DOI:
10.1039/D1RA03724B
(Paper)
RSC Adv., 2021,
11, 22692-22709
Metal- and solvent-free synthesis of aniline- and phenol-based triarylmethanes via Brönsted acidic ionic liquid catalyzed Friedel-Crafts reaction†
Received
12th May 2021
, Accepted 18th June 2021
First published on 30th June 2021
Abstract
A beneficial, scalable and efficient methodology for the synthesis of aniline-based triarylmethanes has been established through the double Friedel-Crafts reaction of commercial aldehydes and primary, secondary or tertiary anilines using Brönsted acidic ionic liquid as a powerful catalyst, namely [bsmim][NTf2]. This protocol was successfully performed under metal- and solvent-free conditions with a broad range of substrates, giving the corresponding aniline-based triarylmethane products in good to excellent yields (up to 99%). In addition, alternative aromatic nucleophiles such as phenols and electron-rich arenes were also studied using this useful approach to achieve a diversity of triarylmethane derivatives in high to excellent yields.
Introduction
Triarylmethanes (TRAMs) are valuable pharmacophores possessing a diversity of biological activities.1 In particular, triarylmethanes are found in natural products2 and are utilized as versatile compounds for photochromic agents,3 dyes,4 fluorescent probes,5 and are employed as building blocks in synthesis.6 Among these compounds, aniline- and phenol-based TRAMs are of growing importance because of their interesting properties4 and shown promising biological activities1a,1q,1x (Fig. 1).
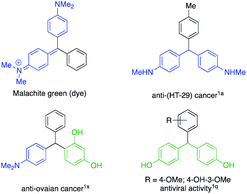 |
| | Fig. 1 Examples of important aniline- and phenol-based triarylmethanes. | |
In particular, primary aniline-based TRAMs were usually employed as monomers for the preparation of polyamides, which are useful in several applications for material science.6 In addition, amino and hydroxy groups on benzene rings of primary aniline- and phenol-based TRAMs can be easily converted into various functional groups that could be precursors for the preparation of diverse TRAMs.7 Therefore, synthetic strategies of such these compounds are highly desirable.
Although, many methods are available for the preparation of aniline-based TRAMs, most are derived from tertiary anilines.4k,8 Whereas, the synthetic methods for aniline-based TRAMs from primary and secondary anilines are still very limited. These syntheses suffer from drawbacks such as corrosive acid or toxic metal catalysts, harsh conditions (high temperature and/or microwave irradiation), using organic solvents and limited substrate scope.1a,6c,6d,6e,6f,6g,9 In 2005, Martínez-Palou and co-workers first reported the synthesis of primary, secondary and tertiary aniline-based triarylmethanes catalyzed by aniline hydrochloride salt (PhNH2·HCl) under microwave irradiation or high reaction temperature9a (Scheme 1A). Recently, Afonso and co-workers reported the synthesis of secondary aniline-based triarylmethanes using ytterbium-catalyzed Friedel-Crafts reaction in acetonitrile solvent under high pressure (9 kbar). However, only unsubstituted secondary anilines were successful and primary and tertiary anilines failed1a (Scheme 1B).
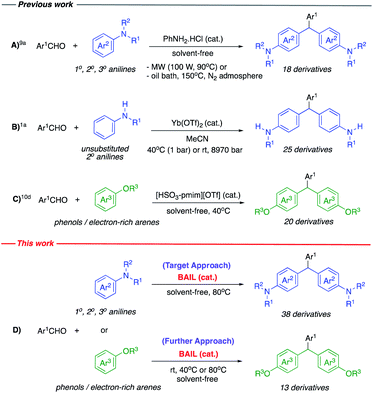 |
| | Scheme 1 Schematic synthesis of aniline- and phenol-based triarylmethanes (A–D). | |
At present, high performance, non-toxic, inexpensive, stable and reusable catalysts are gaining considerable attention for green synthesis. In particular, practical and metal-free reactions are strongly desirable for environmentally and economically sustainable processes. Brönsted acidic ionic liquids (BAILs) are among the most desirable catalysts in the green context. They have evolved as remarkable acid catalysts for numerous organic reactions in past decades.10 BAILs are privileged catalysts with moisture resistance, air tolerance, non-volatility, thermal stability, low-toxicity, environment-friendly, less corrosive, easy to handle, inexpensive, reusable, good solubility with many organic compounds and practical use even on a gram-scale synthesis. In contrast traditional Brönsted and Lewis acids generally involve some disadvantages such as corrosive acids, toxic metal catalysts, unstable, moisture sensitive, environmental contaminants and not reusable.
Friedel-Crafts reactions are classical and valuable tools for the synthesis of a variety of organic compounds.10d,10k,10l,11,12 However, to the best of our knowledge, examples of using BAILs as catalysts in Friedel-Crafts reactions for the synthesis of triarylmethanes are extremely rare to date.10c,10d,10e In 2014 a Brönsted acidic ionic liquid, [HSO3-pmim][OTf] was employed for stimulating the synthesis of triarylmethane derivatives from electron-rich arenes10d (Scheme 1C).
Based on these concepts, aniline-based triarylmethanes become our goals for searching a novel synthetic strategy under green conditions. Herein, a highly efficient and convenient methodology for the synthesis of a variety of aniline-based triarylmethanes via double Friedel-Crafts reaction catalyzed by Brönsted acidic ionic liquid is presented. The reaction was conducted using commercially available aromatic/heteroaromatic aldehydes and various primary, secondary or tertiary anilines under metal- and solvent-free conditions. Furthermore, several kinds of aromatic nucleophiles such as phenols and electron-rich arenes were also studied employing this protocol to achieve a diversity of triarylmethane derivatives (Scheme 1D).
Results and discussion
Brönsted acidic ionic liquid based-imidazonium cations, as shown in Fig. 2, were selected and prepared following a previous procedure10b,10c and employed as catalysts for this approach.
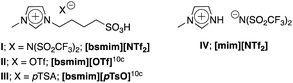 |
| | Fig. 2 Brönsted acidic ionic liquids (BAILs). | |
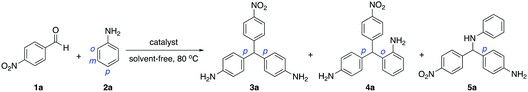
The synthesis of aniline-based triarylmethanes using a Friedel-Crafts reaction catalyzed by a Brönsted acidic ionic liquid was explored using readily available 4-nitrobenzaldehyde (1a) and aniline (2a) as model substrates (Table 1). The possibility preparing a range of products was investigated by including para–para (pp), para–ortho (po) and ortho–ortho (oo) isomers. This methodology afforded the para–para (pp) isomer as a major product and para–ortho (po) isomer as a minor product, whereas the ortho–ortho (oo) isomeric product was not formed.
Table 1 Optimization of the reactiona
|
| Entry |
Substrate (mmol) |
Catalyst (mol%) |
Time (h) |
Yieldb (%) |
| 1a |
2a |
3a |
4a |
5a |
| Reaction conditions: 1a (1.0 mmol), 2a (3.0–7.0 mmol), catalyst (5–20 mol%), solvent-free, 80 °C. Isolated yield. Room temperature. 100 °C. Second run. Water (1.0 mL). Toluene (1.0 mL). |
| 1c |
1.0 |
3.0 |
I (10) |
12.0 |
0 |
Trace |
15 |
| 2 |
1.0 |
3.0 |
I (10) |
12.0 |
58 |
7 |
— |
| 3 |
1.0 |
3.0 |
I (10) |
3.0 |
71 |
9 |
— |
| 4 |
1.0 |
3.0 |
I (10) |
1.5 |
70 |
8 |
— |
| 5 |
1.0 |
3.0 |
I (20) |
1.5 |
78 |
10 |
— |
| 6 |
1.0 |
3.0 |
I (5) |
1.5 |
65 |
10 |
— |
| 7d |
1.0 |
3.0 |
I (20) |
1.5 |
71 |
10 |
— |
| 8 |
1.0 |
5.0 |
I (20) |
1.5 |
90(89)e |
10(11)e |
— |
| 9 |
1.0 |
7.0 |
I (20) |
1.5 |
91 |
9 |
— |
| 10f |
1.0 |
5.0 |
I (20) |
1.5 |
48 |
5 |
25 |
| 11g |
1.0 |
5.0 |
I (20) |
1.5 |
84 |
9 |
2 |
| 12 |
1.0 |
5.0 |
II (20) |
1.5 |
75 |
8 |
Trace |
| 13 |
1.0 |
5.0 |
III (20) |
1.5 |
46 |
5 |
14 |
| 14 |
1.0 |
5.0 |
IV (20) |
1.5 |
42 |
20 |
30 |
| 15 |
1.0 |
5.0 |
HNTf2 (20) |
1.5 |
75 |
19 |
— |
| 16 |
1.0 |
5.0 |
H3PO4 (20) |
1.5 |
0 |
0 |
— |
| 17 |
1.0 |
5.0 |
H2SO4 (20) |
1.5 |
0 |
0 |
— |
| 18 |
1.0 |
5.0 |
HCl (20) |
1.5 |
28 |
Trace |
52 |
| 19 |
1.0 |
5.0 |
Cu(OTf)2 (20) |
1.5 |
85 |
9 |
— |
| 20 |
1.0 |
5.0 |
— |
1.5 |
0 |
0 |
— |
Initial optimization reaction conditions were performed by treatment of 1a (1.0 mmol) and 2a (3.0 mmol) in the presence of 10 mol% of Brönsted acidic ionic liquid (I) under solvent-free conditions (entries 1–4). The results showed that room temperature did not give the desired product (3a) even after 12 h, while a trace amount of isomeric product (4a) was observed along with intermediate (5a) in 15% yield (entry 1). Product 3a was obtained when performing the reaction at elevated temperature (80 °C) giving a moderate yield after 12 h (58%, entry 2). Surprisingly, the chemical yield of 3a was improved when reducing the reaction time, produced 71% at 3.0 h (entry 3) and was unchanged at 1.5 h (70% yield, entry 4). Subsequently, the amount of catalyst I was screened and the results indicated that 20 mol% led to an increase in yield of 3a to 78% (entry 5), whereas 5 mol% gave an inferior result (65%, entry 6). To improve the product yield, a slightly higher reaction temperature (100 °C) was tried with 20 mol% of catalyst I for 1.5 h, resulting in a lower yield (71%, entry 7). However, a higher yield of 3a was achieved when increasing the amount of aniline (2a) (entries 8 and 9), in which 5.0 equivalent of 2a was sufficient to provide the highest yield of 90% (entry 8).
For comparison, the reaction using water and toluene solvent was investigated. In the case of water, a moderate yield of major product (3a) was produced (48%) and minor product (4a) with 5% yield along with intermediate (5a) at 25% (entry 10). This result suggests that the reaction was not completed, probably due to poor solubility of organic substrates in the water system. While toluene afforded a high yield of 3a at 84% but lower than solvent-free conditions (entry 11 vs. 8).
Under the same conditions, various Brönsted acidic ionic liquid catalysts (II–III), which consists of different counter-anions were examined (entries 12–13). Catalyst II having OTf anion and catalyst III having pTsO anion, both gave diminished yields of 3a in 75% and 46%, respectively. This discloses that the activity of catalysts depends on the acidity of the counter anion and the NTf2 anion exhibits a better catalytic activity for this protocol than the OTf and pTsO anions. This could be due to the NTf2 anion, which is derived from traditional acid bis(trifluoromethane)imide (HNTf2) possesses hydrogen atoms that are bound to nitrogen and hence is more acidic.
In addition, catalyst IV, which consists of methylimidazolium cation and NTf2 anion, provided the major product 3a in lower yield (42%) and increased the minor product 4a to 20% yield. The intermediate 5a was also observed in 30% yield (entry 14), most likely due to instability of catalyst IV that can convert the counter-anion back to the traditional acid HNTf2. This result was confirmed with the traditional acid bis(trifluoromethane)imide (HNTf2), which is a very corrosive acid and not easy to use. When the reaction was promoted by HNTf2 a lower yield of the major product 3a was found (75%) and an increased yield of the minor product 4a was obtained (19%), similar to catalyst IV (entry 15). Other traditional acids such as H3PO4, H2SO4 and HCl were examined (entries 16–18). In case of strong acids H3PO4 and H2SO4 that fully reacted to aniline base, provided only imine intermediate and no any products were observed (entries 16–17). While acid HCl gave a lower yield of the major product 3a in 28% yield along with the intermediate 5a in 52% yield (entry 18). This is probably due to HCl react with aniline to form an aniline hydrochloride salt (PhNH2·HCl), which could act as a catalyst.9a A metal catalyst Cu(OTf)2 was tested for comparison and revealed a slightly lower yield of the desired product (3a) in 85% (entry 19 vs. 8). A control experiment was examined in the absence of a catalyst and the result showed that no products were observed (entry 20).
To probe the efficiency of this methodology, the investigation was extended to the reaction of various aldehydes with different kinds of nucleophile substrates including primary, secondary and tertiary anilines, electron-rich arenes and phenols employing the optimal conditions (Table 1, entry 8). Accordingly, a variety of primary anilines were first evaluated and the results are summarized in Scheme 2.
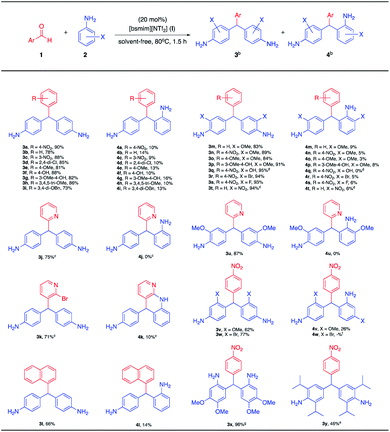 |
| | Scheme 2 Synthesis of triarylmethanes with various aldehyde substrates.a aReaction conditions: 1 (1.0 mmol), 2 (5.0 mmol), [bsmim][NTf2] (I) (20 mol%), solvent-free, 80 °C, 1.5 h. bIsolated yield. c3.0 h. dToluene (0.5 mL), 3.0 h. e100 °C, 24 h. fComplex mixture. g2.8 equivalent of 3,4-dimetoxyaniline was recovered. | |
Unsubstituted primary anilines reacted smoothly with benzaldehydes bearing both electron-withdrawing and electron-donating groups to achieve the excellent yield of the major product 3a (90%), high yield of the major products 3c–3h (81–86%) and good yields of the products 3b (78%) and 3i (73%). The minor isomeric products 4a–4i were found in trace quantities (9–16%). Heteroaromatic aldehydes gave good yields of the major products but with longer reaction times (3 h). Pyridine-2-carbaldehyde produced the major product 3j in 75% yield with no minor product 4j being observed, while 2-bromopyridine-3-carbaldehyde provided the major product 3k in 71% yield and the minor product 4k was formed in 10% yield through intramolecular cyclization of the para–ortho isomeric intermediate. Indeed, aromatic aldehydes with sterically hindered molecules such as 1-naphthaldehyde gave a diminished yield of the major product 3l (66%) and generated the minor product 4l in 14% yield. Subsequently, ortho substituted primary anilines were examined, including electron-donating groups (X = OMe, OH) and electron-withdrawing groups (X = Br, F, NO2) on benzene rings. The results revealed that the corresponding products were produced in high to excellent yields with no electronic influence of substituents. Electron-donating substituents generated high to excellent yields of the major products 3m, 3n, 3o, 3p and 3q in 83, 89, 84, 91 and 95% yields respectively.
Electron-withdrawing substituents also gave excellent yields of the major products 3r (94%), 3s (93%) and 3t (94%). In addition, similar results were obtained in the case of heteroaromatic aldehydes. The reaction of pyridine-2-carbaldehyde with 2-methoxyanilines produced the major product (3u) generally in high yield (87%) with no formative of the minor product 4u. However, the synthesis of compounds 3q and 3t must be carried out in toluene solvent, which was used to dissolve the solid substrates. Significantly, meta substituted primary aniline substrates were found to provide product yields which depended on the electronic effect of the meta position substituent.
In the case of meta-methoxyaniline, the major product (3v) resulted in a relatively lower yield (62% vs. 89% for 3n) and the yield of minor product (4v) was increased to 26% (vs. 4n, 5%). Whereas, meta-bromoaniline gave the major product (3w) in lower yield (77% vs. 94% for 3r) along with complex mixtures of minor products (o,p- and o,o-isomers). This is due to the fact that substitutes at the meta-position on the benzene ring of aniline can enhance the nucleophilicity of aniline substrates at the ortho-position causing an increase in the yield of the para–ortho (po) isomeric product. However, methoxy groups gave a superior electronic effect than bromo groups. Furthermore, whereas 3,4-dimetoxyaniline was employed as a substrate, the compound 3x was isolated as the sole product in 96% yield. Notable, 3,4-dimethoxyaniline substrate could be recovered (2.8 mmol) from the reaction mixture, indicating that only equivalent amounts of aniline substrate (2.2 equivalent) required to achieve the desirable products. Sterically hindered anilines such as 2,6-diisopropylaniline were also examined leading to an acceptable yield of the product 3y of 46%.
Encouraged by the results summarized in above, the scope of aniline nucleophiles was further explored. A variety of N-substituted and N,N-disubstituted anilines were investigated and found to readily undergo the reaction under optimized conditions (Table 1, entry 8) to generate structurally diverse aniline-based triarylmethanes in variable yields (Scheme 3). Similar to primary anilines, secondary N-methylaniline was smoothly performed to generate the major products 3aa–3ad in comparatively high yields (83–89%) along with trace amounts of the minor products 4aa–4ad (5–9%). As expected, heteroaromatic aldehydes such as pyridine-2-carbaldehyde provided good yields of product 3ae (77%) with the minor product 4ae was not being formed. In the case of N-methyl-2-substituted anilines such as N-methyl-2-(trifluromethyl)aniline was successfully performed to furnished the sole product 3af in excellent yield (95%). While N-methyl-3-substituted anilines such as N-methyl-3-fluoroaniline led to a high yield of the major product 3ag (85%) and the minor product 4ag in 13% yield was observed.
