Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, aromatization and cavitates of an oxanorbornene-fused dibenzo[de,qr]tetracene nanobox†
Han
Chen
 ,
Zeming
Xia
,
Zeming
Xia
 and
Qian
Miao
and
Qian
Miao
 *
*
Department of Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China. E-mail: miaoqian@cuhk.edu.hk
First published on 13th January 2022
Abstract
Oxanorbornene-fused double-stranded macrocycles, represented by kohnkene, are not only synthetic precursors toward short segments of zigzag carbon nanotubes but also typical cavitands processing an intrinsic cavity. However, their capability to bind guest molecules in solution remained unexplored. Herein we report a new member of oxanorbornene-fused double-stranded macrocycles, which is named a nanobox herein because of its shape. Reductive aromatization of this oxanorbornene-fused nanobox leads to observation of a new zigzag carbon nanobelt by high resolution mass spectroscopy. The fluorescence titration and NMR experiments indicate that this nanobox encapsulates C70 in solution with a binding constant of (3.2 ± 0.1) × 106 M−1 in toluene and a high selectivity against C60 and its derivatives. As found from the X-ray crystallographic analysis, this nanobox changes the shape of its cross-section from a rhombus to nearly a square upon accommodating C60.
Introduction
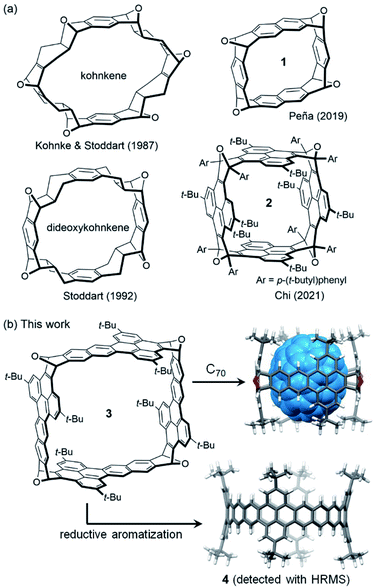
Oxanorbornene-fused double-stranded macrocycles, represented by kohnkene (Fig. 1a), have received considerable attention not only because they are synthetic precursors toward short segments of zigzag carbon nanotubes1–6 but also because they are typical cavitands processing well defined cavities as a result of the intrinsic curvature of the oxanorbornene moieties.7 Kohnkene was synthesized by Kohnke, Stoddart and co-workers in 1987 during the targeted synthesis of [12]cyclacene,8,9 and named after its creator. Partial deoxygenation of kohnkene with low valent titanium gave dideoxykohnkene10 (Fig. 1a), which has a cavity shaped like a Celtic cross. The recently revived interest in the synthesis of carbon nanobelts,11–13 particularly, the efforts to synthesize zigzag carbon nanobelts14–16 by Diels–Alder reactions17–20 gave rise to new members of oxanorbornene-fused double-stranded macrocycles,21 such as 117 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (Fig. 1a). Because these double-stranded macrocycles are shaped like boxes, they are named oxanorbornene-fused nanoboxes herein. Similar to kohnkene and dideoxykohnkene, these nanoboxes all have a well-defined cavity although they lack hydrogen atoms pointing inward the cavity. However, their potential to function as molecular containers is largely unexplored. It is found that kohnkene has a cavity too small to accommodate any molecular guest, while dideoxykohnkene can only accommodate a molecule of water in its cavity in the crystal state. Some oxanorbornene-fused nanoboxes (e.g.1) are found to have their cavities occupied by co-crystallized solvent molecules,17,21 while the others (e.g.2) are found to have empty cavities in the crystals because access to the cavity is blocked by bulky substituting groups.18,20 On the other hand, none of these oxanorbornene-fused macrocycles have demonstrated the capability of accommodating guest molecules in solution.
20 (Fig. 1a). Because these double-stranded macrocycles are shaped like boxes, they are named oxanorbornene-fused nanoboxes herein. Similar to kohnkene and dideoxykohnkene, these nanoboxes all have a well-defined cavity although they lack hydrogen atoms pointing inward the cavity. However, their potential to function as molecular containers is largely unexplored. It is found that kohnkene has a cavity too small to accommodate any molecular guest, while dideoxykohnkene can only accommodate a molecule of water in its cavity in the crystal state. Some oxanorbornene-fused nanoboxes (e.g.1) are found to have their cavities occupied by co-crystallized solvent molecules,17,21 while the others (e.g.2) are found to have empty cavities in the crystals because access to the cavity is blocked by bulky substituting groups.18,20 On the other hand, none of these oxanorbornene-fused macrocycles have demonstrated the capability of accommodating guest molecules in solution.
