Open Access Article
Open Access ArticleAcetone complexes for high-performance perovskite photovoltaics with reduced nonradiative recombination†
Zhen
Wang
a,
Chenguang
Yang
ab,
Yuying
Cui
b,
Li
Xie
a and
Feng
Hao
 *b
*b
aFaculty of Printing, Packaging and Digital Media Technology, Xi’an University of Technology, Xi’an, 710054, China. E-mail: haofeng@uestc.edu.cn
bSchool of Materials and Energy, University of Electronic Science and Technology of China, Chengdu 611731, China
First published on 7th January 2022
Abstract
Solvent engineering has been widely employed in the preparation of high-quality perovskite films for emerging halide perovskite solar cells (PSCs). However, the heavy use of toxic solvents (such as N-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF), etc.) has caused critical concerns for health and the environment. Herein, a greener solvent acetone was applied to partially replace DMF and NMP for depositing high-quality perovskite thin films. During the film deposition, an acetone–MAI–PbI2 intermediate was formed to modulate the crystallization process and reduce the grain boundary gaps to achieve high quality perovskite films. The results of photoluminescence spectroscopy, transient photovoltage decay, and Mott–Schottky analysis indicated that this solvent engineering with acetone enabled suppressed trap-assisted nonradiative recombination and facilitated the charge extraction in the corresponding PSC devices. Consequently, the target devices achieved a power conversion efficiency (PCE) of 19.45%, and the open-circuit voltage was enhanced by 70 mV due to the reduced nonradiative recombination. This work provides an idea to alleviate the toxicity of processing solvents that hinders the mass production of PSCs.
1. Introduction
Perovskite solar cells (PSCs) are attractive candidates for next-generation photovoltaic technology due to their remarkable efficiencies and low-cost fabrication process.1 PSCs have achieved a certified power conversion efficiency (PCE) record of 25.5%, which is attributed to the outstanding properties of perovskite semiconductors, such as high defect tolerance, low exciton binding energy, long diffusion length, strong optical absorption, and ambipolar charge transport.2–7 By means of a low-temperature solution process, perovskite films with low trap density and high crystallinity can be obtained owing to the low activation barrier for crystallization.8,9 The one-step spin coating method is one of the most common deposition methods for preparing high-efficiency PSC devices by virtue of its simplicity and scalability.10,11The solution chemistry of perovskite precursors has a strong influence on the crystallization dynamics.12,13 The growth kinetics of perovskite crystals critically depends on the solution of colloidal precursors with PbXn complexes.14,15 The colloids can serve as nucleation centers, which are further organized on the substrate forming a solid intermediate phase.16 Their structure and stability determine the size and optoelectronic quality of the generated perovskite particles.17 In general, the stability of solvent-induced intermediates is closely associated with their coordination ability with PbI2.18 Polar aprotic solvents, such as N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), N,N-dimethylacetamide (DMAC), and N-methyl-2-pyrrolidone (NMP), are widely used to form intermediates.19–24
However, DMF, DMSO, DMCA and NMP are highly toxic and environmentally hazardous, and are widely utilized in most high-performance PSC devices currently.25–28 A crucial factor limiting the large-scale production of PSCs is the massive use of these toxic solvents.29–31 Recently, some attempts have been made to replace the toxic solvents with less toxic γ-butyrolactone (GBL) and acetonitrile (ACN), although sometimes it is accompanied by the compromise of the device performance.32,33 Ramadan et al. fabricated perovskite films which were deposited from ACN solvent only with a PCE of 18.5%.34 Huang et al. applied a solvent mixture of GBL and DMSO to form efficient PSCs with a PCE of 17.02% in an ambient environment.35 On the way to green production of PSCs, it is crucial and urgent to replace the toxic solvents with green ones.36–38
Acetone is a non-toxic, Lewis base solvent with high volatility and low boiling point. Zhang et al. used acetone to assist the low-temperature crystallization of CsPbI2Br perovskite films. The addition of acetone improved the strength of the lead-Lewis base interaction and the wettability of the perovskite precursors.39 This demonstrated that the introduction of environmentally-friendly solvent acetone is conducive to forming high-quality perovskite films. However, the specific interaction between acetone and perovskite is poorly investigated and not yet understood.
Herein, a solvent mixture of acetone, DMF, and NMP is applied for the deposition of methylammonium lead iodide (MAPbI3) perovskite films. The interaction between acetone and perovskite was systematically probed by Fourier transform infrared spectroscopy (FT-IR) and X-ray diffraction (XRD). It was revealed that the addition of acetone can form acetone–MAI–PbI2 complexes to regulate the crystallization process and reduce the grain boundary gaps, resulting in the formation of high-quality perovskite films. A series of carrier dynamic characterization tests further disclosed that the formation of acetone complexes could not only inhibit the nonradiative recombination but also facilitate the charge extraction. With these effects, the PCE and the open-circuit voltage (Voc) of the corresponding devices were significantly improved. Meanwhile, the target device also showed improved humidity stability owing to the high hydrophobicity of the as-prepared perovskite film.
