Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Systematic investigation of the mechanocatalytic partial depolymerization of cellulose towards oligomeric glycans
Gregor
Meyer
,
Dominique
Lumpp
,
Anne-Kathrin
Stulik
,
Dagmar
Hoffmann
and
Marcus
Rose
 *
*
Technical University of Darmstadt, Department of Chemistry, Chemical Technology II, Peter-Grünberg-Straße 8, 64287 Darmstadt, Germany. E-mail: marcus.rose@tu-darmstadt.de
First published on 5th February 2024
Abstract
The selective depolymerization of cellulose is a major challenge and usually leads to the formation of monosaccharides as main products. Once depolymerized, various platform chemicals such as 5-hydroxymethylfurfural and furfural can be obtained from cellulose. Our work aims to convert cellulose selectively into oligomeric glycans as more valuable products compared to sugars, by using mechanocatalysis in a planetary ball mill. In this work, reaction parameters such as acid content, filling level, rotational speed and grinding duration were investigated systematically and optimized towards a maximum amount of soluble oligomeric species and a minimum of monosaccharides. The systematic investigation of the mechanocatalytic partial depolymerization resulted in a nearly full-soluble fraction containing oligomeric glycans.
1. Introduction
When trying to move away from fossil raw materials and to replace them with renewable raw materials, the development of new efficient processes is essential. Lignocellulose still has untapped potential as renewable raw material, especially the 30–50% cellulose it contains, which makes cellulose the most abundand (bio)-polymer worldwide.1,2 Of the 170 × 109 t lignocellulosic biomass produced annually, only 3–4% are currently used for food and non-food purposes.3 Once digested, various platform chemicals such as 5-hydroxymethylfurfural and furfural can be obtained from cellulose.4,5 Conventional aqueous digestions, like acid hydrolysis, were typically carried out with sulfuric acid or hydrochloric acid at temperatures of 100–180 °C, leading to monomeric sugars.6–8 An alternative to conventional digestions is mechanochemistry,9 which has advanced in recent decades and was acknowledged as one of the top ten emerging technologies in chemistry by IUPAC in 2019.10 As a solvent-free process, mechanocatalysis enables lignocellulose digestion through the combination of mechanical forces and a Brønsted acid.11–13 Mechanocatalytic partial depolymerization of lignocellulose, cellulose and hemicellulose as well as for other polysaccharides such as chitosan14 is carried out with a strong acid (pKa below −1.8 (ref. 13)), e.g. sulfuric acid, and shows remarkable differences regarding the reaction process and the product spectrum.15–19 In contrast to conventional processes, mechanocatalytic treatment produces oligomeric sugars, so-called glycans and xylans, instead of monomeric sugars.20 Hereby a polymerization from glucose itself occurs which inhibits a complete depolymerization to monomeric sugars.13 It has also been shown using solid catalysts and ball milling that the rate for oligomer formation can be drastically increased.21 It should be emphasized that the temperature is limited to about 90 °C due to follow-up reactions starting from monomeric sugars up to carbonization of the substrate.22–25 In addition to the mentioned platform chemicals, glycans represent a promising class of substances based on renewable raw materials. In a previous study we showed that meachanocatalytic partial depolymerization of lignocellulosic materials, e.g. apple pomace, to glycans with a molecular weight between 1–10 kDa is possible, whereby the yield is dependent on grinding duration and acid content.26 In this work, the process of mechanocatalytic partial depolymerization of cellulose as model substance is systematically investigated in order to obtain a high glycan yield. The aim is a systematic study to understand the influence of the various reation parameters in mechanocatalytic depolymerization of cellulose to water-soluble oligomeric glycans.2. Experimental
2.1. Materials
As substrate for mechanocatalytic partial depolymerization α-cellulose (Sigma-Aldrich, C8002-1KG) was used. For impregnation sulfuric acid (Roth, 4623.5) and diethyl ether (Sigma-Aldrich, 24004-2.5L-M) were used.2.2. Cellulose impregnation