Herein we report a new oxanorbornene-fused nanobox (3 in Fig. 1b), which contains four dibenzo[de,qr]tetracene subunits. Density functional theory (DFT) calculations indicate that the cavity of 3 has a cross-section shaped like a square and can accommodate a fullerene, such as C70 (Fig. 1b). As detailed below, reductive aromatization of 3 led to observation of the corresponding zigzag carbon nanobelt 4 (Fig. 1b) by high resolution mass spectroscopy, and the capability of 3 to bind a fullerene (C60 or C70) in both solution and crystal states was demonstrated using different techniques.
Results and discussion
Synthesis and structural analysis
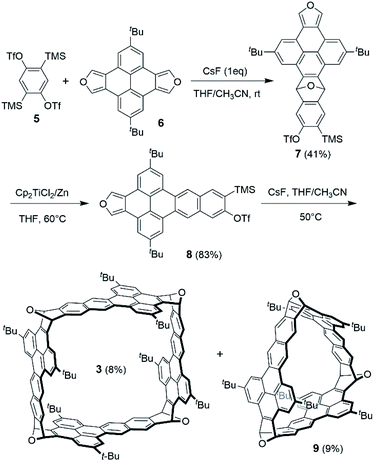
Scheme 1 shows the synthesis of 3 starting from bistriflate 522 and pyrenodifuran 6,18 which were synthesized following the reported procedures. Treatment of 5 with one equivalent of CsF resulted in the corresponding benzyne in situ, which reacted with one equivalent of 6 to afford the Diels–Alder adduct (7) in a yield of 41%. Reductive aromatization of 7 with TiCp2Cl2/Zn23 gave the deoxygenated product (8) in a yield of 83%. In contrast, the attempts to deoxygenate 8 under other conditions including NaI/trimethylsilyl iodide (TMSI)17,18 and NH4ReO4/P(OPh)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 led to decomposition of 7 or a very low yield of 8. Having a diene moiety (in the furan ring) on one end and a potential benzyne (to be formed by desilylation and elimination of triflate) as the dienophile on the other end, 8 was used as a bifunctional building block to construct the nanobox through Diels–Alder reactions. The reaction of 8 with an excess of CsF (5 equivalents) in a dilute solution in THF and acetonitrile at 50 °C gave the cyclic tetramer (3) in a yield of 8% together with the cyclic trimer (9) in a yield of 9%. The yields of 3 and 9 are higher than those of the reported oxanorbornene-fused nanoboxes from a bisbenzyne precursor and a bisfuran in a two-step manner18 (e.g. 4% for compound 117 and 2% for compound 220). The pale-yellow solids of 3 and 9 both form colorless solutions in CH2Cl2, which both exhibit blue luminescence upon irradiation with UV light. As shown in Fig. S2 in the ESI,† the absorption and photoluminescence spectra of 3 are very similar to those of 9, respectively, but have higher intensity, in agreement with the fact that 3 has more dibenzo[de, qr]tetracene subunits than 9. The 1H NMR of 3 in the downfield region shows five singlets (Fig. S31†), which are assigned to the corresponding protons on the basis of the ROESY 2D NMR (Fig. S32†). The 1H NMR of 9 in the downfield region (Fig. S29†), slightly different from that of 3, shows three singlets and two doublets due to observation of the coupling between two meta protons on the same benzene ring.
24 led to decomposition of 7 or a very low yield of 8. Having a diene moiety (in the furan ring) on one end and a potential benzyne (to be formed by desilylation and elimination of triflate) as the dienophile on the other end, 8 was used as a bifunctional building block to construct the nanobox through Diels–Alder reactions. The reaction of 8 with an excess of CsF (5 equivalents) in a dilute solution in THF and acetonitrile at 50 °C gave the cyclic tetramer (3) in a yield of 8% together with the cyclic trimer (9) in a yield of 9%. The yields of 3 and 9 are higher than those of the reported oxanorbornene-fused nanoboxes from a bisbenzyne precursor and a bisfuran in a two-step manner18 (e.g. 4% for compound 117 and 2% for compound 220). The pale-yellow solids of 3 and 9 both form colorless solutions in CH2Cl2, which both exhibit blue luminescence upon irradiation with UV light. As shown in Fig. S2 in the ESI,† the absorption and photoluminescence spectra of 3 are very similar to those of 9, respectively, but have higher intensity, in agreement with the fact that 3 has more dibenzo[de, qr]tetracene subunits than 9. The 1H NMR of 3 in the downfield region shows five singlets (Fig. S31†), which are assigned to the corresponding protons on the basis of the ROESY 2D NMR (Fig. S32†). The 1H NMR of 9 in the downfield region (Fig. S29†), slightly different from that of 3, shows three singlets and two doublets due to observation of the coupling between two meta protons on the same benzene ring.