2. Results and discussion
The precursor solution was prepared by replacing some of the toxic solvent with acetone. The corresponding environmental and health impacts of different solvents are listed in Table S1 (ESI†). Compared with DMF and NMP, acetone shows better environmental friendliness and lower health damage. The control and target were denoted by mixing acetone and a solvent mixture (NMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF = 9
DMF = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (v
8 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v)) at volume ratios of 0
v)) at volume ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and 50
300 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
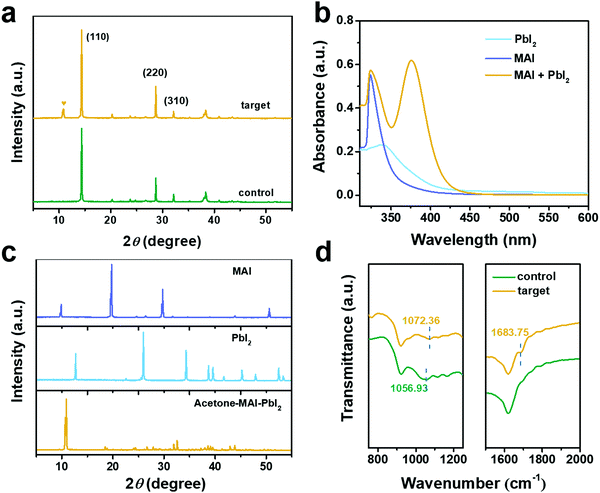
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250, respectively. Then it was spin-coated on a NiOx substrate to form the perovskite layer. X-ray diffraction (XRD) measurements were performed to identify the crystalline phase for the target and control films, as shown in Fig. 1a. It was noted that a new crystalline peak emerged at 10.4° for the perovskite film with acetone. It was conjectured that this peak was ascribed to the complexes of acetone, PbI2, and MAI. To verify this speculation, the UV-Vis absorption spectra of PbI2, MAI, and equivalent PbI2 and MAI dissolved in acetone were collected, as shown in Fig. 1b. A sharp peak at 324 nm was assigned for MAI absorption in acetone solution and a broader peak at 339 nm was ascribed for PbI2 absorption in acetone solution. In particular, a new absorption peak at 374 nm presented in the solution with equivalent PbI2 and MAI dissolved in acetone. This indicated that this emerged absorption peak can be attributed to a new complex formed by acetone with MAI and PbI2. Afterward, a supersaturated solution was prepared by dissolving an equimolar ratio of PbI2 and MAI in acetone, and then dried into a powder. The XRD patterns of acetone–MAI–PbI2, PbI2, and MAI powder are exhibited in Fig. 1c, respectively. The peak of acetone–MAI–PbI2 powder cannot be assigned in the existing database, thus confirming the formation of acetone complexes with PbI2 and MAI (Fig. S4, ESI†). As shown in Fig. 1c, the acetone–MAI–PbI2 powder exhibited a characteristic peak at 10.4°, which is consistent with the extra XRD peak in the target perovskite film (Fig. 1a). The presence of the acetone–MAI–PbI2 complex is further confirmed from the Fourier transform infrared spectroscopy (FT-IR) spectra. As shown in Fig. 1d, compared to the control perovskite film, the target film showed an additional peak at 1683 cm−1, which is ascribed to the C
250, respectively. Then it was spin-coated on a NiOx substrate to form the perovskite layer. X-ray diffraction (XRD) measurements were performed to identify the crystalline phase for the target and control films, as shown in Fig. 1a. It was noted that a new crystalline peak emerged at 10.4° for the perovskite film with acetone. It was conjectured that this peak was ascribed to the complexes of acetone, PbI2, and MAI. To verify this speculation, the UV-Vis absorption spectra of PbI2, MAI, and equivalent PbI2 and MAI dissolved in acetone were collected, as shown in Fig. 1b. A sharp peak at 324 nm was assigned for MAI absorption in acetone solution and a broader peak at 339 nm was ascribed for PbI2 absorption in acetone solution. In particular, a new absorption peak at 374 nm presented in the solution with equivalent PbI2 and MAI dissolved in acetone. This indicated that this emerged absorption peak can be attributed to a new complex formed by acetone with MAI and PbI2. Afterward, a supersaturated solution was prepared by dissolving an equimolar ratio of PbI2 and MAI in acetone, and then dried into a powder. The XRD patterns of acetone–MAI–PbI2, PbI2, and MAI powder are exhibited in Fig. 1c, respectively. The peak of acetone–MAI–PbI2 powder cannot be assigned in the existing database, thus confirming the formation of acetone complexes with PbI2 and MAI (Fig. S4, ESI†). As shown in Fig. 1c, the acetone–MAI–PbI2 powder exhibited a characteristic peak at 10.4°, which is consistent with the extra XRD peak in the target perovskite film (Fig. 1a). The presence of the acetone–MAI–PbI2 complex is further confirmed from the Fourier transform infrared spectroscopy (FT-IR) spectra. As shown in Fig. 1d, compared to the control perovskite film, the target film showed an additional peak at 1683 cm−1, which is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of acetone.40 Similarly, the stretching vibration peak of the C–N bond at 1056 cm−1 of the control film shifted to 1071 cm−1 for the target film owing to the coordination between N and the carbonyl of acetone.41 Therefore, it was confirmed that the acetone–MAI–PbI2 complexes were formed by the coordination reaction between acetone with MAI and PbI2. Moreover, the carbonyl of acetone interacts with the lead ion in PbI2,39 while the carbonyl of acetone interacts with both N and Pb ions in the acetone–MAI–PbI2 complex. The schematic diagram of the acetone–MAI–PbI2 complex is shown in Fig. S5 (ESI†).