For impregnation the required amount of 96% sulfuric acid was weighed in a 500 mL round bottom flask and diluted with about 50 mL diethyl ether per 5 g biomass. Here, the acid content is given in % based on the total mass including acid load itself. After adding the biomass the resulting suspension was stirred for 1 h at room temperature. The solvent was removed under reduced pressure and the product was dried under vacuum on the Schlenk line.2.3. Mechanocatalytic partial depolymerization
A Retsch PM 100 planetary ball mill with a grinding bowl (Retsch 01.0462.0219) made of zirconia is used for the experiments. Depending on the experiments, grinding balls made of zirconia (ZrO2), stainless steel (SS) or tungsten carbide (TC) with diameters of 2 mm, 3 mm or 4 mm, respectively, are used. A list of the grinding balls used can be found in Table 1. Unless stated otherwise, the filling level is 20% of the grinding bowl volume.| Material | Diameter/mm | Density/g cm−3 | Mohs hardness | Supplier |
|---|---|---|---|---|
| Zirconia | 3 | 5.68 | 7.5–8 | Retsch |
| Stainless steel | 2 | 7.8 | 7 | Sturm-Präzision |
| Stainless steel | 3 | 7.8 | 7 | Sturm-Präzision |
| Stainless steel | 4 | 7.8 | 7 | Sturm-Präzision |
| Tungsten carbide | 3 | 15.63 | 9.5 | Tsubaki Nakashima |
The grinding balls are placed in the grinding bowl, 5 g of impregnated cellulose is added and the grinding process is started. The experiments carried out were run with grinding cycles of five minutes grinding followed by two minutes cooling, with the aim that temperatures of 90 °C were not exceeded to prevent subsequent reactions. Possible subsequent products starting from monomeric sugars which were formed in small amounts could be furfural and hydroxymethylfurfural.22,23 For clarity, only the total effective grinding time without the cooling cycles is given. Directly after the grinding process, the temperature inside the grinding bowl is measured and noted with a Scan Temp Pro 440 infrared thermometer in order to exclude subsequent reactions at temperatures above 90 °C.
2.4. Product characterization after amorphization and partial depolymerization
In previous studies the amorphization of cellulose, in absence of depolymerization, was verified by grinding for 86 min followed by a XRD-measurement and comparing it to the literature.25–27 Therefor a Bruker D2 Phaser 2nd Generation was used with a Cu Kα radiation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 Å). The measurements were taken in a 2-theta range from 8° to 60° with a measurement speed of 0.239° (1.1 s)−1 using a background-free silicon sample holder.
406 Å). The measurements were taken in a 2-theta range from 8° to 60° with a measurement speed of 0.239° (1.1 s)−1 using a background-free silicon sample holder.
In addition abrasion of the grinding bowl and the grinding balls is examined based on the experiment series for the comparison of the grinding ball materials. Therefor elemental composition of the samples after depolymerization is determined using X-ray fluorescence analysis. The samples to be analyzed are ground to a fine powder in an agate mortar and transferred into a 2 mm high sample holder made of polyethylene with a diameter of 18 mm. The measurement is carried out with an Epsilon 4 from Malvern Panalytical.
Directly after the grinding process the temperature inside the grinding bowl is measured to confirm that the temperature is below 90 °C and no following reactions e.g. dehydration of sugars to furfural and hydroxymethylfurfural occurs.28 Following mechanocatalytic partial depolymerization, a product amount corresponding to 400 mg without acid loading is extracted in 40 mL deionized water for 1 h at room temperature. The solid residue is filtered off, dried overnight under vacuum at 40 °C and then weighed to determine the insoluble fraction gravimetrically. The filtrate is analyzed by HPLC and furthermore GPC measurements are carried out to determine the molecular weight distribution.
The filtrate was analyzed by HPLC (Agilent 1260 Infinity equipped with a HPX-87H-column at a flow rate of 0.5 mL min−1 at 60 °C). GPC measurements of the samples after grinding were taken to determine the molecular weight distribution of the soluble fraction. Therefore a PSS Suprema column at a flow rate of 1 mL min−1 0.05% NaN3 at 25 °C was used.