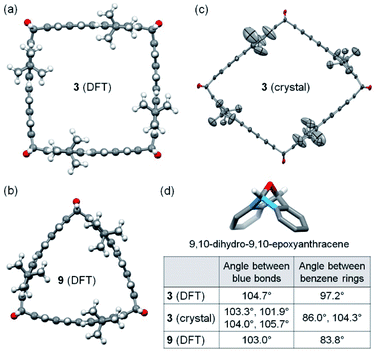
As revealed by the calculations at the B3LYP-D3 level of DFT with the 6-31g(d) basis set, 3 is of C4h symmetry and has a slightly bent square cross-section with essentially flat π-planes of dibenzo[de,qr]tetracene (Fig. 2a), while 9 is of C3h symmetry and its cross-section is shaped like a Reuleaux triangle with bent π-planes of dibenzo[de,qr]tetracene (Fig. 2b). The 9,10-dihydro-9,10-epoxyanthracene moiety at each corner of 3 exhibits the same bond angle of 104.7° between the two blue C–C bonds as shown in Fig. 2d, while that of 9 exhibits a slightly smaller bond angle of 103.0° at each corner. On the basis of the hypothetical homodesmotic reactions shown in Fig. S17 in the ESI,† the strain energy of 3 and 9 is estimated as 7 kcal mol−1 and 16 kcal mol−1, respectively. Although 3 is less strained than 9, 3 was obtained in a slightly lower yield presumably because the formation of 3 requires one more Diels–Alder cycloaddition. Single crystals of 3 were obtained by slow diffusion of isopropanol vapor into its solution in CH2Cl2 and 1,2-dichloroethane. X-ray crystallography revealed a triclinic unit cell containing one molecule of 3 and co-crystallized 1,2-dichloroethane and CH2Cl2.25 It is found that 3 in the crystal has a roughly rhombic cross-section (Fig. 2c) with four different bond angles (101.9–105.7°) in the 9,10-dihydro-9,10-epoxyanthracene moieties as shown in Fig. 2d. The different bond angles (Fig. 2d) and the different dihedral angles between benzene rings in the 9,10-dihydro-9,10-epoxyanthracene moieties in 3 (both DFT-calculated model and crystal structure) and 9 indicate that the oxanorbornene units in these double-stranded macrocycles are not completely rigid but flexible to some degree.
Reductive aromatization of the oxanorbornene-fused nanoboxes
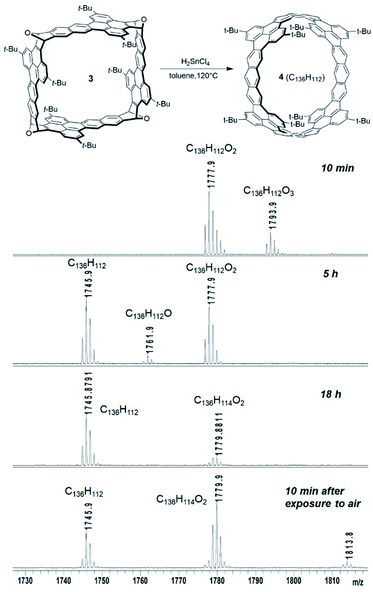
Because reductive aromatization of 3 and 9 can in principle result in the corresponding zigzag carbon nanobelts, the deoxygenation reactions of 3 and 9 were tested under different conditions including H2SnCl4, TiCpCl2/Zn,23 NaI/TMSI,26 TiCl4/LiAlH4,27 and NH4ReO4/P(OPh)3.24 Among these conditions, only treatment with H2SnCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 (freshly prepared from anhydrous SnCl2 and concentrated HCl) at 120 °C under an atmosphere of N2 was able to convert 3 to the corresponding carbon nanobelt (4), which was detected by MALDI-TOF mass spectroscopy from the crude product. As shown in Fig. 3, when 3 was treated with H2SnCl4 in toluene at 120 °C for 10 minutes, the mass spectrum from the reaction mixture indicated the formation of partially deoxygenated products C136H112O3 and C136H112O2. When the reaction time was prolonged, 4 (C136H112) gradually became the major product. The observed molecular ion peak (m/z of 1745.8791) and isotope patterns (Fig. 3 and S24†) are in good agreement with the molecular formula of C136H112 (m/z of 1745.8792). When the crude product was cooled to room temperature and exposed to air, the mass spectrum exhibited a new peak of m/z = 1779.8811 for C136H114O2, which likely resulted from photo-induced oxygenation of 4 by molecular oxygen in air followed by protonation. This peak increased and the peak of 4 decreased quickly as exposure to air was prolonged (Fig. 3). This indicates low stability of 4 toward oxidation by air. Temperature was found important to the reduction of 3 with H2SnCl4. When treated with H2SnCl4 at room temperature for 5 hours, 3 was almost completely recovered. When treated with H2SnCl4 at 80 °C for 5 hours, 3 was partially recovered and a partially deoxygenated product (C136H112O2) was observed by mass spectroscopy. In contrast, other conditions either led to only partial deoxygenation or gave over-reduced products that exhibited molecular ion peaks in the mass spectra in agreement with hydrogenated carbon nanobelts. Unfortunately, our attempts to isolate 4 were not successful. When the reaction mixture was extracted and concentrated, the molecular ion peak for 4 disappeared and the product became less soluble, likely due to oligomerization and oxidation. The nanobelt 4 is less stable than the successfully synthesized zigzag carbon nanobelts19,20 likely because 4 has fewer aromatic sextets.
