Open Access Article
Open Access ArticleSuper silyl-based stable protecting groups for both the C- and N-terminals of peptides: applied as effective hydrophobic tags in liquid-phase peptide synthesis†
An
Wu
 * and
Hisashi
Yamamoto
* and
Hisashi
Yamamoto
 *
*
Peptide Research Centre, Chubu University, 1200 Matsumoto-cho, Kasugai, Aichi 487-8501, Japan. E-mail: awuad@isc.chubu.ac.jp; hyamamoto@isc.chubu.ac.jp
First published on 15th April 2023
Abstract
Tag-assisted liquid-phase peptide synthesis (LPPS) is one of the important processes in peptide synthesis in pharmaceutical discovery. Simple silyl groups have positive effects when incorporated in the tags due to their hydrophobic properties. Super silyl groups contain several simple silyl groups and play an important role in modern aldol reactions. In view of the unique structural architecture and hydrophobic properties of the super silyl groups, herein, two new types of stable super silyl-based groups (tris(trihexylsilyl)silyl group and propargyl super silyl group) were developed as hydrophobic tags to increase the solubility in organic solvents and the reactivity of peptides during LPPS. The tris(trihexylsilyl)silyl group can be installed at the C-terminal of the peptides in ester form and N-terminal in carbamate form for peptide synthesis and it is compatible with hydrogenation conditions (Cbz chemistry) and Fmoc-deprotection conditions (Fmoc chemistry). The propargyl super silyl group is acid-resistant, which is compatible with Boc chemistry. Both tags are complementary to each other. The preparation of these tags requires less steps than previously reported tags. Nelipepimut-S was synthesized successfully with different strategies using these two types of super silyl tags.
Introduction
Peptide therapeutics have played a significant role in clinical medicine and drug discovery,1 making peptide synthesis research more important than ever before. Classical approaches to peptide synthesis can be divided into solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS).2,3 SPPS has been widely used due to simple operation, but it is difficult to analyze the product before the cleavage of the solid support. Compared with SPPS, LPPS usually gives higher purity peptides. However, the application of LPPS strategies to long-chain peptides remains challenging due to solubility and reactivity decrease after several amino acid linkages.2a To solve these problems, many soluble tags were developed to enhance the efficiency of peptide synthesis by increasing the solubility of the substrates in organic solvents (Scheme 1a). In the last century, modified soluble PEGs polymers have been developed.4 Small molecule supports have attracted more attention in recent decades like fluorous supports,5 some phosphorus reagents6 and ‘nanostar’ support.7 Adding a hydrophobic tag is another promising strategy because peptides have a lot of amide bonds which make the whole molecule more hydrophilic. The hydrophobicity usually result from the polycarbon structure in the tags, and these are called polycarbon tags. This was firstly reported by the Tamiaki group in 2001 using benzyl alcohol derivatives with long alkyl chains as the C-terminal protecting groups (Scheme 1b).8 After that, the Chiba,9 Takahashi10 and Sunazuka11 groups focused on polycarbon tags and conducted many excellent studies in this field. Many bioactive peptides were synthesized successfully by them. However, these series of tags usually require multiple steps to synthesize. Apart from this, these tags still belong to a well-developed strategy, which is only installed at the C-terminal to synthesize peptides from the C-terminal to the N-terminal. Very recently, Chiba's group have compared the different cyclization efficiencies of synthesizing cyclic peptides from the chain peptides when the hydrophobic tag is installed at different positions of the peptide chain.12 They tried to install the hydrophobic tag at the N-terminal of the chain peptides, but the chain peptides were still synthesized from the C-terminal instead of starting from the N-terminal. To our knowledge, there is no report focusing on installing hydrophobic tags at the N-terminal to elongate peptides in the reverse direction (N to C) in LPPS.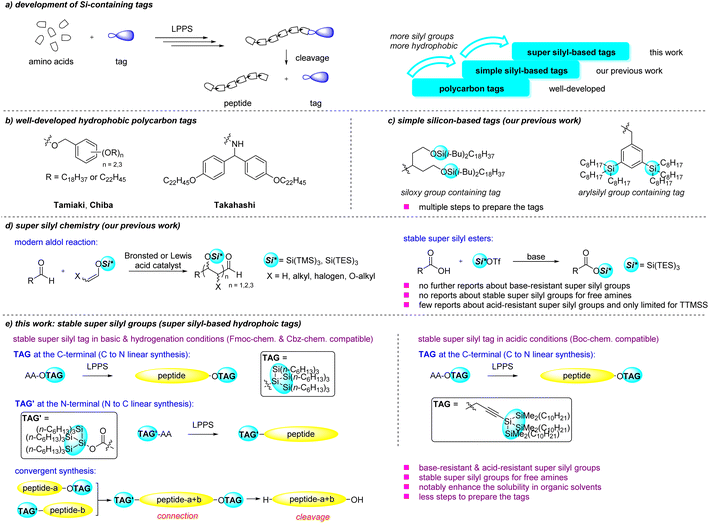 | ||
| Scheme 1 Background of this work (a) development of Si-containing tags; (b) well-developed hydrophobic polycarbon tags; (c and d) our previous work; (e) this work. | ||
Recently, our group has reported two new types of hydrophobic tags containing simple silyl groups (siloxy group and arylsilyl group) (Scheme 1c).13 We found that when the silyl groups were introduced, the hydrophobicity of the tags could be increased and the resulting products became difficult to solidify. Some relevant simple silyl-based tags were also reported by Nishizawa14 and Kubota.15 Kubota has proved that the number of silyl groups could affect the peptide solubility. This information suggested that if more silyl groups were involved in the tags, the hydrophobicity would increase substantially (Scheme 1a).
Super silyl groups are those containing several simple silyl groups connected with Si–Si bonds. Our group has focused on super silyl chemistry over the past two decades. Super silyl groups play an important role in aldehyde aldol reactions.16 The unique structures of the super silyl groups are the key for the success of these methods. Super silyl groups can also protect carboxylic acids to form base-resistant (limited bases) super silyl esters which can be applied in Mannich and Michael reactions (Scheme 1d).16d,g Thus we decided to introduce the super silyl groups in the hydrophobic tags for LPPS. Super silyl groups should be much more hydrophobic molecules because of the number of the simple silyl groups with nine alkyl chains attached. To successfully apply the super silyl tags in the peptide synthesis, the tags should be relatively stable during the peptide elongation. However, apart from the few reported studies,16d,17 there has been no more information about the acid-resistant or base-resistant super silyl groups. Installation of the super silyl groups at the N-terminal to protect the free amine is also an unsolved problem. We only found a few groups reported using a tris(trimethylsilyl)silyl group to form the N–Si bond of their substrates and purified by distillation or sublimation indicating the instability of the compounds.18 In this work, we are also in search of a solution to these problems to enrich the field of stable super silyl groups and apply them in LPPS.