O stretching of acetone.40 Similarly, the stretching vibration peak of the C–N bond at 1056 cm−1 of the control film shifted to 1071 cm−1 for the target film owing to the coordination between N and the carbonyl of acetone.41 Therefore, it was confirmed that the acetone–MAI–PbI2 complexes were formed by the coordination reaction between acetone with MAI and PbI2. Moreover, the carbonyl of acetone interacts with the lead ion in PbI2,39 while the carbonyl of acetone interacts with both N and Pb ions in the acetone–MAI–PbI2 complex. The schematic diagram of the acetone–MAI–PbI2 complex is shown in Fig. S5 (ESI†).
Since the acetone–MAI–PbI2 complexes are presented in the precursor solution, it is noteworthy to explore the influences of the complexes on the crystallization process of the perovskite films. XRD spectra of the perovskite films were collected with different annealing times. The diffraction intensity at the (110), (220), and (310) crystal plane of the control and target perovskite films was significantly enhanced with increasing the annealing time, as shown in Fig. 2a and b. Moreover, the diffraction intensity of the peak at 10.4° gradually diminished during the annealing process for the target film. This indicated that the acetone–MAI–PbI2 complex was in decline with the increase of the annealing time, which may affect the crystallization process of the perovskite film. Fig. 2c and d show the scanning electron microscopy (SEM) images for the control and target perovskite films. The target film with acetone showed reduced grain size and more compact boundaries. The average grain size of the target film with acetone was around 135 nm, while the grain size averaged at 216 nm for the control perovskite film (Fig. S6, ESI†). The formation of the acetone–MAI–PbI2 complexes in the precursor film leads to an increased number of nucleation sites and thus reduced the grain size of the target perovskite film.
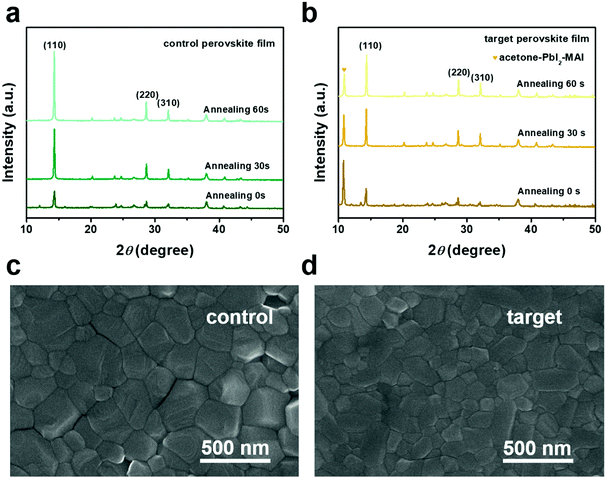 | ||
| Fig. 2 XRD profiles for the control (a) and target (b) films with different annealing times. Top view SEM of the control perovskite film (c) and the target film (d). | ||
Inverted PSCs with FTO/NiOx/perovskite/PC61BM/PEI/Ag structure were assembled to explore the role of the intermediates on the photovoltaic performance. The current density–voltage (J–V) curves were collected under simulated AM 1.5 G (100 mW cm−2) solar illumination (Fig. S7 and Table S2, ESI†). The volume ratio of acetone to NMP/DMF mixture was varied from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 to 60
300 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240, and the best performance was attained at the optimal ratio of 50
240, and the best performance was attained at the optimal ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
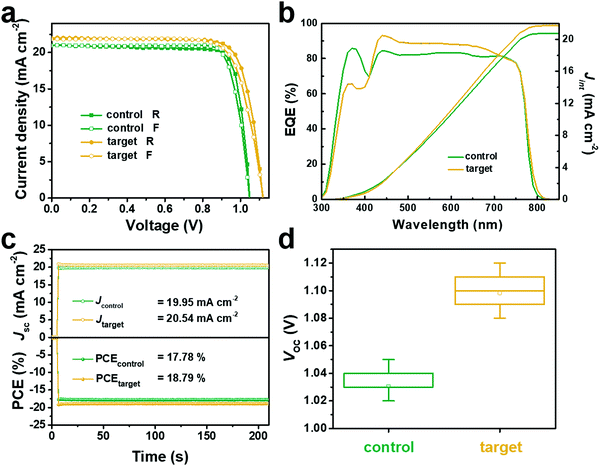
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250. The specific properties of the PSC devices are presented in Fig. 3a and Table 1. The target device exhibited a PCE of 19.45%, with a short-circuit photocurrent density (Jsc) of 22.01 mA cm−2, an open-circuit voltage (Voc) of 1.12 V, and a fill factor (FF) of 79.02%, respectively. While for the control device, it only achieved a PCE of 18.12%, a Jsc of 21.04 mA cm−2, a Voc of 1.05 V, and a FF of 82.14%. The performance enhancement of the target device was manifested in the enlargement of the Voc and Jsc value. The integrated photocurrent densities (Jint) were interpreted from the incident photon-to-electron conversion efficiency (IPCE) spectra, which also can reflect the accuracy of Jsc. The target devices generated a Jint of 21.71 mA cm−2 compared to the control devices with 20.70 mA cm−2, both of which were highly in accordance with the Jsc value, as shown in Fig. 3b. At the maximum power point (MPP), both the stabilized current density and the steady-state power output were monitored in 220 s (Fig. 3c). The control device exhibited a steady Jsc of 19.95 mA cm−2 and a PCE of 17.78% at the MPP of 0.90 V, while the target device showed a steady Jsc of 20.54 mA cm−2 and a PCE of 18.79% at the MPP of 0.94 V. A batch of 25 independent devices for the control and target experiment were prepared to further confirm the repeatability of the photovoltaic performance. The target devices exhibited a significantly higher Voc and PCE than the control devices (Fig. 3d and Fig. S8, ESI†). The improvement of Voc and Jsc may be related to the high-quality perovskite film crystallized from the acetone–MAI–PbI2 complex.