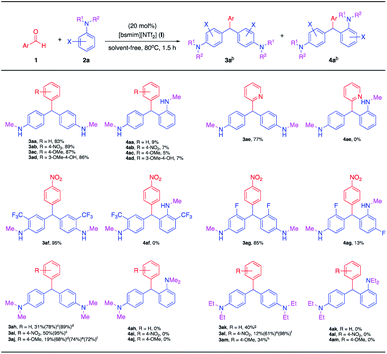 |
| | Scheme 3 Synthesis of triarylmethanes with secondary and tertiary anilines.a aReaction conditions: 1 (1.0 mmol), 2a (5.0 mmol), [bsmim][NTf2] (I) (20 mol%), solvent-free, 80 °C, 1.5 h. bIsolated yield. c6.0 h. d12.0 h. e24.0 h. f100 °C, 6.0 h. g100 °C, 12.0 h. h100 °C, 24.0 h. | |
In addition, this approach can be utilized for tertiary N,N-disubstituted anilines. The reactions specifically provided only para–para (pp) isomeric products (3ah–3am) with no para–ortho (po) isomeric products (4ah–4am) being detected. In contrast a previous report, the reaction did not work well for tertiary anilines and only imine intermediate was formed in case of primary aniline.1a Tertiary aniline reactions proceeded more slowly using this approach, probably due to the different mechanistic transformation involved (Scheme 6). Different products were obtained depending on the electronic effect of the aldehyde substates and the reactivity of the nucleophiles.
In the case of tertiary N,N-dimethylaniline reacting with benzaldehyde the product 3ah in low yield was produced at the optimal reaction time (31%, 1.5 h), indicating that the reaction was incomplete. An increasing yield of 3ah was observed with prolonged reaction times, 78% after 6.0 h and 89% after 12.0 h. In comparison, benzaldehyde with an electron-withdrawing group (4-NO2) proceeded faster and led to a higher yield of the product 3ai (50%) within 1.5 h. This improved to an excellent yield (95%) when the reaction time was increased to 6.0 h, due to the more reactive aldehyde substrate. While benzaldehyde with the electron-donating group (4-OMe) was found to be a deactivating aldehyde, providing the product 3aj in lower 19% yield after 1.5 h, which enhanced to a moderate yield (68%) after 12.0 h and a good yield (74%) after 24 h. However, the yield of product 3aj was unchanged (72%) even at an elevated reaction temperature (100 °C). Furthermore, tertiary N,N-diethylaniline, which is a virtually unreactive nucleophile, could convert to the desirable products but required longer reaction times. Indeed, under optimized reaction conditions (80 °C, 1.5 h), the products 3ak and 3am derived from benzaldehyde and 4-methoxybenzaldehyde substrates, respectively, were not formed, while the product 3al was obtained in very low yield (12%). Gratifyingly, better results were found with acceptable yields when raising the reaction temperature to 100 °C and extending the reaction time, 40% yield of the product 3ak after 12 h and 34% yield of 3am after 24 h. The product 3al was successfully achieved in moderate yield (61%) at 80 °C for 24 h and eventually improved to excellent yield (98%) at 100 °C for 6.0 h.
Further attempts, in which phenols and electron-rich arenes were employed as alternative nucleophiles for rating the scope of this approach and the results are given in Scheme 4. It should be noted that the reactions proceeded depending on the electronic effect of the aldehyde substrates. Previously, a number of methods were reported for the synthesis of triarylmethanes from phenols and electron-rich arenes using Brönsted or Lewis acid catalysts.10d,12 Herein the reactions were carried out using Brönsted acidic ionic liquid as a catalyst and results with different chemical yields of the desirable products were observed. Unsubstituted phenol provided the desirable product in higher yields when the reaction was carried out with aromatic aldehydes bearing electron-withdrawing groups, while a lower yield was observed in the case of aromatic aldehydes bearing electron-donating groups, including benzaldehyde.
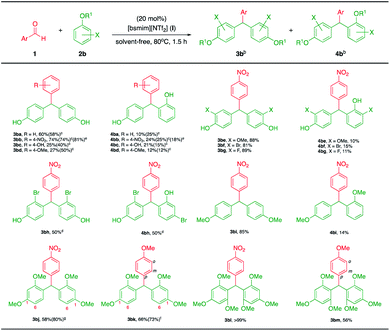 |
| | Scheme 4 Synthesis of triarylmethanes with various phenols and electron-rich arenes.a aReaction conditions: 1 (1.0 mmol), 2b (5.0 mmol), [bsmim][NTf2] (I) (20 mol%), solvent-free, 80 °C, 1.5 h. bIsolated yield. c0.5 h. d3.0 h. ert, 3 h. f40 °C, 0.5 h. g40 °C, 1 h. | |
Under optimal conditions (80 °C, 1.5 h), the major product 3bb (R = 4-NO2) was obtained in good yield (74%) and found a higher yield than the other substituents, 3ba (R = H, 60%), 3bc (R = 4-OH, 25%) and 3bd (R = 4-OMe, 27%). Surprisingly, increased yields of the major products 3bc and 3bd were observed (40 and 50%, respectively) when the reaction time was slightly reduced to 0.5 h, while the major products 3ba and 3bb were unchanged (58 and 74%, respectively). The highest yield of the major product 3bb was found to be 81% when performing the reaction at room temperature for 3.0 h. It is noteworthy to mention that the products having phenol moieties may be susceptible to undergo side reactions and form unidentified products depending the reaction conditions. Increased yields of the minor products were also observed in these results (4ba–4bd, 12–25%). In comparison, ortho substituted phenols could provide more stable products, presented in high isolated yields for 3be (X = o-OMe, 88%), 3bf (X = o-Br, 81%) and 3bg (X = o-F, 89%) along with small amount of the minor products (4be–4bg, 10–15%). Unfortunately, meta substituted phenols afforded the corresponding products not-selectively. For example, meta-bromophenol provided two isomeric products 3bh and 4bh in equally satisfactory yields (50%) with slightly longer reaction time (3.0 h).
In addition, in the case of electron-rich arenes, the corresponding products were obtained in moderate to excellent yields depending on the aldehyde substrates. Aromatic aldehydes with electron-withdrawing group are beneficial for improving the product yield. For example, 4-nitrobenzaldehyde furnished the major product 3bi in high yield (85%) along with trace amounts of the minor product 3bi (14%).
When increasing the number of methoxy groups on the benzene ring of arenes such as 1,3-dimethoxybenzene, the reaction processed more quickly, this is due to the increased electron density on the benzene ring providing highly reactive nucleophiles. However, the products were obtained in only moderate yields (58% for 3bj and 66% for 3bk). By-products were observed as a complex mixture of polar compounds, likely due to over nucleophilic addition at C-6 positions of 3bj and 3bk and ortho-positions of 3bk. While, improved yields of the products 3bj and 3bk were obtained in high yields (80% for 3bj and 73% for 3bk) when performing the reaction at lower reaction temperature (40 °C). In addition, sterically hindered groups of electron-rich arenes such as 1,3,5-trimethoxybenzene was explored using this protocol. Aromatic aldehyde with electron-withdrawing group such as 4-nitrobenzaldehyde provided an excellent yield of the product 3bl (>99%). While aromatic aldehyde with electron-donating group, 4-methoxybenzaldehyde provided lower acceptable yields of the product 3bm (56%). This is due to a less reactive electrophile substrate and over nucleophilic addition at ortho-positions of 3bm led to a complex mixture of by-products.
From the above investigation, it can be concluded that this approach shows excellent tolerance to a wide range of aldehyde and aromatic nucleophile substrates providing the desirable products in considerable yields under metal- and solvent-free conditions.
To demonstrate the practical usefulness of this approach for gram-scale synthesis, the reaction was performed using 10 mmol of 1a treated with 50 mmol of 2a under optimal conditions. The results showed that compound 3a (para–para isomeric adduct) was exclusively obtained as a sole product in excellent yield (94%). Surprisingly, there was no detection of para–ortho isomeric adduct 4a (Scheme 5). This result suggests that an enlarged scale synthesis will be an efficient process to produce specific products.
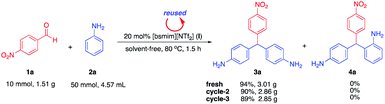 |
| | Scheme 5 Gram-scale synthesis and recyclability. | |
Recycling performance of catalyst I was examined with gram scale synthesis under the optimum reaction (Table 1, entry 8). After the first run, water (10 mL) was added to the reaction mixture and organic residues were extracted with EtOAc (3 × 50 mL) leaving catalyst I in the water layer. Then, after removing the water under high vacuum, recovered catalyst I was reused without purification by simply adding substrates for next run, giving the desirable product 3a in slightly lower yields with 94, 90 and 89% for three cycles, respectively (Scheme 5). Reused catalyst I was confirmed the structure using NMR analysis and showed its retained structure.
A plausible mechanism for the formation of aniline-based triarylmethanes catalyzed by BAIL can be rationalized by considering the following mechanism (Scheme 6). Activation of the carbonyl group of the aldehyde substrate (1) by BAIL (I) in order to easily react with the aniline nucleophile. Then rapid addition of 1° and 2° anilines (2) by N-nucleophilic addition to form an iminium ion intermediate1a (Scheme 6A). This intermediate acted as an activated electrophile (a), promoting addition of a second molecule of aniline by C-nucleophilic addition to generate intermediate c, which could be isolated from the reaction mixture. Then, BAIL (I) activated intermediate c leaving the aniline moiety to afford the iminium intermediate d, which on addition of an aniline nucleophile accomplished the target product (3). In contrast, 3° anilines directly react to activating aldehyde (1) through C-nucleophilic addition at para-position of aniline. Indeed, it is much slower to form iminium intermediate d′, which usually requires high temperature conditions8 and subsequent access to the target product (3) (Scheme 6B).11a
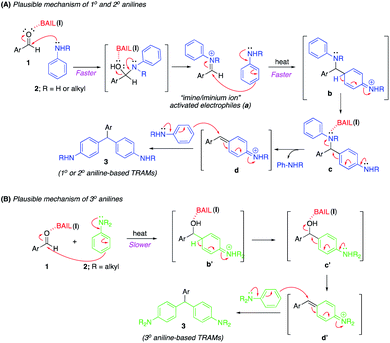 |
| | Scheme 6 Plausible mechanism for the formation of 1° and 2° aniline-based TRAMs (A) and 3° aniline-based TRAMs (B). | |
Conclusions
In summary, this work has developed a convenient, scalable and highly efficient methodology for the synthesis of diverse symmetric triarylmethane derivatives via double Friedel-Crafts reaction of commercial aldehydes and a wide range of aromatic nucleophiles, including primary, secondary and tertiary anilines as well as phenols and electron-rich arenes. The reaction was achieved under metal- and solvent-free conditions catalyzed by Brönsted acidic ionic liquids. Notable [bsmim][NTf2] was found to be the most efficient catalyst to furnish a variety of the corresponding products in considerable yields. In addition, this useful approach features green, stable and reusable catalyst, mild conditions, a wild range of substrates, considerable yields and is environmental benign and excellent sustainability.
Experimental section
General remarks
All chemicals were purchased from commercial sources and used without further purification. 1H and 13C NMR spectra were recorded using a BRUKER AVANC (400 MHz) spectrometer from Burapha University. High-resolution mass spectra (HRMS) data were recorded using a Bruker Daltonics-micrOTOF-Q at Mahidol University and an Agilent Technologies Q-TOF at Naresuan University. Infrared spectra were determined on a PERKIN ELMER FT/IR-2000S spectrophotometer. Analytical thin-layer chromatography (TLC) was conducted on pre-coated TLC plates; silica gel 60 F-254 [E. Merck, Darmstadt, Germany]. Open-column chromatography was carried out using silica gel 60 (0.063–0.200 mm) [E. Merck, Darmstadt, Germany]. Melting points were measured using a melting point apparatus (Griffin) from Burapha University.
General procedure for the preparation of acidic ionic liquids (I, II and III)
To a solution of 1-methylimidazole (4.00 mL, 0.050 mol) in acetonitrile (15.0 mL) was added 1,4-butanesultone (5.7 mL, 0.056 mol) in portions at room temperature. The reaction mixture was heated to 80 °C with stirring for 24 h, and then cooled to room temperature resulting in white precipitate. The white precipitate was filtered and washed with ethyl acetate (3 × 5 mL) to remove any unreacted starting materials, and then the precipitate was dried in a vacuum to give the zwitter ionic compound10c as a white solid in 99% yield (10.8189 g); 1H-NMR (400, CD3OD): d 9.05 (s, 1H), 7.74 (brt, J = 2.0 Hz, 1H), 7.66 (brt, J = 1.6 Hz, 1H), 4.34 (t, J = 7.2 Hz, 2H), 3.99 (s, 3H), 2.88 (t, J = 7.6 Hz, 2H), 2.09 (quint, J = 7.6 Hz, 2H), 1.82 (quint, J = 7.6 Hz, 2H). A mixture of zwitter ionic compound (1.00 g, 4.52 mmol) in anhydrous toluene (2.00 mL) was added a stoichiometric amount of acid (4.52 mmol, 1 equiv.) in portions at room temperature. The reaction was then stirred at 80 °C for 24 h. After complete the reaction, anhydrous toluene was removed by rotary evaporator to obtain acidic ionic liquid products.
1-Butylsulfonic-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (I)10ah. >99% yield (2.2720 g) as a colorless oil; 1H-NMR (400 MHz, DMSO-d6): δ 9.10 (s, 1H), 7.71 (d, J = 1.2 Hz, 1H), 7.64 (d, J = 1.6 Hz, 1H), 6.33 (brs, 1H), 4.16 (t, J = 6.8 Hz, 2H), 3.82 (s, 3H), 2.63 (t, J = 7.6 Hz, 2H), 1.87 (quint, J = 7.2 Hz, 2H), 1.57 (quint, J = 7.2 Hz, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 137.0, 124.0, 122.6, 119.9 (q, JC–F = 320 Hz, CF3SO2N), 35.0, 50.8, 48.9, 36.1, 28.8, 21.8.
1-Butylsulfonic-3-methylimidazolium trifluoromethanesulfonate (II)10c. 95% (1.5902 g) as a colorless oil; 1H-NMR (400 MHz, DMSO-d6): δ 9.13 (s, 1H), 7.77 (s, 1H), 7.70 (s, 1H), 4.40 (brs, 1H), 4.18 (t, J = 7.2 Hz, 2H), 3.85 (s, 3H), 2.53–2.47 (m, 2H), 1.87 (quint, J = 7.2 Hz, 2H), 1.54 (quint, J = 7.2 Hz, 2H).
1-Butylsulfonic-3-methylimidazolium p-toluenesulfonate (III)10c. 97% (1.7300 g) as a colorless oil; 1H-NMR (400 MHz, DMSO-d6): δ 9.13 (s, 1H), 7.76 (s, 1H), 7.69 (s, 1H), 7.48 (d, J = 7.6 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 4.17 (t, J = 6.8 Hz, 2H), 3.95 (brs, 1H), 3.84 (s, 3H), 2.55–2.48 (m, 2H), 2.28 (s, 3H), 1.87 (quint, J = 7.2 Hz, 2H), 1.54 (quint, J = 7.2 Hz, 2H).
General procedure for the preparation of acidic ionic liquid (IV)10ai,10aj
A mixture of 1-methylimidazole (0.5 mL, 6.27 mmol) in anhydrous toluene (2.00 mL) was added a stoichiometric amount of bis(trifluoromethanesulfonyl)imide (1.7628 g, 6.27 mmol) in portions at room temperature. The reaction mixture was heated to 80 °C with stirring for 24 h, and then cooled to room temperature. After complete the reaction, toluene was removed by rotary evaporator to obtain 1-methyl-1H-imidazolium bis(trifluoromethylsulfonyl)imide (IV) as a colorless oil in 86% yield (1.946 g); 1H-NMR (400 MHz, DMSO-d6): δ 8.68 (s, 1H), 8.33 (brs, 1H), 7.45 (s, 1H), 7.43 (s, 1H), 3.81 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 136.4, 122.8, 121.4, 119.8 (q, JC–F = 319 Hz, CF3SO2N), 35.0.
General procedure for the synthesis of triarylmethanes
A mixture of aldehyde 1 (1.0 mmol), nucleophile 2 (5.0 mmol) and [bsmim][NTf2]‡ (20 mol%) was stirred at 80 °C for the period of time. The reaction was monitored by thin layer chromatography (TLC). After the reaction was completed, the crude residue was extracted with ethyl acetate (3 × 5 mL). The combined organic layer was dried over anhydrous sodium sulfate and concentrated using a rotary evaporator. The crude product was purified by column chromatography (SiO2, 5–50% ethyl acetate/n-hexane as eluent depending on the derivatives) to give the desired product (3).
4,4′-((4-Nitrophenyl)methylene)dianiline (3a)9a,9c. CAS number 47334-87-2; 90% yield (0.2867 g) as a yellow solid; mp: 78–80 °C; Rf = 0.23 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.13 (d, J = 8.8 Hz, 2H), 7.32 (d, J = 8.8 Hz, 2H), 6.73 (d, J = 8.4 Hz, 4H), 6.49 (d, J = 8.4 Hz, 4H), 5.35 (s, 1H), 4.96 (brs, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 154.0, 147.0, 145.6, 130.4, 130.0, 129.4, 123.3, 113.9, 54.2; IR (neat): 3419, 3340, 3210, 3019, 1611, 1509, 1311, 1170, 1074, 811, 554 cm−1.
2-((4-Aminophenyl)(4-nitrophenyl)methyl)aniline (4a). CAS number 47334-87-2; 10% yield (0.0315 g) as a yellow solid; mp: 64–66 °C; Rf = 0.33 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.15 (d, J = 8.8 Hz, 2H), 7.30 (d, J = 8.8 Hz, 2H), 6.94 (td, J = 7.0, 2.0 Hz, 1H), 6.71 (d, J = 8.4 Hz, 2H), 6.65 (d, J = 8.0 Hz, 1H), 6.54–6.45 (m, 4H), 5.53 (s, 1H), 5.00 (brs, 2H), 4.73 (brs, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 152.5, 147.3, 145.81, 145.78, 130.2, 129.7, 129.1, 128.6, 127.1, 126.8, 123.3, 116.2, 115.3, 114.0, 49.4; IR (neat): 3442, 3361, 3220, 3065, 3030, 2958, 2921, 2854, 1621, 1510, 1487, 1452, 1341, 1274, 1186, 1105, 1013, 851, 751 cm−1; HRMS (ESI-TOF) m/z calcd for C19H18N3O2 [M + H]+ 320.1394, found 320.1395.
N-((4-Aminophenyl)(4-nitrophenyl)methyl)aniline (5a) (intermediate). 15% yield (0.0479 g) as a yellow solid; mp: 148–152 °C; Rf = 0.38 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.17 (d, J = 8.8 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.00 (t, J = 8.8 Hz, 4H), 6.60 (d, J = 7.6 Hz, 2H), 6.52–6.49 (m, 3H), 6.33 (d, J = 6.8 Hz, 1H), 5.57 (d, J = 6.4 Hz, 1H), 5.04 (brs, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 152.6, 148.0, 147.6, 146.2, 129.2, 128.7, 128.3, 128.1, 123.6, 116.3, 113.9, 113.2, 60.2; IR (neat): 3456, 3367, 1619, 1502, 1345, 1178, 742 cm−1; HRMS (ESI-TOF) m/z calcd for C19H18N3O2 [M + H]+ 320.1394, found 320.1394.
