (6 mg, 0.05 mmol), H2SO4 (10 μL), DMF (1.0 mL), and MeCN (3.0 mL) was sealed in a 20 mL Pyrex tube and kept in an oven at 85 °C for 1 day. After washing with DMF, yellow block crystals were obtained. Yield: 2.5 mg (6%). Selected IR (cm−1): 3036, 2666, 2554, 1713, 1586, 1441, 1398, 1330, 1297, 1202, 1170, 1119, 1090, 1054, 996, 908, 837, 749, 695, 667, 568. Elemental analysis (%) calcd. for Cu2I2C5H4N2O2: C 11.89, H 0.80, N 5.54; found: C 11.96, H 1.00, N 5.52.Synthesis of NJU-Bai61
A single crystal of NJU-Bai61p (10 mg), Dabco (4 mg, 0.036 mmol) and CuI (20 mg, 0.11 mmol) were added to 1.0 mL of DMF and 3.0 mL of MeCN. To this was added 60 μL of HCOOH with stirring. The mixture was sealed in a Pyrex tube and heated to 85 °C for 2 d. Dark-red octahedral crystals were obtained and further characterized by PXRD and the results are shown in Fig. S1 (ESI†). Yield: 8.8 mg (25%). Selected IR (cm−1): 3392, 3108, 2952, 2883, 2840, 1681, 1652, 1587, 1435, 1377, 1319, 1218, 1170, 1087, 1050, 1000, 924, 840, 805, 764, 700, 612, 583, 468, 420. Elemental analysis (%) calcd. for Cu13I11C44.5H68.5N16.5O9.5: C 16.66, H 2.15, N 7.20; found: C 16.87, H 2.30, N 6.98.Sample activation
The as-synthesized sample of NJU-Bai61 was soaked in MeOH for 5 d with refreshing of the MeOH every 8 h. Then, the solvent-exchanged sample was activated at 70 °C and under vacuum for 10 h to obtain the activated NJU-Bai61.Photocatalytic reaction
The photocatalytic CO2 reduction experiments were carried out on an evaluation system (CEL-SPH2N, CEAULIGHT, China), in a 100 mL quartz container. A 300 W xenon arc lamp (300 < λ < 2500 nm) was utilized as the irradiation source. The 20 mg MOFs (NJU-Bai61p or the activated NJU-Bai61) were dispersed in 50 mL of a solution of triethylamine and water (TEA/H2O = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 v/v). The suspension was pre-degassed with CO2 (99.999%) for 30 min to remove the air before irradiation. The reaction was stirred constantly with a magnetic bar to ensure the photocatalyst particles remained in suspension. The temperature of the reaction was maintained at 25 °C by a circulating cooling water system. The gaseous product was measured by gas chromatography (GC-7900, CEAULIGHT, China) with a flame ionization detector (FID) and a thermal conductivity detector (TCD). An ion chromatography (LC-2010 Plus, Shimadzu, Japan) was used for the detection of HCOO−. The concentration of Cu in the solution before and after catalysis was determined using an ICP-OES system (Optima 5300 DV, PerkinElmer). Before the photocatalytic reaction, the suspension of the activated NJU-Bai61 (220 mg), TEA (5 mL) and H2O (45 mL) was pre-degassed with CO2 (99.999%) for 30 min to remove the air, then 2 mL of the filtrate was removed and a Cu concentration of 0.6 mg L−1 was detected. Thus, the concentration of dissolved Cu ions of the activated NJU-Bai61 was 0.05% before catalysis. After the photocatalytic reaction, 2 mL of filtrate was also removed and the concentration of Cu in the filtrate was determined to be 13.8 mg L−1. Thus, the concentration of dissolved Cu ions of the activated NJU-Bai61 was 1.1%. The cycling experiment was carried out as follows: at the end of each run, the suspension was centrifuged and the supernatant was removed. Then the recovered catalyst was washed with distilled water and dried in air at 60 °C before the next cycle.