250. The specific properties of the PSC devices are presented in Fig. 3a and Table 1. The target device exhibited a PCE of 19.45%, with a short-circuit photocurrent density (Jsc) of 22.01 mA cm−2, an open-circuit voltage (Voc) of 1.12 V, and a fill factor (FF) of 79.02%, respectively. While for the control device, it only achieved a PCE of 18.12%, a Jsc of 21.04 mA cm−2, a Voc of 1.05 V, and a FF of 82.14%. The performance enhancement of the target device was manifested in the enlargement of the Voc and Jsc value. The integrated photocurrent densities (Jint) were interpreted from the incident photon-to-electron conversion efficiency (IPCE) spectra, which also can reflect the accuracy of Jsc. The target devices generated a Jint of 21.71 mA cm−2 compared to the control devices with 20.70 mA cm−2, both of which were highly in accordance with the Jsc value, as shown in Fig. 3b. At the maximum power point (MPP), both the stabilized current density and the steady-state power output were monitored in 220 s (Fig. 3c). The control device exhibited a steady Jsc of 19.95 mA cm−2 and a PCE of 17.78% at the MPP of 0.90 V, while the target device showed a steady Jsc of 20.54 mA cm−2 and a PCE of 18.79% at the MPP of 0.94 V. A batch of 25 independent devices for the control and target experiment were prepared to further confirm the repeatability of the photovoltaic performance. The target devices exhibited a significantly higher Voc and PCE than the control devices (Fig. 3d and Fig. S8, ESI†). The improvement of Voc and Jsc may be related to the high-quality perovskite film crystallized from the acetone–MAI–PbI2 complex.
| J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | ||
|---|---|---|---|---|---|
| Control | Reverse | 21.04 | 1.05 | 82.14 | 18.12 |
| Forward | 20.99 | 1.05 | 81.95 | 18.02 | |
| Average | 21.02 ± 0.02 | 1.05 ± 0.01 | 82.04 ± 0.10 | 18.07 ± 0.05 | |
| Target | Reverse | 22.01 | 1.12 | 79.02 | 19.45 |
| Forward | 21.84 | 1.12 | 76.43 | 18.71 | |
| Average | 21.93 ± 0.09 | 1.12 ± 0.01 | 77.73 ± 1.29 | 19.08 ± 0.37 |
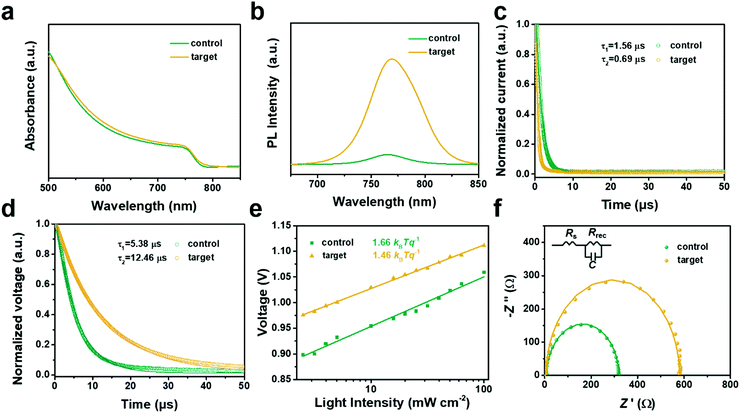
Ultraviolet and visible (UV-Vis) spectra of the perovskite films were collected in Fig. 4a. Typically, the target film showed slightly enhanced absorption at the wavelength of 550–750 nm, which was mainly related to the more compact morphology compared to the control film, as presented in Fig. 2. Moreover, based on the steady-state photoluminescence (PL) spectra, it showed that the PL intensity of the target film was enhanced about 10 times compared to the control one (Fig. 4b), thus indicating the superior crystalline quality for the target film.42,43 Furthermore, transient photocurrent decay (TPC) was measured to monitor the charge transport in the device. As shown in Fig. 4c, it demonstrated that the photocurrent decay time of the target device (0.69 μs) was prominently shorter than that of the control one (1.57 μs). It suggested that faster charge transport occurred in the target devices.44 Besides, transient photovoltage decay (TPV) was further measured to investigate the charge recombination lifetime. It showed that the target device reached up to 12.46 μs compared to 5.38 μs of the control one, which was presented in Fig. 4d. This suggested that the charge recombination in the target device was substantially diminished.45
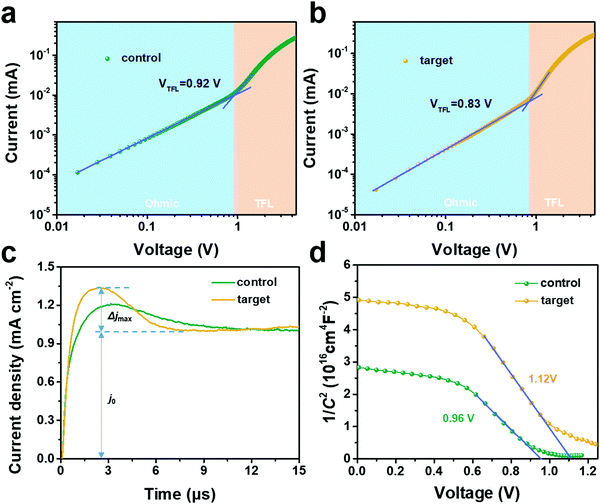
In addition, the J–V property was probed at different light intensities (1–100 mW cm−2) to further investigate the charge recombination kinetics. The curves of Vocversus light intensity are shown in Fig. 4e. Notably, a slope of 1.46 kBTq−1 was acquired for the target device. While a slope of 1.66 kBTq−1 was attained for the control device. Consequently, the slope of the target device was more closer to 1, thus reflecting the suppressed Shockley–Read–Hall (SRH) recombination in the target device.46 Finally, the charge transport properties of the PSCs were elucidated by electrochemical impedance spectroscopy (EIS). Fig. 4f and Table S3 (ESI†) depicted the Nyquist plots under dark conditions. The target device displayed a higher recombination resistance (Rrec) of 575 Ω compared to the 310 Ω for the control device, which implied that the target device was more restrained in charge recombination than the control device.47 A slight reduced series resistance (Rs) indicated better crystalline quality in the target device.48
