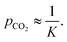Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Accelerated screening of carbon dioxide capture by liquid sorbents†
Ryan J. R.
Jones
 a,
Yungchieh
Lai
a,
Kevin
Kan
a,
Dan
Guevarra
a,
Joel A.
Haber
a,
Yungchieh
Lai
a,
Kevin
Kan
a,
Dan
Guevarra
a,
Joel A.
Haber
 a,
Natalia M.
Ramirez
b,
Alessandra
Zito
a,
Natalia M.
Ramirez
b,
Alessandra
Zito
 b,
Clarabella
Li
b,
Clarabella
Li
 b,
Jenny Y.
Yang
b,
Jenny Y.
Yang
 b,
Aaron M.
Appel
b,
Aaron M.
Appel
 c and
John M.
Gregoire
c and
John M.
Gregoire
 *a
*a
aDivision of Engineering and Applied Science, California Institute of Technology, Pasadena, CA 91125, USA. E-mail: gregoire@caltech.edu
bDepartment of Chemistry, University of California, Irvine, California 92697, USA
cInstitute for Integrated Catalysis, Pacific Northwest National Laboratory, Richland, Washington 99352, USA
First published on 28th February 2024
Abstract
The ideation of carbon capture, concentration, and utilization technologies is establishing a need for carbon dioxide sorbents with specific binding, release, and chemical specifications. While computational workflows can help navigate the broad search space of molecular sorbents, automated experimental screening platforms are relatively underdeveloped. We present a carbon capture screening instrument to characterize the carbon dioxide binding and sorption capacity of liquid sorbent media. We discuss the extension of this capability to characterize the loaded liquid sorbent as well as the headspace to facilitate study of the carbon dioxide adduct, for example its electrochemical activation. The fabrication and computer automation instructions are provided so that the experimental technique can be implemented in a broad range of materials acceleration platforms involving gas sorption.
Introduction
The efficient sorption of CO2 from dilute gas streams is a critical process for enabling carbon sequestration and sustainable energy technologies.1,2 McDannald et al.3 presented the value proposition for developing a materials acceleration platform (MAP)4 for discovery of CO2 sorbents, and recent advancements in computational predictions5 highlight the need for an automated experimental screening instrument. Thermogravimetric analysis (TGA)6 is a traditional method for measurement of CO2 sorption, where the weight-change of a sorbent in a CO2-containing atmosphere is assumed to be due to CO2 sorption after calibrating and accounting for solvent evaporation. TGA is particularly convenient for studying sorbents for thermal CO2 release, as the temperature-dependence of CO2 binding is readily measured via temperature control in the system. For sorbents that are intended to be electrochemically regenerated7 and/or used in reactive capture and conversion (RCC) schemes via electrochemical reduction to carbon-containing chemicals or fuels,8 it is desirable to couple the sorption experiment with an electrochemical cell. While a wetted wall column9 system may be adapted for such purposes, the engineering in such systems is focused on quantitative control of the liquid–gas interface to monitor sorption kinetics, requiring relatively large liquid sorbent volumes and posing challenges for rapid system cleaning. We present herein the carbon capture screening instrument (CCSI), which characterizes CO2 sorption via an infrared measurement of the partial pressure of gas-phase CO2. While detailed infrared spectroscopy measurements can be used to observe the speciation of sorbed CO2 as well as molecular CO2,10 the CCSI employs a relatively inexpensive sensor from Gas Sensing Solutions. The CCSI instrument also interfaces to automated preparation of liquid sorbent media. The target use is for screening of molecular sorbents. While robotic coupling to molecular synthesis instruments may be developed in the future, the present work discusses the mixture of sorbent-loaded solvent with co-solvents or additives, which have been shown to considerably modify the sorption behavior.11 After the custom preparation of the sorbent media and characterization of its CO2 sorption characteristics, the CCSI is design to enabled automated transfer of both the liquid and gas phases to instrumentation for further analysis. For RCC experiments, this liquid analysis is intended to be electrochemical characterization, although that capability is not demonstrated in the present work. Herein we introduce the CCSI design, demonstrate its operation with hydroxide solutions, and present an initial screening experiment for a phenoxide sorbent. The ESI† includes pseudo-code for operation, Python code for incorporation into the HELAO-async12 instrument control platform, a fabrication guide, machining instructions for custom parts, computer drawings, and a parts list with estimated pricing.Results and discussion