Herein, we would like to report two types of stable super silyl-based groups as the hydrophobic tags applied for the LPPS (Scheme 1e). One is the (tris(trihexylsilyl)silyl) group, which can be installed at the C-terminal of peptides in ester form and the N-terminal in carbamate form in LPPS. This super silyl tag precursor only needs one step to prepare compared with multiple steps in preparations of previously reported simple silyl-based tags.13 When it is installed at the C-terminal in ester form, it is compatible with hydrogenation conditions (Cbz chemistry) and Fmoc peptide synthesis conditions (Fmoc chemistry). It can also be applied for the convergent synthesis of peptides. The other is the propargyl super silyl group which is stable under the Boc-deprotection conditions (Boc chemistry) when it is installed at the C-terminal. The solubility of the peptides in organic solvents was enhanced by using these tags. Nelipepimut-S, a peptide cancer vaccine, was synthesized successfully with both Fmoc-chemistry and Boc-chemistry using these two types of super silyl tags.
Results and discussion
Initially, we used the tris(triethylsilyl)silyl group (TAG1) as the tag molecule to install at the C-terminal of alanine which was generated from commercially available tris(triethylsilyl)silane (HTAG1) according to reported work.16d It was found that the super silyl group could be removed under both the Boc-deprotection and Fmoc-deprotection conditions (Scheme 2a). A larger super silyl group with longer alkyl chains (tris(trihexylsilyl)silyl group, TAG2) was tested. It was prepared from trichloro(phenyl)silane and chlorotrihexylsilane (see ESI† and Scheme 4a). The tag could tolerate the Fmoc-deprotection and hydrogenation (Cbz-deprotection) conditions (Scheme 2b). The Rf value of H–Ala–OTAG2 on thin layer chromatography is 0.85 in hexane/EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which is higher than previously reported simple silyl-based tags (0.61 and 0.64),13 roughly indicating the more hydrophobicity of the super silyl group due to more simple silyl groups. For the peptide elongation method, we chose to use active amino acid esters like para-nitrophenyl esters19 or pentafluorophenyl esters20 without adding additional reagents to simplify the reaction system. When we began to test the peptide elongation from H–Ala–OTAG2 using para-nitrophenyl ester, unfortunately, no dipeptide was isolated (Scheme 2b). This might indicate that the bulkiness of the super silyl group prevents the peptide bond formation because the bulk super silyl groups were too close to the reaction center. If a short alkyl chain were used as a bridge between the C-terminal and the super silyl group, the results should be better. With this assumption in mind, TAG3 was prepared. The esterification steps proceeded well and it was tolerant of Fmoc-deprotection and Cbz-deprotection conditions (Scheme 2c). However, when we tried to extend the TES group in TAG3 to trihexylsilyl groups to increase the hydrophobicity, we failed to synthesize this silyl ether type tag. At this stage, TAG4 was developed. The esterification steps also proceeded well and it was tolerant of Fmoc-deprotection and Cbz-deprotection conditions (Scheme 2d). Dipeptide formation was tested for H–Ala–OTAG4 to give 87% yield product, which indicated the necessity of the short alkyl bridge. From the above results, TAG4 might be the best tag containing super silyl group. We also found that most compounds containing the super silyl tags were liquid oil and did not easily solidify in the operation. This might be an advantage when these tags are applied to synthesize peptides in continuous flow.
1 which is higher than previously reported simple silyl-based tags (0.61 and 0.64),13 roughly indicating the more hydrophobicity of the super silyl group due to more simple silyl groups. For the peptide elongation method, we chose to use active amino acid esters like para-nitrophenyl esters19 or pentafluorophenyl esters20 without adding additional reagents to simplify the reaction system. When we began to test the peptide elongation from H–Ala–OTAG2 using para-nitrophenyl ester, unfortunately, no dipeptide was isolated (Scheme 2b). This might indicate that the bulkiness of the super silyl group prevents the peptide bond formation because the bulk super silyl groups were too close to the reaction center. If a short alkyl chain were used as a bridge between the C-terminal and the super silyl group, the results should be better. With this assumption in mind, TAG3 was prepared. The esterification steps proceeded well and it was tolerant of Fmoc-deprotection and Cbz-deprotection conditions (Scheme 2c). However, when we tried to extend the TES group in TAG3 to trihexylsilyl groups to increase the hydrophobicity, we failed to synthesize this silyl ether type tag. At this stage, TAG4 was developed. The esterification steps also proceeded well and it was tolerant of Fmoc-deprotection and Cbz-deprotection conditions (Scheme 2d). Dipeptide formation was tested for H–Ala–OTAG4 to give 87% yield product, which indicated the necessity of the short alkyl bridge. From the above results, TAG4 might be the best tag containing super silyl group. We also found that most compounds containing the super silyl tags were liquid oil and did not easily solidify in the operation. This might be an advantage when these tags are applied to synthesize peptides in continuous flow.
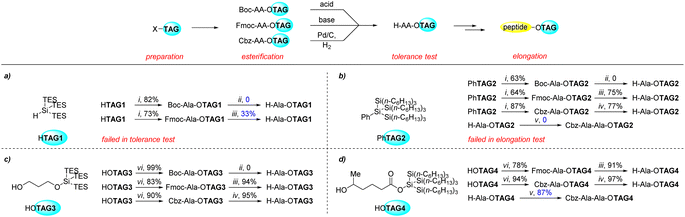 | ||
| Scheme 2 Exploration of the hydrophobic tags (a) TAG1; (b) TAG2; (c) ether type TAG3; (d) ester type TAG4. Reaction conditions (for detailed conditions, please see ESI†): (i) HOTf, PG–Ala–OH, 1-methylimidazole, DCM, 0 °C to r.t.; (ii) 4 M HCl in dioxane, r.t.; (iii) Et2NH, DCM, r.t.; (iv) 10% Pd/C (10 mol%), H2 (1 atm), EtOAc, 50 °C; (v) Cbz–Ala–ONp, CHCl3, r.t.; (vi) PG–Ala–OH, DCC, DMAP, DCM, r.t. | ||
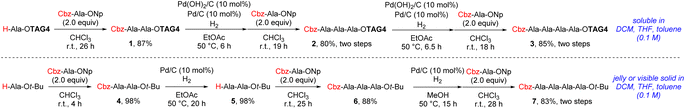
We then started the peptide elongation using Cbz-protected peptides (Cbz chemistry) to test the effect of the hydrophobic tag. Although Fmoc chemistry has been widely used in peptide elongation, using Cbz as N-protecting group is also important due to its stability under both acidic and basic conditions. Additionally, the catalysts used to deprotect the Cbz group can be removed by general filtration to easily give the pure products. We firstly applied TAG4 in the synthesis of an alanine chain. Alanine chains can be described as ‘difficult peptide sequences’ due to the aggregation during the synthesis.21 We can quickly know the effect of the hydrophobic tag by testing the alanine chain synthesis. From Scheme 3, TAG4 can improve the solubility of tetrapeptide (3) in dichloromethane, tetrahydrofuran and toluene compared with tert-butyl ester protected (7). It was also found that compound 6 was difficult to dissolve in EtOAc and the solvent should be changed to MeOH during the next hydrogenation step in contrast with compounds 2. These results show the positive effects when super silyl group is used in the peptide synthesis.