45 v/v). The suspension was pre-degassed with CO2 (99.999%) for 30 min to remove the air before irradiation. The reaction was stirred constantly with a magnetic bar to ensure the photocatalyst particles remained in suspension. The temperature of the reaction was maintained at 25 °C by a circulating cooling water system. The gaseous product was measured by gas chromatography (GC-7900, CEAULIGHT, China) with a flame ionization detector (FID) and a thermal conductivity detector (TCD). An ion chromatography (LC-2010 Plus, Shimadzu, Japan) was used for the detection of HCOO−. The concentration of Cu in the solution before and after catalysis was determined using an ICP-OES system (Optima 5300 DV, PerkinElmer). Before the photocatalytic reaction, the suspension of the activated NJU-Bai61 (220 mg), TEA (5 mL) and H2O (45 mL) was pre-degassed with CO2 (99.999%) for 30 min to remove the air, then 2 mL of the filtrate was removed and a Cu concentration of 0.6 mg L−1 was detected. Thus, the concentration of dissolved Cu ions of the activated NJU-Bai61 was 0.05% before catalysis. After the photocatalytic reaction, 2 mL of filtrate was also removed and the concentration of Cu in the filtrate was determined to be 13.8 mg L−1. Thus, the concentration of dissolved Cu ions of the activated NJU-Bai61 was 1.1%. The cycling experiment was carried out as follows: at the end of each run, the suspension was centrifuged and the supernatant was removed. Then the recovered catalyst was washed with distilled water and dried in air at 60 °C before the next cycle.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We wish to acknowledge the Cheung Kong Scholars Program, the Hundred Talents Program of Shaanxi Province, the National Natural Science Foundation of China (21771121, 21673111) for their support. This work was also supported by the National Key Research and Development Program of China (2019YFC0408303).Notes and references
- (a) O. M. Yaghi, M. J. Kalmutzki and C. S. Diercks, Introduction to Reticular Chemistry: Applications of Metal-Organic Frameworks, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany 2019, p. 285 CrossRef; (b) C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS; (c) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS; (d) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS; (e) L. Zou, Y. Sun, S. Che, X. Yang, X. Wang, M. Bosch, Q. Wang, H. Li, M. Smith, S. Yuan, Z. Perry and H. C. Zhou, Adv. Mater., 2017, 29, 1700229 CrossRef; (f) P. G. Boyd, A. Chidambaram, E. Garcia-Diez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gladysiak, P. Schouwink, S. M. Moosavi, M. M. Maroto-Valer, J. A. Reimer, J. A. R. Navarro, T. K. Woo, S. Garcia, K. C. Stylianou and B. Smit, Nature, 2019, 576, 253 CrossRef CAS; (g) W. D. Jones, J. Am. Chem. Soc., 2020, 142, 4955 CrossRef CAS.
- (a) S. Berardi, S. Drouet, L. Francas, C. Gimbert-Surinach, M. Guttentag, C. Richmond, T. Stoll and A. Llobet, Chem. Soc. Rev., 2014, 43, 7501 RSC; (b) V. P. Indrakanti, J. D. Kubicki and H. H. Schobert, Energy Environ. Sci., 2009, 2, 745 RSC; (c) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982 RSC.
- (a) T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature, 1979, 277, 637 CrossRef CAS; (b) H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229 CrossRef CAS; (c) A. Dhakshinamoorthy, Z. Li and H. Garcia, Chem. Soc. Rev., 2018, 47, 8134 RSC; (d) H. Rao, L. C. Schmidt, J. Bonin and M. Robert, Nature, 2017, 548, 74 CrossRef CAS; (e) Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Chem. Rev., 2014, 114, 9987 CrossRef CAS.
- Y.-H. Luo, L.-Z. Dong, J. Liu, S.-L. Li and Y.-Q. Lan, Coord. Chem. Rev., 2019, 390, 86 CrossRef CAS.
- M. Tahir and N. S. Amin, Energy Convers. Manage., 2013, 76, 194 CrossRef CAS.
- (a) C. S. Diercks, Y. Liu, K. E. Cordova and O. M. Yaghi, Nat. Mater., 2018, 17, 301 CrossRef CAS; (b) O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. O. Yazaydin and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016 CrossRef CAS; (c) S. Wang and X. Wang, Small, 2015, 11, 3097 CrossRef CAS; (d) A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Angew. Chem., Int. Ed., 2016, 55, 5414 CrossRef CAS; (e) M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783 RSC.