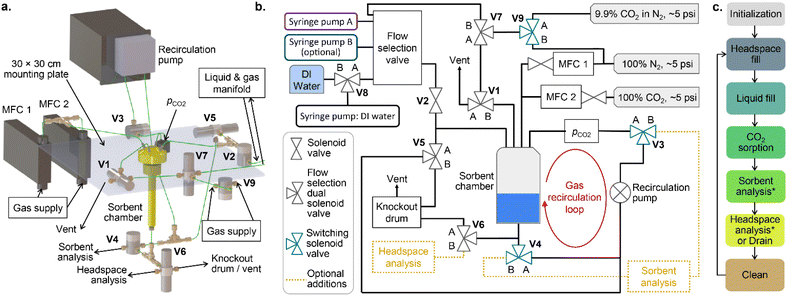
The carbon capture screening instrument
The CCSI instrument is shown in Fig. 1a with the liquid and gas handling diagram shown in Fig. 1b. The system is designed to measure the partial pressure of CO2 in the headspace (pCO2) using an infrared sensor while the headspace is bubbled through the liquid sorbent media via the recirculation pump, which accelerates gas–liquid equilibration. Starting from a given amount of CO2 in the headspace, the CCSI can be operated in a “constant moles” mode in which the sensor measures the rate of sorption of CO2 from the headspace. A thermodynamic assessment of the CO2 sorption in this operating mode relies on precise measurement of pCO2, especially at low partial pressures. Alternatively, CCSI can be operated in a “constant pressure” mode by implementing a feedback loop to inject CO2 into the headspace to maintain a constant pCO2. This mode of operation is preferred for characterizing sorption thermodynamics, as discussed further below. We also note our anecdotal observation that reliable measurement of the low pCO2 values encountered in “constant moles” mode required frequent sensor calibration, which is undesirable for deployment of CCSI for accelerated sorbent screening. While CCSI may accommodate experiments at various total pressures, the present work is limited to near-ambient (1 atm) headspace pressure. A total pressure gauge in included in the system, although the data from this sensor is not used in the present work.The high-level steps of the CCSI instrument for each experiment cycle are shown in Fig. 1c, and the pseudo-code showing the granular execution of these steps is provided in the ESI.† The liquid sorbent for each CCSI experiment is automatically prepared via programmed injection into the sorption chamber from 1 or more syringe pumps. The headspace is prepared prior to the injection of the liquid sorbent, and the sorption experiment is considered to start at the initiation of the sorbent injection into the recirculation cell. After liquid injection, the recirculation pump is activated for the duration of the measurement, which is 1 hour for most measurements in the present work. We note that within a couple minutes, the data exhibits whether there is substantial CO2 sorption from the given headspace, although for the present work we conduct longer experiments to observe the path toward gas–liquid equilibration. As noted in Fig. 1c, the experiment cycle concludes by extraction of the liquid and purging of the instrument.
The headspace for each CCSI experiment is prepared in one of 2 ways. A gas cylinder with 9.85 ± 0.2% CO2 in N2 from Airgas provides the standard process gas. Flushing the system with this gas, injecting the liquid sorbent, and sealing the system provides the initial state where the moles of CO2 in the system is limited to the headspace volume at 0.099 atm CO2. The amount of CO2 sorbed by the liquid (mtot) is then determined by the CO2 added to the system via the mass flow controller (MFC) to maintain this pCO2.
If there are multiple values of pCO2 that will be routinely used, custom gases may be prepared accordingly and selected as the source gas for this primary method of preparing the headspace. In our standard operation, we occasionally use lower pressures of CO2, e.g. 1% or 0.1%, which we prepare via an alternative method wherein the atmosphere is initialized with pure N2. After liquid injection and sealing of the system, the feedback loop for maintaining the setpoint pCO2 is started, where low CO2 in the headspace results in immediate CO2 injection. From here, the experiment proceeds as described above, and the calculation of mtot accounts for the CO2 from the MFC that is in the gas phase.