4,4′-(Phenylmethylene)dianiline (3b)6a,6g,9a,9c,9d,9e. CAS number 603-40-7; 78% yield (0.2133 g) as a yellow solid; mp: 130–132 °C; Rf = 0.33 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.24 (t, J = 7.2 Hz, 2H), 7.14 (t, J = 7.2 Hz, 1H) 7.06 (d, J = 7.2 Hz, 2H), 6.73 (d, J = 8.4 Hz, 4H), 6.47 (d, J = 8.4 Hz, 4H), 5.17 (s, 1H), 4.88 (brs, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 146.6, 145.8, 131.9, 129.4, 128.9, 128.0, 125.6, 113.7, 54.6; IR (neat): 3420, 3338, 3211, 3021, 1739, 1618, 1509, 1268, 1177, 813, 698, 574, 556 cm−1.
2-((4-Aminophenyl)(phenyl)methyl)aniline (4b)9e. CAS number 13168-31-5; 14% yield (0.0379 g) as a brown oil; Rf = 0.45 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.27 (t, J = 7.2 Hz, 2H), 7.17 (t, J = 7.6 Hz, 1H), 7.05 (d, J = 7.2 Hz, 2H), 6.90 (td, J = 8.0, 1.6 Hz, 1H), 6.71 (d, J = 8.0 Hz, 2H), 6.62 (dd, J = 8.0, 1.2 Hz, 1H), 6.54–6.44 (m, 4H), 5.30 (s, 1H), 4.92 (brs, 2H), 4.57 (brs, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 146.9, 145.6, 144.0, 129.7, 129.6, 129.1, 129.0, 128.2, 128.1, 126.6, 125.9, 116.0, 115.1, 113.9, 49.8; IR (neat): 3365, 300, 3209, 3001, 1739, 1618, 1509, 1268, 1177, 813, 698, 574, 556 cm−1.
4,4′-((3-Nitrophenyl)methylene)dianiline (3c)9d. CAS number 82646-09-1; 88% yield (0.2800 g) as a viscous brown oil; Rf = 0.26 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.04 (dt, J = 7.2, 2.4 Hz, 1H), 7.86 (brs, 1H), 7.55–7.51 (m, 2H), 6.75 (d, J = 8.0 Hz, 4H), 6.51 (d, J = 8.4 Hz, 4H), 5.39 (s, 1H), 4.97 (brs, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 148.3, 147.7, 147.0, 135.7, 130.6, 129.5, 129.4, 123.0, 120.9, 113.9, 53.9; IR (neat): 3415, 3335, 3205, 3011, 1620, 1511, 1313, 1244, 1080, 745, 521 cm−1.
2-((4-Aminophenyl)(3-nitrophenyl)methyl)aniline (4c). 9% yield (0.0299 g) as a yellow solid; mp: 70–72 °C; Rf = 0.37 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.07 (ddd, J = 8.0, 2.4, 1.2 Hz, 1H), 7.85 (t, J = 2.0 Hz, 1H), 7.58 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 7.6 Hz, 1H), 6.94 (td, J = 7.6, 1.6 Hz, 1H), 6.72 (d, J = 8.4 Hz, 2H), 6.65 (dd, J = 8.0, 0.8 Hz, 1H), 6.56–6.51 (m, 4H), 5.56 (s, 1H), 5.00 (brs, 2H), 4.76 (brs, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 147.7, 147.2, 146.6, 145.8, 135.9, 129.6, 129.5, 129.0, 128.7, 127.1, 126.8, 123.3, 121.1, 116.1, 115.2, 114.0, 48.9; IR (neat): 3442, 3361, 3205, 3011, 1620, 1511, 1311, 1170, 1074, 811, 554, 527, 447 cm−1; HRMS (ESI-TOF) m/z calcd for C19H18N3O2 [M + H]+ 320.1394, found 320.1394.
4,4′-((2,4-Dichlorophenyl)methylene)dianiline (3d)6r. CAS number 2451434-53-8; 85% yield (0.2915 g) as a yellow oil; Rf = 0.42 (40% EtOAc/n-hexane);1-NMR (400 MHz, DMSO-d6): δ 7.54 (d, J = 2.4 Hz, 1H), 7.33 (dd, J = 8.4, 2.0 Hz, 1H), 6.92 (d, J = 8.4 Hz, 1H), 6.64 (d, J = 8.4 Hz, 4H), 6.47 (d, J = 8.4 Hz, 4H), 5.46 (s, 1H), 3.95 (brs, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 147.0, 142.2, 134.1, 132.0, 131.2, 129.6, 129.5, 128.7, 127.0, 113.9, 51.0; IR (neat): 3443, 3355, 3210, 2924, 1727, 1619, 1510, 1465, 1274, 1045, 819, 573, 508 cm−1
2-((4-Aminophenyl)(2,4-dichlorophenyl)methyl)aniline (4d). 10% yield (0.0340 g) as a white solid; mp: 120–122 °C; Rf = 0.56 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.57 (d, J = 2.0 Hz, 1H), 7.34 (dd, J = 8.4, 2.0 Hz, 1H), 6.94 (td, J = 8.0, 1.2 Hz, 1H), 6.85 (d, J = 8.4 Hz, 1H), 6.72–6.65 (m, 3H), 6.51 (d, J = 8.4 Hz, 2H), 6.47 (td, J = 7.2, 0.8 Hz, 1H), 6.39 (dd, J = 7.6, 1.2 Hz, 1H), 5.48 (s, 1H), 5.01 (s, 2H), 4.52 (s, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 147.4, 145.6, 140.8, 134.5, 131.8, 131.6, 129.8, 128.9, 128.4, 127.3, 127.1, 127.0, 126.2, 116.3, 115.4, 114.1, 46.9; IR (neat): 3431, 3343, 2923, 1734, 1619, 1514, 1454, 1380, 1273, 1104, 1046, 820, 752, 549, 640 cm−1; HRMS (ESI-TOF) m/z calcd for C19H17Cl2N2 [M + H]+ 343.0763, found 343.0763.
4,4′-((4-Methoxyphenyl)methylene)dianiline (3e)9a,9c. CAS number 848126-11-4; 81% yield (0.2468 g) as a brown solid; mp: 130–132 °C; Rf = 0.25 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.95 (d, J = 8.8 Hz, 2H), 6.81 (d, J = 8.8 Hz, 2H), 6.70 (d, J = 8.4 Hz, 4H), 6.46 (d, J = 8.4 Hz, 4H), 5.11 (s, 1H), 4.85 (s, 4H), 3.69 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.2, 146.5, 137.8, 132.3, 129.8, 129.3, 113.8, 113.4, 55.0, 53.8; IR (neat): 3408, 3333, 3002, 2970, 1739, 1606, 1505, 1232, 1026, 830, 559, 507 cm−1.
2-((4-Aminophenyl)(4-methoxyphenyl)methyl)aniline (4e). 13% yield (0.0403 g) as a yellow solid; mp: 120–122 °C; Rf = 0.31 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.95 (d, J = 8.4 Hz, 2H), 6.90 (td, J = 8.0, 1.6 Hz, 1H), 6.83 (d, J = 8.8 Hz, 2H), 6.70 (d, J = 8.4 Hz, 2H). 6.61 (dd, J = 8.0, 0.8 Hz, 1H), 6.53 (dd, J = 7.6, 1.6 Hz, 1H), 6.48 (d, J = 8.4 Hz, 2H), 6.46 (td, J = 8.0, 0.8 Hz, 1H), 5.23 (s, 1H), 4.90 (s, 2H), 4.53 (s, 2H), 3.71 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.5, 146.8, 145.5, 135.8, 130.2, 130.0, 129.5, 129.0, 128.6, 126.6, 116.1, 115.1, 113.9, 113.5, 56.0, 49.1; IR (neat): 3365, 3001, 2926, 2853, 1894, 1618, 1507, 1242, 1175, 1029, 834, 751, 624, 580, 570, 548, 530, 509, 471, 446 cm−1; HRMS (ESI-TOF) m/z calcd for C20H21N2O [M + H]+ 305.1648, found 305.1645.
4-(Bis(4-aminophenyl)methyl)phenol (3f)6b,6h,6i,6j,6k,6l,9a. CAS number 110146-05-9; 88% yield (0.2552 g) as a brown solid; mp: 198–200 °C; Rf = 0.36 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.14 (s, 1H), 6.83 (d, J = 8.4 Hz, 2H), 6.69 (d, J = 8.4 Hz, 4H), 6.63 (d, J = 8.4 Hz, 2H), 6.45 (d, J = 8.4 Hz, 4H), 5.04 (s, 1H), 4.85 (brs, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 155.2, 146.4, 136.0, 132.6, 129.7, 129.3, 114.7, 113.7, 53.8; IR (neat): 3233, 3021, 2876, 2704, 1612, 1600, 1442, 1359, 1171, 1184, 1107, 831, 815, 603, 567 cm−1.
4-((2-Aminophenyl)(4-aminophenyl)methyl)phenol (4f). 10% yield (0.0296 g) as a brown solid; mp: 184–186 °C; Rf = 0.48 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.20 (s, 1H), 6.89 (td, J = 7.2, 1.6 Hz, 1H), 6.83 (d, J = 8.4 Hz, 2H), 6.70 (d, J = 8.4 Hz, 2H), 6.65 (d, J = 8.4 Hz, 2H), 6.60 (dd, J = 8.0, 0.8 Hz, 1H), 6.53 (dd, J = 7.6, 1.2 Hz, 1H), 6.50–6.44 (m, 3H), 5.16 (s, 1H), 4.89 (brs, 2H), 4.49 (brs, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 155.5, 146.8, 145.5, 134.0, 130.4, 130.0, 129.5, 129.0, 129.0, 126.5, 116.1, 115.0, 114.9, 113.9, 49.2; IR (neat): 3369, 3302, 3018, 2923, 2853, 2807, 2684, 2612, 1894, 1713, 1609, 1508, 1489, 1453, 1242, 1175, 1158, 1103, 1013, 939, 866, 834, 819, 797, 752, 707, 640, 626, 549, 527, 447 cm−1; HRMS (ESI-TOF) m/z calcd for C19H17N2O [M − H]− 289.1346, found 289.1334.
4-(Bis(4-aminophenyl)methyl)-2-methoxyphenol (3g)13a. CAS number 114163-79-0; 82% yield (0.2620 g) as a brown solid; mp: 160–162 °C; Rf = 0.23 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.71 (s, 1H), 6.71 (d, J = 8.4 Hz, 4H), 6.64 (d, J = 8.4 Hz, 1H), 6.61 (d, J = 2.0 Hz, 1H), 6.45 (d, J = 8.4 Hz, 4H), 6.40 (dd, J = 8.4, 2.0 Hz, 1H), 5.05 (s, 1H), 4.85 (s, 4H), 3.63 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 147.2, 146.4, 144.4, 136.7, 132.5, 129.3, 121.2, 115.0, 113.7, 113.33, 113.31, 55.6, 54.2; IR (neat): 3005, 2971, 1738, 1366, 1216 cm−1.
4-((2-Aminophenyl)(4-aminophenyl)methyl)-2-methoxyphenol (4g). 16% yield (0.0520 g) as a brown solid; mp: 108–110 °C; Rf = 0.32 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.77 (s, 1H), 6.89 (td, J = 7.6, 1.6 Hz, 1H), 6.71 (d, J = 8.4 Hz, 2H), 6.66 (d, J = 8.0 Hz, 1H), 6.64 (d, J = 2.0 Hz, 1H), 6.61 (dd, J = 7.6, 0.8 Hz, 1H), 6.55 (dd, J = 7.6, 1.2 Hz, 1H), 6.50–6.45 (m, 3H), 6.38 (dd, J = 8.0, 2.0 Hz, 1H), 5.16 (s, 1H), 4.89 (s, 2H), 4.49 (s, 2H), 3.64 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 147.8, 147.2, 146.0, 145.2, 135.1, 130.8, 129.9, 129.4, 129.3, 127.0, 121.8, 116.5, 115.6, 115.5, 114.3, 114.0, 56.0, 50.0; IR (neat): 3346, 2923, 2853, 1742, 1614, 1509, 1492, 1454, 1374, 1262, 1223, 1178, 1122, 1031, 941, 801, 637, 594, 509, 528 cm−1; HRMS (ESI-TOF) m/z calcd for C20H20N2O2Na [M + Na]+ 343.141, found 343.1417.
4,4′-((3,4,5-Trimethoxyphenyl)methylene)dianiline (3h). 86% yield (0.3124 g) as a white solid; mp: 186–188 °C; Rf = 0.28 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.74 (d, J = 8.4 Hz, 4H), 6.47 (d, J = 8.4 Hz, 4H), 6.35 (s, 2H) 5.10 (s, 1H), 4.87 (s, 4H), 3.63 (s, 6H), 3.61 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 152.5, 146.6, 141.5, 135.6, 131.8, 129.3, 113.8, 106.4, 60.0, 55.8, 54.8; IR (neat): 3434, 3347, 3003, 2935, 2836, 1738, 1511, 1230, 1123, 1002 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O3 [M + H]+ 365.1860, found 356.1881.
2-((4-Aminophenyl)(3,4,5-trimethoxyphenyl)methyl)aniline (4h). 10% yield (0.0364 g) as a brown solid; mp: 174–176 °C; Rf = 0.45 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.91 (td, J = 8.0, 1.6 Hz, 1H), 6.74 (d, J = 8.4 Hz, 2H), 6.64–6.57 (m, 2H), 6.52–6.46 (m, 3H), 6.36 (s, 2H), 5.23 (s, 1H), 4.93 (s, 2H), 4.55 (s, 2H), 6.63 (s, 3H), 3.62 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 152.6, 146.9, 145.6, 139.6, 135.8, 129.7, 129.5, 128.9, 128.2, 126.7, 116.1, 115.0, 113.9, 106.6, 60.0, 55.8, 50.1; IR (neat): 3430, 3337, 3103, 2934, 2840, 1730, 1522, 1230, 1125, 1002, 975, 775 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O3 [M + H]+ 365.1860, found 356.1883.
4,4′-((3,4-Bis(benzyloxy)phenyl)methylene)dianiline (3i). 73% yield (0.3540 g) as a yellow solid; mp: 114–116 °C; Rf = 0.19 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.44–7.28 (m, 10H), 6.93 (d, J = 8.4 Hz, 1H), 6.76 (d, J = 2.0 Hz, 1H), 6.66 (d, J = 8.4 Hz, 2H), 6.54 (dd, J = 8.4, 2.0 Hz, 1H), 6.45 (d, J = 8.4 Hz, 2H), 5.06 (s, 3H), 4.98 (s, 2H), 4.86 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 147.7, 146.5, 136.8, 137.5, 137.2, 132.1, 129.3, 128.4, 128.3, 127.8, 127.7, 127.6, 121.6, 116.0, 114.2, 113.8, 70.2, 54.1; IR (neat): 3433, 3357, 3216, 3029, 2932, 2873, 1739, 1622, 1508, 1261, 1214, 1126, 1003, 745, 696 cm−1; HRMS (ESI-TOF) m/z calcd for C33H31N2O2 [M + H]+ 487.2380, found 487.2417.
2-((4-Aminophenyl)(3,4-bis(benzyloxy)phenyl)methyl)aniline (4i). 13% yield (0.0611 g) as a brown oil; Rf = 0.31 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.45–7.29 (m, 10H), 6.96 (d, J = 8.4 Hz, 1H), 6.90 (td, J = 7.2, 1.6 Hz, 1H), 6.78 (d, J = 1.6 Hz, 1H), 6.67 (d, J = 8.4 Hz, 2H), 6.61 (dd, J = 8.0, 0.8 Hz, 1H), 6.54 (dd, J = 8.4, 2.0 Hz, 1H), 6.50–6.44 (m, 4H), 5.18 (s, 1H), 5.07 (s, 2H), 4.98 (s, 2H), 4.92 (s, 2H), 4.51 (s, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 147.7, 146.8, 146.8, 145.5, 137.4, 137.1, 136.9, 130.0, 129.5, 129.0, 128.5, 128.4, 128.3, 127.8, 127.8, 127.6, 126.6, 121.8, 116.3, 116.1, 115.1, 114.2, 113.9, 70.3, 70.2, 49.4; IR (neat): 3443, 3350, 3220, 2940, 2932, 2873, 1735, 1621, 1508, 1265, 1214, 1127, 1003, 745, 600, 696 cm−1; HRMS (ESI-TOF) m/z calcd for C33H31N2O2 [M + H]+ 487.2380, found 487.2414.
4,4′-(Pyridin-2-ylmethylene)dianiline (3j). 75% yield (0.2065 g) as an orange solid; mp: 165–170 °C; Rf = 0.31 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.46 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 7.66 (td, J = 8.0, 2.0 Hz, 1H), 7.16 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H), 6.78 (d, J = 8.4 Hz, 4H), 6.46 (d, J = 8.4 Hz, 4H), 5.24 (s, 1H), 4.88 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 164.4, 148.8, 146.6, 136.3, 131.0, 129.4, 123.1, 121.1, 113.7, 57.1; IR (neat): 3425, 3321, 3200, 3004, 2923, 2881, 1890, 1623, 1588, 1568, 1513, 1434, 1295, 1176, 871, 757 cm−1; HRMS (ESI-TOF) m/z calcd for C18H18N3 [M + H]+ 276.1495, found 276.1496.
4,4′-((2-Bromopyridin-3-yl)methylene)dianiline (3k). 71% yield (0.2532 g) as a yellow solid; mp: 170–172 °C; Rf = 0.29 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.21 (dd, J = 4.8, 2.0 Hz, 1H), 7.36, (dd, J = 7.6, 4.4 Hz, 1H), 7.30 (dd, J = 8.0, 2.0 Hz, 1H), 6.66 (d, J = 8.4H, 4H), 6.48 (d, J = 8.4, 4H), 5.41 (s, 1H), 4.97 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 147.7, 147.1, 144.3, 141.9, 139.2, 129.6, 129.2, 123.3, 114.0, 53.4; IR (neat): 3467, 3378, 3352, 3039, 2997, 2920, 1613, 1550, 1512, 1391, 1178, 1049, 799, 570 cm−1; HRMS (ESI-TOF) m/z calcd for C18H17BrN3 [M + H]+ 354.0600, found 354.0600.