Although TAG4 can enhance the solubility of the peptides in LPPS, we still hope to install the TAG2 at the C-terminal directly because the TAG4 was synthesized viaTAG2 precursor and needed multiple steps (Scheme 4a). As it was mentioned before, if the super silyl group were installed at the C-terminal directly, peptide elongation would be inhibited (Scheme 2b). In previous studies, we focused on starting peptide elongation from monopeptide. If we first install the TAG2 at the C-terminal of a dipeptide followed by peptide elongation, the bulk tag may not inhibit the peptide bond formation because the reaction center and the tag have a two-amino-acid separation. It is just like treating one amino acid as the short bridge in TAG4 (Scheme 4b). Additionally, synthesis of dipeptides is well reported and even some of them are commercially available. To confirm this assumption, we tried to install tris(trihexylsilyl)silyl group (TAG2) at the C-terminal of the dipeptide Cbz–Ala–Ala–OH in ester form. Fortunately, the super silyl group tagged dipeptide 8 was successfully isolated in 68% yield (Scheme 4c). After hydrogenation of the dipeptide, the crude product was mixed with pentafluorophenyl ester to isolate the tripeptide 9 in high yield (83%) (Scheme 4a). These results indicated that the peptide elongation was no problem and the assumption was reasonable. The tris(trihexylsilyl)silyl group (TAG2) can be used as the hydrophobic tag directly and is better than TAG4 containing an alkyl bridge.
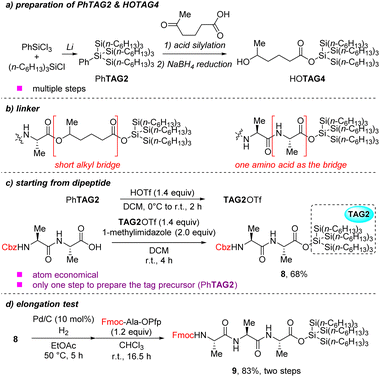 | ||
| Scheme 4 Further studies of super silyl tags (a) preparation of PhTAG2 and HOTAG4; (b) different linker bridges; (c) starting from dipeptide; (d) elongation test. | ||
After obtaining compound 9, to further confirm our hypothesis in Scheme 1a that more silyl groups in the super silyl structure help increase the solubility compared to the simple silyl-based tags, we synthesized Cbz–A4–OTAG2 from 9 and test its solubility in CPME (cyclopentyl methyl ether)15 to compare with previously reported simple silyl-based tag Cbz–A4–OTAGSimple. As it was shown in Scheme 5, the two compounds have similar molecule weights, but the solubility in CMPE was quite different. Cbz–A4–OTAG2 can dissolve in CMPE in almost twice amount compared with Cbz–A4–OTAGSimple. This supports our hypothesis that super silyl-based tag has advantage over the simple silyl-based tag.
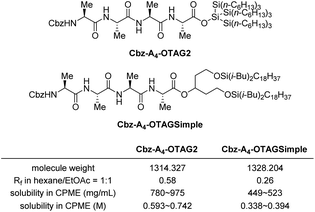 | ||
| Scheme 5 Solubility comparison of the tagged peptides in CPME (room temperature) (for detailed measurement procedure, please see ESI†). | ||
To date, we have successfully developed the tris(trihexylsilyl)silyl group as the hydrophobic tag installed at the C-terminal in ester form. In general, the developed tag is installed at the C-terminal and used for elongation to the N-terminal as it was mentioned before. In SPPS, resins installed at the C-terminal are well developed while those installed at the N-terminal to synthesize peptides in the reverse direction (N to C) are not widely used.22 In LPPS, researchers focused on developing different structures of tags at the C-terminal and using these tags to conduct the peptide synthesis in a linear or convergent way.6–11 Epimerization and diketopiperazine (DKP) formation are the major reasons why peptide synthesis in the reverse direction is not widely used. Our group has given an initial test for simple silyl-based tags at the N-terminal.13 We hope to install the super silyl group at the N-terminal to conduct the LPPS in the reverse direction (N to C). Additionally, in the reported convergent synthesis strategy,9c one of the fragments in the convergent connection step is free carboxylic acid without tag (Scheme 6a). If the untagged carboxylic acid fragment is a long chain, the solubility in organic solvents or the reactivity might be problematic in the connection step. However, if a super silyl group is installed at the N-terminal of the carboxylic acid fragment, the following connection step will be easier because both fragments are tagged. After the convergent connection, the final peptide will be protected by the super silyl groups at both the C- and N-terminals. At this stage, using fluoride ion can remove both tags in one step (Scheme 6b). This will be useful in especially long peptide chain synthesis. Herein, we report efforts towards the installation of the super silyl groups at the N-terminal.