While mtot quantifies the amount of CO2 in the liquid phase, the goal of the CCSI experiment is to quantify the chemically bound CO2. Thus, mtot is modelled as the sum of the physisorbed unbound CO2 (mphys), as dictated by Henry's Law coefficient for the solvent, and the chemisorbed CO2 (mchem).
Demonstration with hydroxide sorbent
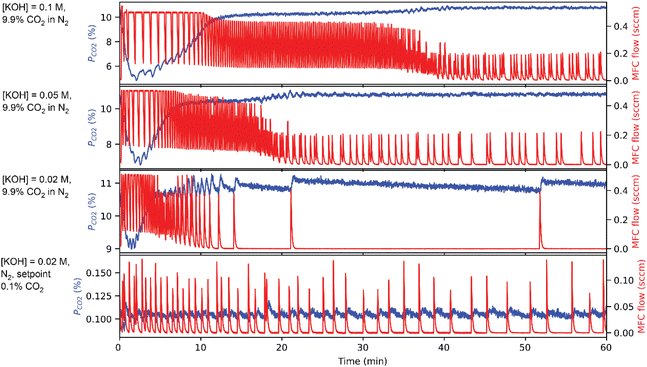
To demonstrate the operation of CCSI, two syringe pumps were loaded with deionized water and aqueous 0.2 M KOH solution. The programmed mixing of solutions from these syringe pumps enabled characterization of sorption from aqueous solutions containing 0.02, 0.05, and 0.1 M KOH.Fig. 2 shows example data for select combinations of [KOH] and pCO2 where the CO2 sorption is evidenced by an initial decrease in the pCO2 signal, which triggers periodic injection of CO2 to eventually restore the headspace to its initial value of pCO2. For the aqueous KOH experiments, mphys is calculated as the product of the Henry's coefficient for CO2 in water (0.033 M atm−1) and the pCO2 for the respective experiment. The reaction of CO2 and OH− can result in either carbonate or bicarbonate, whose relative concentration may be determined by measuring the pH of the liquid, where carbonate is the dominant species above pH ∼ 10 and bicarbonate is the dominant species below pH ∼ 10. Fig. 3 shows the value of mchem with respect to the loading of KOH for each of the 4 experiments. For 3 of these experiments, the pH was measured after the CCSI experiment. The measurements with 9.9% CO2 in the headspace follow the expected trend for reaction of OH− and CO2 to form KHCO3, which is corroborated by the measurement of near-neutral pH for each liquid. The measurement with 0.1% CO2 more closely matches the expected value of mchem for the formation of K2CO3. Due the anticipated poor kinetics for equilibration due to the low pCO2 and low initial [KOH], this measurement was extended to 5 hours, after which the measured pH of 12.15 corroborates the formation of carbonate as the primary species of dissolved inorganic carbon.
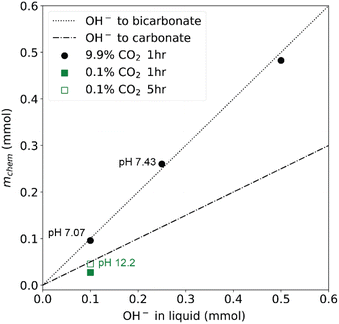 | ||
Fig. 3 The data from the four 1 hour experiments in Fig. 2 are summarized to show the relationship between the inferred amount of chemisorbed CO2 and the amount of OH− in the initial liquid sorbent. The experiment with lowest [KOH] and partial pressure was extended to 5 hours, where the measured pH of the liquid sorbent (pH 12.2) corroborates the approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of the chemisorbed CO2 and hydroxide expected for formation of carbonate as the primary dissolved inorganic carbon species. For the other 3 experiments, this ratio is approximately 1 2 ratio of the chemisorbed CO2 and hydroxide expected for formation of carbonate as the primary dissolved inorganic carbon species. For the other 3 experiments, this ratio is approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, corresponding to the formation of bicarbonate. 1, corresponding to the formation of bicarbonate. | ||
Screening a molecular sorbent
The primary purpose of the CCSI measurement is to characterize the chemisorption of CO2 of a sorbent molecule R dissolved into a liquid solvent. If the chemisorbed state of the CO2 is RCO2, the equilibrium of RCO2 with the sorbent R under a CO2 partial pressure pCO2 is described by the binding constant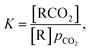 | (1) |
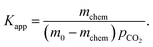 | (2) |
The error and uncertainty of this quantity will be minimized when the concentrations of R and RCO2 are approximately equal, which for a given expected binding constant K motivates the choice of
However, for a binding constant larger than 103, which is required for capture from dilute sources of CO2, the poor transfer kinetics at the corresponding low pCO2 may lead to undesirably long experiment times to equilibrate the liquid sorbent and headspace. For primary screening, we propose using a ca. 10% CO2 atmosphere, which is sufficient to identify the sorbents with binding constants between approximately 10−1 and 102. If mRCO2 is found to be approximately equal to m0, then the apparent binding constant is too large to be adequately characterized with pCO2 = 9.9%, motivating additional experiments with lower values, such as the 0.1% CO2 atmospheres used in Fig. 2.