28 (freshly prepared from anhydrous SnCl2 and concentrated HCl) at 120 °C under an atmosphere of N2 was able to convert 3 to the corresponding carbon nanobelt (4), which was detected by MALDI-TOF mass spectroscopy from the crude product. As shown in Fig. 3, when 3 was treated with H2SnCl4 in toluene at 120 °C for 10 minutes, the mass spectrum from the reaction mixture indicated the formation of partially deoxygenated products C136H112O3 and C136H112O2. When the reaction time was prolonged, 4 (C136H112) gradually became the major product. The observed molecular ion peak (m/z of 1745.8791) and isotope patterns (Fig. 3 and S24†) are in good agreement with the molecular formula of C136H112 (m/z of 1745.8792). When the crude product was cooled to room temperature and exposed to air, the mass spectrum exhibited a new peak of m/z = 1779.8811 for C136H114O2, which likely resulted from photo-induced oxygenation of 4 by molecular oxygen in air followed by protonation. This peak increased and the peak of 4 decreased quickly as exposure to air was prolonged (Fig. 3). This indicates low stability of 4 toward oxidation by air. Temperature was found important to the reduction of 3 with H2SnCl4. When treated with H2SnCl4 at room temperature for 5 hours, 3 was almost completely recovered. When treated with H2SnCl4 at 80 °C for 5 hours, 3 was partially recovered and a partially deoxygenated product (C136H112O2) was observed by mass spectroscopy. In contrast, other conditions either led to only partial deoxygenation or gave over-reduced products that exhibited molecular ion peaks in the mass spectra in agreement with hydrogenated carbon nanobelts. Unfortunately, our attempts to isolate 4 were not successful. When the reaction mixture was extracted and concentrated, the molecular ion peak for 4 disappeared and the product became less soluble, likely due to oligomerization and oxidation. The nanobelt 4 is less stable than the successfully synthesized zigzag carbon nanobelts19,20 likely because 4 has fewer aromatic sextets.
Cavitates of the nanobox with fullerenes
Host–guest chemistry of the nanobox 3 in solution with different fullerenes including C60, C70, [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) and indene-C60 bisadduct (ICBA) was studied using different techniques. From a solution containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3 and C60 or a derivative of C60 in toluene, the high resolution MALDI-TOF mass spectra (Fig. S20–S22 in the ESI†) revealed both free 3 and the corresponding cavitate: C60⊂3 (m/z: 2530.8703), PCBM⊂3 (m/z: 2720.9606), or ICBA⊂3 (m/z: 2762.9836). In contrast, from the solution of 3 and C70 (1
1 mixture of 3 and C60 or a derivative of C60 in toluene, the high resolution MALDI-TOF mass spectra (Fig. S20–S22 in the ESI†) revealed both free 3 and the corresponding cavitate: C60⊂3 (m/z: 2530.8703), PCBM⊂3 (m/z: 2720.9606), or ICBA⊂3 (m/z: 2762.9836). In contrast, from the solution of 3 and C70 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
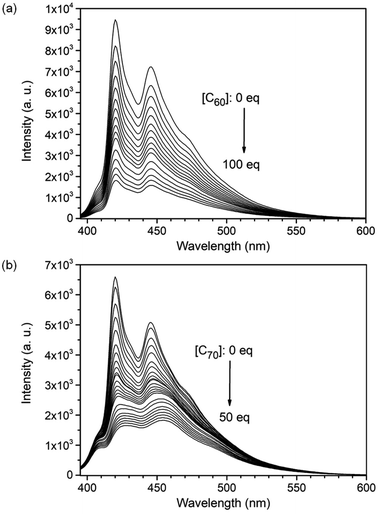
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in toluene, only the molecular ion peak for C70⊂3 (m/z: 2650.8664) was observed in the mass spectrum (Fig. S23†). This suggests that 3 binds C70 more strongly than C60. Upon gradual addition of a fullerene to the solution of 3, the intensity of the blue fluorescence of 3 decreased dramatically as shown in Fig. 4, S9 and S11.† The Job's plots with an unchanged total concentration of 3 and fullerene in toluene showed a maximum fluorescence change when the ratio of 3:fullerene reached 1
1) in toluene, only the molecular ion peak for C70⊂3 (m/z: 2650.8664) was observed in the mass spectrum (Fig. S23†). This suggests that 3 binds C70 more strongly than C60. Upon gradual addition of a fullerene to the solution of 3, the intensity of the blue fluorescence of 3 decreased dramatically as shown in Fig. 4, S9 and S11.† The Job's plots with an unchanged total concentration of 3 and fullerene in toluene showed a maximum fluorescence change when the ratio of 3:fullerene reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as shown in Fig. S3–S6.† On the basis of the 1