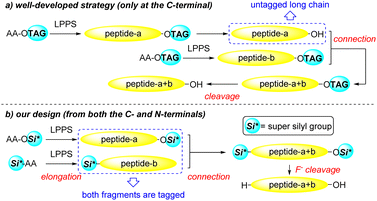 | ||
| Scheme 6 Advantage of the super silyl group installed at the N-terminal in convergent synthesis (a) well-developed strategy; (b) our design. | ||
Firstly, we decided to connect the super silyl group and nitrogen atom directly. This type of N–Si bond formation has been rarely reported. We tried to apply similar N–Si bond formation conditions as MacMillan's work18c to install tris(triethylsilyl)silyl group (TAG1) at the N-terminal, however, we failed to isolate a stable product (Scheme 7a). It was possibly because the tris(triethylsilyl)silyl group was not bulky enough to stabilize the N–Si bond. The bulkier super silyl group di(triisopropylsilyl)phenylsilyl (TAG5) was then used and the product TAG5–Phe–OMe was stable during the purification. Unfortunately, the methyl ester was difficult to remove under the hydrolysis conditions to give free carboxylic acid for the following elongation step. When the methyl ester was changed to benzyl ester (TAG5–Phe–OBn), the N–Si bond was broken under the hydrogenation conditions when removing the benzyl group. Meanwhile, the yields of the silylation products TAG5–Phe–OMe/Bn are lower than 50% and difficult to purify due to the similar Rf value of the byproducts on the TLC. We then tried other super silyl groups, di(triisobutylsilyl)phenylsilyl (TAG6) and di(diisopropyloctylsilyl)phenylsilyl (TAG7), for which steric hindrance was between TAG1 and TAG5. We found the stable products were difficult to isolate (Scheme 7a). At this stage, direct N–Si bond formation might not be a good approach for installing the super silyl group. In 1999, Lipshutz's group reported a silyl carbamate group for amine protection and they applied this structure in SPPS as a linker between solid support and the N-terminal of peptides (Scheme 7b).22e,23 According to these reports, we decided to apply the super silyl carbamate group as the hydrophobic tag at the N-terminal. Fortunately, starting from HTAG1, tris(triethylsilyl)silyl carbamate group (TAG1′) was successfully installed at the N-terminal to give the stable product TAG1′–Phe–OMe in 85% yield (Scheme 8). With this result, more complicated or larger super silyl groups (TAG2, TAG6 and TAG7) were tested as the super silyl carbamates at the N-terminals. The yields of the products were moderate to good (Scheme 8). These newly formed super silyl carbamate tags (TAG2′, TAG6′ and TAG7′) were as useful as TAG1′, which enriched the family of super silyl carbamate tags. When the side chain of the amino acids has functional groups like H–Lys(Boc)′OBn, the super silyl groups can also form the carbamates at the N-terminals (Scheme 8, TAG1′–Lys(Boc)–OBn and TAG6′–Lys(Boc)–OBn). Apart from these, we found that the super silyl group could be installed at the silde chain of some special amino acids in moderate yield (Scheme 8, Boc–Lys(TAG6′)–OBn). This might be useful when synthesizing a peptide containing these special amino acids.
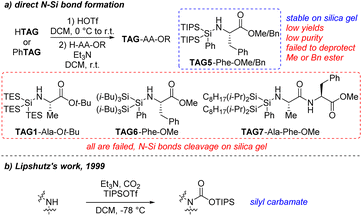 | ||
| Scheme 7 Development of stable super silyl tags at the N-terminal (a) direct N–Si bond formation; (b) reported work. | ||
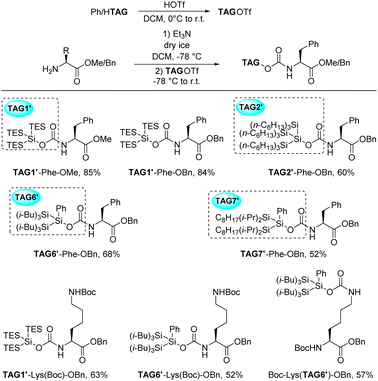 | ||
| Scheme 8 Examples of different stable super silyl groups at the N-terminal or side chain of different amino acids (for detailed reaction conditions, please see ESI†). | ||
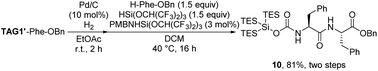
We then began the elongation test of these super silyl carbamate groups. The smallest carbamate (TAG1′) was chosen because it was the least stable structure compared with other large super silyl carbamates. When the hydrogenation reaction was conducted to remove the benzyl group, the pure carboxylic acid product could be obtained without destroying the super silyl carbamate tag. To test the peptide elongation with this tag, we preferred to use silyl ester-mediated peptide bond formation conditions developed by our group.24 This method proved to have no epimerization or DKP formation in our previous studies.13,24 The dipeptide 10 was isolated in good yield by using the hydrosilane reagent (81%, Scheme 9) and no diastereoisomer was detected.
From these results, we believe that these super silyl carbamate tags at the N-terminal can promote linear peptide synthesis in the reverse direction (N to C) and also can improve the solubility of the fragment elaborated with these tags in convergent synthesis (Scheme 6b). To confirm this, we initially tried to synthesize a simple pentapeptide. We chose the larger super silyl carbamate tagged monopeptide TAG2′–Phe–OBn. From this monopeptide, we could successfully elongate it to the dipeptide 11 in 85% yield by using the hydrosilane eagent (Scheme 10a). After coupling with a deprotected C-tagged tripeptide 12, a pentapeptide 13 which was protected by the super silyl groups at both the C- and N-terminals was successfully prepared in 67% yield using the hydrosilane reagent. After treating with fluoride ion (HF/Py), the free pentapeptide 14 was isolated in >99% yield and the dr was >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from 1H-NMR. The purity was 95% confirmed by reversed-phase high performance liquid chromatography (RP-HPLC) (Scheme 10b and Fig. 1). The high dr and purity indicated that no epimerization in the convergent connection step.
1 from 1H-NMR. The purity was 95% confirmed by reversed-phase high performance liquid chromatography (RP-HPLC) (Scheme 10b and Fig. 1). The high dr and purity indicated that no epimerization in the convergent connection step.
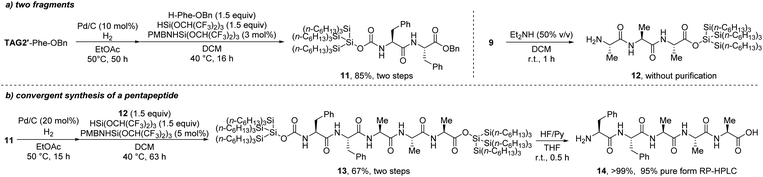 | ||
| Scheme 10 Convergent synthesis of a pentapeptide (a) preparation of two fragments; (b) convergent connection and removal of the tags. | ||
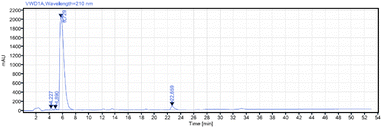 | ||
| Fig. 1 RP-HPLC analysis of crude pentapeptide (14) (for detailed conditions, please see ESI†). | ||
The results in Scheme 10 indicate the utility of the super silyl groups as the hydrophobic tags in both C- and N-terminals in linear and convergent peptide synthesis to some degree, however, the final product is only a short pentapeptide and contains no functional groups in the side chain of the residues. It is necessary to synthesize longer peptides with some hydrophilic residues or some functional groups to further prove the utility of this method. A peptide cancer vaccine nelipepimut-S was chosen to provide a further test with the super silyl tags.25 We plan to divide this peptide chain into two fragments containing 7 and 2 residues and finally combine them in a convergent step. Considering that the super silyl tag installed at the C-terminal in ester form is sensitive to the Boc-deprotection conditions but stable under the Fmoc-deprotection conditions (Scheme 2b and d), a linear peptide elongation system from C- to N-terminal with Fmoc chemistry was used to synthesize the fragment with 7 residues (Scheme 11a). This elongation system is slightly simplified from what we used before.13 The pentafluorophenol esters were used as building blocks and most of the impurities or by-products were removed with 2-aminoethanol, saturated sodium carbonate solution and water. As shown in Scheme 11b, we firstly installed the super silyl tag (TAG2) at the C-terminal of the dipeptide 16. Then, the peptide elongation was started according to the elongation system to give the 7-residue-fragment 18 in 42% isolated total yield after ten steps. For the other fragment, we started from the TAG6′–Lys(Boc)–OBn to elongate into the dipeptide 19 with the hydrosilane reagent in 81% yield followed by removing the benzyl group (Scheme 11c). To connect the two fragments, traditional coupling reagents were chosen for the coupling,9c because using the hydrosilane reagent would require a long time for the coupling of long peptides (such as 63 h from Scheme 10b). The final two fragments were connected in a convergent step26 to give the product 21 in 48% yield which was protected by the super silyl groups at both the C- and N-terminals (Scheme 11d). Considering that the super silyl tags were unstable under the acidic conditions and removing the protecting groups on the side chain (Boc and t-Bu) also needed the acidic conditions, we could remove all the tags and the protecting groups in one step without using fluoride ion. After treating 21 in TFA/TIPS/H2O, the final nelipepimut-S (22) was formed in >99% yield with 73% purity, which was confirmed by reversed-phase high performance liquid chromatography (RP-HPLC) (Scheme 11d and Fig. 2).