4-(5,10-Dihydrobenzo[b][1,8]naphthyridine-5-yl)aniline (4k). 10% yield (0.0283 g) as a yellow solid; mp: 164–168 °C; Rf = 0.39 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.39 (s, 1H), 7.97 (d, J = 1.6 Hz, 1H), 7.36 (dd, J = 8.0, 1.6 Hz, 1H), 7.07 (td, J = 8.4, 1.6 Hz, 1H), 7.02–6.97 (m, 2H), 6.80–6.73 (m, 4H), 6.41 (d, J = 8.4 Hz, 2H), 5.14 (s, 1H). 4.88 (s, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 151.6, 147.0, 146.0, 138.8, 136.8, 135.1, 129.0, 127.8, 127.0, 123.4, 120.6, 118.9, 116.2, 114.4, 113.9, 45.4; IR (neat): 3373, 3179, 3097, 3010, 2922, 2852, 1737, 1580, 1608, 1510, 1496, 1445, 1270, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777, 1124, 1096, 808, 738, 512, 406 cm−1; HRMS (ESI-TOF) m/z calcd for C18H16N3 [M + H]+ 274.1339, found 274.1343.
777, 1124, 1096, 808, 738, 512, 406 cm−1; HRMS (ESI-TOF) m/z calcd for C18H16N3 [M + H]+ 274.1339, found 274.1343.
4,4′-(Naphthalen-1-ylmethylene)dianiline (3l)6c,9d. CAS number 1132762-70-9; 66% yield (0.2151 g) as a yellow solid; mp: 210–212 °C; Rf = 0.38 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.03 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 7.6 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.46–7.36 (m, 3H), 6.92 (d, J = 6.8 Hz, 1H), 6.72 (d, J = 8.4 Hz, 4H), 6.46 (d, J = 8.4 Hz, 4H), 5.94 (s, 1H), 4.88 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 146.6, 141.7, 133.6, 131.6, 131.4, 129.6, 128.5, 126.54, 126.53, 125.8, 125.4, 125.2, 124.5, 113.9, 50.8; IR (neat): 3438, 3415, 3339, 3211, 2924, 1611, 1508, 1268, 1177, 787, 573, 501 cm−1.
2-((4-Aminophenyl)(naphthalen-1-yl)methyl)aniline (4l). 14% yield (0.0452 g) as a white solid; mp: 154–156 °C; Rf = 0.53 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.90 (d, J = 8.0 Hz, 2H), 7.78 (d, J = 8.0 Hz, 1H), 7.47–7.37 (m, 3H). 6.93–88 (m, 2H), 6.79 (d, J = 8.4 Hz, 2H), 6.69 (d, J = 7.6 Hz, 1H), 6.51 (d, J = 8.4 Hz, 2H), 6.44–6.38 (m, 2H), 5.99 (s, 1H), 4.95 (s, 2H), 4.66 (s, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 147.0, 145.3, 140.4, 133.6, 131.5, 130.0, 129.1, 129.0, 128.5, 128.1, 126.8, 126.7, 126.2, 125.9, 125.5, 125.2, 124.4, 116.2, 115.3, 114.0, 46.1; IR (neat): 3461, 3424, 3371, 3031, 2923, 2853, 1736, 1620, 1513, 1493, 1454, 1392, 1277, 1246, 1182, 838, 794, 749, 506, 411 cm−1; HRMS (ESI-TOF) m/z calcd for C23H21N2 [M + H]+ 325.1699, found 325.1705.
4,4′-(Phenylmethylene)bis(2-methoxyaniline) (3m)9a. CAS number 6259-05-8; 83% yield (0.2757 g) as a brown solid; mp: 134–136 °C; Rf = 0.33 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.25 (t, J = 7.6 Hz, 2H), 7.15 (t, J = 7.6 Hz, 1H), 7.08 (d, J = 7.2 Hz, 2H), 6.57 (d, J = 1.6 Hz, 2H), 6.54 (d, J = 8.0 Hz, 2H), 6.37 (dd, J = 8.0, 1.6 Hz, 2H), 5.25 (s, 1H), 4.55 (s, 4H), 3.63 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 146.2, 145.6, 135.6, 132.6, 128.9, 128.0, 125.7, 121.3, 113.5, 111.6, 55.3, 55.2; IR (neat): 3470, 3373, 3005, 2970, 2938, 2856, 1737, 1616, 1515, 1279, 1227, 1149, 1032, 740, 706 cm−1.
4-((2-Amino-3-methoxyphenyl)(phenyl)methyl)-2-methoxyaniline (4m). 9% yield (0.0287 g) as a brown solid; mp: 128–130 °C; Rf = 0.43 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.27 (t, J = 7.2 Hz, 2H), 7.18 (t, J = 7.2 Hz, 1H), 7.06 (d, J = 7.4 Hz, 2H), 6.71 (dd, J = 8.0, 0.8 Hz, 1H), 6.57 (d, J = 2.0 Hz, 1H), 6.53 (d, J = 7.6 Hz, 1H), 6.49 (t, J = 7.6 Hz, 1H), 6.32 (dd, J = 8.0, 2.0 Hz, 1H), 6.23 (dd, J = 8.0, 0.8 Hz, 1H), 5.39 (s, 1H), 4.58 (s, 2H), 4.20 (s, 2H), 3.75 (s, 3H), 3.62 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 146.6, 146.3, 143.8, 135.9, 134.4, 130.6, 129.1, 128.6, 128.1, 126.0, 121.5, 121.4, 115.7, 113.6, 111.8, 108.4, 55.4, 55.2, 50.3; IR (neat): 3428, 3036, 2923, 2855, 1618, 1512, 1483, 1455, 1176, 1073, 1010, 780, 743, 423 cm−1; HRMS (ESI-TOF) m/z calcd for C21H23N2O2 [M + H]+ 335.1754, found 335.1757.
4,4′-((4-Nitrophenyl)methylene)bis(2-methoxyaniline) (3n)13b. CAS number 1429648-38-3; 89% yield (0.3373 g) as a brown solid; mp: 82–84 °C; Rf = 0.26 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.14 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 8.8 Hz, 2H), 6.58 (d, J = 2.0 Hz, 2H), 6.55 (d, J = 7.6 Hz, 2H), 6.37 (dd, J = 8.0, 1.6 Hz, 2H), 5.43 (s, 1H), 4.62 (s, 4H), 3.65 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 153.8, 146.3, 145.6, 136.02, 131.1, 130.0, 123.3, 121.3, 113.5, 111.5, 55.2, 54.9; IR (neat): 3430, 3321, 3210, 2924, 1611, 1508, 1445, 1291, 1121, 1028, 903, 702, 511 cm−1.
4-((2-Amino-3-methoxyphenyl)(4-nitrophenyl)methyl)-2-methoxyaniline (4n). 5% yield (0.0188 g) as a brown oil; Rf = 0.32 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.15 (d, J = 8.8 Hz, 2H), 7.30 (d, J = 8.8 Hz, 2H), 6.74 (dd, J = 8.0, 1.2 Hz, 1H), 6.58 (d, J = 1.6 Hz, 1H), 6.56 (d, J = 8.0 Hz, 1H), 6.52 (t, J = 8.0 Hz, 1H), 6.31 (dd, J = 8.0, 2.0 Hz, 1H), 6.22 (dd, J = 7.6, 0.8 Hz, 1H), 5.62 (s, 1H), 4.65 (s, 2H), 4.36 (s, 2H), 3.76 (s, 3H), 3.64 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 152.3, 146.7, 146.4, 145.8, 136.3, 134.7, 130.2, 129.4, 127.1, 123.3, 121.44, 121.38, 115.8, 113.6, 111.8, 108.7, 55.5, 55.2, 49.8; IR (neat): 3457, 3422, 3053, 2924, 2852, 1592, 1505, 1455, 1335, 1245, 1215, 1093, 1010, 736, 596, 425 cm−1; HRMS (ESI-TOF) m/z calcd for C21H21N3O4 [M]+ 379.1532, found 379.1533.
4,4′-((4-Methoxyphenyl)methylene)bis(2-methoxyaniline) (3o)13d,13e. CAS number 1012062-39-3; 84% yield (0.3077 g) as a brown solid; mp: 128–132 °C; Rf = 0.30 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.98 (d, J = 8.8 Hz, 2H), 6.82 (d, J = 8.8 Hz, 2H), 6.54 (d, J = 1.6 Hz, 2H), 6.52 (d, J = 8.0 Hz, 2H), 6.5 (dd, J = 8.0, 1.6 Hz, 2H), 5.18 (s, 1H), 4.53 (s, 4H), 3.70 (s, 3H), 3.63 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 157.2, 146.2, 137.5, 135.5, 133.0, 129.8, 121.2, 113.5, 113.4, 111.5, 55.2, 54.9, 54.5; IR (neat): 3463, 3422, 3322, 3012, 2958, 2835, 1617, 1506, 1280, 1229, 1029, 742, 631, 579 cm−1.
4-((2-Amino-3-methoxyphenyl)(4-methoxyphenyl)methyl)12-methoxyaniline (4o). 3% yield (0.0107 g) as a brown oil; Rf = 0.39 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.96 (d, J = 8.8 Hz, 2H), 6.83 (d, J = 8.8 Hz, 2H), 6.71 (dd, J = 8.0, 1.2 Hz, 1H), 6.55 (d, J = 1.6 Hz, 1H), 6.53 (d, J = 8.0 Hz, 1H), 6.49 (t, J = 8.0 Hz, 1H), 6.30 (dd, J = 8.0, 2.0 Hz, 1H), 6.22 (dd, J = 7.6, 0.8 Hz, 1H), 5.31 (s, 1H), 4.57 (s, 2H), 4.16 (s, 2H), 3.75 (s, 3H), 3.71 (s, 3H), 3.62 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.5, 146.6, 146.3, 135.8, 135.6, 134.3, 131.0, 129.9, 128.9, 121.4, 121.2, 115.7, 113.5, 111.7, 108.3, 55.4, 55.2, 55.0, 49.5; IR (neat): 3444, 3363, 2927, 2835, 1609, 1508, 1477, 1243, 1032 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O3 [M + H]+ 365.1860, found 365.1860.
4-(Bis(4-amino-3-methoxyphenyl)methyl)-2-methoxyphenol (3p). 91% yield (0.03462 g) as a black solid; mp: 138–140 °C; Rf = 0.25 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.73 (s, 1H), 6.67–6.65 (m, 2H), 6.55 (d, J = 1.6 Hz, 2H), 6.52 (d, J = 8.0 Hz, 2H), 6.44 (dd, J = 8.4, 2.0 Hz, 1H), 6.37 (dd, J = 8.0, 1.6 Hz, 2H), 5.13 (s, 1H), 4.52 (brs, 4H), 3.64 (s, 9H); 13C-NMR (100 MHz, DMSO-d6): δ 147.1, 146.1, 144.5, 136.4, 135.4, 133.2, 121.2, 115.0, 113.4, 113.3, 111.5, 55.6, 55.2, 54.9; IR (neat): 3749, 3370, 2939, 2838, 1737, 1592, 1512, 1217, 1029 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O4 [M + H]+ 381.1809, found 381.1809.
4-((2-Amino-3-methoxyphenyl)(4-amino-3-methoxyphenyl)methyl)-2-methoxyphenol (4p). 8% yield (0.0302 g) as a brown solid; mp: 128–130 °C; Rf = 0.31 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.78 (s, 1H), 6.71–6.65 (m, 3H), 6.55–6.47 (m, 3H), 6.39 (d, J = 8.4 Hz, 1H), 6.32 (d, J = 8.0 Hz, 1H), 6.25 (d, J = 7.6 Hz, 1H), 5.24 (s, H), 4.56 (s, 2H), 4.13 (s, 2H), 3.75 (s, 3H), 3.64 (s, 3H), 3.63 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 147.3, 146.6, 146.3, 144.8, 135.8, 134.5, 134.3, 131.2, 129.2, 121.5, 121.3, 115.8, 115.1, 113.6, 111.8, 108.3, 55.6, 55.5, 55.2, 50.1; IR (neat): 3747, 3470, 2839, 2838, 1737, 1592, 1512, 1217, 1029, 952, 847, 554, 447 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O4 [M + H]+ 381.1809, found 381.1805.
5,5′-((4-Nitrophenyl)methylene)bis(2-aminophenol) (3q). 95% yield (0.3354 g) as a brown solid; mp: 154–156 °C; Rf = 0.40 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.91 (s, 2H), 8.15 (d, J = 8.8 Hz, 2H), 7.32 (d, J = 8.8 Hz, 2H), 6.50 (d, J = 7.6 Hz, 2H), 6.38 (d, J = 2.0 Hz, 2H), 6.26 (dd, J = 8.0, 2.0 Hz, 2H), 5.28 (s, 1H), 4.43 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 154.1, 145.6, 143.9, 134.9, 131.6, 130.1, 123.3, 121.2, 120.1, 117.9, 115.3, 114.2, 54.5; IR (neat): 3374, 3297, 3045, 2703, 2587, 1592, 1441, 1507, 1348, 1201, 1134 cm−1; HRMS (ESI-TOF) m/z calcd for C19H18N3O4 [M + H]+ 352.1292, found 352.1292.
4,4′-((4-Nitrophenyl)methylene)bis(2-bromoaniline) (3r). 94% yield (0.4528 g) as a yellow solid; mp: 128–130 °C; Rf = 0.27 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.16 (d, J = 8.8 Hz, 2H), 7.34 (d, J = 8.4 Hz, 2H), 7.03 (d, J = 2.0 Hz, 2H), 6.79 (d, J = 8.4, 2.0 Hz, 2H), 6.74 (d, J = 8.4 Hz, 2H), 5.48 (s, 1H), 5.27 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 152.4, 145.9, 144.4, 132.2, 131.9, 130.0, 128.9, 123.6, 115.5, 107.4, 52.9; IR (neat): 3464, 3371, 3207, 2926, 1616, 1497, 1342, 1309, 1243, 1157, 1108, 1035, 853, 810, 699, 672, 564, 413 cm−1; HRMS (ESI-TOF) m/z calcd for C19H16Br2N3O2 [M + H]+ 475.9604, found 475.9604.
4-((2-Amino-3-bromophenyl)(4-nitrophenyl)methyl)-2-bromoaniline (4r). 5% yield (0.0259 g) as a yellow solid; mp: 118–120 °C; Rf = 0.30 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.18 (d, J = 8.8 Hz, 2H), 7.32 (dd, J = 7.6, 1.6 Hz, 1H), 7.29 (d, J = 9.2 Hz, 2H), 7.02 (brs, 1H), 6.76 (d, J = 0.8 Hz, 2H), 6.54 (dd, J = 8.0, 2.0 Hz, 1H), 6.49 (t, J = 8.0 Hz, 1H), 5.76 (s, 1H), 5.30 (s, 2H), 5.00 (s, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 151.1, 146.1, 144.7, 124.8, 132.4, 130.9, 130.2, 130.0, 129.2, 128.7, 128.2, 123.6, 117.4, 115.5, 109.2, 107.4, 49.2; IR (neat): 3461, 3410, 3370, 3340, 2958, 2924, 2852, 1742, 1616, 1510, 1496, 1441, 1344, 1276, 1253, 855, 825, 767, 749 cm−1; HRMS (ESI-TOF) m/z calcd for C19H16Br2N3O2 [M + H]+ 475.9604, found 475.9605.
4,4′-((4-Nitrophenyl)methylene)bis(2-fluoroaniline) (3s). 93% yield (0.3305 g) as a yellow solid; mp: 120–122 °C; Rf = 0.17 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.15 (d, J = 8.8 Hz, 2H), 7.34 (d, J = 8.8 Hz, 2H), 6.73–6.65 (m, 4H), 6.61 (dd, J = 8.4, 2.0 Hz, 2H), 5.46 (s, 1H), 5.06 (s, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 152.7, 150.4 (d, 1JC–F = 236.0 Hz), 145.9, 134.9 (d, 2JC–F = 13.0 Hz), 130.9 (d, 3JC–F = 5.0 Hz), 130.0, 124.9 (d, 4JC–F = 2.0 Hz), 123.5, 116.2 (d, 3JC–F = 5.0 Hz), 115.3 (d, 2JC–F = 19.0 Hz), 53.4; IR (neat): 3428, 3380, 2923, 2885, 1618, 1512, 1483, 1455, 1315, 1274, 1227, 1176, 1010, 806, 780, 743, 549, 485, 423 cm−1; HRMS (ESI-TOF) m/z calcd for C19H16F2N3O2 [M + H]+ 356.1205, found 356.1207.
4-((2-Amino-3-fluorophenyl)(4-nitrophenyl)methyl)-2-fluoroaniline (4s). 6% yield (0.0210 g) as a yellow solid; mp: 114–116 °C; Rf = 0.27 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.17 (d, J = 9.2 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 6.94 (ddd, J = 11.2, 8.0, 1.2 Hz, 1H), 6.72 (t, J = 8.0 Hz, 1H), 6.64 (dd, J = 12.4, 1.6 Hz, 1H), 6.59 (dd, J = 8.0, 2.0 Hz, 1H), 6.50 (td, J = 8.0, 5.6 Hz, 1H), 6.39 (d, J = 7.6 Hz, 1H), 5.74 (s, 1H), 5.10 (s, 2H), 4.86 (s, 2H); 13C-NMR (100 MHz, DMSO-d6): δ 151.8, 151.5 (d, 1JC–F = 235.0 Hz), 150.9 (d, 1JC–F = 236.0 Hz), 146.5, 135.5 (d, 2JC–F = 13.0 Hz), 134.4 (d, 2JC–F = 12.0 Hz), 130.6, 129.5 (d, 3JC–F = 5.0 Hz), 129.5 (d, 4JC–F = 2.0 Hz), 125.6 (d, 3JC–F = 3.0 Hz), 125.2 (d, 4JC–F = 2.0 Hz), 123.91, 116.7 (d, 3JC–F = 5.0 Hz), 116.0 (d, 2JC–F = 18.0 Hz), 115.9 (d, 3JC–F = 7.0 Hz), 113.5 (d, 2JC–F = 18.0 Hz), 49.0; IR (neat): 3444, 3310, 2974, 9875, 1650, 1510, 1420, 1311, 1201, 1110, 874, 854, 700, 654, 542 cm−1; HRMS (ESI-TOF) m/z calcd for C19H16F2N3O2 [M + H+] 356.1205, found 356.1205.
4,4′-(Phenylmethylene)bis(2-nitroaniline) (3t). 94% yield (0.3430 g) as a yellow solid; mp: 160–162 °C; Rf = 0.23 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.62 (d, J = 2.0 Hz, 2H), 7.43 (brs, 4H), 7.33 (t, J = 7.2 Hz, 2H), 7.24 (t, J = 7.6 Hz, 1H), 7.20 (dd, J = 8.8, 2.0 Hz, 2H), 7.13 (d, J = 7.2 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 5.47 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 145.1, 142.9, 136.8, 130.8, 129.7, 128.9, 128.7, 126.6, 124.6, 119.8, 52.9; IR (neat): 3415, 3335, 3205, 3011, 1620, 1511, 1313, 1244, 1080, 745, 521 cm−1; HRMS (ESI-TOF) m/z calcd for C19H17N4O4 [M + H]+ 365.1244, found 365.1244.