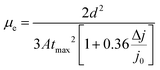
Additionally, the trap state density (Nt) can be evaluated based on the space charge-limited current (SCLC),49 which can be determined via the trap-filled limit voltage (VTFL) according to the equation:
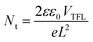 | (1) |
 | (2) |
The electronic and doping characteristics of the devices were investigated via Mott–Schottky (M–S) analysis.53 Under dark conditions, the junction capacitance is calculated based on the function of bias voltage, as shown in eqn (3):
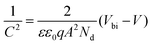 | (3) |
3. Conclusions
In summary, high-quality perovskite thin films were deposited using the eco-friendly solvent acetone to replace some of the toxic NMP/DMF as the solvent. It demonstrated that the formation of acetone–MAI–PbI2 complexes could obviously improve the quality of the perovskite thin films by modulating the crystallization process. The as-prepared perovskite films exhibited reduced grain size and grain boundary gaps, with a more compact morphology. The PL, TPC, TPV, and light intensity dependence results of Voc demonstrated that the target films were able to suppress the trap-assisted nonradiative recombination. Also, the built-in potential (Vbi) was enhanced for the target device, which can serve to increase the charge extraction process. Therefore, the target devices exhibited favorable improvement in Voc and Jsc, and the inverted MAPbI3 PSCs exhibited a substantial improvement of the PCE (19.45%). The combination of improved hydrophobicity and decreased trap density further contributed to better stability in a humid environment. The green solvent acetone is expected to be extendable to other perovskite compositions, which provides a greener route towards the environmentally friendly manufacturing of PSCs.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China NSFC (51702038 and 52102145), the Xi’an Science and Technology Bureau Planning project (Grant No. 21XJZZ0054), the Postdoctoral Science Foundation of Shaanxi Province (Grant No. 2018BSHEDZZ101), the Science and Technology Bureau of Beilin District, Xi’an (Grant No. GX2035), the Key Project of the Science & Technology Department of Sichuan Province (2020YFG0061) and the Recruitment Program for Young Professionals.References
- B. Wilk, S. Öz, E. Radicchi, F. Ünlü, T. Ahmad, A. P. Herman, F. Nunzi, S. Mathur, R. Kudrawiec and K. Wojciechowski, Green Solvent-Based Perovskite Precursor Development for Ink-Jet Printed Flexible Solar Cells, ACS Sustainable Chem. Eng., 2021, 9, 3920–3930 CrossRef CAS.
- H. J. Snaith, Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells, J. Phys. Chem. Lett., 2013, 4, 3623–3630 CrossRef CAS.
- Z. Yang, A. Surrente, K. Galkowski, N. Bruyant, D. K. Maude, A. A. Haghighirad, H. J. Snaith, P. Plochocka and R. J. Nicholas, Unraveling the Exciton Binding Energy and the Dielectric Constant in Single-Crystal Methylammonium Lead Triiodide Perovskite, J. Phys. Chem. Lett., 2017, 8, 1851–1855 CrossRef CAS PubMed.
- J. Chen and N. G. Park, Causes and Solutions of Recombination in Perovskite Solar Cells, Adv. Mater., 2019, 31, 1803019 CrossRef CAS PubMed.
- H. Zhang, M. K. Nazeeruddin and W. C. H. Choy, Perovskite Photovoltaics: The Significant Role of Ligands in Film Formation, Passivation, and Stability, Adv. Mater., 2019, 31, 1805702 CrossRef PubMed.
- W. Xu, Y. Gao, W. Ming, F. He, J. Li, X. H. Zhu, F. Kang, J. Li and G. Wei, Suppressing Defects-Induced Nonradiative Recombination for Efficient Perovskite Solar Cells through Green Antisolvent Engineering, Adv. Mater., 2020, 32, 2003965 CrossRef CAS PubMed.
- Best Research-Cell Efficiencies. https://www.nrel.gov/pv/cell-efficiency.html (accessed November 2021).
- D. T. Moore, H. Sai, K. W. Tan, D. M. Smilgies, W. Zhang, H. J. Snaith, U. Wiesner and L. A. Estroff, Crystallization Kinetics of Organic-Inorganic Trihalide Perovskites and the Role of the Lead Anion in Crystal Growth, J. Am. Chem. Soc., 2015, 137, 2350–2358 CrossRef CAS PubMed.