3. Results and discussion
3.1. Influence of grinding duration
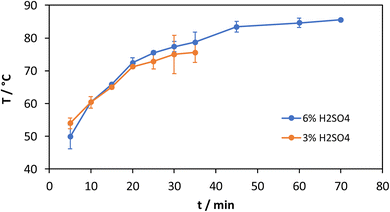
The time-resolved mechanocatalytic partial depolymerization of cellulose is carried out at 500 rpm and a filling level of 20% with 3 mm zirconia grinding balls for 3% and 6% acid content. The time range for the experiments with 6% acid content is up to 70 min and for the experiments with 3% acid content up to 35 min. The temperature, the product composition and the molecular weight are measured at time intervals of 5 min each, which corresponds to one grinding cycle. The reactions are carried out anew for each measuring point in order to exclude any influence of the sampling. The product composition and the temperature are determined as mean values of three experiments each, including the standard deviation as error bar. Due to the increasing energy input over time and the increased number of impacts of the grinding balls, both an increase in the soluble oligomer fraction and an increase in temperature are expected.As expected, the temperature for an acid content of 3% and 6% increases with increasing grinding time (Fig. 1). At first, a sharp increase in temperature can be seen, which decreases with increasing grinding time and approaches a maximum. The acid loading has no influence on the temperature and the curves for 3% and 6% sulfuric acid content run parallel within the standard deviation. The limit is reached after 50 min at a temperature of around 85 °C. This shows that the selected grinding cycle intervals of 5 min grinding and 2 min cooling protect against overheating and subsequent reactions under the given reaction conditions.
An often addressed issue is the disentanglement of thermal and mechanical effects on the catalytic performance. With the herein applied ball mill it is not possible to deduce this difference conclusively. However, as the milling parameter studies in the following are carried out for milling times >30 min the average temperature is always in the range of 75–85 °C, hence, excluding thermal effects based on the average temperature.
The yield of the experiments with 6% acid content is shown in Fig. 2. The mean results with the standard deviations of the oligomers and the solid residue are shown. As expected, the oligomer fraction increases with an increase in grinding time, while the solid residue fraction decreases. An increasing reaction time leads to more impacts of the grinding balls, which can lead to reactions. The significant increase of the oligomer fraction from 17% to 56% between 15 min and 20 min is particularly striking. A possible explanation is the complete amorphization of the samples, which increases solubility or the formation of oligomer chains that are short enough to become soluble in water. Amorphization itself can be ruled out based on previous results.26
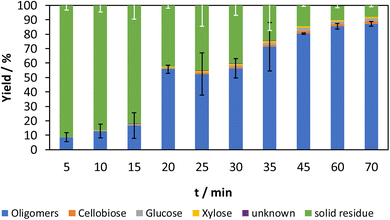 | ||
| Fig. 2 Time-resolved mechanocatalytic partial depolymerization of cellulose with 6% acid content. Reaction conditions: 20% filling level with 3 mm zirconia grinding balls, 500 rpm. | ||
The influence of the acid content on the mechanocatalytic partial depolymerization is investigated with an acid content of 3%. Fig. 3 shows the yields with the corresponding standard deviations for an acid content of 3%.
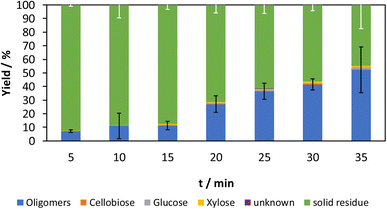 | ||
| Fig. 3 Time-resolved mechanocatalytic partial depolymerization of cellulose with 3% acid content. Reaction conditions: 20% filling level with 3 mm zirconia grinding balls, 500 rpm. | ||
As in the experiments with 6% acid content, an increase of the oligomer fraction during the observed time range can be observed here as well. The largest increase of the oligomer fraction can be observed between 15 min and 20 min. During this period, the oligomer fraction increases from 11% to 27%. It is noticeable that the proportion in the following grinding interval at 25 min increases again significantly to 36%, whereby this is within the scope of the standard deviation. Compared to the experiments with 6% acid content, the proportion of oligomer is lower for the same grinding time. After 30 min, the oligomer fraction is 56% with 6% acid content and 42% with 3% acid content. This is due to the higher amount of catalyst, which accelerates the reaction.