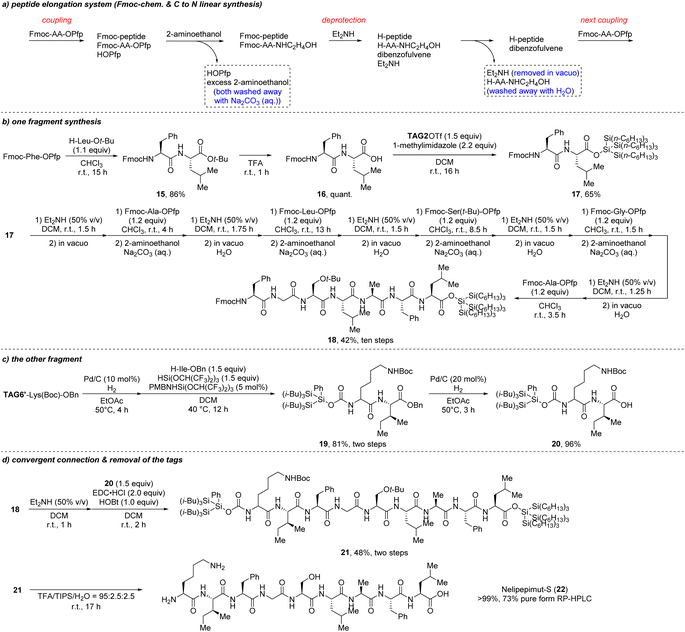 | ||
| Scheme 11 Convergent synthesis of nelipepimut-S with Fmoc-chemistry (a) peptide elongation system; (b and c) preparation of two fragments; (d) convergent connection and removal of the tags. | ||
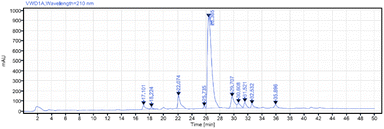 | ||
| Fig. 2 RP-HPLC analysis of crude nelipepimut-S (22) (for detailed conditions, please see ESI†). | ||
At this stage, we have proved the utility of the super silyl tags in both C- and N-terminals in linear and convergent peptide synthesis. However, these tags are sensitive to the acidic conditions whether they were installed at the C-terminal in ester form or at the N-terminal in carbamate form. For peptide elongation, using Fmoc-protected amino acids (Fmoc chemistry) and Boc-protected amino acids (Boc chemistry) are the two most important strategies.9h It is necessary to develop an ‘acid-resistant’ tag for Boc chemistry as supplement to extend the field of the super silyl-based tags in LPPS.
We began to explore the strategy to install the super silyl groups in acid-resistant form. From the above results, the instability might result from the Si–O bonds in ester form or the carbamate form. If we can connect the super silyl groups in Si–C bonds, the acid-resistance would be increased. In 1977, Sieber and Gerlach developed a 2-(trimethylsilyl)ethyl (TMSE) ester as a protecting group for carboxylic acids that can be easily removed by fluoride ion (Scheme 12a).27 We hope to develop a 2-(super silyl)ethyl ester, in which the super silyl group is connected by a Si–C bond. After the initial test of the 2-(tris(trimethylsilyl)silyl) ester (TAG8), we found it was partly resistant to the Boc-deprotection conditions (Scheme 12b). Only about 43% deprotected product was detected, but it still revealed that a Si–C bond was more stable than a Si–O bond under the Boc-deprotection conditions. We turned to use the larger super silyl group to increase the stability of the Si–C bond, however, we failed to construct the Si–C bond when the tris(trimethylsilyl)silyl group was changed to tris(triethylsilyl)silyl group after testing many methods like a Reformatsky reagent,28 a Grignard reagent17d or a radical pathway.17b,c At this stage, a propargyl structure was developed. The Si–C(sp) bond might be more easily formed due to less steric effects compared with sp3 carbon. It would be resistant to acid conditions. With this assumption in mind, we began to test the smallest tris(trimethylsilyl)silyl group first. As shown in Scheme 12c, propargyl tris(trimethylsilyl)silyl tag (TAG9) was synthesized successfully and quantitative yield of the Boc-deprotection product was obtained after installed at the C-terminal of Boc–Ala–OH indicating the stability in acidic conditions.
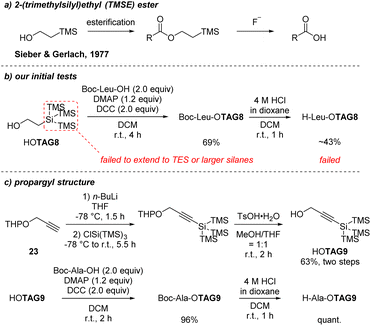 | ||
| Scheme 12 Development of acid-resistant super silyl tags at the C-terminal (a) TMSE ester; (b) our initial tests of TAG8; (c) propargyl structure of TAG9. | ||
With this positive result, we started to explore larger super silyl groups to make the propargyl tag as hydrophobic as possible. To simplify the reaction, ethynylmagnesium chloride was used to test the formation of Si–C(sp) bond. However, we found a similar problem when changing the tris(trimethylsilyl)silyl group to tris(triethylsilyl)silyl group (Scheme 13a, 25a and 25b). A slightly longer alkyl group on the silicon atom inhibited the bond formation thoroughly. Further exploration informed us that the two methyl groups on the silicon atoms were important for the Si–C(sp) bond formation. With only one methyl group, the reactivity decreased a lot due to the steric effect (Scheme 13a, 25c and 25d). From these results, we decided to extend only one alkyl chain on the silicon atom to increase the hydrophobicity of the propargyl tag. When the alkyl chain was extended too far, the reactivity also decreased because of the increase of the steric hindrance (Scheme 13a, 25f). Finally, tris(decyldimethylsilyl)silyl (Scheme 13a, 25e) was chosen to prepare the propargyl super silyl tag in moderated yield (TAG10, Scheme 13b). After obtaining the TAG10, we tested whether it was applicable in LPPS or not. Both Boc–Ala–OTAG10 and Fmoc–Ala–OTAG10 could be prepared in high yield and the H–Ala–OTAG10 was obtained in quantitative yield after the deprotection of Boc group while 87% H–Ala–OTAG10 was isolated when deprotecting the Fmoc group (Scheme 13c). This gave evidence for the stability of TAG10 under the Boc-deprotection conditions but slight instability under the Fmoc-deprotection conditions.