- J. Huang, Y. Yuan, Y. Shao and Y. Yan, Understanding the Physical Properties of Hybrid Perovskites for Photovoltaic Applications, Nat. Rev. Mater., 2017, 2, 17042 CrossRef CAS.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Compositional Engineering of Perovskite Materials for High-Performance Solar Cells, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- G. Zheng, C. Zhu, J. Ma, X. Zhang, G. Tang, R. Li, Y. Chen, L. Li, J. Hu, J. Hong, Q. Chen, X. Gao and H. Zhou, Manipulation of Facet Orientation in Hybrid Perovskite Polycrystalline Films by Cation Cascade, Nat. Commun., 2018, 9, 2793 CrossRef PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Solvent Engineering for High-Performance Inorganic-Organic Hybrid Perovskite Solar Cells, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- J. Zhang, L. Zhang, X. Li, X. Zhu, J. Yu and K. Fan, Binary Solvent Engineering for High-Performance Two-Dimensional Perovskite Solar Cells, ACS Sustainable Chem. Eng., 2019, 7, 3487–3495 CrossRef CAS.
- K. Yan, M. Long, T. Zhang, Z. Wei, H. Chen, S. Yang and J. Xu, Hybrid Halide Perovskite Solar Cell Precursors: Colloidal Chemistry and Coordination Engineering Behind Device Processing for High Efficiency, J. Am. Chem. Soc., 2015, 137, 4460–4468 CrossRef CAS PubMed.
- E. Radicchi, E. Mosconi, F. Elisei, F. Nunzi and F. De Angelis, Understanding the Solution Chemistry of Lead Halide Perovskites Precursors, ACS Appl. Energy Mater., 2019, 2, 3400–3409 CrossRef CAS.
- N. S. Dutta, N. K. Noel and C. B. Arnold, Crystalline Nature of Colloids in Methylammonium Lead Halide Perovskite Precursor Inks Revealed by Cryo-Electron Microscopy, J. Phys. Chem. Lett., 2020, 11, 5980–5986 CrossRef CAS PubMed.
- B. Li, M. Li, C. Fei, G. Cao and J. Tian, Colloidal Engineering for Monolayer CH3NH3PbI3 Films Toward High Performance Perovskite Solar Cells, J. Mater. Chem. A, 2017, 5, 24168–24177 RSC.
- Z. Li, Y. Sun, H. Yao, J. Zhao, Q. Wang, L. Ding and Z. Jin, Intermediates Transformation for Efficient Perovskite Solar Cells, J. Energy Chem., 2021, 52, 102–114 CrossRef.
- N. Ahn, D. Y. Son, I. H. Jang, S. M. Kang, M. Choi and N. G. Park, Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide, J. Am. Chem. Soc., 2015, 137, 8696–8699 CrossRef CAS PubMed.
- D. Shen, X. Yu, X. Cai, M. Peng, Y. Ma, X. Su, L. Xiao and D. Zou, Understanding The Solvent-Assisted Crystallization Mechanism Inherent In Efficient Organic–Inorganic Halide Perovskite Solar Cells, J. Mater. Chem. A, 2014, 2, 20454–20461 RSC.
- X. Deng, Z. Cao, Y. Yuan, M. Oliver Lam Chee, L. Xie, A. Wang, Y. Xiang, T. Li, P. Dong, L. Ding and F. Hao, Coordination Modulated Crystallization and Defect Passivation in high Quality Perovskite Film for Efficient Solar Cells, Coord. Chem. Rev., 2020, 420, 213408 CrossRef CAS.
- Y. Jo, K. S. Oh, M. Kim, K. H. Kim, H. Lee, C. W. Lee and D. S. Kim, High Performance of Planar Perovskite Solar Cells Produced from PbI2(DMSO) and PbI2(NMP) Complexes by Intramolecular Exchange, Adv. Mater. Interfaces, 2016, 3, 1500768 CrossRef.
- K. Liao, L. Xie, Y. Cui, S. Wang, C. Li, A. Wang, X. Deng, Y. Xiang, L. Ding and F. Hao, Aqueous Solvent-Regulated Crystallization and Interfacial Modification in Perovskite Solar Cells With Enhanced Stability and Performance, J. Power Sources, 2020, 471, 228447 CrossRef CAS.
- S. Wang, J. Hu, A. Wang, Y. Cui, B. Chen, X. Niu and F. Hao, Facile Lattice Tensile Strain Compensation in Mixed-Cation Halide Perovskite Solar Cells, J. Energy Chem., 2022, 66, 422–428 CrossRef.
- L. Xie, X. Deng, C. Li, Y. Cui, Z. Cao, A. Wang, S. Wang, Y. Chen, Z. Wang, Y. Liu, Q. Bao, L. Ding and F. Hao, Tetrazole Modulated Perovskite Films for Efficient Solar Cells with Improved Moisture Stability, Chem. Eng. J., 2021, 420, 127579 CrossRef CAS.
- M. Kim, G.-H. Kim, T. K. Lee, I. W. Choi, H. W. Choi, Y. Jo, Y. J. Yoon, J. W. Kim, J. Lee, D. Huh, H. Lee, S. K. Kwak, J. Y. Kim and D. S. Kim, Methylammonium Chloride Induces Intermediate Phase Stabilization for Efficient Perovskite Solar Cells, Joule, 2019, 3, 2179–2192 CrossRef CAS.
- J. Li, J. Duan, X. Yang, Y. Duan, P. Yang and Q. Tang, Review on Recent Progress of Lead-Free Halide Perovskites in Optoelectronic Applications, Nano Energy, 2021, 80, 105526 CrossRef CAS.