4-((2-Amino-3-nitrophenyl)(phenyl)methyl)-2-nitroaniline (4t). 6% yield (0.0240 g) as an orange solid; mp: 88–90 °C; Rf = 0.30 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.97 (d, J = 8.8, 1.6 Hz, 1H), 7.58 (d, J = 2.0 Hz, 1H), 7.45 (brs, 2H), 7.34 (t, J = 7.2 Hz, 2H), 7.26 (t, J = 7.2 Hz, 1H), 7.15 (dd, J = 8.8, 2.4 Hz, 1H), 7.11–7.06 (m, 4H), 7.50 (d, J = 8.8 Hz, 1H), 6.91 (dd, J = 7.2, 1.2 Hz, 1H), 6.64 (dd, J = 8.4, 7.2 Hz, 1H), 5.78 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 145.3, 143.5, 141.5, 137.0, 136.1, 131.9, 131.5, 129.8, 129.1, 128.9, 128.7, 127.0, 124.8, 124.5, 119.8, 115.1, 48.1; IR (neat): 3447, 3334, 3207, 3015, 1640, 1538, 1315, 1249, 1080, 740, 647, 501, 478, 324 cm−1; HRMS (ESI-TOF) m/z calcd for C19H17N4O4 [M + H]+ 365.1244, found 365.1236.
4,4′-(Pyridin-2-ylmethylene)(bis(2-methoxyaniline)) (3u). 87% yield (0.2924 g) as a brown solid; mp: 124–126 °C; Rf = 0.32 (60% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.48 (dd, J = 4.8, 0.8 Hz, 1H), 7.67 (td, J = 7.6, 1.6 Hz, 1H), 7.18–7.14 (m, 2H), 6.65 (d, J = 1.6 Hz, 2H), 6.54 (d, J = 7.6 Hz, 2H), 6.45 (dd, J = 8.0, 1.6 Hz, 2H), 5.33 (s, 1H), 4.56 (brs, 4H), 3.64 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 164.2, 148.8, 146.2, 136.4, 135.7, 131.7, 123.2, 121.3, 121.2, 113.5, 111.6, 51.8, 55.2; IR (neat): 3443, 3351, 2949, 1739, 1513, 1231, 1030, 756, 627 cm−1; HRMS (ESI-TOF) m/z calcd for C20H21N3O2 [M]+ 335.1634, found 335.1633.
4,4′-((4-Nitrophenyl)methylene)bis(3-methoxyaniline) (3v). 62% yield (0.2356 g) as a yellow solid; mp: 164–166 °C; Rf = 0.26 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.07 (d, J = 8.8 Hz, 2H), 7.15 (d, J = 8.4 Hz, 2H), 6.32 (d, J = 8.4 Hz, 2H), 6.22 (d, J = 2.0 Hz, 2H), 6.05 (dd, J = 8.0, 2.0 Hz, 2H), 5.83 (s, 1H), 4.99 (brs, 4H), 3.55 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 157.3, 154.9, 148.6, 145.3, 130.0, 129.6, 123.0, 118.1, 105.6, 97.8, 55.1, 41.5; IR (neat): 3412, 3310, 3210, 2935, 2833, 1736, 1611, 1504, 1341, 1204, 1039 cm−1; HRMS (ESI-TOF) m/z calcd for C21H22N3O4 [M + H]+ 380.1605, found 380.1603.
2-((4-Amino-2-methoxyphenyl)(4-nitrophenyl)methyl)-5-methoxyaniline (4v). 26% yield (0.0988 g) as a yellow solid; mp: 88–90 °C; Rf = 0.32 (50% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.12 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.8 Hz, 2H), 6.33 (d, J = 8.0 Hz, 1H), 6.30 (d, J = 8.8 Hz, 1H), 6.25 (d, J = 2.4 Hz, 2H), 6.08–6.05 (m, 2H), 5.58 (s, 1H), 5.06 (brs, 2H), 4.57 (brs, 2H), 3.63 (s, 3H), 3.59 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 158.6, 157.4, 153.0, 149.0, 146.7, 145.7, 130.1, 129.8, 129.6, 123.1, 119.5, 116.5, 105.8, 101.8, 100.5, 97.6, 55.1, 54.6, 42.8; IR (neat): 3444, 3366, 2936, 2834, 1610, 1505, 1463, 1341, 1205, 1172, 1030, 829, 744, 570 cm−1; HRMS (ESI-TOF) m/z calcd for C21H22N3O4 [M + H]+ 380.1605, found 380.1605.
4,4′-((4-Nitrophenyl)methylene)bis(3-bromoaniline) (3w). 77% yield (0.3667 g) as a yellow solid; mp: 198–200 °C; Rf = 0.17 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.16 (d, J = 8.8 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 6.84 (d, J = 2.4 Hz, 2H), 6.49 (dd, J = 8.4, 2.4 Hz, 2H), 6.40 (d, J = 8.4 Hz, 2H), 5.85 (s, 1H), 5.35 (brs, 4H); 13C-NMR (100 MHz, DMSO-d6): δ 151.3, 149.0, 146.0, 130.8, 130.3, 127.2, 125.0, 123.6, 117.5, 113.1, 53.5; IR (neat): 3471, 3413, 3375, 3317, 3216, 1625, 1601, 1508, 1487, 1344, 1262, 1026, 867, 816, 741, 696 cm−1; HRMS (ESI-TOF) m/z calcd for C19H16Br2N3O2 [M + H]+ 475.9604, found 475.9595.
6,6′-((4-Nitrophenyl)methylene)bis(3,4-dimethoxyaniline) (3x). 96% yield (0.4242 g) as a brown solid; mp: 184–186 °C; Rf = 0.47 (100% EtOAc); 1H-NMR (400 MHz, DMSO-d6): δ 8.17 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 6.40 (s, 2H), 6.16 (s, 2H), 5.51 (s, 1H), 4.49 (brs, 4H), 3.68 (s, 6H), 3.44 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 151.6, 148.9, 145.9, 140.8, 140.09, 140.08, 130.3, 123.2, 116.8, 115.6, 100.98, 100.96, 57.0, 55.2, 44.1; IR (neat): 3463, 3422, 3322, 3012, 2958, 2835, 1617, 1506, 1280, 1229, 1029, 742, 631, 579 cm−1; HRMS (ESI-TOF) m/z calcd for C23H26N3O6 [M + H]+ 440.1816, found 440.1817.
4,4′-((4-Nitrophenyl)methylene)bis(2,6-diisopropylaniline) (3y)6p,6q. CAS number 870633-59-3; 46% yield (0.2230 g) as a brown oil; Rf = 0.33 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.13 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 6.70 (s, 4H), 5.35 (s, 1H), 4.47 (brs, 4H), 3.03–2.90 (m, 4H), 1.06 (d, J = 6.8 Hz, 12H), 1.05 (d, J = 6.8 Hz, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 155.1, 145.4, 139.8, 131.2, 130.8, 129.8, 123.2, 122.9, 55.5, 26.7, 22.7, 22.5; IR (neat): 3397, 2959, 2927, 2869, 1622, 1588, 1520, 1462, 1441, 1342, 1310, 1260, 1213, 1185, 1158, 1067, 1013, 993, 970, 855, 848, 748, 725, 725, 527, 469 cm−1.
4,4′-(Phenylmethylene)bis(N-methylaniline) (3aa)1a,4j,4k. CAS number 3808-38-6; 83% yield (0.2502 g) as a yellow oil; Rf = 0.39 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.25 (t, J = 7.2 Hz, 2H), 7.14 (t, J = 7.2 Hz, 1H), 7.06 (d, J = 7.2 Hz, 2H), 6.80 (d, J = 8.4 Hz, 4H), 6.44 (d, J = 8.4 Hz, 4H), 5.45 (q, J = 5.2 Hz, 2H), 5.22 (s, 1H), 2.62 (d, J = 4.8 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 148.1, 145.8, 131.7, 129.4, 128.9, 128.0, 125.6, 111.5, 54.6, 29.9; IR (neat): 3412, 2926, 2880, 2812, 1736, 1612, 1509, 1341, 803 cm−1.
N-Methyl-2-((4-(methylamino)phenyl)(phenyl)methyl)aniline (4aa). 9% yield (0.0260 g) as a brown solid; mp: 128–130 °C; Rf = 0.55 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.26 (t, J = 7.2 Hz, 2H), 7.17 (t, J = 7.2 Hz, 1H), 7.09–7.00 (m, 3H), 6.76 (d, J = 8.4 Hz, 2H), 6.57–6.47 (m, 3H), 6.45 (d, J = 8.4 Hz, 2H), 5.50 (q, J = 5.2 Hz, 1H), 5.40 (s, 1H), 4.70 (q, J = 4.8 Hz, 1H), 2.64 (d, J = 4.8 Hz, 3H), 2.63 (d, J = 4.8 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 148.3, 146.6, 144.1, 129.65, 129.58, 129.1, 129.0, 128.5, 128.1, 127.0, 125.9, 115.4, 111.5, 109.4, 49.1, 30.3, 29.8; IR (neat): 3407, 3019, 2877, 2810, 1883, 1736, 1612, 1515, 1316, 1262, 1179, 1151, 1106, 1059, 806, 747, 535 cm−1; HRMS (ESI-TOF) m/z calcd for C21H23N2 [M + H]+ 303.1856, found 303.1855.
4,4′-((4-Nitrophenyl)methylene)bis(N-methylaniline) (3ab). 89% yield (0.3090 g) as a viscous brown oil; Rf = 0.39 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.14 (d, J = 8.8 Hz, 2H), 7.32 (d, J = 8.8 Hz, 2H), 6.81 (d, J = 8.4 Hz, 4H), 6.46 (d, J = 8.4 Hz, 4H), 5.54 (q, J = 5.2 Hz, 2H), 5.40 (s, 1H), 2.63 (d, J = 5.2 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 154.1, 148.4, 145.6, 130.2, 130.0, 129.4, 123.3, 111.6, 54.2, 29.8; IR (neat): 3412, 2926, 2880, 2812, 1736, 1612, 1509, 1341, 803 cm−1; HRMS (ESI-TOF) m/z C21H22N3O2 [M + H]+ calcd 348.1707, found 348.1707.
N-Methyl-2-((4-(methylamino)phenyl)(4-nitrophenyl)methyl)aniline (4ab). 7% yield (0.0251 g) as a yellow solid; mp: 130–132 °C; Rf = 0.52 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.14 (d, J = 8.8 Hz, 2H), 7.26 (d, J = 8.8 Hz, 2H), 7.11–7.06 (m, 1H), 6.76 (d, J = 8.4 Hz, 2H), 6.56–6.52 (m, 3H), 6.47 (d, J = 8.4 Hz, 2H), 5.61 (s, 1H), 5.58 (q, J = 5.2 Hz, 1H), 4.93 (q, J = 4.8 Hz, 1H), 2.64 (d, J = 4.8 Hz, 3H), 2.63 (d, J = 5.2 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 152.5, 148.6, 146.7, 145.5, 130.2, 129.7, 128.9, 128.3, 127.5, 127.2, 123.2, 115.5, 111.6, 109.6, 48.8, 30.3, 29.7; IR (neat): 3434, 2936, 2881, 2811, 1736, 1610, 1509, 1341, 803, 751, 665 cm−1; HRMS (ESI-TOF) m/z calcd for C21H22N3O2 [M + H]+ 348.1707, found 348.1704.
4,4′-((4-Methoxyphenyl)methylene)bis(N-methylaniline) (3ac)1a,9a. CAS number 848126-21-6; 87% yield (0.2901 g) as a viscous green oil; Rf = 0.32 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.96 (d, J = 8.4 Hz, 2H), 6.81 (d, J = 8.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 4H), 6.43 (d, J = 8.8 Hz, 4H), 5.43 (q, J = 5.2 Hz, 2H), 5.16 (s, 1H), 3.70 (s, 3H), 2.62 (d, J = 5.2 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 157.2, 148.0, 137.7, 132.0, 129.8, 129.3, 113.4, 111.4, 55.0, 53.7, 29.9; IR (neat): 3407, 2971, 2879, 2811, 1737, 1611, 1507, 1240, 1175, 1031, 810, 546 cm−1.
2-((4-Methoxyphenyl)(4-(methylamino)phenyl)methyl)-N-methylaniline (4ac). 5% yield (0.0149 g) as a yellow oil; Rf = 0.48 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.04 (t, J = 7.2 Hz, 1H), 6.92 (d, J = 8.8 Hz, 2H), 6.82 (d, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 2H), 6.57–6.47 (m, 3H), 6.44 (d, J = 8.8 Hz, 2H), 5.47 (q, J = 5.6 Hz, 1H), 5.32 (s, 1H), 4.63 (q, J = 5.6 Hz, 1H), 3.71 (s, 3H), 2.64 (d, J = 4.4 Hz, 3H), 2.62 (d, J = 4.4 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.4, 148.2, 146.6, 135.8, 130.0, 129.6, 128.9, 126.9, 115.4, 113.5, 111.5, 109.4, 55.0, 48.3, 30.3, 29.8; IR (neat): 3408, 3333, 3002, 2970, 1606, 1505, 1242, 1175, 1029, 834, 751, 624, 580, 570, 548, 530, 509, 471, 446 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O [M + H]+ 333.1961, found 333.1966.
4-(Bis(4-(methylamino)phenyl)methyl)-2-methoxyphenol (3ad). 86% yield (0.2995 g) as a purple solid; mp: 74–76 °C; Rf = 0.19 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.72 (s, 1H), 6.79 (d, J = 8.4 Hz, 4H), 6.67–6.62 (m, 2H), 6.46–6.38 (m, 5H), 5.42 (q, J = 5.2 Hz, 2H), 5.10 (s, 1H), 3.63 (s, 3H), 2.62 (d, J = 5.2 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 147.9, 147.2, 144.5, 136.7, 132.3, 129.4, 121.2, 115.0, 113.38, 113.35, 111.4, 55.6, 55.6, 54.2, 29.9; IR (neat): 3404, 2970, 2929, 2811, 1736, 1612, 1508, 1121, 1031, 814 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O2 [M + H]+ 349.1911, found 349.1911.
2-Methoxy-4-((2-(methylamino)phenyl)(4-(methylamino)phenyl)methyl)phenol (4ad). 7% yield (0.0238 g) as a brown oil; Rf = 0.29 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.78 (s, 1H), 7.04 (td, J = 7.0, 1.2 Hz, 1H), 6.76 (d, J = 8.4 Hz, 2H), 6.66 (d, J = 8.0 Hz, 1H), 6.62 (d, J = 1.6 Hz, 1H), 6.58 (dd, J = 8.0, 1.6 Hz, 1H), 6.53–6.48 (m, 2H), 6.44 (d, J = 8.4 Hz, 2H), 6.35 (dd, J = 8.0, 1.6 Hz, 1H), 5.46 (q, J = 6.4 Hz, 1H), 5.26 (s, 1H), 4.54 (q, J = 5.2 Hz, 1H), 3.63 (s, 3H), 2.64 (d, J = 4.8 Hz, 3H), 2.63 (d, J = 4.0 Hz, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 148.2, 147.3, 146.6, 144.8, 134.7, 130.1, 129.6, 129.1, 128.8, 126.9, 121.3, 115.4, 115.1, 113.6, 111.5, 109.4, 55.6, 48.9, 30.4, 29.9; IR (neat): 3531, 3411, 3327, 3088, 3012, 2970, 1615, 1514, 1475, 1456, 1435, 1266, 1244, 1121, 1067, 1051, 1033, 765, 756, 670, 503 cm−1; HRMS (ESI-TOF) m/z calcd for C22H25N2O2 [M + H]+ 349.1911, found 349.1915.
4,4′-(Pyridin-2-ylmethylene)bis(N-methylaniline) (3ae)1a. CAS number 1810046-99-1; 77% yield (0.2335 g) as a white soil; mp: 132–134 °C; Rf = 0.33 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.47 (dd, J = 4.8, 1.2 Hz, 1H), 7.66 (td, J = 7.6, 2.0 Hz, 1H), 7.16 (ddd, J = 7.6, 5.2, 1.2 Hz, 1H), 7.13 (d, J = 8.0 Hz, 1H), 6.87 (d, J = 8.4 Hz, 4H), 6.44 (d, J = 8.4 Hz, 4H), 5.44 (q, J = 5.6 Hz, 2H), 5.30 (s, 1H), 5.24 (d, J = 4.4 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 164.4, 148.8, 148.1, 136.3, 130.8, 129.4, 123.2, 121.1, 111.4, 57.1, 29.9; IR (neat): 3419, 3255, 3024, 2971, 1739, 1520, 1432, 1365, 1217, 806, 762, 519 cm−1.
4,4′-((4-Nitrophenyl)methylene)bis(N-methyl-2-(trifluoromethyl)aniline) (3af). 95% yield (0.4609 g) as a yellow oil; Rf = 0.43 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.18 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 7.15–7.13 (m, 4H), 6.72 (d, J = 9.2 Hz, 2H), 5.68 (s, 1H), 5.64 (q, J = 4.8 Hz, 2H), 2.74 (d, J = 4.8 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 152.2, 146.0, 145.21, 145.20, 134.0, 130.0, 128.9, 126.3 (q, 4JC–F = 4.0 Hz), 123.7, 111.9, 111.1 (q, JC–F = 29.0 Hz), 52.8, 30.0; IR (neat): 3479, 2937, 2824, 1735, 1624, 1605, 1583, 1515, 1467, 1422, 1345, 1320, 1298, 1259, 1239, 1181, 1142, 1092, 1072, 1043, 1015, 855, 810, 788, 739, 687, 548, 434 cm−1; HRMS (ESI-TOF) m/z calcd for C23H20F6N3O2 [M + H]+ 484.1454, found 484.1454.
4,4′-((4-Nitrophenyl)methylene)bis(3-fluoro-N-methylaniline) (3ag). 85% yield (0.3272 g) as a yellow solid; mp: 152–154 °C; Rf = 0.20 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.16 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 6.62–6.55 (m, 2H), 6.34–6.26 (m, 4H), 5.94 (q, J = 4.8 Hz, 2H), 5.75 (s, 1H), 2.64 (d, J = 5.2 Hz, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 160.9 (d, 1JC–F = 241.0 Hz), 151.4, 150.8 (d, 3JC–F = 11.0 Hz), 146.0, 130.3 (d, 4JC–F = 6.0 Hz), 129.6, 123.5, 114.6 (d, 2JC–F = 15.0 Hz), 107.8, 98.0 (d, 2JC–F = 26.0 Hz), 41.0, 29.6; IR (neat): 3434, 2902, 1735, 1625, 1580, 1511, 1471, 1408, 1341, 1260, 1191, 1175, 1151, 1097, 1054, 1013, 877, 839, 802, 746, 697, 529 cm−1; HRMS (ESI-TOF) m/z calcd for C21H20F2N3O2 [M + H]+ 384.1518, found 384.1518.