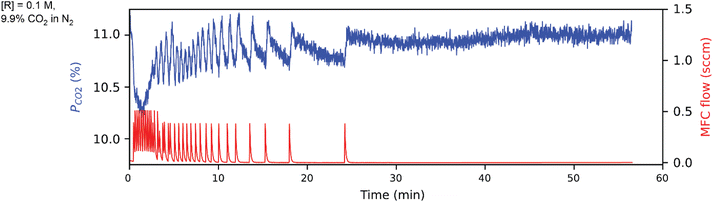
Tetramethylammonium pentafluorophenoxide is a novel molecular sorbent, whose synthesis is characterized by nuclear magnetic resonance (NMR) and Fourier transform infrared spectroscopy (FTIR) in Fig. S1–S5.†Fig. 4 shows the CCSI data for 9.9% CO2 headspace with aqueous 0.1 M tetramethylammonium pentafluorophenoxide sorbent, corresponding to m0 = 0.5 mmol in the 5 mL aqueous solvent. The pCO2 and CO2 injection data are shown in Fig. 4, whose analysis provides mtot = 0.07 (mmol) after the ∼1 h experiment, with mphys = 0.0165 (mmol) from the presumed free CO2 physisorbed in the aqueous solvent. We note that mtot reached 80% and 90% of this value after ca. 550 and 750 s, respectively, highlighting the opportunity to accelerate sorbent screening as needed.
Following the analysis of eqn (2), the mchem = 0.0542 mmol corresponds to an apparent binding constant Kapp = 1.35. While this value is insufficient for applications such as direct air capture, the rapid acquisition of this value from an automated system demonstrates the ability of the CCSI instrument to accelerate sorbent discovery efforts.
Safety and build validation
Provided adherence to standard safety practices regarding compressed gasses, the chemical components of the sorbent media, and the control electronics, we do not envision any safety hazards that emerge from the CCSI design. While the CCSI in intended to operate at total pressures near 5 psi above ambient, the use of pressure-relieving regulators can help mitigate exposure of the user and equipment to unsafe pressures. Given the intended use as a screening tool for novel chemical sorbents, the primary safety evaluation for implementation of the CCSI in research workflows involves any chemical hazards of the sorbent media. For example, the KOH-based sorbent media demonstrated herein requires adherence to handling of caustic media.While component-level operation instructions are provided in the ESI,† we recommend the following evaluations to validate the CCSI assembly. The first validation is against atmospheric leaks, which can be performed by purging the system with N2 gas until the CO2 sensor reaches its noise floor, following by sealing the headspace and recirculating the gas using the recirculation pump. The CO2 sensor time series should then characterize the leak rate of CO2 in the system, which should be below 10 ppm s−1, although the acceptable rate depends on the intended system use with respect to sorption strength, pCO2, and measurement duration. The analogous experiment can be performed with the standard gas intended for sorbent screening, i.e. 9.9% CO2 in the present work, where any decline in the measured pCO2 during sealed headspace recirculation indicates a leak to atmosphere. In both cases, we note that the diffusion of gasses through plastic tubing may provide an apparent nonzero leak rate. With the gas handling validated, we recommend that the first set of sorption experiments involve a known strong sorbent. For example, reproduction of Fig. 3 by performing the experiments shown in Fig. 2 should provide sufficient quantitative characterization of the CCSI to enable subsequent characterization of novel sorbents.