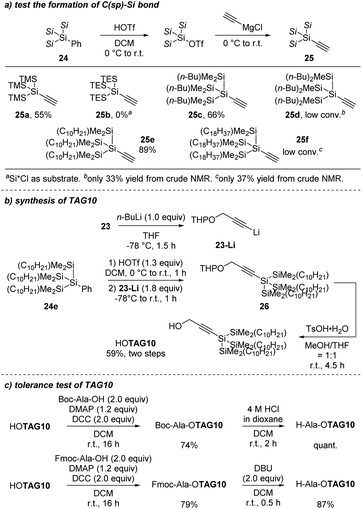 | ||
| Scheme 13 Extension of the alkyl chains on the silicon atoms (a) test the formation of C–Si bond; (b) synthesis of TAG10; (c) tolerance test of TAG10. | ||
We believed that TAG10 could be used as an ‘acid-resistant’ tag for Boc chemistry in LPPS according to the above results. To confirm this, we applied it in the synthesis of nelipepimut-S in Boc chemistry. A linear peptide elongation system from C- to N-terminal with Boc chemistry is described in Scheme 14a. Traditional coupling reagents were used and the by-product urea had no influence on the following reactions which can be removed in the final purification step. As shown in Scheme 14b, the propargyl super silyl tag (TAG10) was successfully installed at the C-terminal of the Boc–Leu–OH. After finishing the peptide elongation using the system in Scheme 13a, the fully protected nelipepimut-S (27) was successfully synthesized in 62% isolated total yield in sixteen steps. To remove the tag, lithium hydroxide was used to give the protected nelipepimut-S (28) in 93% yield with 83% purity, which was confirmed by reversed-phase high performance liquid chromatography (RP-HPLC) (Scheme 14c and Fig. 3).
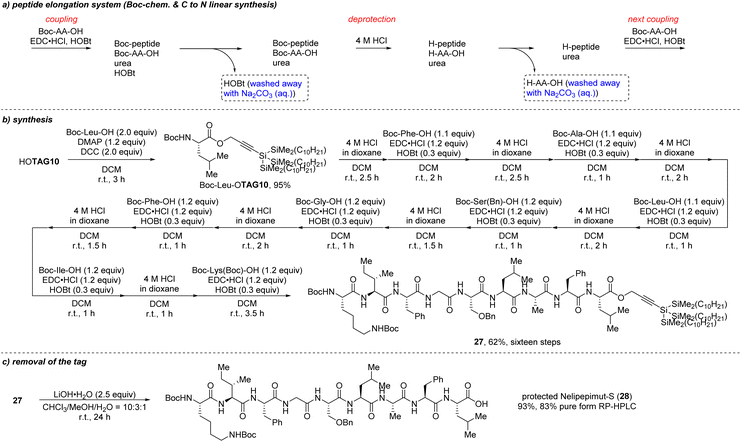 | ||
| Scheme 14 Synthesis of protected nelipepimut-S with Boc-chemistry (a) peptide elongation system; (b) synthesis with TAG10; (c) removal of the tag. | ||
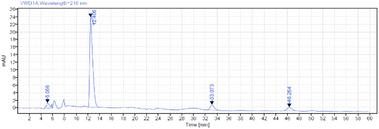 | ||
| Fig. 3 RP-HPLC analysis of crude protected nelipepimut-S (28) (for detailed conditions, please see ESI†). | ||
Conclusions
To sum up, considering that silyl groups play important roles in the hydrophobicity of silicon derivatives, we have developed super silyl groups as hydrophobic tags installed at both the C-terminal in ester form and N-terminal in carbamate form for increasing the solubility in organic solvents and the reactivity during peptide synthesis. The tags can be easily removed by fluoride ion or strong acids. When the super silyl group is installed at the C-terminal, it is stable under hydrogenation and Fmoc-deprotection conditions. For the super silyl carbamate tags at the N-terminal, it was the first reported application of the hydrophobic tags at the N-terminal in LPPS to synthesize peptides in the reverse direction (N to C). This can provide a new synthetic strategy for LPPS and help increase the solubility of the substrates in convergent peptide synthesis. A pentapeptide and nelipepimut-S were successfully synthesized in this convergent way. Apart from this, we have also developed a propargyl super silyl group which is stable under Boc-deprotection conditions when installed at the C-terminal of peptides to make it possible to elongate the peptides in Boc chemistry. Protected nelipepimut-S was also prepared by using this propargyl super silyl tag in Boc chemistry. All of these tags could be prepared in several steps compared with the reported tags. Further studies are continuing in our lab, such as testing the peptide synthesis in non-halogenated solvents and applying the tags in a flow system.Data availability
Experimental procedures, characterisation data and the NMR & HPLC spectra for all compounds have been included in the ESI.†Author contributions
A. W. and H. Y. conceived and designed the project. A. W. conducted all the experiments and collected the data. A. W. prepared the manuscript with input from all contributing authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by Scientific Research S (JP17H06142) from the Japan Society for the Promotion of Science (JSPS), Takeda Science Foundation, and Naito Foundation. We would like to thank Dr Isai Ramakrishna for providing the hydrosilane reagent for the amide bonds formation.Notes and references
- (a) P. Vlieghe, V. Lisowsli, J. Martinez and M. Khrestchatisky, Drug Discovery Today, 2010, 15, 40–56 CrossRef CAS PubMed; (b) J. L. Lau and M. K. Dunn, Bioorg. Med. Chem., 2018, 26, 2700–2707 CrossRef CAS PubMed; (c) A. Henninot, J. C. Collins and J. M. Nuss, J. Med. Chem., 2018, 61, 1382–1414 CrossRef CAS PubMed.