5-Fluoro-2-((2-fluoro-4-(methylamino)phenyl)(4-nitrophenyl)methyl)-N-methylaniline (4ag). 13% yield (0.0485 g) as a yellow solid; mp: 120–22 °C; Rf = 0.31 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.15 (d, J = 8.8 Hz, 2H), 7.26 (d, J = 8.8 Hz, 2H), 6.48 (t, J = 8.4 Hz, 1H), 6.41 (t, J = 8.8 Hz, 1H), 6.34–6.25 (m, 4H), 5.94 (q, J = 5.2 Hz, 1H), 5.73 (s, 1H), 5.42–5.37 (m, 1H), 2.65–2.62 (m, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 162.7 (d, 1JC–F = 238.0 Hz), 161.2 (d, 1JC–F = 241.0 Hz), 151.0, 150.9 (d, 3JC–F = 11.0 Hz), 148.7 (d, 3JC–F = 11.0 Hz), 146.1, 130.3 (d, 3JC–F = 6.0 Hz), 130.1, 130.0 (d, 3JC–F = 7.0 Hz), 123.3, 121.6 (d, 4JC–F = 2.0 Hz), 114.6 (2JC–F = 16.0 Hz), 107.7, 100.98 (2JC–F = 21.0 Hz), 98.2 (2JC–F = 25.0 Hz), 96.6 (2JC–F = 26.0 Hz), 42.2, 30.2, 29.6; IR (neat): 3422, 3324, 2977, 2874, 1612, 1514, 1487, 1312, 1251, 1181, 1155, 1110, 1081, 880, 840, 801, 756, 741, 664, 543 cm−1; HRMS (ESI-TOF) m/z calcd for C21H20F2N3O2 [M + H]+ 384.1518, found 384.1514.
4,4′-(Phenylmethylene)bis(N,N-dimethylaniline) (3ah)4k,8a,8b,8c,8i,10e. CAS number 129-73-7; 89% yield (0.2939 g) as a white solid; mp: 94–96 °C; Rf = 0.36 (5% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.26 (t, J = 7.2 Hz, 2H), 7.16 (t, J = 7.2 Hz, 1H), 7.07 (d, J = 7.2 Hz, 2H), 6.89 (d, J = 8.8 Hz, 4H), 6.64 (d, J = 8.8 Hz, 4H), 5.30 (s, 1H), 2.83 (s, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 148.8, 145.4, 132.2, 129.4, 128.9, 128.1, 125.7, 112.4, 54.2, 40.3; IR (neat): 3029, 2882, 2803, 1611, 1517, 1442, 1349, 1215, 1127, 944, 790, 699 cm−1.
4,4′-((4-Nitrophenyl)methylene)bis(N,N-dimethylaniline) (3ai)8a,8c,8i,10e,12c. CAS number 5327-39-9; 95% yield (0.3580 g) as a yellow solid; mp: 176–178 °C; Rf = 0.38 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.16 (d, J = 8.8 Hz, 2H), 7.34 (d, J = 8.4 Hz, 2H), 6.91 (d, J = 8.4 Hz, 4H), 6.67 (d, J = 8.8 Hz, 4H), 5.51 (s, 1H), 2.85 (s, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 153.7, 149.0, 145.7, 130.8, 130.0, 129.4, 123.4, 112.5, 53.9, 40.2; IR (neat): 2920, 2878, 2803, 1931, 1882, 1606, 1512, 1332, 1201, 940, 809, 797, 565 cm−1.
4,4′-((4-Methoxyphenyl)methylene)bis(N,N-dimethylaniline) (3aj)4k,8c,8i,10e. CAS number 641-59-8; 72% yield (0.2583 g) as a white solid; mp: 98–102 °C; Rf = 0.47 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 6.98 (d, J = 8.4 Hz, 2H), 6.89 (d, J = 8.4 Hz, 4H), 6.83 (d, J = 8.8 Hz, 2H), 6.64 (d, J = 8.8 Hz, 4H), 5.25 (s, 1H), 3.71 (s, 3H), 2.84 (s, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 157.3, 148.7, 137.4, 132.6, 129.8, 129.3, 113.5, 112.4, 55.0, 53.4, 40.3; IR (neat): 3006, 2862, 2802, 1739, 1608, 1509, 1347, 1245, 1031, 812, 560 cm−1.
4,4′-(Phenylmethylene)bis(N,N-diethylaniline) (3ak)4k,8j,8k,10e. CAS number 82-90-6; 40% yield (0.1546 g) as a brown oil; Rf = 0.37 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 7.24 (t, J = 7.2 Hz, 2H), 7.14 (t, J = 7.2 Hz, 1H), 7.09 (d, J = 7.2 Hz, 2H), 6.87 (d, J = 8.8 Hz, 4H), 6.55 (d, J = 8.8 Hz, 4H), 5.24 (s, 1H), 3.26 (q, J = 6.8 Hz, 8H), 1.04 (t, J = 6.8 Hz, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 146.1, 146.0, 131.5, 130.1, 129.3, 128.5, 126.1, 111.8, 54.8, 44.1, 12.9; IR (neat): 3074, 2971, 2929, 2889, 2866, 1606, 1267, 1197, 1078, 785, 713 cm−1.
4,4′-((4-Nitrophenyl)methylene)bis(N,N-diethylaniline) (3al)10e. CAS number 81713-52-2; 98% yield (0.4250 g) as a yellow solid; mp: 108–112 °C; Rf = 0.43 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.15 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 8.8 Hz, 4H), 6.58 (d, J = 8.8 Hz, 4H), 5.42 (s, 1H), 3.28 (q, J = 6.8 Hz, 8H), 1.05 (t, J = 6.8 Hz, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 153.9, 145.9, 145.6, 130.0, 129.7, 129.5, 123.3, 111.4, 53.9, 43.6, 12.4; IR (neat): 3074, 2971, 2929, 2889, 2866, 1606, 1510, 1339, 1267, 1197, 1078, 785, 713 cm−1.
4,4-((4-methoxyphenyl)methylene)bis(N,N-diethylaniline) (3am)10e. CAS number 123095-07-8; 34% yield (0.1416 g) as a yellow oil; Rf = 0.43 (10% EtOAc/n-hexane); 1H NMR (400 MHz, DMSO-d6): δ 6.98 (d, J = 8.4 Hz, 2H), 6.84 (d, J = 8.8 Hz, 4H), 6.82 (d, J = 8.8 Hz, 2H), 6.55 (d, J = 8.8 Hz, 4H), 5.17 (s, 1H), 3.70 (s, 3H), 3.27 (q, J = 7.2 Hz, 8H), 1.04 (t, J = 7.2 Hz, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 157.3, 145.6, 137.6, 131.4, 129.8, 129.6, 113.4, 111.4, 55.0, 53.4, 43.6, 12.5; IR (neat): 3064, 2941, 2929, 1739, 1608, 1509, 1347, 1245, 1068, 785, 760, 611, 520 cm−1.
4,4′-(Phenylmethylene)diphenol (3ba)10d,12k,12n,12o,13c. CAS number 4081-02-1; 60% yield (0.1653 g) as a white solid; mp: 146–148 °C; Rf = 0.21 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.24 (s, 2H), 7.26 (t, J = 7.6 Hz, 2H), 7.16 (t, J = 7.2 Hz, 1H), 7.07 (d, J = 7.2 Hz, 2H), 6.87 (d, J = 8.4 Hz, 4H), 6.67 (d, J = 8.8 Hz, 4H), 5.43 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 155.5, 145.0, 134.6, 129.8, 128.9, 128.1, 125.9, 115.0, 54.4; IR (neat): 3181, 3020, 2698, 2592, 1601, 1503, 1445, 1360, 1232, 1171, 818, 715, 572, 549 cm−1.
2-((4-Hydroxyphenyl)(phenyl)methyl)phenol (4ba)12l,13c. CAS number 60054-61-7; 10% yield (0.0283 g) as a white solid; mp: 120–122 °C; Rf = 0.27 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.38 (s, 1H), 9.25 (s, 1H), 7.25 (t, J = 7.6 Hz, 2H), 7.16 (t, J = 7.2 Hz, 1H), 7.08–6.99 (m, 3H), 6.88–6.79 (m, 3H), 6.76–6.65 (m, 4H), 5.72 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 155.6, 154.7, 144.6, 134.0, 130.7, 130.1, 129.8, 129.1, 128.1, 127.2, 125.8, 118.7, 115.1, 115.0, 48.4; IR (neat): 3527, 3334, 2923, 2854, 1738, 1590, 1508, 1455, 1356, 1231, 1170, 1091, 838, 748, 703, 605, 548, 520 cm−1.
4,4′-((4-Nitrophenyl)methylene)diphenol (3bb)12c,12k,12m. CAS number 63647-37-0; 81% yield (0.2603 g) as a yellow solid; mp: 178–180 °C; Rf = 0.44 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.36 (s, 2H), 8.15 (d, J = 8.8 Hz, 2H), 7.33 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.4 Hz, 4H), 6.70 (d, J = 8.8 Hz, 4H), 5.54 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 155.9, 153.2, 145.8, 133.4, 130.1, 129.9, 123.4, 115.3, 54.0; IR (neat): 3359, 6022, 2970, 1891, 1703, 1594, 1508, 1342, 1442, 1218, 1171, 1107, 1042, 1014, 811, 744, 696, 558 cm−1.
2-((4-Hydroxyphenyl)(4-nitrophenyl)methyl)phenol (4bb)12k,12m. CAS number 63118-62-7; 18% yield (0.0578 g) as a viscous yellow oil; Rf = 0.53 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.50 (s, 1H), 9.33 (s, 1H), 8.14 (d, J = 8.8 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 7.06 (t, J = 7.8 Hz, 1H), 6.86 (d, J = 8.4 Hz, 2H), 6.81 (d, J = 8.0 Hz, 1H), 6.80–6.60 (m, 4H), 5.79 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 155.9, 154.7, 152.8, 145.8, 132.4, 130.2, 130.0, 129.6, 129.4, 127.7, 123.4, 118.9, 115.3, 115.2, 48.6; IR (neat): 3344, 3109, 3080, 3019, 2923, 2852, 1898, 1596, 1502, 1455, 1341, 1225, 1176, 1088, 836, 848, 755, 699, 635, 465 cm−1.
4,4′,4′′-Methanetriyltriphenol (3bc)6s,6t,6v,12n. CAS number 603-44-1; 40% yield (0.1169 g) as a white solid; mp: 220–224 °C; Rf = 0.24 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.21 (s, 3H), 6.84 (d, J = 8.4 Hz, 6H), 6.65 (d, J = 8.4 Hz, 6H), 5.21 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 155.4, 135.2, 129.7, 114.9, 53.6; IR (neat): 3238, 3020, 2880, 1601, 1503, 1440, 1356, 1215, 1173, 1099, 1017, 827 cm−1.
4,4′-((2-Hydroxyphenyl)methylene)diphenol (4bc)6u. CAS number 51728-14-4; 15% yield (0.0438 g) as a white solid; mp: 182–184 °C; Rf = 0.30 (40% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.33 (s, 1H), 9.22 (s, 2H), 7.00 (td, J = 8.0, 2.0 Hz, 1H), 6.86–6.64 (m, 11H), 5.61 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 155.4, 154.6, 134.8, 131.4, 130.0, 129.8, 127.0, 118.7, 115.1, 115.0, 47.5; IR (neat): 3509, 3242, 3062, 2922, 1587, 1505, 1447, 1211, 1082, 750 cm−1.
4,4′-((4-Methoxyphenyl)methylene)diphenol (3bd)1q,12m. CAS number 63074-92-0; 50% yield (0.1532 g) as a yellow solid; mp: 122–124 °C; Rf = 0.26 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.22 (s, 2H), 6.96 (d, J = 8.8 Hz, 2H), 6.84 (d, J = 8.4 Hz, 4H), 6.83 (d, J = 8.8 Hz, 2H), 6.66 (d, J = 8.4 Hz, 4H), 5.28 (s, 1H), 3.70 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.4, 155.5, 137.1, 135.1, 129.84, 129.79, 115.0, 113.6, 55.0, 53.6; IR (neat): 3273, 3020, 2952, 2936, 2842, 1592, 1508, 1445, 1218, 1166, 1033, 827, 556 cm−1.
2-((4-Hydroxyphenyl)(4-methoxyphenyl)methyl)phenol (4bd)12m. CAS number 63074-86-2; 12% yield (0.0368 g) as a yellow oil; Rf = 0.31 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.33 (s, 1H), 9.22 (s, 1H), 7.03–6.91 (m, 3H), 6.84–6.76 (m, 5H), 6.73–6.63 (m, 4H), 5.64 (s, 1H), 3.70 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.4, 155.4, 154.6, 136.4, 134.4, 131.0, 129.9, 129.7, 129.5, 127.0, 118.6, 115.1, 115.0, 114.9, 113.5, 55.0, 47.4; IR (neat): 3325, 3028, 2932, 2836, 1894, 1736, 1608, 1507, 1452, 1360, 1291, 1232, 1171, 1105, 1089, 1026, 938, 874, 832, 818, 752, 643, 621, 572, 548, 512, 403 cm−1.
4,4′-((4-Nitrophenyl)methylene)bis(2-methoxyphenol) (3be). 88% yield (0.3375 g) as a yellow solid; mp: 68–70 °C; Rf = 0.23 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.93 (brs, 2H), 8.15 (d, J = 8.8 Hz, 2H), 7.36 (d, J = 8.8 Hz, 2H), 6.72 (d, J = 8.0 Hz, 2H), 6.72 (s, 2H), 6.46 (d, J = 8.0 Hz, 2H), 5.55 (s, 1H), 3.66 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 153.0, 147.5, 145.8, 145.2, 133.8, 130.1, 123.4, 121.3, 115.4, 113.3, 55.6, 54.6; IR (neat): 3481, 2934, 2844, 1593, 1551, 1342, 1268, 1217, 1065, 1031, 856, 824, 753, 728, 424 cm−1; HRMS (ESI-TOF) m/z calcd for C21H19NO6Na [M + Na]+ 404.1105, found 404.1104.
4-((2-Hydroxy-3-methoxyphenyl)(4-nitrophenyl)methyl)-2-methoxyphenol (4be). 10% yield (0.0403 g) as a yellow solid; mp: 46–48 °C; Rf = 0.31 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.91 (s, 1H), 8.70 (s, 1H), 8.14 (d, J = 8.8 Hz, 2H), 7.28 (d, J = 8.8 Hz, 2H), 6.86 (dd, J = 8.0, 1.2 Hz, 1H), 6.73–6.60 (m, 3H), 6.44–6.30 (m, 2H), 5.84 (s, 1H), 3.78 (s, 3H), 3.65 (s, 3H); 13C-NMR (100 MHz, DMSO-d6); δ 152.7, 147.6, 147.5, 145.8, 145.2, 143.7, 133.1, 130.0, 129.6, 123.3, 121.5, 118.6, 115.3, 113.6, 110.2, 55.8, 55.6, 48.9; IR (neat): 3402, 3054, 2970, 2926, 2850, 1660, 1587, 1484, 1354, 1211, 1201, 1092, 1009, 772, 740, 422 cm−1; HRMS (ESI-TOF) m/z calcd for C21H19NO6Na [M + Na]+ 404.1105, found 404.1105.
4,4′-((4-Nitrophenyl)methylene)bis(2-bromophenol) (3bf). 81% yield (0.3873 g) as a yellow oil; Rf = 0.17 (30% EtOAc/n-hexane); 1H NMR (400 MHz, DMSO-d6): δ 10.27 (s, 2H), 8.18 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 7.18 (s, 2H), 6.91 (s, 4H), 5.64 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 152.8, 151.7, 146.1, 134.8, 133.0, 130.1, 129.2, 123.7, 116.5, 109.4, 52.8; IR (neat): 3448, 3069, 2927, 2847, 1601, 1510, 1494, 1494, 1337, 1258, 1176, 1038, 848, 811, 699 cm−1; HRMS (ESI-TOF) m/z calcd for C19H14Br2NO4 [M + H]+ 477.9284, found 477.9284.
2-Bromo-4-((3-bromo-2-hydroxyphenyl)(4-nitrophenyl)methyl)phenol (4bf). 15% yield (0.0728 g) as a yellow oil; Rf = 0.34 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 10.27 (s, 1H), 9.25 (s, 1H), 8.17 (d, J = 8.8 Hz, 2H), 7.43 (dd, J = 7.6, 1.6 Hz, 1H), 7.30 (d, J = 8.8 Hz, 2H), 7.14 (d, J = 2.0 Hz, 1H), 6.92 (d, J = 8.4 Hz, 1H), 6.88 (dd, J = 8.4, 2.4 Hz, 1H), 6.77 (t, J = 7.6 Hz, 1H), 6.71 (dd, J = 8.0, 1.6 Hz, 1H), 5.95 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 152.9, 151.3, 151.2, 146.1, 133.9, 133.1, 132.5, 131.5, 130.1, 129.5, 129.2, 123.6, 121.2, 116.5, 111.8, 109.4, 48.8; IR (neat): 3461, 2923, 2853, 1736, 1594, 1513, 1492, 1468, 1447, 1342, 1283, 1239, 1108, 1071, 1043, 1014, 957, 906, 894, 824, 767, 734, 708, 690, 666, 554, 489 cm−1; HRMS (ESI-TOF) m/z calcd for C19H14Br2NO4 [M + H]+ 477.9284, found 477.111.
4,4′-((4-Nitrophenyl)methylene)bis(2-fluorophenol) (3bg). 89% yield (0.3192 g) as a colorless oil; Rf = 0.17 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.86 (s, 2H), 8.16 (d, J = 8.8 Hz, 2H), 7.36 (d, J = 8.8 Hz, 2H), 6.90 (t, J = 8.4 Hz, 2H), 6.84 (dd, J = 12.4, 2.4 Hz, 2H), 6.72 (dd, J = 8.0, 2.0 Hz, 2H), 5.61 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 151.87, 150.9 (d, 1JC–F = 240.0 Hz), 146.1, 143.6 (d, 2JC–F = 12.0 Hz), 134.0 (d, 3JC–F = 6.0 Hz), 130.2, 125.1 (d, 4JC–F = 3.0 Hz), 123.7, 117.9 (d, 4JC–F = 3.0 Hz), 116.6 (d, 2JC–F = 19.0 Hz), 53.4; IR (neat): 3351, 1700, 1624, 1595, 1509, 1439, 1287, 1274, 1234, 1188, 1105, 1015, 957, 859, 811, 756, 733, 697, 576, 526, 452 cm−1; HRMS (ESI-TOF) m/z calcd for C19H14F2NO4 [M + H]+ 358.0885, found 365.0899.