Adaptions and additional considerations
We note that for all experiments in the present work, pCO2 was determined by using the premixed 9.9% CO2 gas or by dosing a pure N2 to obtain 0.1% CO2. In the former preparation, the reading from the CO2 sensor is recorded after the headspace is prepared, providing the setpoint value for the feedback control of the CO2 injections. With this method, the absolute accuracy of the CO2 sensor is inconsequential. We anecdotally observed that the absolute reading can drift. For automated execution of lower pCO2 experiments, e.g. 0.1%, the zero-level of the sensor should be calibrated in the pure N2 atmosphere to ensure accurate preparation of the gas via MFC feedback to the setpoint pCO2.The MFC control algorithm used in the present work is relatively simple and designed to mitigate the over-pressurization of CO2 in the system. We believe that the gas–liquid exchange kinetics are the limiting factor for experiment throughput, although we note the opportunity for increasing the efficiency of pCO2 through, for example, a proportional–integral–derivative (PID) controller.
The CCSI system provides the ability for post-sorption analysis of the sorbent media. In the present work, this analysis is limited to manual pH measurements. Fig. 1b illustrates the valve configuration for implementing post-sorption characterization of the CO2-loaded liquid and headspace gas. These steps are noted as optional in Fig. 1c, and the pseudo-code provided in the ESI† includes the steps for executing such post-sorption analyses, as well as the additional cleaning steps.
To the best of our knowledge, there is no commercial analogue to the CCSI instrument, with automated preparation of sorbent media, execution of sorption characterization, and triggering subsequent liquid and gas analyses. The design employed herein offers a relatively inexpensive set of components, as detailed in the ESI.† Compared to a TGA instrument, which can cost upwards of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U.S.D., the CCSI components are comparable and possibly lower depending on factors such as availability of an existing computer, the chemical compatibility of components (the present build can handle aqueous and nonaqueous solvents, while several valve and tubing components may be replaced with less expensive alternatives for aqueous-only operation), and the sorbent media preparation manifold. The biggest variable in the system cost is the choice of recirculation pump, which can be ca. 1000 U.S.D. for an inexpensive peristaltic pump using tubing with limited chemical compatibility, to ca. 4000 U.S.D. for a diaphragm pump with PEEK tubing, which increases chemical compatibility and enables pumping of viscous sorbent media. We note that in the present work the recirculation pump acts only on the headspace, but we envision pumping on the liquid sorbent in subsequent electrochemical characterization using a recirculation cell design that we previously reported.14 We have built multiple versions of the CCSI with different choices of pumps to meet different specifications of the sorbent media. To our knowledge, no similar instrument has been replicated in an independent laboratory.
000 U.S.D., the CCSI components are comparable and possibly lower depending on factors such as availability of an existing computer, the chemical compatibility of components (the present build can handle aqueous and nonaqueous solvents, while several valve and tubing components may be replaced with less expensive alternatives for aqueous-only operation), and the sorbent media preparation manifold. The biggest variable in the system cost is the choice of recirculation pump, which can be ca. 1000 U.S.D. for an inexpensive peristaltic pump using tubing with limited chemical compatibility, to ca. 4000 U.S.D. for a diaphragm pump with PEEK tubing, which increases chemical compatibility and enables pumping of viscous sorbent media. We note that in the present work the recirculation pump acts only on the headspace, but we envision pumping on the liquid sorbent in subsequent electrochemical characterization using a recirculation cell design that we previously reported.14 We have built multiple versions of the CCSI with different choices of pumps to meet different specifications of the sorbent media. To our knowledge, no similar instrument has been replicated in an independent laboratory.