1 as shown in Fig. S3–S6.† On the basis of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, the binding constant (Ka) of 3 for C60 is determined as (3.3 ± 0.8) × 104 M−1 at room temperature by fitting the data from three independent fluorescence titration experiments using the equation: F/F0 = (1 + (kf/ks)Ka[C60])/(1 + Ka[C60]),29,30 where F and F0 are the fluorescence intensity of nanobelts with and without addition of C60, respectively; kf and ks are proportionality constants for the complex and nanobox 3, respectively; and Ka is the binding constant of nanobox 3 for C60. Using the same method, the binding constants of 3 for C60 derivatives, PCBM and ICBA, are determined as (3.3 ± 0.9) × 104 M−1 and (3.1 ± 0.7) × 104 M−1, respectively, which are essentially the same as that of C60⊂3 likely as a result of arranging the substituting groups outside the cavity of 3. The binding constant of 3 for C70 at room temperature is determined using the same method as (3.2 ± 0.1) × 106 M−1, which is larger than that of C60⊂3 by two orders of magnitude. The binding constant of 3 for C70 in toluene is larger than the reported values of [10]cycloparaphenylene ((8.4 ± 0.3) × 104 M−1, measured from UV-vis titration),31 [11]cycloparaphenylene ((1.5 ± 0.1) × 105 M−1, measured from UV-vis titration),31 and [4]cyclo(2,11-hexa-peri-hexabenzocoronene) (1.07 × 106 M−1, measured from fluorescence quenching)32 but lower than that of porphyrin nanohoops (2 × 107 M−1, measured from UV-vis titration)33 in the same solvent. Moreover, 3 exhibits a higher selectivity between two fullerenes (C70/C60 = 97) than [10]cycloparaphenylene (C60/C70 = 33),30,31 the porphyrin nanohoop (C60/C70 = 15),33 and (12,8)-[4]cyclo-2,8-anthanthrenylene (C70/C60 = 1.3 in o-dichlorobenzene)34 as well as the self-assembled capsule (C70/C60 = 21 in C2H2Cl4 as measured from UV-vis titration or C70/C60 = 4.2 in C2H2Cl4 as measured by isothermal titration calorimetry).35
1 stoichiometry, the binding constant (Ka) of 3 for C60 is determined as (3.3 ± 0.8) × 104 M−1 at room temperature by fitting the data from three independent fluorescence titration experiments using the equation: F/F0 = (1 + (kf/ks)Ka[C60])/(1 + Ka[C60]),29,30 where F and F0 are the fluorescence intensity of nanobelts with and without addition of C60, respectively; kf and ks are proportionality constants for the complex and nanobox 3, respectively; and Ka is the binding constant of nanobox 3 for C60. Using the same method, the binding constants of 3 for C60 derivatives, PCBM and ICBA, are determined as (3.3 ± 0.9) × 104 M−1 and (3.1 ± 0.7) × 104 M−1, respectively, which are essentially the same as that of C60⊂3 likely as a result of arranging the substituting groups outside the cavity of 3. The binding constant of 3 for C70 at room temperature is determined using the same method as (3.2 ± 0.1) × 106 M−1, which is larger than that of C60⊂3 by two orders of magnitude. The binding constant of 3 for C70 in toluene is larger than the reported values of [10]cycloparaphenylene ((8.4 ± 0.3) × 104 M−1, measured from UV-vis titration),31 [11]cycloparaphenylene ((1.5 ± 0.1) × 105 M−1, measured from UV-vis titration),31 and [4]cyclo(2,11-hexa-peri-hexabenzocoronene) (1.07 × 106 M−1, measured from fluorescence quenching)32 but lower than that of porphyrin nanohoops (2 × 107 M−1, measured from UV-vis titration)33 in the same solvent. Moreover, 3 exhibits a higher selectivity between two fullerenes (C70/C60 = 97) than [10]cycloparaphenylene (C60/C70 = 33),30,31 the porphyrin nanohoop (C60/C70 = 15),33 and (12,8)-[4]cyclo-2,8-anthanthrenylene (C70/C60 = 1.3 in o-dichlorobenzene)34 as well as the self-assembled capsule (C70/C60 = 21 in C2H2Cl4 as measured from UV-vis titration or C70/C60 = 4.2 in C2H2Cl4 as measured by isothermal titration calorimetry).35
In order to study the encapsulation of C60 and C70 by 3 with NMR spectroscopy, o-C6D4Cl2, a better solvent for fullerenes, was used. Addition of excessive C60 (3.5 eq.) into the solution of 3 in o-C6D4Cl2 led to a broadened and slightly down-field shifted peak for H-a as shown in Fig. 5. In contrast, addition of 0.4 equivalent of C70 into the solution of 3 resulted in two apparently different sets of peaks, which are attributed to the free host (3) and the complex (C70⊂3), respectively. In the presence of excessive C70 (2.0 eq.), the peaks for the free host disappeared and only the peaks for C70⊂3 were observed. The same 1H NMR spectrum (Fig. 5) was observed when 3.5 eq. of C70 was added into a solution that already contained 3 and 3.5 equivalent of C60 in o-C6D4Cl2. These results indicate that 3 binds C60 weakly in o-C6D4Cl2 with a fast exchange at the NMR time scale but binds C70 strongly with a slow exchange under the same conditions. In agreement with the NMR experiments, from the solution containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3, C60 and C70 in o-C6H4Cl2, the high-resolution mass spectrum revealed the complex of C70⊂3 only. These results indicate selective encapsulation of C70 by 3 in the presence of C60. From the NMR titration experiments (Fig. S36 in the ESI†), the binding constant of 3 for C70 in o-C6H4Cl2 at room temperature is determined as 2.5 × 104 M−1, which is smaller than the binding constant in toluene presumably because C70 and 3 have a higher degree of solvation in o-C6H4Cl2 than in toluene.
1 mixture of 3, C60 and C70 in o-C6H4Cl2, the high-resolution mass spectrum revealed the complex of C70⊂3 only. These results indicate selective encapsulation of C70 by 3 in the presence of C60. From the NMR titration experiments (Fig. S36 in the ESI†), the binding constant of 3 for C70 in o-C6H4Cl2 at room temperature is determined as 2.5 × 104 M−1, which is smaller than the binding constant in toluene presumably because C70 and 3 have a higher degree of solvation in o-C6H4Cl2 than in toluene.
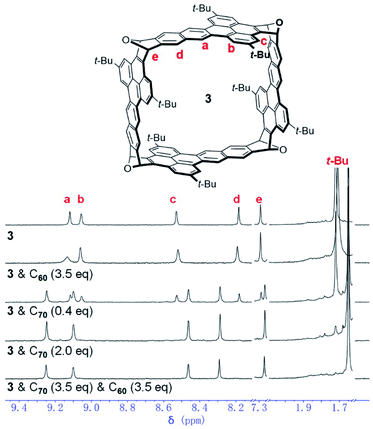 | ||
| Fig. 5 1H NMR spectrum of 3 in o-C6D4Cl2 in comparison to those of 3 with C60 or C70 in o-C6D4Cl2 (400 MHz, 298 K). | ||
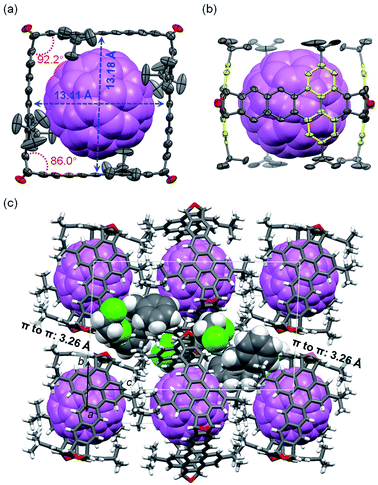
The structure of C60⊂3 was unambiguously determined by X-ray crystallographic analysis of the single crystals of C60⊂3·2(C2H4Cl2)·2(C6H5CH3),25 which were grown by slow diffusion of isopropanol vapor into the solution of C60 and 3 in o-dichloroethane (C2H4Cl2) and toluene (C6H5CH3). However, our attempts to grow single crystals of C70⊂3 suitable for X-ray diffraction were not successful. As shown in Fig. 6a, the cross section of 3 in the crystal of C60⊂3 is close to a square, which has a side length of about 13.1 Å as measured from the distance between the center carbon atoms of dibenzo[de,qr]tetracene on the opposite sides. Sixteen short intermolecular C-to-C contacts in the range of 3.11–3.36 Å are observed between 3 and C60. The cross-section of 3 in C60⊂3 has inner angles of 92.2° and 86.0° as measured from the dihedral angle between the two benzene rings in each 9,10-dihydro-9,10-epoxyanthracene moiety (see Fig. 2d). Another finding from comparing the structures of 3 in the crystals with and without C60 is that the yellow benzene rings in 3 bend inward to form concave–convex π–π interactions with C60 as shown in Fig. 6b. As a result, the two yellow benzene rings in the same dibenzo[de,qr]tetracene unit form a dihedral angle of 13.7°. Further detailed analysis on the Hirshfeld surface36 of C60 in the complex shows short contacts (CH–π interactions) between the t-butyl group of 3 and C60 (Fig. S1 in the ESI†). Fig. 6c shows the molecular packing in a unit cell of C60⊂3·2(C2H4Cl2)·2(C6H5CH3), where two adjacent molecules of 3 exhibit face-to-face π-stacking with a distance of 3.26 Å between π-planes (defined by the tetracene moiety). In contrast, no π–π interactions are observed between molecules of C60, which are in fact separated by the t-butyl groups of 3. The π–π stacking between molecules of 3 and the relatively high HOMO energy level of 3 (−5.18 eV as calculated at the B3LYP/6-31-g(d) level of DFT) suggest that the crystals of C60⊂ 3 can, in principle, function as hole-transporting organic semiconductors for application in phototransistors or photodetectors37 on the basis of photo-induced electron transfer38 from 3 to C60. Unfortunately, our preliminary efforts to drop-cast or dip-coat a solution of C60⊂3 onto a substrate failed to give films suitable for device fabrication.
Conclusions
In summary, the above study has put forth a new oxanorbornene-fused nanobox (3), which contains four dibenzo[de, qr]tetracene subunits. It was synthesized through one-pot iterative Diels–Alder reactions, which also gave a Reuleaux triangle-shaped double-stranded macrocycle (9). Reductive aromatization of 3 with H2SnCl4 led to observation of the corresponding zigzag carbon nanobelt by high resolution mass spectroscopy. The host-guest chemistry of 3 with different fullerenes was studied in both solution and crystal states using different techniques. The fluorescence titration experiments indicate that 3 encapsulates C70 in toluene with a binding constant of (3.2 ± 0.1) × 106 M−1 and a high selectivity against C60 and its derivatives. The NMR titration experiments indicate that 3 encapsulates C70 with a slow exchange at the NMR time scale and a binding constant of 2.5 × 104 M−1 in o-dichlorobenzene. The X-ray crystallographic analysis shows that 3 changes the shape of its cross-section from a rhombus to nearly a square upon accommodating C60. On the basis of the above results, 3 is the first member of oxanorbornene-fused double-stranded macrocycles demonstrating the capability to accommodate molecular guests in solution. This aromatic nanobox may find potential application for crystallization of fullerene derivatives, which is still a challenge in fullerene chemistry due to the limited solubility of fullerenes and the geometrical similarities of the carbon spheroids.39–41Data availability
All the data are provided in ESI.†Author contributions
H. Chen and Q. Miao conceived the project, and Q. Miao directed the project. H. Chen performed most of the experiments and calculations, and Z. Xia contributed to the fluorescence tritration experiments and data analysis. Q. Miao and H. Chen wrote the manuscript, and all authors checked the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Ms. Hoi Shan Chan for the single crystal crystallography. This work was supported by the Research Grants Council of Hong Kong (GRF 14300218).Notes and references
- W. D. Neudorff, D. Lentz, M. Anibarro and A. D. Schlüter, Chem.–Eur. J., 2003, 9, 2745–2757 CrossRef CAS PubMed.
- Q.-H. Guo, Y. Qiu, M.-X. Wang and J. Fraser Stoddart, Nat. Chem., 2021, 13, 402–419 CrossRef CAS PubMed.
- K. Y. Cheung, Y. Segawa and K. Itami, Chem.–Eur. J., 2020, 26, 14791–14801 CrossRef CAS PubMed.
- H. Chen and Q. Miao, J. Phys. Org. Chem., 2020, 33, e4145 CAS.
- T.-H. Shi and M.-X. Wang, CCS Chem., 2020, 2, 916–931 Search PubMed.
- R. Gleiter, B. Esser and S. C. Kornmayer, Acc. Chem. Res., 2009, 42, 1108–1116 CrossRef CAS PubMed.
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, John Wiley & Sons, 2nd edn, 2009, ch. 6 Search PubMed.
- F. H. Kohnke, A. M. Z. Slawin, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed., 1987, 26, 892–894 CrossRef.
- U. Girreser, D. Giuffrida, F. H. Kohnke, J. P. Mathias, D. Philp and J. F. Stoddart, Pure Appl. Chem., 1993, 65, 119–125 CAS.
- P. R. Ashton, G. R. Brown, N. S. Isaacs, D. Giuffrida, F. H. Kohnke, J. P. Mathias, A. M. Z. Slawin, D. R. Smith, J. F. Stoddart and D. J. Williams, J. Am. Chem. Soc., 1992, 114, 6330–6353 CrossRef CAS.
- G. Povie, Y. Segawa, T. Nishihara, Y. Miyauchi and K. Itami, Science, 2017, 356, 172–175 CrossRef CAS PubMed.
- K. Y. Cheung, S. Gui, C. Deng, H. Liang, Z. Xia, Z. Liu, L. Chi and Q. Miao, Chem, 2019, 5, 838–847 CAS.
- H. M. Bergman, G. R. Kiel, R. C. Handford, Y. Liu and T. D. Tilley, J. Am. Chem. Soc., 2021, 143, 8619–8624 CrossRef CAS PubMed.
- T. H. Shi, Q. H. Guo, S. Tong and M. X. Wang, J. Am. Chem. Soc., 2020, 142, 4576–4580 CrossRef CAS PubMed.
- T. H. Shi, S. Tong and M. X. Wang, Angew. Chem., Int. Ed., 2020, 59, 7700–7705 CrossRef CAS PubMed.
- Z. Xia, S. H. Pun, H. Chen and Q. Miao, Angew. Chem., Int. Ed., 2021, 60, 10311–10318 CrossRef CAS PubMed.
- F. Schulz, F. García, K. Kaiser, D. Pérez, E. Guitián, L. Gross and D. Peña, Angew. Chem., Int. Ed., 2019, 58, 9038–9042 CrossRef CAS PubMed.
- H. Chen, S. Gui, Y. Zhang, Z. Liu and Q. Miao, CCS Chem., 2020, 2, 613–619 Search PubMed.
- K. Y. Cheung, K. Watanabe, Y. Segawa and K. Itami, Nat. Chem., 2021, 13, 255–259 CrossRef CAS PubMed.
- Y. Han, S. Dong, J. Shao, W. Fan and C. Chi, Angew. Chem., Int. Ed., 2021, 60, 2658–2662 CrossRef CAS PubMed.
- J. Wang and Q. Miao, Org. Lett., 2019, 21, 10120–10124 CrossRef CAS PubMed.
- N. Pavliček, B. Schuler, S. Collazos, N. Moll, D. Pérez, E. Guitián, G. Meyer, D. Peña and L. Gross, Nat. Chem., 2015, 7, 623–628 CrossRef PubMed.
- T. V. RajanBabu and W. A. Nugent, J. Am. Chem. Soc., 2002, 116, 986–997 CrossRef.
- M. Murai, T. Ogita and K. Takai, Chem. Commun., 2019, 55, 2332–2335 RSC.
- CCDC 2116508 and 2090735 contain the supplementary crystallographic data for 3·5(C2H4Cl2)·2(CH2Cl2) and C60⊂3·2(C2H4Cl2)·2(C6H5CH3), respectively..
- K. G. Kumarasinghe, F. R. Fronczek, H. U. Valle and A. Sygula, Org. Lett., 2016, 18, 3054–3057 CrossRef CAS PubMed.
- J. E. McMurry and M. P. Fleming, J. Org. Chem., 2002, 40, 2555–2556 CrossRef.
- V. K. Patel, E. Kayahara and S. Yamago, Chem.–Eur. J., 2015, 21, 5742–5749 CrossRef CAS PubMed.
- K. A. Connors, Binding Constants: The Measurement of Molecular Complex Stability, John Wiley & Sons, New York, 1987 Search PubMed.
- T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed.
- T. Iwamoto, Y. Watanabe, H. Takaya, T. Haino, N. Yasuda and S. Yamago, Chem.–Eur. J., 2013, 19, 14061–14068 CrossRef CAS PubMed.
- D. Lu, S. Cui and P. Du, Synlett, 2017, 28, 1671–1677 CrossRef CAS.
- Y. Xu, S. Gsanger, M. B. Minameyer, I. Imaz, D. Maspoch, O. Shyshov, F. Schwer, X. Ribas, T. Drewello, B. Meyer and M. von Delius, J. Am. Chem. Soc., 2019, 141, 18500–18507 CrossRef CAS PubMed.
- T. Matsuno, S. Sato, R. Iizuka and H. Isobe, Chem. Sci., 2015, 6, 909–916 RSC.
- E. Huerta, G. A. Metselaar, A. Fragoso, E. Santos, C. Bo and J. de Mendoza, Angew. Chem., Int. Ed., 2007, 46, 202–205 CrossRef CAS PubMed.
- J. J. McKinnon, M. A. Spackman and A. S. Mitchell, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 627–668 CrossRef PubMed.
- Q. Huang, G. Zhuang, H. Jia, M. Qian, S. Cui, S. Yang and P. Du, Angew. Chem., Int. Ed., 2019, 58, 6244–6249 CrossRef CAS PubMed.
- N. J. Tremblay, A. A. Gorodetsky, M. P. Cox, T. Schiros, B. Kim, R. Steiner, Z. Bullard, A. Sattler, W. Y. So, Y. Itoh, M. F. Toney, H. Ogasawara, A. P. Ramirez, I. Kymissis, M. L. Steigerwald and C. Nuckolls, Chemphyschem, 2010, 11, 799–803 CrossRef CAS PubMed.
- V. G. Jiménez, A. H. G. David, J. M. Cuerva, V. Blanco and A. G. Campaña, Angew. Chem., Int. Ed., 2020, 59, 15124–15128 CrossRef PubMed.
- K. Tashiro and T. Aida, Chem. Soc. Rev., 2007, 36, 189–197 RSC.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis and characterization, fluorescence titration, crystal structures, DFT calculations, mass spectra and NMR spectra. CCDC 21165082090735. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc06553j |
| This journal is © The Royal Society of Chemistry 2022 |