2-Fluoro-4-((3-fluoro-2-hydroxyphenyl)(4-nitrophenyl)methyl)phenol (4bg). 11% yield (0.0400 g) as a colorless oil; Rf = 0.26 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.83 (s, 1H), 9.76 (s, 1H), 8.16 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 7.08 (ddd, J = 10.4, 8.0, 1.6 Hz, 1H), 6.91 (t, J = 8.8 Hz, 1H), 6.82 (dd, J = 12.4, 2.0 Hz, 1H), 6.76 (td, J = 7.6, 5.2 Hz, 1H), 6.70 (dd, J = 8.4, 2.0 Hz, 1H), 6.56 (d, J = 7.6 Hz, 1H), 5.89 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 151.6 (d, 1JC–F = 237.0 Hz), 151.5, 150.9 (d, 1JC–F = 239.0 Hz), 146.0, 143.6 (d, 2JC–F = 12.0 Hz), 142.2 (d, 2JC–F = 14.0 Hz), 133.1 (d, 4JC–F = 5.0 Hz), 132.5 (d, 3JC–F = 3.0 Hz), 130.0, 125.2 (d, 4JC–F = 2.0 Hz), 125.0 (d, 4JC–F = 2.0 Hz), 123.5, 119.0 (d, 3JC–F = 7.0 Hz), 117.8 (d, 3JC–F = 3.0 Hz), 116.7 (d, 2JC–F = 19.0 Hz), 114.4 (d, 2JC–F = 19.0 Hz), 48.4; IR (neat): 3369, 2928, 1699, 1594, 1514, 1474, 1344, 1262, 1192, 1108, 1066, 825, 756, 728, 454, 418 cm−1; HRMS (ESI-TOF) m/z calcd for C19H14F2NO4 [M + H]+ 358.0885, found 365.00904.
4,4′-((4-Nitrophenyl)methylene)bis(3-bromophenol) (3bh). 50% yield (0.2394 g) as a white solid; mp: 142–144 °C; Rf = 0.23 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 9.92 (s, 2H), 8.18 (d, J = 8.8 Hz, 2H), 7.23 (d, J = 8.8 Hz, 2H), 7.05 (d, J = 2.8 Hz, 2H), 6.74 (dd, J = 8.4, 2.4 Hz, 2H), 6.55 (d, J = 8.4 Hz, 2H), 5.96 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 157.2, 150.1, 146.2, 131.2, 130.9, 130.4, 124.8, 123.7, 119.7, 115.0, 53.6; IR (neat): 3406, 3205, 2957, 2922, 2849, 1603, 1484, 1512, 1339, 1211, 1031, 855, 806, 692, 551 cm−1; HRMS (ESI-TOF) m/z calcd for C19H14Br2NO4 [M + H]+ 477.9284, found 477.9104.
5-Bromo-2-((2-bromo-4-hydroxyphenyl)(4-nitrophenyl)methyl)phenol (4bh). 50% yield (0.2390 g) as a colorless oil; Rf = 0.31 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 10.11 (brs, 1H), 9.87 (brs, 1H), 8.17 (d, J = 8.4 Hz, 2H), 7.23 (d, J = 8.8 Hz, 2H), 7.04 (d, J = 2.8 Hz, 1H), 6.70 (d, J = 2.0 Hz, 1H), 6.94 (dd, J = 8.0, 1.6 Hz, 1H), 6.74 (dd, J = 8.4, 2.4 Hz, 1H), 6.61 (d, J = 8.4 Hz, 1H), 6.48 (d, J = 8.0 Hz, 1H), 5.98 (s, 1H); 13C-NMR (100 MHz, DMSO-d6): δ 157.1, 156.0, 150.6, 146.1, 131.2, 131.1, 130.9, 130.2, 128.0, 124.6, 123.6, 121.7, 120.2, 119.6, 117.8, 114.9, 48.1; IR (neat): 3359, 3101, 3022, 2970, 1611, 1594, 1508, 1432, 1342, 1218, 1171, 1107, 1042, 811, 744, 696, 558 cm−1; HRMS (ESI-TOF) m/z calcd for C19H14Br2NO4 [M + H]+ 477.9284, found 477.9105.
4,4′-((4-Nitrophenyl)methylene)bis(methoxybenzene) (3bi)10d,12c,12k,12m,12n. CAS number 128527-22-0; 85% yield (0.2970 g) as a colorless oil; Rf = 0.37 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.16 (d, J = 8.8 Hz, 2H), 7.34 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.8 Hz, 4H), 6.88 (d, J = 8.8 Hz, 4H), 5.67 (s, 1H), 3.71 (s, 6H); 13C-NMR (100 MHz, DMSO-d6): δ 157.9, 152.6, 145.9, 134.9, 130.1, 130.0, 123.5, 114.0, 55.0, 53.8; IR (neat): 3001, 2933, 2835, 1606, 1582, 1506, 1462, 1343, 1243, 1175, 1110, 1030, 821, 801, 564 cm−1.
1-Methoxy-2-((4-methoxyphenyl)(4-nitrophenyl)methyl)benzene (4bi)12k,12m. CAS number 936568-71-7; 14% yield (0.0489 g) as a colorless oil; Rf = 0.43 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.14 (d, J = 8.8 Hz, 2H), 7.27 (d, J = 8.8 Hz, 2H), 7.27–7.23 (m, 1H), 7.01 (d, J = 7.6 Hz, 1H), 6.97 (d, J = 8.8 Hz, 2H), 6.90–6.85 (m, 3H), 6.77 (dd, J = 7.6, 1.6 Hz, 1H), 5.89 (s, 1H), 3.72 (s, 3H), 3.67 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 157.9, 156.6, 152.3, 145.9, 133.9, 130.8, 130.2, 130.0, 129.5, 128.2, 123.4, 120.3, 113.9, 111.3, 55.5, 55.0, 48.4; IR (neat): 3007, 2944, 2935, 3840, 1850, 1602, 1507, 1450, 1344, 1244, 1210, 1170, 1121, 1029, 820, 800, 740, 695 cm−1.
4,4′-((4-Nitrophenyl)methylene)bis(1,3-dimethoxybenzene) (3bj)10d,12c. 80% yield (0.3280 g) as a white solid; mp: 146–148 °C; Rf = 0.43 (30% EtOAc/n-hexane); 1H-NMR (400 MHz, CDCl3): d 8.08 (d, J = 8.8 Hz, 2H), 7.18 (d, J = 8.4 Hz, 2H), 6.63 (d, J = 8.4 Hz, 2H), 6.47 (d, J = 2.4 Hz, 2H), 6.38 (dd, J = 8.4, 2.4 Hz, 2H), 6.05 (s, 1H), 3.79 (s, 6H), 3.68 (s, 6H); 13C-NMR (100 MHz, CDCl3): δ 159.7, 158.0, 153.2, 146.1, 130.3, 129.7, 123.4, 123.1, 103.8, 98.8, 55.5, 55.3, 42.5; IR (neat): 2993, 2962, 2933, 2837, 1734, 1604, 1586, 1514, 1504, 1435, 1342, 1291, 1208, 1192, 1175, 1103, 1029, 937, 822, 697 cm−1; HRMS (ESI-TOF) m/z calcd for C23H24NO6 [M + H]+ 410.1598, found 410.1596.
4,4′-((4-Methoxyphenyl)methylene)bis(1,3-dimethoxybenzene) (3bk)12i. CAS number 1578250-27-7; 73% yield (0.2875 g) as a white solid; mp: 122–124 °C; Rf = 0.26 (10% EtOAc/n-hexane); 1H-NMR (400 MHz, CDCl3): δ 6.95 (d, J = 8.8 Hz, 2H), 6.77 (d, J = 8.8 Hz, 2H), 6.68 (d, J = 8.4 Hz, 2H), 6.45 (d, J = 2.4 Hz, 2H), 6.36 (dd, J = 8.4, 2.8 Hz, 2H), 5.95 (s, 1H), 3.78 (s, 6H), 3.77 (s, 3H), 3.68 (s, 6H); 13C-NMR (100 MHz, CDCl3): δ 159.1, 158.0, 157.5, 136.6, 130.2, 130.0, 125.7, 113.2, 103.5, 98.7, 55.7, 55.2, 55.1, 41.3; IR (neat): 2994, 2957, 2932, 2836, 1608, 1584, 1501, 1455, 1435, 1243, 1205, 1110, 1030, 936, 829, 821, 795, 564 cm−1.
2,2′-((4-Nitrophenyl)methylene)bis(1,3,5-trimethoxybenzene) (3bl)12k. >99% yield (0.4751 g) as a white solid; mp: 138–140 °C; Rf = 0.23 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): δ 8.01 (d, J = 8.8 Hz, 2H), 7.08 (d, J = 8.4 Hz, 2H), 6.17 (s, 1H), 6.15 (s, 4H), 3.74 (s, 6H), 3.48 (s, 12H); 13C-NMR (100 MHz, DMSO-d6): δ 159.5, 159.2, 154.9, 144.7, 128.5, 122.4, 111.1, 111.0, 91.4, 55.7, 55.1, 36.7; IR (neat): 2995, 2964, 2961, 2823, 2811, 1605, 1581, 1504, 1430, 1341, 1211, 1110, 1102, 1050, 951, 842, 612 cm−1; HRMS (ESI-TOF) m/z calcd for C25H28NO8 [M + H]+ 470.1809, found 470.1809.
2,2′-((4-Methoxyphenyl)methylene)bis(1,3,5-trimethoxybenzene) (3bm). 56% yield (0.2545 g) as a white solid; mp: 114–116 °C; Rf = 0.35 (20% EtOAc/n-hexane); 1H-NMR (400 MHz, DMSO-d6): d 6.78 (d, J = 8.4 Hz, 2H), 6.65 (d, J = 8.8 Hz, 2H), 6.11 (s, 4H), 6.01 (s, 1H), 3.72 (s, 6H), 3.67 (s, 3H), 3.46 (s, 12H); 13C-NMR (100 MHz, DMSO-d6): d 159.3, 158.8, 156.2, 137.3, 128.6, 113.1, 112.2, 91.4, 55.7, 54.9, 54.8, 35.6; IR (neat): 3001, 2959, 2935, 2836, 1585, 1509, 1451, 1410, 1332, 1226, 1200, 1110, 1057, 1033, 949, 810, 791, 750, 632, 542, 519 cm−1; HRMS (ESI-TOF) m/z calcd for C26H31O7 [M + H]+ 455.2064, found 455.2065.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was financially supported by the Research Grant of Burapha University through National Research Council of Thailand (Grant no. 162/2561), the Office of National Higher Education Science Research and Innovation Policy Council (NXPO) (Grant no. BO5F630030), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), and the Research Unit in Synthetic Compounds and Synthetic Analogues from Natural Product for Drug Discovery (RSND), Burapha University. Special thanks to Dr Ron Beckett, Faculty of Science, Burapha University for his comments and proofreading the English manuscript.
Notes and references
-
(a) R. F. A. Gomes, J. A. S. Coelho, R. F. M. Frade, A. F. Trindade and C. A. M. Afonso, J. Org. Chem., 2015, 80, 10404–10411 CrossRef CAS PubMed;
(b) R. Palchaudhuri, V. Nesterenko and P. J. Hergenrother, J. Am. Chem. Soc., 2008, 130, 10274–10281 CrossRef CAS PubMed;
(c) Shagufta, A. K. Srivastava, R. Sharma, R. Mishra, A. K. Balapure, P. S. R. Murthy and G. Panda, Bioorg. Med. Chem., 2006, 14(5), 1497–1505 CrossRef CAS PubMed;
(d) R. S. Dothager, K. S. Putt, B. J. Allen, B. J. Leslie, V. Nesterenko and P. J. Hergenrother, J. Am. Chem. Soc., 2005, 127, 8686–8696 CrossRef CAS PubMed;
(e) Q. Li, K. W. Woods, W. Wang, N.-H. Lin, A. Claiborne, W. Gu, J. Cohen, V. S. Stoll, C. Hutchins, D. Frost, S. H. Rosenberg and H. L. Sham, Bioorg. Med. Chem. Lett., 2005, 15, 2033–2039 CrossRef CAS PubMed;
(f) S. B. Bodendiek, C. Rubinos, M. P. Trelles, N. Coleman, D. P. Jenkins, H. Wulff and M. Srinivas, Front. Pharmacol., 2012, 3, 106 CAS;
(g) R. A. Al-Qawasmeh, Y. Lee, M.-Y. Cao, X. Gu, A. Vassilakos, J. A. Wright and A. Young, Bioorg. Med. Chem. Lett., 2004, 14, 347–350 CrossRef CAS PubMed;
(h) A. Natarajan, Y.-H. Fan, H. Chen, Y. Guo, J. Iyasere, F. Harbinski, W. J. Christ, H. Aktas and J. A. Halperin, J. Med. Chem., 2004, 47, 1882–1885 CrossRef CAS PubMed;
(i) C. Ricco, F. Abdmouleh, C. Riccobono, L. Guenineche, F. Martin, E. Goya-Jorge, N. Lagarde, B. Liagre, M. B. Ali, C. Ferroud, M. E. Arbi and M. S.-I. Veitía, Bioorg. Chem., 2020, 96, 103591 CrossRef CAS PubMed;
(j) G. Panda, J. Shagufta, K. Mishra, V. Chaturvedi, A. K. Srivastave, R. Srivastava and B. S. Srivastava, Bioorg. Med. Chem., 2004, 12, 5269–5276 CrossRef CAS PubMed;
(k) G. Panda, M. K. Parai, S. K. Das, Shagufta, M. Sinha, V. Chaturvedi, A. K. Srivastava, Y. S. Manju, A. N. Gaikwad and S. Sinha, Eur. J. Med. Chem., 2007, 42, 410–419 CrossRef CAS PubMed;
(l) M. K. Parai, G. Panda, V. Chaturvedi, Y. K. Manju and S. Sinha, Bioorg. Med. Chem. Lett., 2008, 18, 289–292 CrossRef CAS PubMed;
(m) P. Singh, S. K. Manna, A. K. Jana, T. Saha, P. Mishra, S. Bera, M. K. Parai, M. S. L. Kumar, S. Mondal, P. Trivedi, V. Chaturvedi, S. Singh, S. Sinha and G. Panda, Eur. J. Med. Chem., 2015, 95, 357–368 CrossRef CAS PubMed;
(n) R. S. Upadhayaya, J. K. Vandavasi, N. R. Vasireddy, V. Sharma, S. S. Dixit and J. Chattopadhyaya, Bioorg. Med. Chem., 2009, 17, 2830–2841 CrossRef CAS PubMed;
(o) M.-P. Lézé, M. L. Borgne, P. Marchand, D. Loquet, M. Kogler, G. L. Baut, A. Palusczak and R. W. Hartmann, J. Enzyme Inhib. Med. Chem., 2004, 19, 549–557 CrossRef PubMed;
(p) M. Nibu, K. Yokomizo, M. Uyeda and K. Sumoto, Chem. Pharm. Bull., 2005, 53, 1171–1174 CrossRef PubMed;
(q) N. Mibu, K. Yokomizo, M. Uyeda and K. Sumoto, Chem. Pharm. Bull., 2003, 11, 1325–1327 CrossRef PubMed;
(r) M. L. Borgne, P. Marchand, B. Delevoye-Seiller, J.-M. Robert, G. L. Baut, R. W. Hartmann and M. Palzer, Bioorg. Med. Chem. Lett., 1999, 9, 333–336 CrossRef PubMed;
(s) T. Küçükkılınç and I. Özer, Arch. Biochem. Biophys., 2005, 440, 118–122 CrossRef PubMed;
(t) T. T. Kucukkilinc and I. Ozer, Chem.–Biol. Interact., 2008, 175, 309–311 CrossRef CAS PubMed;
(u) Y. Y. Yücel, Y. O. Tacal and I. Özer, Arch. Biochem. Biophys., 2008, 478, 201–205 CrossRef PubMed;
(v) R. A. Schnick, Prog. Fish-Cult., 1988, 50, 190–196 CrossRef;
(w) J. M. Bastidas, P. Pinilla, E. Cano, J. L. Polo and S. Miguel, Corros. Sci., 2003, 45, 427–449 CrossRef;
(x) J. Finer, J. Chabala and L. Evan, US Pat., WO2002056880A1, 2002.
-
(a) R. D. Bindal, J. T. Golab and J. A. Katzenellenbogen, J. Am. Chem. Soc., 1990, 112, 7861–7868 CrossRef CAS;
(b) L. Bai, N. Masukawa, M. Yamaki and S. Takagi, Phytochemistry, 1998, 47, 1637–1640 CrossRef CAS;
(c) C. Jin, R. G. Michetich and M. Daneshtalab, Phytochemistry, 1999, 50, 505–508 CrossRef CAS.
- D. F. Duxbury, Chem. Rev., 1993, 93, 381–433 CrossRef CAS.
-
(a) P. F. Gordon and P. Gregory, Organic Chemistry in Colour, Springer, New York, 1983, p. 242 Search PubMed;
(b) K. Venkata-raman, in The Chemistry of Synthetic Dyes, Academic, New York, 1971, vol. 4 Search PubMed;
(c) E. Gurr, Synthetic Dyes in Biology, Medicine and Chemistry, Academic, New York, 1971 Search PubMed;
(d) R. Muthyala, A. R. Katritzky and X. Lan, Dyes Pigm., 1994, 25, 303–324 CrossRef CAS;
(e) M. Irie, J. Am. Chem. Soc., 1983, 105, 2078–2079 CrossRef CAS;
(f) V. V. Ghaisas, B. J. Kane and F. F. Nord, J. Org. Chem., 1958, 23, 560–565 CrossRef CAS;
(g) C. D. Mason and F. F. Nord, J. Org. Chem., 1951, 16, 722–727 CrossRef CAS;
(h) D. Fisher, W. R. Caseri and G. Hähner, J. Colloid Interface Sci., 1998, 198, 337–346 CrossRef;
(i) D. R. Doerge, H. C. Chang, R. L. Divi and M. I. Churchwell, Chem. Res. Toxicol., 1998, 11, 1098–1110 Search PubMed;
(j) D. R. Doerge, M. I. Churchwell, T. A. Gehring, Y. M. Pu and S. M. Plakas, Rapid Commun. Mass Spectrom., 1998, 12, 1625–1634 CrossRef CAS PubMed;
(k) C.-p. Dong, S. Kodama, A. Uematsu, A. Nomoto, M. Ueshima and A. Ogawa, J. Org. Chem., 2017, 82, 12530–12538 CrossRef CAS PubMed.