Experimental
The parts list and assembly instructions are provided in the ESI.† The tubing used for the primary data include primarily polyether ether ketone tubing with a short segment of Viton tubing used in the 300 rpm Masterflex C/L 77122-24 peristaltic pump for recirculation. To avoid the use of the soft-walled tubing and to expand the chemical compatibility of the system, the pump can be replaced with, for example, a KNF SIMDOS 02 diaphragm pump. The CO2 sensor is a Gas Sensing Solutions SprintIR6S-20. Reported pH measurements were acquired with an OAKTON PC700 pH meter. Additional custom machined parts are described in the ESI.†The 18.2 MΩ water used to prepare all sorbent media was from a Millipore Milli-Q Advantage A10. The KOH solution was prepared via dissolution of KOH pellets (Macron Fine Chemicals, AR-ACS). The two pure gases are 99.999%, 99.998% for CO2 and N2, respectively, and the gas mixture is 9.85 ± 0.2% CO2 in N2 (airgas).
Synthetic work was carried out in ambient air and environment. All solvents and reagents were purchased from commercial vendors and used without further purification unless otherwise noted. Deuterated DMSO used for NMR characterization was purchased from Cambridge Isotope Laboratories, Inc., as was degassed via the free–pump–thaw method and stored over activated 3 Å molecular sieves in a glovebox.
Fourier Transform Infrared (FTIR) spectroscopy was performed using a Thermo Scientific Nicolet iS5 FTIR Spectrometer with iD5 diamond ATR. 1H, 13C{1H}, and 19F{1H} NMR and spectra were recorded on a 600 MHz Varian instrument. 1H and 13C NMR spectra chemical shifts are reported as δ values in ppm relative to the residual solvent (CD3)2SO (2.50 ppm, 1H, and 39.52 ppm, 13C).
Tetramethylammonium 2,3,4,5,6-pentafluorophenolate was synthesized and characterized as follows. Pentafluorophenol (1.672 g, 9 mmol) was weighed out and added to a vial containing 25% w/w tetra(n-methyl)ammonium hydroxide (3.298 g, 9 mmol) and methanol (2 mL). This mixture was stirred at room temperature overnight. The resulting solution was dried using a rotary evaporator and then dried further on a high vacuum pump on a Schlenk line overnight. The product is a fine white powder. The product was stored in a nitrogen atmosphere until use, which involved air exposure during the dissolution in water to create the sorbent media. Yield: 2.259 g (97%). 1H NMR (600 MHz d-DMSO) δ 3.14 (TMA) ppm. 13C{1H} NMR (151 MHz, d-DSMO) δ 147.8 (tt, J = 14.1, 4.0 Hz), 140.9 (dtdd, J = 231.9, 9.2, 4.6, 1.9 Hz), 138.6 (ddtd, J = 235.9, 19.2, 10.6, 2.3 Hz), 123.7 (dtt, J = 222.4, 14.8, 5.4 Hz), 54.4–54.3 (m, TMA) ppm. 13C{1H} NMR (151 MHz, D2O) δ 142.0–141.7 (m), 140.8 (dm, J = 231.6 Hz), 138.2 (dm, J = 240.7 Hz), 129.8 (dm, J = 232.4 Hz), 55.3–55.2 (m, TMA) ppm. 19F{1H} (565 MHz, d-DMSO) δ −172.0–172.1 (m, 2F), −172.1–172.3 (m, 2F), −196.3–196.5 (m, 1F) ppm. FTIR (ATR)/cm−1v = 3032.41 (w), 1690.86 (w), 1644.75 (w), 1599.17 (w), 1504.13 (s), 1487.98 (s), 1467.75 (s), 1248.42 (m), 1230.19 (m), 1167 (w), 1084.29 (w), 1046.34 (w), 1001.62 (s), 967.89 (s), 723.23 (w), 604.91 (w), 577.75 (w).