- (a) A. Isidro-Llobet, M. N. Kenworthy, S. Mukherjee, M. E. Kopach, K. Wegner, F. Gallou, A. G. Smith and F. Roschangar, J. Org. Chem., 2019, 84, 4615–4628 CrossRef CAS PubMed; (b) A. Sharma, A. Kumar, B. G. de la Torre and F. Albericio, Chem. Rev., 2022, 122, 13516–13546 CrossRef CAS PubMed.
- Some researchers divided the peptide synthesis into three types including CSPS, SPPS and LPPS (see ref. 2b).
- (a) E. Bayer and M. Mutter, Nature, 1972, 237, 512–513 CrossRef CAS PubMed; (b) V. N. R. Pillai, M. Mutter, E. Bayer and I. Gatfield, J. Org. Chem., 1980, 45, 5364–5370 CrossRef CAS; (c) D. J. Gravert and K. D. Janda, Chem. Rev., 1997, 97, 489–509 CrossRef CAS PubMed; (d) D. E. Bergbreiter, J. Tian and C. Hongfa, Chem. Rev., 2009, 109, 530–582 CrossRef CAS PubMed.
- M. Mizuno, T. Miura, K. Goto, D. Hosaka and T. Inazu, Chem. Commun., 2003, 39, 972–973 RSC.
- (a) F. Stazi, D. Marcoux, J.-C. Poupon, D. Latassa and A. B. Charette, Angew. Chem., Int. Ed., 2007, 46, 5011–5014 CrossRef CAS PubMed; (b) J. Wu, G. An, S. Lin, J. Xie, W. Zhou, H. Sun, Y. Pan and G. Li, Chem. Commun., 2014, 50, 1259–1261 RSC; (c) C. W. Seifert, A. Paniagua, G. A. White, L. Cai and G. Li, Eur. J. Org. Chem., 2016, 1714–1719 CrossRef CAS PubMed; (d) H. Li, J. Chao, G. Tian, J. Hasan, Y. Jin, Z. Zhang and C. Qin, Org. Chem. Front., 2020, 7, 689–696 RSC; (e) H. Li, J. Chao, J. Hasan, G. Tian, Y. Jin, Z. Zhang and C. Qin, J. Org. Chem., 2020, 85, 6271–6280 CrossRef CAS PubMed; (f) H. Li, J. Chao, Z. Zhang, G. Tian, J. Li, N. Chang and C. Qin, Org. Lett., 2020, 22, 3323–3328 CrossRef CAS PubMed; (g) H. Li, J. Ren, J. Li, Z. Zhang, N. Chang and C. Qin, Org. Biomol. Chem., 2020, 18, 8433–8442 RSC.
- J. Yeo, L. Peeva, S. Chung, P. Gaffney, D. Kim, C. Luciani, S. Tsukanov, K. Seibert, M. Kopach, F. Albericio and A. Livingston, Angew. Chem., Int. Ed., 2021, 60, 7786–7795 CrossRef CAS PubMed.
- H. Tamiaki, T. Obata, Y. Azefu and K. Toma, Bull. Chem. Soc. Jpn., 2001, 74, 733–738 CrossRef CAS.
- (a) K. Chiba, Y. Kono, K. Nishimoto, Y. Kitano and M. Tada, Chem. Commun., 2002, 38, 1766–1767 RSC; (b) S. Kitada, S. Fujita, Y. Okada, S. Kim and K. Chiba, Bioorg. Med. Chem. Lett., 2011, 21, 4476–4479 CrossRef CAS PubMed; (c) Y. Okada, H. Suzuki, T. Nakae, S. Fujita, H. Abe, K. Nagano, T. Yamada, N. Ebata, S. Kim and K. Chiba, J. Org. Chem., 2013, 78, 320–327 CrossRef CAS PubMed; (d) S. Kitada, S. Fujita, Y. Okada, S. Kim and K. Chiba, Tetrahedron, 2013, 69, 2555–2559 CrossRef CAS; (e) Y. Fujita, S. Fujita, Y. Okada and K. Chiba, Org. Lett., 2013, 15, 1155–1157 CrossRef CAS PubMed; (f) E. Matsumoto, Y. Fujita, Y. Okada, E. I. Kauppinen, H. Kamiya and K. Chiba, J. Pept. Sci., 2015, 21, 691–695 CrossRef CAS PubMed; (g) Y. Okada, H. Wakamatsu, M. Sugai, E. I. Kauppinen and K. Chiba, Org. Lett., 2015, 17, 4264–4267 CrossRef CAS PubMed; (h) H. Wakamatsu, Y. Okada, M. Sugai, S. R. Hussaini and K. Chiba, Asian J. Org. Chem., 2017, 6, 1584–1588 CrossRef CAS; (i) Y. Okada, R. Takasawa, D. Kubo, N. Iwanaga, S. Fujita, K. Suzuki, H. Suzuki, H. Kamiya and K. Chiba, Org. Process Res. Dev., 2019, 23, 2576–2581 CrossRef CAS; (j) S. Nagahara, Y. Okada and K. Chiba, Chem. Sci., 2021, 12, 12911–12917 RSC.
- (a) D. Takahashi and T. Yamamoto, Tetrahedron Lett., 2012, 53, 1936–1939 CrossRef CAS; (b) D. Takahashi, T. Yano and T. Fukui, Org. Lett., 2012, 14, 4514–4517 CrossRef CAS PubMed; (c) K. Aihara, C. Komiya, A. Shigenaga, T. Inokuma, D. Takahashi and A. Otaka, Org. Lett., 2015, 17, 696–699 CrossRef CAS PubMed; (d) D. Takahashi, T. Inomata and T. Fukui, Angew. Chem., Int. Ed., 2017, 56, 7803–7807 CrossRef CAS PubMed; (e) Y. Hayashi, W. Fukasawa, T. Hirose, M. Iwatsuki, R. Hokari, A. Ishiyama, M. Kanaida, K. Nonaka, A. Také, K. Otoguro, S. Ōmura, K. Shiomi and T. Sunazuka, Org. Lett., 2019, 21, 2180–2184 CrossRef CAS PubMed.
- Y. Hayashi, T. Hirose, M. Iwatsuki, S. Ōmura and T. Sunazuka, Org. Lett., 2019, 21, 8229–8233 CrossRef CAS PubMed.
- S. Yamagami, Y. Okada, Y. Kitano and K. Chiba, Eur. J. Org. Chem., 2021, 3133–3138 CrossRef CAS.
- A. Wu, I. Ramakrishna, T. Hattori and H. Yamamoto, Org. Biomol. Chem., 2022, 20, 8685–8692 RSC.
- A. Nagaya, S. Murase, Y. Mimori, K. Wakui, M. Yoshino, A. Matsuda, Y. Kobayashi, H. Kurasaki, D. R. Cary, K. Masuya, M. Handa and N. Nishizawa, Org. Process Res. Dev., 2021, 25, 2029–2038 CrossRef CAS.