- Y. Cui, S. Wang, L. Ding and F. Hao, Green-Solvent-Processable Perovskite Solar Cells, Adv. Energy Sustainability Res., 2020, 2, 2000047 CrossRef.
- T. Bu, L. Wu, X. Liu, X. Yang, P. Zhou, X. Yu, T. Qin, J. Shi, S. Wang, S. Li, Z. Ku, Y. Peng, F. Huang, Q. Meng, Y.-B. Cheng and J. Zhong, Synergic Interface Optimization with Green Solvent Engineering in Mixed Perovskite Solar Cells, Adv. Energy Mater., 2017, 7, 1700576 CrossRef.
- M. Zhang, D. Xin, X. Zheng, Q. Chen and W.-H. Zhang, Toward Greener Solution Processing of Perovskite Solar Cells, ACS Sustainable Chem. Eng., 2020, 8, 13126–13138 CrossRef CAS.
- Y. Cui, S. Wang, C. Li, A. Wang, J. Ren, C. Yang, B. Chen, Z. Wang and F. Hao, Eco-Friendly Antisolvent Enabled Inverted MAPbI3 Perovskite Solar Cells with Fill Factors over 84%, Green Chem., 2021, 23, 3633–3641 RSC.
- L. Li, Y. Chen, Z. Liu, Q. Chen, X. Wang and H. Zhou, The Additive Coordination Effect on Hybrids Perovskite Crystallization and High-Performance Solar Cell, Adv. Mater., 2016, 28, 9862–9868 CrossRef CAS PubMed.
- H. B. Kim, H. Choi, J. Jeong, S. Kim, B. Walker, S. Song and J. Y. Kim, Mixed Solvents for the Optimization of Morphology in Solution-Processed, Inverted-Type Perovskite/Fullerene Hybrid Solar Cells, Nanoscale, 2014, 6, 6679–6683 RSC.
- A. J. Ramadan, N. K. Noel, S. Fearn, N. Young, M. Walker, L. A. Rochford and H. J. Snaith, Unravelling the Improved Electronic and Structural Properties of Methylammonium Lead Iodide Deposited from Acetonitrile, Chem. Mater., 2018, 30, 7737–7743 CrossRef CAS.
- S. H. Huang, K. Y. Tian, H. C. Huang, C. F. Li, W. C. Chu, K. M. Lee, Y. C. Huang and W. F. Su, Controlling the Morphology and Interface of the Perovskite Layer for Scalable High-Efficiency Solar Cells Fabricated Using Green Solvents and Blade Coating in an Ambient Environment, ACS Appl. Mater. Interfaces, 2020, 12, 26041–26049 CrossRef CAS PubMed.
- G. Giuliano, A. Bonasera, M. Scopelliti, D. Chillura Martino, T. Fiore and B. Pignataro, Boosting the Performance of One-Step Solution-Processed Perovskite Solar Cells Using a Natural Monoterpene Alcohol as a Green Solvent Additive, ACS Appl. Electron. Mater., 2021, 3, 1813–1825 CrossRef CAS.
- M. Yavari, M. Mazloum-Ardakani, S. Gholipour, M. M. Tavakoli, S.-H. Turren-Cruz, N. Taghavinia, M. Grätzel, A. Hagfeldt and M. Saliba, Greener, Nonhalogenated Solvent Systems for Highly Efficient Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 1800177 CrossRef.
- M. Wang, Q. Fu, L. Yan, J. Huang, Q. Ma, M. Humayun, W. Pi, X. Chen, Z. Zheng and W. Luo, Systematic Optimization of Perovskite Solar Cells via Green Solvent Systems, Chem. Eng. J., 2020, 387, 123966 CrossRef CAS.
- W. Tang, Y. Chen, J. Yang, R. Yuan, Y. Lv, Q. Ma, Y. Wu, P. Zhang and W.-H. Zhang, Acetone-Assisted Precursor Engineering Enables Low-Temperature Fabrication of CsPbI2Br Perovskite for Efficient Solar Cells, J. Power Sources, 2021, 482, 228965 CrossRef CAS.
- Y. Cai, J. Cui, M. Chen, M. Zhang, Y. Han, F. Qian, H. Zhao, S. Yang, Z. Yang, H. Bian, T. Wang, K. Guo, M. Cai, S. Dai, Z. Liu and S. Liu, Multifunctional Enhancement for Highly Stable and Efficient Perovskite Solar Cells, Adv. Funct. Mater., 2020, 31, 2005776 CrossRef.
- J. Zhu, M. Tang, B. He, K. Shen, W. Zhang, X. Sun, M. Sun, H. Chen, Y. Duan and Q. Tang, Ultraviolet Filtration and Defect Passivation for Efficient and Photostable CsPbBr3 Perovskite Solar Cells by Interface Engineering with Ultraviolet Absorber, Chem. Eng. J., 2021, 404, 126548 CrossRef CAS.
- N. Chen, X. Yi, J. Zhuang, Y. Wei, Y. Zhang, F. Wang, S. Cao, C. Li and J. Wang, An Efficient Trap Passivator for Perovskite Solar Cells: Poly(propylene glycol) bis(2-aminopropyl ether), Nano-Micro Lett., 2020, 12, 177 CrossRef CAS PubMed.