Conclusions
We present an automated instrument for characterization of carbon dioxide capture from a headspace into liquid sorbent media. The instrument interfaces sorption characterization with automated preparation of the liquid sorbent and headspace gas, as well as extension of the instrument for subsequent characterization of the liquid and gas. Characterization of aqueous tetramethylammonium pentafluorophenoxide revealed an apparent binding constant (on a pressure basis) of 1.35, demonstrating deployment of the instrument to characterize novel sorbent–solvent combinations, a critical component of a materials acceleration platform for accelerating the development of carbon capture, concentration, and utilization technologies.Data availability
The instrument control software is available at https://github.com/High-Throughput-Experimentation/helao-async, with dependencies in https://github.com/High-Throughput-Experimentation/helao-core. The computer drawings of the instrument, instrumentation control software (helao snapshot), the data files acquired for the present work, and the source code for the analysis and plotting of those data are provided via CaltechData at https://data.caltech.edu/records/5z5zz-m9r81 (doi: 10.22002/5z5zz-m9r81). The license for the CCSI hardware is included alongside the design files in that repository, as well as a ESI document† for this manuscript.Author contributions
R. J. J. and J. M. G. designed the CCSI with input from J. Y. Y., A. M. A., and J. A. H. R. J. J. and K. K. assembled and validated the CCSI. R. J. R., K. K., and D. G. developed the instrument control software with validation experiments by K. K. and Y. L. Y. L. operated CCSI and analyzed its data. C. L. and N. M. R. synthesized and characterized the phenoxide sorbent under supervision of J. Y. Y. J. M. G. was the primary author of the manuscript with contributions from all authors. R. J. R, K. K., and Y. L. were the primary authors of the assembly and operation instructions.Conflicts of interest
A patent application for the CCSI has been filed. J. M. G. is an industrial consultant for experiment automation.Acknowledgements
This material is based upon work performed by the Center for Closing the Carbon Cycle, which is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences Energy Frontier Research Centers program under Award Number DE-SC0023427.Notes and references
- X. Wang and C. Song, Front. Energy Res., 2020, 8, 560849 CrossRef.
- A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- A. McDannald, H. Joress, B. DeCost, A. E. Baumann, A. G. Kusne, K. Choudhary, T. Yildirim, D. W. Siderius, W. Wong-Ng, A. J. Allen, C. M. Stafford and D. L. Ortiz-Montalvo, Cell Rep. Phys. Sci., 2022, 3, 101063 CrossRef CAS.
- M. M. Flores-Leonar, L. M. Mejía-Mendoza, A. Aguilar-Granda, B. Sanchez-Lengeling, H. Tribukait, C. Amador-Bedolla and A. Aspuru-Guzik, Curr. Opin. Green Sustainable Chem., 2020, 25, 100370 CrossRef.
- Z. Zhang, A. L. Kummeth, J. Y. Yang and A. N. Alexandrova, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2123496119 CrossRef CAS PubMed.
- M. Plaza, C. Pevida, B. Arias, J. Fermoso, A. Arenillas, F. Rubiera and J. Pis, J. Therm. Anal. Calorim., 2008, 92, 601–606 CrossRef CAS.
- J. M. Barlow, L. E. Clarke, Z. Zhang, D. Bím, K. M. Ripley, A. Zito, F. R. Brushett, A. N. Alexandrova and J. Y. Yang, Chem. Soc. Rev., 2022, 51, 8415–8433 RSC.
- M. A. Sabri, S. Al Jitan, D. Bahamon, L. F. Vega and G. Palmisano, Sci. Total Environ., 2021, 790, 148081 CrossRef CAS PubMed.
- H. Karlsson and H. Svensson, Energy Procedia, 2017, 114, 2009–2023 CrossRef CAS.
- X. Wang, V. Schwartz, J. C. Clark, X. Ma, S. H. Overbury, X. Xu and C. Song, J. Phys. Chem. C, 2009, 113, 7260–7268 CrossRef CAS.
- J. M. Barlow and J. Y. Yang, J. Am. Chem. Soc., 2022, 144, 14161–14169 CrossRef CAS PubMed.
- D. Guevarra, K. Kan, Y. Lai, R. J. R. Jones, L. Zhou, P. Donnelly, M. Richter, H. S. Stein and J. M. Gregoire, Digital Discovery, 2023, 2, 1806–1812 RSC.
- M. Özcan, J. Phys. Chem. Lett., 2022, 13, 3507–3509 CrossRef PubMed.
- R. J. R. Jones, Y. Wang, Y. Lai, A. Shinde and J. M. Gregoire, Rev. Sci. Instrum., 2018, 89, 124102 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Molecular sorbent synthesis data, CCSI assembly instructions, CCSI automation using helao-async, and CCSI bill of materials. See DOI: https://doi.org/10.1039/d3dd00232b |
| This journal is © The Royal Society of Chemistry 2024 |