-
(a) A. C. Bhasikuttan, J. Mohanty, W. M. Nau and H. Pal, Angew. Chem., 2007, 119, 4198–4200 CrossRef;
(b) Y. Urano, M. Kamiya, K. Kanda, T. Ueno, K. Hirose and T. Nagano, J. Am. Chem. Soc., 2005, 127, 4888–4894 CrossRef CAS PubMed;
(c) H. Abe, J. Wang, K. Furukawa, K. Oki, M. Uda, S. Tsuneda and Y. Ito, Bioconjugate Chem., 2008, 19, 1219–1226 CrossRef CAS PubMed;
(d) M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC;
(e) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
-
(a) D. Likhatchev, L. Alexandrova, M. Tlenkopatchev, R. Vilar and R. Vera-Graziano, J. Appl. Polym. Sci., 1995, 57, 37–44 CrossRef CAS;
(b) T. Akhter, S. C. Mun, S. Saeed, O. O. Park and H. M. Siddiqi, RSC Adv., 2015, 5(87), 71183–71189 RSC;
(c) T. Li, H. Huang, L. Wang and Y. Chen, RSC Adv., 2017, 7, 40996–41003 RSC;
(d) R. Hariharan, S. Bhuvana, N. Amutha and M. Sarojadevi, High Perform. Polym., 2006, 18, 893–905 CrossRef CAS;
(e) X. H. Huang, W. Huang, J. Y. Liu, L. L. Meng and D. Y. Yan, Polym. Int., 2012, 61, 1503–1509 CrossRef CAS;
(f) W. Huang, D. Y. Yan and Q. H. Lu, Macromol. Rapid Commun., 2001, 22, 1481–1484 CrossRef CAS;
(g) M. Yan, F. Jiang, X. Zhao and N. Liu, J. Appl. Polym. Sci., 2008, 109, 2460–2464 CrossRef CAS;
(h) B.-K. Chen, T.-M. Chiu and S.-Y. Tsay, J. Appl. Polym. Sci., 2004, 94, 382–393 CrossRef CAS;
(i) T. Nakayama, A. Mochizuki and M. Ueda, React. Funct. Polym., 1996, 30, 109–115 CrossRef CAS;
(j) Y.-T. Chang, C.-F. Shu, C.-M. Leu and K.-H. Wei, J. Polym. Sci., 2003, 41, 3726–3735 CrossRef CAS;
(k) C. Hamciuc, I.-D. Carja, E. Hamciuc, T. Vlad-Bubulac and M. Ignat, Polym. Adv. Technol., 2013, 24, 258–265 CrossRef CAS;
(l) Y. Huang, J. Qin and Y. Gu, J. Appl. Polym. Sci., 2004, 93, 1198–1202 CrossRef CAS;
(m) D. Likhachev, L. Alexandrova, M. Tlenkopatchev, A. Martinez-Richa and R. Vera-Graziano, J. Appl. Polym. Sci., 1996, 61, 815–818 CrossRef;
(n) M. Ueda and T. Nakayama, Macromolecules, 1996, 29, 6427–6431 CrossRef CAS;
(o) N. Batina, M. Kunitake and K. Itaya, J. Electroanal. Chem., 1996, 405, 245–250 CrossRef;
(p) B. K. Bahuleyan, G. W. Son, D.-W. Park, C.-S. Ha and I. Kim, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1066–1082 CrossRef CAS;
(q) B. K. Bahuleyan, U. Lee, C.-S. Ha and I. Kim, Appl. Catal., A, 2008, 351, 36–44 CrossRef CAS;
(r) R. J. Kamel, M. A. Mutar and F. S. Khlewee, Int. J. Res. Pharm. Sci., 2019, 10(3), 1936–1941 CrossRef CAS;
(s) L. Luo, Y. Meng, T. Qiu and X. Li, J. Appl. Polym. Sci., 2013, 130(2), 1064–1073 CrossRef CAS;
(t) T. Liu, Y. Nie, R. Chen, L. Zhang, Y. Meng and X. Li, J. Mater. Chem. A, 2015, 3(3), 1188–1198 RSC;
(u) T. L. Caskey, T. L. Parker and P. H. Martin, US Pat., US4447598A, 1984;
(v) T. Fukuoka, H. Uyama and S. Kobayashi, Macromolecules, 2004, 37, 5911–5915 CrossRef CAS.
-
(a) D. Qiu, H. Meng, L. Jin, S. Wang, S. Tang, X. Wang, F. Mo, Y. Zhang and J. Wang, Angew. Chem., 2013, 125, 11795–11798 CrossRef;
(b) J.-J. Dai, C. Fang, B. Xiao, J. Yi, Z.-J. Liu, X. Lu, L. Liu and Y. Fu, J. Am. Chem. Soc., 2013, 135, 8436–8439 CrossRef CAS PubMed;
(c) D. P. Hari and B. Konig, Angew. Chem., Int. Ed., 2013, 52, 4734–4743 CrossRef CAS PubMed;
(d) Y. Xu, B. Li, X. Zhang and X. Fan, J. Org. Chem., 2017, 82, 9637–9646 CrossRef CAS PubMed.
-
(a) C. S. Reddy, A. Nagaraj, A. Srinivas and G. P. Reddy, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2009, 48, 248–254 Search PubMed;
(b) M. Alvaro, H. García, A. Sanjuán and M. Esplá, Appl. Catal., A, 1998, 175, 105–112 CrossRef CAS;
(c) G. R. Bardajee, Beilstein J. Org. Chem., 2011, 7, 135–144 CrossRef PubMed;
(d) J. Liu, T. He and L. Wang, Tetrahedron, 2011, 67, 3420–3426 CrossRef CAS;
(e) S. S. Ganesan and A. Ganesan, Tetrahedron Lett., 2014, 55, 694–698 CrossRef;
(f) J. Kothandapani, A. Ganesan, P. Vairaprakash and S. S. Ganesan, Tetrahedron Lett., 2015, 56, 2238–2242 CrossRef CAS;
(g) J. Kothandapani, A. Ganesan and S. S. Ganesan, Tetrahedron Lett., 2015, 56, 5568–5572 CrossRef CAS;
(h) X. Zhang, X. Zhang, N. Li, X. Xu, R. Qiu and S. Yin, Tetrahedron Lett., 2014, 55, 120–123 CrossRef CAS;
(i) H. M. Bachhav, B. S. Takale and V. N. Telvekar, Synth. Commun., 2013, 43, 1909–1914 CrossRef CAS;
(j) M. Periasamy, N. Kishorebabu and K. N. Jayakumar, Tetrahedron Lett., 2007, 48, 1955–1958 CrossRef CAS;
(k) M. Periasamy, N. Jayakumar and P. Bharathi, J. Org. Chem., 2000, 65, 3548–3550 CrossRef CAS PubMed.
-
(a) D. Guzmán-Lucero, J. Guzmán, D. Likhatchev and R. Martínez-Palou, Tetrahedron Lett., 2005, 46, 1119–1122 CrossRef;
(b) R. Nallagonda, M. Rehan and P. Ghorai, J. Org. Chem., 2014, 79, 2934–2943 CrossRef CAS PubMed;
(c) S. J. Ahmadi, M. Hosseinpour and S. Sadjadi, Green Chem. Lett. Rev., 2012, 5(3), 403–407 CrossRef CAS;
(d) S. Ajaikumar and A. Pandurangan, J. Mol. Catal. A: Chem., 2008, 286, 21–30 CrossRef CAS;
(e) P. J. Grisdale, J. C. Doty, T. H. Regan and J. L. R. Williams, J. Org. Chem., 1967, 32(8), 2401–2405 CrossRef CAS.
-
(a) A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed;
(b) A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed;
(c) W. Senapak, R. Saeeng, J. Jaratjaroonphong, T. Kasemsuk and U. Sirion, Org. Biomol. Chem., 2016, 14, 1302–1310 RSC;
(d) A. Wang, X. Zheng, Z. Zhao, C. Li, Y. Cui, X. Zheng, J. Yin and G. Yang, Appl. Catal., A, 2014, 482, 198–204 CrossRef CAS;
(e) C. Mukhopadhyay, A. Datta and P. K. Tapaswi, Synth. Commun., 2012, 42, 2453–2463 CrossRef CAS;
(f) Y. Gu, F. Shi and Y. Deng, J. Mol. Catal. A: Chem., 2004, 212, 71–75 CrossRef CAS;
(g) G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang and D. Sun, Green Chem., 2004, 6, 75–77 RSC;
(h) A. Zare, F. Abi, A. R. Moosavi-Zare, M. H. Beyzavi and M. A. Zolfigol, J. Mol. Liq., 2013, 178, 113–121 CrossRef CAS;
(i) A. S. Smarasekara and O. S. Owereh, Catal. Commun., 2010, 11, 1072–1075 CrossRef;
(j) A. Taheri, B. Lai, C. Cheng and Y. Gu, Green Chem., 2015, 17, 812–816 RSC;
(k) K. Qiao and C. Yokoyama, Chem. Lett., 2004, 33, 472–473 CrossRef CAS;
(l) V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615–2665 CrossRef PubMed;
(m) Z. Duan, Y. Gu, J. Zhang, L. Zhu and Y. Deng, J. Mol. Catal. A: Chem., 2006, 250, 163–168 CrossRef CAS;
(n) S. Sahoo, T. Joseph and S. B. Halligudi, J. Mol. Catal. A: Chem., 2006, 250, 179–182 CrossRef;
(o) D. Fang, Q.-R. Shi, J. Cheng, K. Gong and Z.-L. Liu, Appl. Catal., A, 2008, 345, 158–163 CrossRef CAS;
(p) X. Lui, L. Xiao, H. Wu, Z. Li, J. Chen and C. Xia, Catal. Commun., 2009, 10, 424–427 CrossRef;
(q) F. Dong, F. Zhenghao and L. Zuliang, Catal. Commun., 2009, 10, 1267–1270 CrossRef CAS;
(r) X. Liang and C. Qi, Catal. Commun., 2011, 12, 808–812 CrossRef CAS;
(s) M. Vafaeezadeh and H. Alinezhad, J. Mol. Liq., 2016, 218, 95–105 CrossRef CAS;
(t) A. K. Bagdi and A. Hajra, RSC Adv., 2014, 4, 23287–23291 RSC;
(u) T. Chang, L. He, L. Bian, H. Han, M. Yuan and X. Gao, RSC Adv., 2014, 4, 727–731 RSC;
(v) A. R. Hajipour, Y. Ghayeb, N. Sheikhan and A. E. Ruoho, Tetrahedron Lett., 2009, 50, 5649–5651 CrossRef CAS;
(w) R. Kore and R. Srivastava, J. Mol. Catal. A: Chem., 2011, 345, 117–126 CrossRef CAS;
(x) F. Han, L. Yang, Z. Li and C. Xia, Org. Biomol. Chem., 2012, 10, 346–354 RSC;
(y) L. He, S. Qin, T. Chang, Y. Sun and X. Gao, Catal. Sci. Technol., 2013, 3, 1102–1107 RSC;
(z) Z. Chen, Q. Zhu and W. Su, Tetrahedron Lett., 2011, 52, 2601–2604 CrossRef CAS;
(a
a) D. Forbes and K. J. Weaver, J. Mol. Catal. A: Chem., 2004, 214, 129–132 CrossRef CAS;
(a
b) J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J. P. Bazureau and J. Hamelin, Catal. Commun., 2002, 3, 185–190 CrossRef CAS;
(a
c) H. Xing, T. Wang, Z. Zhou and Y. Dai, Ind. Eng. Chem. Res., 2005, 44, 4147–4150 CrossRef CAS;
(a
d) F. Dong, J. Luo, X.-L. Zhou and Z.-L. Liu, Catal. Lett., 2007, 116, 76–80 CrossRef;
(a
e) K. Qiao, H. Hagiwara and C. Yokoyama, J. Mol. Catal. A: Chem., 2006, 246, 65–69 CrossRef CAS;
(a
f) S. Kitaoka, K. Nobuoka and Y. Ishikawa, Chem. Commun., 2004, 1902–1903 RSC;
(a
g) H. Xing, T. Wang, Z. Zhou and Y. Dai, J. Mol. Catal. A: Chem., 2007, 264, 53–59 CrossRef CAS;
(a
h) T. Huang, P.-F. Yan, Z. Xu, X. Liu, Q. Xin, H. Liu and Z. C. Zhang, J. Phys. Chem. B, 2018, 122(22), 6007–6016 CrossRef CAS PubMed;
(a
i) M. H. Kowsari and M. Fakhraee, J. Chem. Eng. Data, 2015, 60(3), 551–560 CrossRef CAS;
(a
j) H. Watanabe, H. Doi, S. Saito, M. Matsugami, K. Fujii, R. Kanzaki, Y. Kameda and Y. Umebayashi, J. Mol. Liq., 2016, 217, 35–42 CrossRef CAS;
(a
k) C. Wu, L.-H. Lu, A.-Z. Peng, G.-K. Jia, C. Peng, Z. Cao, Z. Tang, W.-M. He and X. Xu, Green Chem., 2018, 20(16), 3683–3688 RSC;
(a
l) Z. Cao, Q. Zhu, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019, 30, 2132–2138 CrossRef CAS;
(a
m) M. Li, F. Wu and Y. Gu, Chin. J. Catal., 2019, 40, 1135–1140 CrossRef CAS;
(a
n) J. Jiang, F. Xiao, W.-M. He and L. Wang, Chin. Chem. Lett., 2021, 32, 1637–1644 CrossRef.
-
(a) R. Hekmatshoar, F. Mousavizadeh and R. Rahanamafar, J. Chem. Sci., 2013, 125, 1009–1013 CrossRef CAS;
(b) G. Sartori and R. Maggi, Chem. Rev., 2006, 106, 1077–1104 CrossRef CAS PubMed;
(c) P. H. Gore, Chem. Rev., 1955, 55, 229–281 CrossRef CAS;
(d) M. H. Valkenberg, C. deCastro and W. F. Hölderich, Appl. Catal., A, 2001, 215, 185–190 CrossRef CAS;
(e) J. E. Appleton, K. N. Dack, A. D. Green and J. Steele, Tetrahedron Lett., 1993, 34, 1529–1532 CrossRef CAS;
(f) J. Esquivias, R. Gómez Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2006, 45, 629–633 CrossRef CAS PubMed;
(g) P. Thirupathi and S. S. Kim, J. Org. Chem., 2009, 74, 7755–7761 CrossRef CAS PubMed;
(h) P. Thirupathi and S. S. Kim, Eur. J. Org. Chem., 2010, 1798–1808 CrossRef CAS;
(i) P. Thirupathi and S. S. Kim, J. Org. Chem., 2010, 75, 5240–5249 CrossRef CAS PubMed.
-
(a) J. Jaratjaroonphong, S. Sathalalai, P. Techasauvapak and V. Reutrakul, Tetrahedron Lett., 2009, 50, 6012–6015 CrossRef CAS;
(b) S. G. K. Prakash, G. Fogassy and G. A. Olah, Catal. Lett., 2010, 138, 155–159 CrossRef CAS;
(c) Y. Leng, F. Chen, L. Zuo and W. Duan, Tetrahedron Lett., 2010, 51, 2370–2373 CrossRef CAS;
(d) M. Wilsdorf, D. Leichnitz and H.-U. Reissig, Org. Lett., 2013, 15(10), 2494–2497 CrossRef CAS PubMed;
(e) G. K. S. Prakash, C. Panja, A. Shakhmin, E. Shah, T. Mathew and G. A. Olah, J. Org. Chem., 2009, 74, 8659–8668 CrossRef CAS PubMed;
(f) Z. Li, Z. Duan, J. Kang, H. Wang, L. Yu and Y. Wu, Tetrahedron, 2008, 64, 1924–1930 CrossRef CAS;
(g) M. Kodomari, M. Nagamatsu, M. Akaike and T. Aoyama, Tetrahedron Lett., 2008, 49, 2537–2540 CrossRef CAS;
(h) K. Mohammadiannejad-Abbasabadi, I. Mohammadpoor-Baltork, S. Tangestaninejad, M. Moghadam, V. Mirkhani and R. Kia, Tetrahedron, 2016, 72, 1433–1439 CrossRef CAS;
(i) B. M. Babu, P. B. Thakur, N. N. Rao, G. S. Kumar and H. M. Meshram, Tetrahedron Lett., 2014, 55, 1868–1872 CrossRef CAS;
(j) X. Wang, Y. Wang, D.-M. Du and J. Xu, J. Mol. Catal. A: Chem., 2006, 255, 31–35 CrossRef CAS;
(k) S. Podder, J. Choudhury, U. K. Roy and S. Roy, J. Org. Chem., 2007, 72, 3100–3103 CrossRef CAS PubMed;
(l) A. Pochini and R. Ungaro, J. Chem. Soc., Chem. Commun., 1976, 309–310 RSC;
(m) M. Barbero, S. Cadamuro, S. Dughera, C. Magistris and P. Venturello, Org. Biomol. Chem., 2011, 9, 8393–8399 RSC;
(n) C. R. Liu, M.-B. Li, C.-F. Yang and S.-K. Tian, Chem. Commun., 2008, 1249–1251 RSC;
(o) K. H. Bhadra and G. D. Yadav, Mol. Catal., 2018, 455, 150–158 CrossRef CAS.
-
(a) D. H. Grangaard, Tappi, 1961, 44, 433–439 CAS;
(b) G. Guedes, M. López-Rodríguez, A. G. Ravelo and A. Estévez-Braun, Eur. J. Org. Chem., 2012, 29, 5757–5766 CrossRef;
(c) I. Tooru, T. Rikio and Z. Kazuo, Japan Patent, JP06032756A, 1994;
(d) L. Hasemann, European Patent, EP2078737A1, 2009;
(e) A. W. Bassett and J. F. Stanzione, US Pat., US20200199087A1, 2018.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03724b |
| ‡ Recycling experiment. After the first run, water (1 mL) was added to the reaction mixture and organic residues were extracted with EtOAc (3 × 5 mL) leaving catalyst I in the water layer. Then, after removing the water under high vacuum, recovered catalyst I was reused without purification by simply adding substrates for the second run. |
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ab and
Uthaiwan Sirion
ab and
Uthaiwan Sirion *ab
*ab

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777, 1124, 1096, 808, 738, 512, 406 cm−1; HRMS (ESI-TOF) m/z calcd for C18H16N3 [M + H]+ 274.1339, found 274.1343.
777, 1124, 1096, 808, 738, 512, 406 cm−1; HRMS (ESI-TOF) m/z calcd for C18H16N3 [M + H]+ 274.1339, found 274.1343.