- Y. Zhao, P. Zhu, S. Huang, S. Tan, M. Wang, R. Wang, J. Xue, T. H. Han, S. J. Lee, A. Zhang, T. Huang, P. Cheng, D. Meng, J. W. Lee, J. Marian, J. Zhu and Y. Yang, Molecular Interaction Regulates the Performance and Longevity of Defect Passivation for Metal Halide Perovskite Solar Cells, J. Am. Chem. Soc., 2020, 142, 20071–20079 CrossRef CAS PubMed.
- R. Wang, J. Xue, L. Meng, J.-W. Lee, Z. Zhao, P. Sun, L. Cai, T. Huang, Z. Wang, Z.-K. Wang, Y. Duan, J. L. Yang, S. Tan, Y. Yuan, Y. Huang and Y. Yang, Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells, Joule, 2019, 3, 1464–1477 CrossRef CAS.
- Z. Yao, W. Zhao, S. Chen, Z. Jin and S. F. Liu, Mn Doping of CsPbI3 Film Towards High-Efficiency Solar Cell, ACS Appl. Energy Mater., 2020, 3, 5190–5197 CrossRef CAS.
- L. Xie, Z. Cao, J. Wang, A. Wang, S. Wang, Y. Cui, Y. Xiang, X. Niu, F. Hao and L. Ding, Improving Energy Level Alignment By Adenine for Efficient and Stable Perovskite Solar Cells, Nano Energy, 2020, 74, 104846 CrossRef CAS.
- L. Xie, J. Xie, S. Wang, B. Chen, C. Yang, Z. Wang, X. Liu, J. Chen, K. Jia and F. Hao, Fluorinated Oligomer Wrapped Perovskite Crystals for Inverted MAPbI3 Solar Cells with 21% Efficiency and Enhanced Stability, ACS Appl. Mater. Interfaces, 2021, 13, 26093–26101 CrossRef CAS PubMed.
- X. Deng, Z. Cao, C. Li, S. Wang and F. Hao, Benzotriazole Derivative Inhibits Nonradiative Recombination and Improves the UV-Stability of Inverted MAPbI3 Perovskite Solar Cells, J. Energy Chem., 2022, 65, 592–599 CrossRef.
- A. A. Zhumekenov, M. I. Saidaminov, M. A. Haque, E. Alarousu, S. P. Sarmah, B. Murali, I. Dursun, X.-H. Miao, A. L. Abdelhady, T. Wu, O. F. Mohammed and O. M. Bakr, Formamidinium Lead Halide Perovskite Crystals with Unprecedented Long Carrier Dynamics and Diffusion Length, ACS Energy Lett., 2016, 1, 32–37 CrossRef CAS.
- L. Qiu, K. Xing, J. Zhang, Y. Yang, W. Cao, X. Zhou, K. Zhu, D. Xia and R. Fan, Two-Dimensional Metal-Organic Frameworks-Based Grain Termination Strategy Enables High-Efficiency Perovskite Photovoltaics with Enhanced Moisture and Thermal Stability, Adv. Funct. Mater., 2021, 31, 2010368 CrossRef CAS.
- J. Peng, Y. Sun, Y. Chen, Y. Yao and Z. Liang, Light and Thermally Induced Evolutional Charge Transport in CH3NH3PbI3 Perovskite Solar Cells, ACS Energy Lett., 2016, 1, 1000–1006 CrossRef CAS.
- Y. Liu, H. Zhang, Y. Zhang, B. Xu, L. Liu, G. Chen, C. Im and W. Tian, Influence of Hole Transport Layers on Internal Absorption, Charge Recombination and Collection in HC(NH2)2PbI3 Perovskite Solar Cells, J. Mater. Chem. A, 2018, 6, 7922–7932 RSC.
- W. T. Wang, P. Chen, C. H. Chiang, T. F. Guo, C. G. Wu and S. P. Feng, Synergistic Reinforcement of Built-In Electric Fields for Highly Efficient and Stable Perovskite Photovoltaics, Adv. Funct. Mater., 2020, 30, 1909755 CrossRef CAS.
- J.-H. Lee, J. Kim, G. Kim, D. Shin, S. Y. Jeong, J. Lee, S. Hong, J. W. Choi, C.-L. Lee, H. Kim, Y. Yi and K. Lee, Introducing Paired Electric Dipole Layers for Efficient and Reproducible Perovskite Solar Cells, Energy Environ. Sci., 2018, 11, 1742–1751 RSC.
- E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T. Y. Yang, J. H. Noh and J. Seo, Efficient, Stable and Scalable Perovskite Solar Cells Using Poly(3-hexylthiophene), Nature, 2019, 567, 511–515 CrossRef CAS PubMed.
- H. Zhang, X. Ren, X. Chen, J. Mao, J. Cheng, Y. Zhao, Y. Liu, J. Milic, W.-J. Yin, M. Grätzel and W. C. H. Choy, Improving the Stability and Performance of Perovskite Solar Cells via Off-the-Shelf Post-Device Ligand Treatment, Energy Environ. Sci., 2018, 11, 2253–2262 RSC.
- S. Wang, B. Yang, J. Han, Z. He, T. Li, Q. Cao, J. Yang, J. Suo, X. Li, Z. Liu, S. Liu, C. Tang and A. Hagfeldt, Polymeric Room-Temperature Molten Salt as a Multifunctional Additive Toward Highly Efficient and Stable Inverted Planar Perovskite Solar Cells, Energy Environ. Sci., 2020, 13, 5068–5079 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma01165k |
| This journal is © The Royal Society of Chemistry 2022 |