- S. Yano, T. Mori and H. Kubota, Molecules, 2021, 26, 3947 CrossRef PubMed.
- Selected papers about super silyl groups: (a) M. B. Boxer and H. Yamamoto, J. Am. Chem. Soc., 2006, 128, 48–49 CrossRef CAS PubMed; (b) Y. Yamaoka and H. Yamamoto, J. Am. Chem. Soc., 2010, 132, 5354–5356 CrossRef CAS PubMed; (c) P. B. Brady and H. Yamamoto, Angew. Chem., Int. Ed., 2012, 51, 1942–1946 CrossRef CAS PubMed; (d) J. Tan, M. Akakura and H. Yamamoto, Angew. Chem., Int. Ed., 2013, 52, 7198–7202 CrossRef CAS PubMed; (e) A. Izumiseki and H. Yamamoto, J. Am. Chem. Soc., 2014, 136, 1308–1311 CrossRef CAS PubMed; (f) M. Sai, M. Akakura and H. Yamamoto, Chem. Commun., 2014, 50, 15206–15208 RSC; (g) A. Izumiseki and H. Yamamoto, Angew. Chem., Int. Ed., 2015, 54, 8697–8699 CrossRef CAS PubMed; (h) W. Gati and H. Yamamoto, Chem. Sci., 2016, 7, 394–399 RSC; (i) W. Gati and H. Yamamoto, Acc. Chem. Res., 2016, 49, 1757–1768 CrossRef CAS PubMed.
- (a) N. Auner, J. Organomet. Chem., 1987, 336, 59–81 CrossRef CAS; (b) B. Kopping, C. Chatgilialoglu, M. Zehnder and B. Giese, J. Org. Chem., 1992, 57, 3994–4000 CrossRef CAS; (c) A. Postigo, S. Kopsov, C. Ferreri and C. Chatgilialoglu, Org. Lett., 2007, 9, 5159–5162 CrossRef CAS PubMed; (d) H. Wachenfeldt and D. Strand, J. Org. Chem., 2013, 78, 12268–12273 CrossRef PubMed.
- (a) M. Zirngast, J. Baumgartner and C. Marschner, Eur. J. Inorg. Chem., 2008, 1078–1087 CrossRef CAS; (b) T. Kerackian, A. Reina, D. Bouyssi, N. Monteiro and A. Amgoune, Org. Lett., 2020, 22, 2240–2245 CrossRef CAS PubMed; (c) H. A. Sakai, W. Liu, C. C. Le and D. W. C. MacMillan, J. Am. Chem. Soc., 2020, 142, 11691–11697 CrossRef CAS PubMed.
- M. Bodánszky, Nature, 1955, 175, 685 CrossRef PubMed.
- Selected papers about pentafluorophenyl esters: (a) E. Atherton and R. C. Sheppard, J. Chem. Soc., Chem. Commun., 1985, 165–166 RSC; (b) E. Atherton, L. R. Cameron and R. C. Sheppard, Tetrahedron, 1988, 44, 843–857 CrossRef CAS; (c) W. D. G. Brittain and C. R. Coxon, Chem.–Eur. J., 2020, 28, 10–23 Search PubMed.
- (a) S. A. Kates, N. A. Solé, M. Beyermann, G. Barany and F. Albericio, Pept. Res., 1996, 9, 106–113 CAS; (b) D. M. M. Jaradat, Amino Acids, 2018, 50, 39–68 CrossRef CAS PubMed.
- Selected papers about reverse direction SPPS: (a) B. Henkel, L. Zhang and E. Bayer, Liebigs Ann./Recl., 1997, 2161–2168 CrossRef CAS; (b) R. Léger, R. Yen, M. W. She, V. J. Lee and S. J. Hecker, Tetrahedron Lett., 1998, 39, 4171–4174 CrossRef; (c) A. Johansson, E. Åkerblom, K. Ersmark, G. Lindeberg and A. Hallberg, J. Comb. Chem., 2000, 2, 496–507 CrossRef CAS PubMed; (d) N. Thieriet, F. Guibé and F. Albericio, Org. Lett., 2000, 2, 1815–1817 CrossRef CAS PubMed; (e) B. H. Lipshutz and Y.-J. Shin, Tetrahedron Lett., 2001, 42, 5629–5633 CrossRef CAS; (f) W. G. Gutheil and Q. Xu, Chem. Pharm. Bull., 2002, 50, 688–691 CrossRef CAS PubMed; (g) R. Sasubilli and W. G. Gutheil, J. Comb. Chem., 2004, 6, 911–915 CrossRef CAS PubMed; (h) J. Li, Y. Zhu, B. Liu, F. Tang, X. Zheng and W. Huang, Org. Lett., 2021, 23, 7571–7574 CrossRef CAS PubMed.
- B. H. Lipshutz, P. Papa and J. M. Keith, J. Org. Chem., 1999, 64, 3792–3793 CrossRef CAS.
- W. Muramatsu, C. Manthena, E. Nakashima and H. Yamamoto, ACS Catal., 2020, 10, 9594–9603 CrossRef CAS.
- M. Keshavarz-Fathi and N. Rezaei, Vaccines for Cancer Immunotherapy, Elsevier Ltd, 2018, pp. 145–152 Search PubMed.
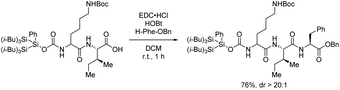
- Traditional coupling reagents were chosen for the coupling according to ref. 9c, because using the hydrosilane reagent would require a long time for the coupling of long peptides (such as 63 h from Scheme 10b). We made a pretest by coupling compound 20 and H–Phe–OBn with traditional coupling reagent and found that our tag can be tolerant under this widely used coupling reagent. The yield is good and the dr is excellent
 .
. - (a) P. Sieber, Helv. Chim. Acta, 1977, 60, 2711–2716 CrossRef CAS; (b) H. Gerlach, Helv. Chim. Acta, 1977, 60, 3039–3044 CrossRef CAS; (c) L. A. Carpino, J.-H. Tsao, H. Ringsdorf, E. Fell and G. Hettrich, J. Chem. Soc., Chem. Commun., 1978, 358–359 RSC; (d) S. M. Weinreb, D. M. Demko and T. A. Lessen, Tetrahedron Lett., 1986, 27, 2099–2102 CrossRef CAS.
- (a) P. Stangier, M. M. Palcic and D. R. Bundle, Carbohydr. Res., 1995, 267, 153–159 CrossRef CAS PubMed; (b) S. N. Greszler and J. S. Johnson, Angew. Chem., Int. Ed., 2009, 48, 3689–3691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc01239e |
| This journal is © The Royal Society of Chemistry 2023 |