- C. Wang, Z. Xie, K. E. DeKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445 CrossRef CAS.
- (a) R. Li, W. Zhang and K. Zhou, Adv. Mater., 2018, 30, 1705512 CrossRef; (b) Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126 RSC; (c) Y. Fu, D. Sun, Y. Chen, R. Huang, Z. Ding, X. Fu and Z. Li, Angew. Chem., Int. Ed., 2012, 51, 3364 CrossRef CAS; (d) D. Chen, H. Xing, C. Wang and Z. Su, J. Mater. Chem. A, 2016, 4, 2657 RSC; (e) Y. Lee, S. Kim, J. K. Kang and S. M. Cohen, Chem. Commun., 2015, 51, 5735 RSC; (f) L.-Y. Wu, Y.-F. Mu, X.-X. Guo, W. Zhang, Z.-M. Zhang, M. Zhang and T.-B. Lu, Angew. Chem., Int. Ed., 2019, 58, 9491 CrossRef CAS; (g) R. Li, J. Hu, M. Deng, H. Wang, X. Wang, Y. Hu, H.-L. Jiang, J. Jiang, Q. Zhang, Y. Xie and Y. Xiong, Adv. Mater., 2014, 26, 4783 CrossRef CAS; (h) Z.-C. Kong, J.-F. Liao, Y.-J. Dong, Y.-F. Xu, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, ACS Energy Lett., 2018, 3, 2656 CrossRef CAS.
- J. W. Maina, C. Pozo-Gonzalo, L. Kong, J. Schutz, M. Hill and L. F. Dumee, Mater. Horiz., 2017, 4, 345 RSC.
- (a) X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Nat. Energy, 2019, 4, 690 CrossRef CAS; (b) Y. Ji and Y. Luo, ACS Catal., 2016, 6, 2018 CrossRef CAS; (c) X. Chang, T. Wang and J. Gong, Energy Environ. Sci., 2016, 9, 2177 RSC; (d) W. Tu, Y. Zhou and Z. Zou, Adv. Mater., 2014, 26, 4607 CrossRef CAS.
- (a) E.-X. Chen, M. Qiu, Y.-F. Zhang, Y.-S. Zhu, L.-Y. Liu, Y.-Y. Sun, X. Bu, J. Zhang and Q. Lin, Adv. Mater., 2018, 30, 1704388 CrossRef; (b) H. Zhang, J. Wei, J. Dong, G. Liu, L. Shi, P. An, G. Zhao, J. Kong, X. Wang, X. Meng, J. Zhang and J. Ye, Angew. Chem., Int. Ed., 2016, 55, 14310 CrossRef CAS.
- (a) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781 CrossRef CAS; (b) J. Bai, E. Leiner and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 783 CrossRef CAS; (c) J. Bai, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 1737 CrossRef CAS; (d) R. Peng, M. Li and D. Li, Coord. Chem. Rev., 2010, 254, 1 CrossRef CAS; (e) Y. Kang, F. Wang, J. Zhang and X. Bu, J. Am. Chem. Soc., 2012, 134, 17881 CrossRef CAS; (f) P. C. Ford, E. Cariati and J. Bourassa, Chem. Rev., 1999, 99, 3625 CrossRef CAS; (g) T. Okubo, K. Himoto, K. Tanishima, S. Fukuda, Y. Noda, M. Nakayama, K. Sugimoto, M. Maekawa and T. Kuroda-Sowa, Inorg. Chem., 2018, 57, 2373 CrossRef CAS.
- D. Shi, R. Zheng, M.-J. Sun, X. Cao, C.-X. Sun, C.-J. Cui, C.-S. Liu, J. Zhao and M. Du, Angew. Chem., Int. Ed., 2017, 56, 14637 CrossRef CAS.
- (a) X. Lin, Y. Gao, M. Jiang, Y. Zhang, Y. Hou, W. Dai, S. Wang and Z. Ding, Appl. Catal., B, 2018, 224, 1009 CrossRef CAS; (b) P. D. Dietzel, R. E. Johnsen, H. Fjellvag, S. Bordiga, E. Groppo, S. Chavan and R. Blom, Chem. Commun., 2008, 5125 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1958778 and 1958779. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc03754k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |