Open Access Article
Open Access ArticleSynthesis of ethyl furfuryl ether via etherification of furfuryl alcohol with ethanol over montmorillonite K10†
Nobutaka Yamanaka *,
Koji Nishi,
Kenji Yasunaga and
Hiroshi Yamada
*,
Koji Nishi,
Kenji Yasunaga and
Hiroshi Yamada
Department of Applied Chemistry, National Defense Academy, 1-10-20 Hashirimizu, Yokosuka, Kanagawa 239-8686, Japan. E-mail: yamanaka@nda.ac.jp
First published on 12th August 2024
Abstract
Ethyl furfuryl ether (EFE), which is synthesized via etherification of furfuryl alcohol (FFalc) with ethanol over Brønsted acid catalysts, is used as an additive in gasoline to reduce its consumption and CO2 emission. In this work, we demonstrate that the performance of this synthesis route can be improved by using commercially available, low-cost, and environmentally friendly montmorillonite K10, which produces EFE in a relatively high yield of 45.3% and a FFalc conversion of 94.2% at a low reaction temperature of 393 K within 1 h. Other commercially available clay minerals showing Brønsted acidity, namely, kaolinite and halloysite, were also used in the etherification reaction under identical conditions. The catalytic performance followed the order of montmorillonite K10 > halloysite > kaolinite, which is consistent with that of the Brønsted acidities determined via acid–base titration. The spent montmorillonite K10 showed a catalytic performance comparable to that of the fresh catalyst after calcination.
Introduction
Owing to the depletion of non-renewable fossil fuel reserves and increasing concern about global climate change, tremendous efforts have been devoted to convert renewable biomass resources into fuels and valuable chemicals.1–4 The most abundant and renewable biomass on the Earth is lignocellulose, which is mainly composed of cellulose, hemicellulose, and lignin.3–5 Furfural, which is obtained from the decomposition of hemicellulose, is an important platform chemical with an annual production volume of more than 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.3,4,6 The reduction of furfural produces furfuryl alcohol (FFalc), which is not fully utilized and over-supplied in the chemical market.1,2,7 One promising method to exploit FFalc is its etherification with alkyl alcohols over Brønsted acid catalysts to synthesize alkyl furfuryl ethers (Scheme 1),8–10 which have been used as additives in gasoline to reduce its consumption and CO2 emission.8,9,11–14 Moreover, alkyl furfuryl ethers can be further converted to other valuable fuel additives such as alkyl tetrahydrofurfuryl ethers and alkyl levulinates.8,11–13,15
000 tons.3,4,6 The reduction of furfural produces furfuryl alcohol (FFalc), which is not fully utilized and over-supplied in the chemical market.1,2,7 One promising method to exploit FFalc is its etherification with alkyl alcohols over Brønsted acid catalysts to synthesize alkyl furfuryl ethers (Scheme 1),8–10 which have been used as additives in gasoline to reduce its consumption and CO2 emission.8,9,11–14 Moreover, alkyl furfuryl ethers can be further converted to other valuable fuel additives such as alkyl tetrahydrofurfuryl ethers and alkyl levulinates.8,11–13,15
H. Nguyen et al. developed an etherification of FFalc with iso-propanol using metal chlorides, namely CrCl3 and YCl3,16 with CrCl3 affording iso-propyl furfuryl ether as the product in a higher yield of approximately 35%. In this system, the metal chlorides generated Brønsted acid species upon alcoholysis, enabling the etherification reaction to proceed. Although effective for the etherification reaction, the homogeneous Brønsted acid catalysts present some drawbacks, such as a cumbersome product separation, catalyst recycling, and environmental problems. To overcome these issues, various heterogeneous catalysts, including zeolites, have been used in the etherification of FFalc with alkyl alcohols to synthesize alkyl furfuryl ethers. However, the etherification reaction over heterogeneous catalysts often requires high reaction temperatures of 398 K or above and long reaction times of 4 h or above, which should be addressed for their industrial applications.8,11,15,17–21
Recently, we demonstrated that montmorillonite K10 is an effective heterogeneous Brønsted acid catalyst for the esterification of FFalc with ethanol to ethyl levulinate (EL).1 During the conversion, ethyl furfuryl ether (EFE) was formed in a maximum yield of 31.0% and behaved as an intermediate (Fig. S1†). In this work, we aimed to increase the yield of the EFE product by optimizing the reaction conditions. As a result, montmorillonite K10 afforded EFE from FFalc and ethanol in a shorter reaction time at a lower reaction temperature compared with the heterogeneous Brønsted acid catalysts mentioned above. While montmorillonite K10 has been used in various etherification reactions, to the best of our knowledge, there are no reports of its use in the etherification reaction of FFalc.22–25 Then, other commercially available clay minerals showing Brønsted acidity, namely, kaolinite and halloysite, were also applied to the etherification of FFalc with ethanol under the optimized reaction conditions, and their catalytic performance was correlated to their acid strength expressed according to the Hammett acidity function and their Brønsted acidities determined via acid–base titration. Finally, the reusability of montmorillonite K10 was investigated because this clay mineral showed a much higher EFE yield than kaolinite and halloysite.
Experimental
Materials
FFalc, EL, and p-nitroaniline were purchased from Tokyo Chemical Industry Co., Ltd. EFE was purchased from Fluorochem. Ethanol and n-dodecane were purchased from Wako Pure Chemical Ind., Ltd. All organic chemicals were used as received without further purification. Montmorillonite K10, halloysite, and kaolinite were purchased from Sigma-Aldrich.Catalyst characterization
Powder X-ray diffractograms (XRD) were recorded on a Bruker D8 ADVANCE diffractometer operated at 40 kV and 40 mA using CuKα radiation (λ = 0.15418 nm) and identified by using Crystallography Open Database (COD).26 The clay minerals were scanned at a step width of 0.02° and a speed of 6° min−1 over the range 5° ≤ 2θ ≤ 80°.The acid scale of the clay minerals was measured on a UV–vis spectrophotometer (GENESYS 10S UV–VIS, Thermo Scientific) over the spectral range of 300–650 nm using p-nitroaniline as a basic indicator.2 In a typical procedure, clay minerals (25 mg) and p-nitroaniline (1.0 mg) were added to ethanol (10 mL), and the mixture was stirred for 0.5 h with a magnetic stirrer. The clay mineral was then separated from the mixture by centrifugation, and the UV spectrum of the solution was recorded. The Hammett acidity function (H0) was calculated according to the equation H0 = pKa(In) + log([In]/[InH+]), where pKa(In) is the pKa value of the p-nitroaniline solution (0.99), and [In] and [InH+] are the molar concentrations of the unprotonated and protonated forms of p-nitroaniline, respectively.
Typical experimental procedures
The etherification of FFalc with ethanol over the clay minerals was performed in a 30 mL high-pressure autoclave (TVS-N2 type, Taiatsu Techno Co.) with a magnetic stirrer, a pressure gauge, and an automatic temperature controller. In a typical procedure, clay mineral (50 mg), FFalc (1.0 mmol), n-dodecane (0.30 mmol) as an internal standard material, and ethanol (3.0 mL) were charged into the autoclave. After being sealed, the autoclave was purged with N2 to remove the air and maintained at 1.0 MPa at room temperature. The autoclave was placed in a preheated oil bath at 393 K for 1 h. After completion of the reaction, the autoclave was removed from the oil bath and quickly cooled in a water bath to stop the reaction. The autoclave was placed in a water bath for at least 0.5 h until the catalyst settled to the bottom of the autoclave.13 The remaining N2 was then discharged, and the supernatant solution was analyzed using a gas chromatograph (Nexis GC-2030, Shimadzu, using a flame ionization detector) equipped with a capillary column (Rxi-5ms, 30 m × 0.25 mm). The carrier gas was helium, and its flow rate was 1.72 mL min−1. The detector temperature was 553 K. The conversion and product yields were calculated using the internal standard method.Results and discussion
Catalyst characterization
The specific surface area, total pore volume, and Brønsted acidity of the clay minerals were investigated in our previous work and are summarized in Table S1.† Their Brønsted acidities determined via acid–base titration follow the order: montmorillonite K10 (0.378 mmol g−1) > halloysite (0.181 mmol g−1) > kaolinite (0.093 mmol g−1).In this study, the acid strength of the clay minerals was measured using the Hammett acidity function (H0), and the results are shown in Table 1. There is the following relationship between H0 value and acid strength: the lower the H0 value of a catalyst, the higher its acid strength.2,27 From this relationship, we obtained the acid strength order of the clay minerals with the following H0 values: halloysite (1.07) > kaolinite (1.11) > montmorillonite K10 (1.92).
Catalytic tests
Because montmorillonite K10 showed a much higher Brønsted acidity than kaolinite and halloysite, the former was first used in the etherification of FFalc with ethanol to synthesize EFE.First, the effect of reaction temperature was investigated from 353 K to 413 K under 1.0 MPa N2 for 1 h, and the results are shown in Table 2. At 353 K, the target product EFE was obtained in 15.0% yield along with a small amount of EL (1.0% yield) and the FFalc conversion was 34.7% (Table 2, entry 1). When the reaction temperature was increased from 353 K to 393 K, the catalytic performance of montmorillonite K10 was greatly enhanced, giving EFE in 45.6% yield and a FFalc conversion of 93.4% (Table 2, entries 1–3). The increase in the reaction temperature also had a positive effect on the EL yield, which indicates that increasing the reaction temperature facilitated the conversion of EFE to EL. Further increasing the reaction temperature to 413 K resulted in a substantial decrease in the yield of EFE (Table 2, entry 4). Therefore, 393 K was selected as the optimum reaction temperature for further experiments. At any reaction temperature, a large difference between the FFalc conversion and total yield of EFE and EL was observed. This is most likely due to the formation of dark-colored insoluble polymeric byproducts such as humin because FFalc easily undergoes self-polymerization in the presence of acid catalysts.12,28,29
| Entry | Reaction temp./K | Conv./% | Yield/% | |
|---|---|---|---|---|
| EFE | EL | |||
| a Reaction conditions: montmorillonite K10, 50 mg; FFalc, 1.0 mmol; n-dodecane, 0.30 mmol; ethanol, 3.0 mL; N2 pressure, 1.0 MPa; reaction time, 1 h. | ||||
| 1 | 353 | 34.7 | 15.0 | 1.0 |
| 2 | 373 | 73.8 | 35.3 | 4.5 |
| 3 | 393 | 93.4 | 45.6 | 8.8 |
| 4 | 413 | 99.7 | 29.6 | 26.1 |
Next, the effect of the ethanol volume was investigated by varying the ethanol volume from 1.5 mL to 6.0 mL while keeping other reaction parameters constant. The results are shown in Table 3. When the etherification of FFalc over montmorillonite K10 was performed in 1.5 mL ethanol, EFE was formed in 38.2% yield together with a large amount of EL (12.7% yield) and the FFalc conversion was 99.1% (Table 3, entry 1). By increasing the ethanol volume to 3.0 mL, a higher EFE yield of 45.6% and a lower FFalc conversion of 93.4% were obtained, accompanied by a lower EL yield of 8.8% (Table 3, entry 2). Therefore, a dilute concentration of FFalc was required to increase the yield and selectivity of the target product EFE. A similar yield of EFE was obtained using 4.5 mL ethanol (Table 3, entry 3). Further increasing the ethanol volume to 6.0 mL resulted in a decrease in the catalytic performance of montmorillonite K10 (Table 3, entry 4). This result implies a mass transfer limitation,30 which was supported by the fact that a much lower catalytic performance was observed when the ethanol volume was increased to 7.5 mL (Table 3, entry 5). Accordingly, the optimum ethanol volume of 3.0 mL was selected for further experiments.
| Entry | Ethanol volume/mL | Conv./% | Yield/% | |
|---|---|---|---|---|
| EFE | EL | |||
| a Reaction conditions: montmorillonite K10, 50 mg; FFalc, 1.0 mmol; n-dodecane, 0.30 mmol; N2 pressure, 1.0 MPa; reaction temperature, 393 K; reaction time, 1 h. | ||||
| 1 | 1.5 | 99.1 | 38.2 | 12.7 |
| 2 | 3.0 | 93.4 | 45.6 | 8.8 |
| 3 | 4.5 | 94.6 | 46.0 | 10.2 |
| 4 | 6.0 | 84.9 | 40.4 | 5.5 |
| 5 | 7.5 | 74.9 | 34.7 | 4.8 |
Then, the catalyst amount was optimized, and the results are shown in Table 4. Upon increasing the catalyst amount from 10 mg to 50 mg, the FFalc conversion and EFE yield increased from 39.3% to 93.4% and from 17.9% to 45.6%, respectively (Table 4, entries 1–3). Further increasing the catalyst amount to 70 mg and 90 mg increased slightly the FFalc conversion to 99.1%, whereas the yield of EFE gradually decreased to 43.2% (Table 4, entries 4 and 5). Considering that the yield of EL steadily increased from 8.8% to 14.0%, the decrease in the EFE yield can be ascribed to the increase in the number of available Brønsted acid sites that facilitate the conversion of the target product to the byproduct. Therefore, 50 mg was selected as the optimum catalyst amount for further experiments.
| Entry | Catalyst amount/mg | Conv./% | Yield/% | |
|---|---|---|---|---|
| EFE | EL | |||
| a Reaction conditions: FFalc, 1.0 mmol; n-dodecane, 0.30 mmol; ethanol, 3.0 mL; N2 pressure, 1.0 MPa; reaction temperature, 393 K; reaction time, 1 h. | ||||
| 1 | 10 | 39.3 | 17.9 | 1.7 |
| 2 | 30 | 81.0 | 40.2 | 6.5 |
| 3 | 50 | 93.4 | 45.6 | 8.8 |
| 4 | 70 | 97.6 | 44.6 | 10.3 |
| 5 | 90 | 99.1 | 43.2 | 14.0 |
The effect of the N2 pressure was investigated from 0.1 MPa to 1.0 MPa, and the results are shown in Table 5. Increasing the N2 pressure from 0.1 MPa to 0.7 MPa improved the catalytic performance of montmorillonite K10 (Table 5, entries 1–3). A similar behavior was reported by S. Shimazu et al.21 Performing the etherification reaction under a higher N2 pressure of 1.0 MPa hardly improved the EFE yield (Table 5, entry 4). Accordingly, a N2 pressure of 0.7 MPa was selected as the optimum condition for further experiments.
| Entry | N2 pressure/MPa | Conv./% | Yield/% | |
|---|---|---|---|---|
| EFE | EL | |||
| a Reaction conditions: montmorillonite K10, 50 mg; FFalc, 1.0 mmol; n-dodecane, 0.30 mmol; ethanol, 3.0 mL; reaction temperature, 393 K; reaction time, 1 h. | ||||
| 1 | 0.1 | 89.5 | 43.2 | 6.5 |
| 2 | 0.4 | 93.5 | 43.0 | 8.4 |
| 3 | 0.7 | 94.2 | 45.3 | 8.3 |
| 4 | 1.0 | 93.4 | 45.6 | 8.8 |
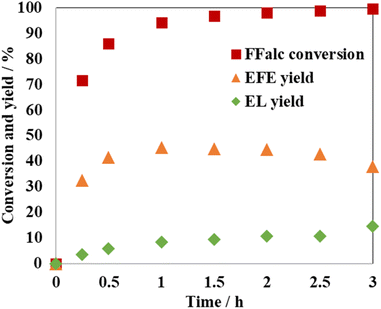
Fig. 1 shows the effect of the reaction time on the etherification of FFalc with ethanol over montmorillonite K10. After 0.25 h, the FFalc conversion and EFE yield were 71.6% and 32.6%, respectively. By prolonging the reaction time to 0.5 h, the FFalc conversion and EFE yield increased to 85.9% and 41.4%, respectively. A maximum EFE yield of 45.3% and a FFalc conversion of 94.2% were obtained after 1 h. Further increasing the reaction time caused a decrease in the yield of EFE, whereas the yield of EL increased. Therefore, the reaction time of 1 h was found to be optimal.
In our previous work, EFE was formed in 31.0% yield after 1 h at 393 K (Fig. S1†), whereas a much higher EFE yield of 45.3% was obtained in this work under identical reaction conditions except for a lower N2 pressure and a larger amount of FFalc.
Under the optimized conditions, other commercially available clay minerals showing Brønsted acidity, namely, kaolinite and halloysite, were also applied to the etherification reaction. As shown in Table 6, the two clay minerals exhibited much lower catalytic performance than montmorillonite K10. A comparison of their catalytic performance with their acid strength and Brønsted acidities shown in Tables 1 and S1,† respectively, revealed that the order of their catalytic performance was consistent with that of their Brønsted acidities. Thus, the higher the Brønsted acidity of a catalyst, the higher its catalytic performance in the etherification reaction.
| Entry | Catalyst | Conv./% | Yield/% | |
|---|---|---|---|---|
| EFE | EL | |||
| a Reaction conditions: catalyst, 50 mg; FFalc, 1.0 mmol; n-dodecane, 0.30 mmol; ethanol, 3.0 mL; N2 pressure, 0.7 MPa; reaction temperature, 393 K; reaction time, 1 h.b n.d. represents not detected. | ||||
| 1 | Kaolinite | 15.2 | 0.6 | n.d.b |
| 2 | Halloysite | 23.1 | 3.7 | 0.2 |
| 3 | Montmorillonite K10 | 94.2 | 45.3 | 8.3 |
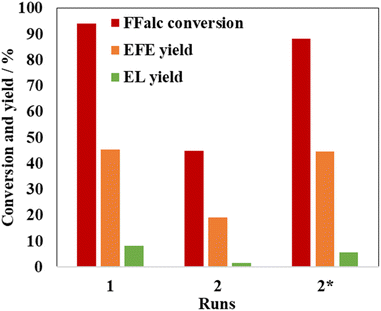
The reusability of montmorillonite K10 for the etherification reaction was investigated under the optimized conditions (Fig. 2). After completion of the reaction, the reaction mixture was transferred to a centrifuge tube. The wall of the autoclave was washed with acetone two or three times, and the resulting mixture was also transferred to the tube. The mixture was centrifuged to separate the spent montmorillonite K10 from the solution. The majority of the solution was discarded, and fresh acetone was added to the tube. The mixture was then stirred and centrifuged again. This process was repeated several times. The washed montmorillonite K10 was dried under vacuum overnight, collected from the centrifuge tube, and reused for the next run. After the first run, the surface of the spent catalyst became black, whereas that of the original catalyst was white. This surface color change to black indicates the adsorption and deposition of the abovementioned insoluble polymeric byproducts such as humin on the catalyst surface, which decreased the accessibility of the Brønsted acid sites required for the etherification reaction to proceed.6 This was expected to decrease the catalytic performance of montmorillonite K10 after the second run. In fact, a substantial decrease in the catalytic performance was observed in the second run, with the yield of EFE and FFalc conversion being 19.1% and 44.9%, respectively. To remove unwanted insoluble polymeric byproducts, the spent montmorillonite K10 after the first run was calcined at 673 K for 4 h in air. The calcined catalyst afforded an EFE yield of 44.6% and a FFalc conversion of 88.2%, which were comparable to those of the fresh catalyst (Fig. 2, runs 1 and 2*). Therefore, montmorillonite K10 could be reused for the etherification reaction after thermal treatment.
Finally, the catalytic results of montmorillonite K10 for the etherification of FFalc with ethanol were compared with those of some heterogeneous Brønsted acid catalysts (Table 7). The clay mineral showed an EFE yield comparable to that of the heterogeneous catalysts within a shorter time at a lower reaction temperature, demonstrating its superiority. In addition, montmorillonite K10 has the advantages of being commercially available, low-cost, and environmentally benign compared with other heterogeneous catalysts. Therefore, the newly developed catalytic system based on commercially available, low-cost, and environmentally friendly montmorillonite K10 is effective for the synthesis of EFE via the etherification of FFalc with ethanol.
Conclusions
In this study, we demonstrated that commercially available, low-cost, and environmentally friendly montmorillonite K10 is an effective heterogeneous Brønsted acid catalyst for the etherification of FFalc with ethanol. The clay mineral produced the target product EFE in 45.3% yield and a FFalc conversion of 94.2% at a reaction temperature of 393 K within 1 h. This catalytic performance is superior to those of previously reported heterogeneous Brønsted acid catalysts. In the etherification reaction under the optimized conditions, montmorillonite K10 was superior to other commercially available clay minerals showing Brønsted acidity, namely, kaolinite and halloysite, probably because of its much higher Brønsted acidity. Furthermore, it could be reused without a considerable loss of its catalytic performance after calcination. Our catalytic system offers a substantial improvement of the synthesis of EFE from FFalc and ethanol in terms of product separation, cost, and performance, thereby providing an environmentally friendly, economic, and effective route to biomass utilization.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Nobutaka Yamanaka: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, visualization, writing – original draft. Koji Nishi: methodology, formal analysis, investigation, writing – review & editing. Kenji Yasunaga and Hiroshi Yamada: data curation, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI (Grant Numbers 23K13787 and 23K03705).References
- N. Yamanaka, D. Abe, M. Miwaka-Saiga, K. Yasunaga, H. Yamada and S. Shimazu, Sustainable Energy Fuels, 2022, 6, 5153 RSC.
- G. Wang, Z. Zhang and L. Song, Green Chem., 2014, 16, 1436 RSC.
- N. Yamanaka and S. Shimazu, Reactions, 2023, 4, 667 CrossRef CAS.
- S. Zhu, Y. Cen, J. Guo, J. Chai, J. Wang and W. Fan, Green Chem., 2016, 18, 5667 RSC.
- A. Démolis, N. Essayem and F. Rataboul, ACS Sustain. Chem. Eng., 2014, 2, 1338 CrossRef.
- L. Peng, X. Gao, X. Yu, H. Li, J. Zhang and L. He, Energy Fuels, 2019, 33, 330 CrossRef CAS.
- G. Zhao, M. Liu, X. Xia, L. Li and B. Xu, Molecules, 2019, 24, 1881 CrossRef CAS PubMed.
- N. Mulik and V. Bokade, Mol. Catal., 2022, 531, 112689 CrossRef CAS.
- M. Song, C. Qiu, P. Ma, J. Zhong, Z. Zhang, W. Fang, W. Song, J. Fan and W. Lai, Renewable Energy, 2023, 212, 468 CrossRef CAS.
- A. Saotta, A. Allegri, F. Liuzzi, G. Fornasari, N. Dimitratos and S. Albonetti, ChemEngineering, 2023, 7, 23 CrossRef CAS.
- D. R. Chaffey, T. E. Davies, S. H. Taylor and A. E. Graham, ACS Sustain. Chem. Eng., 2018, 6, 4996 CrossRef CAS.
- N. L. Mulik, P. S. Niphadkar and V. V. Bokade, Res. Chem. Intermed., 2020, 46, 2309 CrossRef CAS.
- Q. Cao, W. Zhang, S. Luo, R. Guo and D. Xu, Energy Fuels, 2021, 35, 12725 CrossRef CAS.
- T. A. Natsir and S. Shimazu, Fuel Process. Technol., 2020, 200, 106308 CrossRef CAS.
- Q. Cao, J. Guan, G. Peng, T. Hou, J. Zhou and X. Mu, Catal. Commun., 2015, 58, 76 CrossRef CAS.
- H. Nguyen, N. Xiao, S. Daniels, N. Marcella, J. Timoshenko, A. Frenkel and D. G. Vlachos, ACS Catal., 2017, 7, 7363 CrossRef CAS.
- Y. A. Topolyuk and A. I. Nekhaev, Mendeleev Commun., 2018, 28, 93 CrossRef CAS.
- J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150 CrossRef CAS.
- M. M. Antunes, R. F. Mendes, F. A. A. Paz and A. A. Valente, Catalysts, 2021, 11, 190 CrossRef CAS.
- P. Neves, M. M. Antunes, P. A. Russo, J. P. Abrantes, S. Lima, A. Fernandes, M. Pillinger, S. M. Rocha, M. F. Ribeiro and A. A. Valente, Green Chem., 2013, 15, 3367 RSC.
- T. A. Natsir, T. Hara, N. Ichikuni and S. Shimazu, ACS Appl. Energy Mater., 2018, 1, 2460 CrossRef CAS.
- B. Lu, L.-J. Li, T.-S. Li and J.-T. Li, J. Chem. Res., Synop., 1998, 604 RSC.
- M. Roze, V. Kampars, K. Teivena, R. Kampare and E. Liepins, Mater. Sci. Appl. Chem., 2013, 28, 67 CrossRef CAS.
- A. M. Bahmanpour, F. Héroguel, C. J. Baranowski, J. S. Luterbacher and O. Kröcher, Appl. Catal., A, 2018, 560, 165 CrossRef CAS.
- A. M. Tomkiel, A. D. Majewski, L. Siergiejczyk and J. W. Morzycki, Molecules, 2023, 28, 7068 CrossRef CAS PubMed.
- A. Altomare, N. Corriero, C. Cuocci, A. Falcicchio, A. Moliterni and R. Rizzi, J. Appl. Crystallogr., 2015, 48, 598 CrossRef CAS.
- B. M. Matsagar and P. L. Dhepe, New J. Chem., 2017, 41, 6137 RSC.
- K. Tang, S. Xie, G. R. Cofield, X. Yang, E. Tian and H. Lin, Energy Technol., 2018, 6, 1826 CrossRef CAS.
- X. Gao, L. Peng, H. Li and K. Chen, BioResources, 2015, 10, 6548 CAS.
- K. Y. Nandiwale, S. K. Sonar, P. S. Niphadkar, P. N. Joshi, S. S. Deshpande, V. S. Patil and V. V. Bokade, Appl. Catal., A, 2013, 460–461, 90 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03921a |
| This journal is © The Royal Society of Chemistry 2024 |