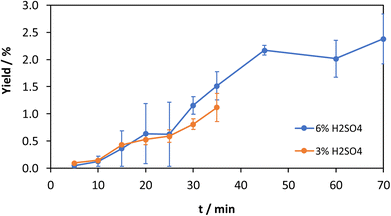
It is observed that the glucose yield (Fig. 4) increases at 6% acid content and a grinding time of up to 45 min. What is striking is the initially small increase in yield, which increases up to 45 min. The reason for this is the presence of longer cellulose chains on average with shorter grinding times, which is reflected in the lower soluble fraction of the samples. When the grinding time increases, more cellulose chains with a lower degree of polymerization are formed. This, in turn, statistically leads to more cleavages at the chain ends, which lead to the formation of glucose. Above a grinding time of 45 min, the glucose yield is constant at about 2.2%, which can be attributed to the establishment of an equilibrium between depolymerization and polymerization. The glucose yield at 3% acid content is analogous but with a lower slope. Equilibrium is expected to occur with longer reaction times.
The number and weight average molecular weights as well as a comparison of the GPC chromatograms after 35 min of both measurement series are shown in Fig. 5 and 6.
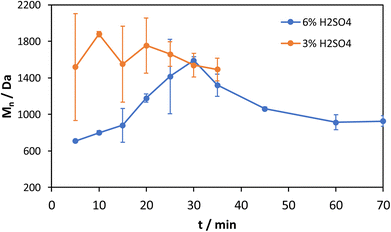
The number average molecular weight of the test series with 3% acid content is constant, considering the high standard deviations. At 6% acid content the number average molecular weight increases from about 700 Da to 1500 Da at 30 min and then levels off to 1000 Da where it can be considered constant after 45 min. This fact agrees with the glucose yield, which also takes on a constant value at 45 min. This trend can be explained in relation to the trend in Fig. 2 that shows a strong increase of the soluble fraction at around 20 min and further increasing after 30 min. In the initial phase, the majority of the solid substrate is slowly depolymerized with mainly short oligomeric fragments that increase in size as the depolymerization proceeds explaining the increasing average molecular weight. At this point after ca. 30 min a majority of 50–60% was solubilized. From this point on, although the depolymerization of the solid residue continues, the depolymerization of the already soluble fragments from the first phase dominates, which explains the decreasing molecular weight. The levelling off to 1000 Da is then a result of an equilibrium between depolymerization and repolymerization of short chain fragments.
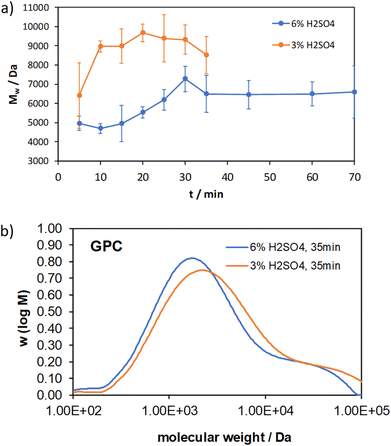
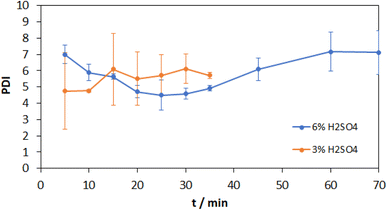
The weight average molecular weights of the test series with 3% acid content increase after the first grinding cycle and remain constant within the standard deviation, which is consistent with the number average molecular weights. For 6% acid content, the weight average molecular weight increases from 5000 Da at 5 min to 7000 Da at 30 min and then levels off at 6000 Da as a constant value. This matches with the glucose yield and the number average molecular weight. The comparison of the GPC chromatograms after 35 min illustrates the influence of the higher acidity towards lower molecular weights. What is striking is the significantly higher molecular weight compared to the number average molecular weight, which is typical for a broad molecular weight distribution. The resulting polydispersity index (PDI) is plotted in Fig. 7.
A high PDI in the range from 4 to 7 can be observed over the entire observation period, due to the weight average molecular weight, which is significantly higher than the number average molecular weight. This results from a broad molecular weight distribution. Since the yield of oligomers with the highest mass fraction is crucial, the weight average molecular weight is considered in the following discussion.
3.2. Influence of grinding ball material
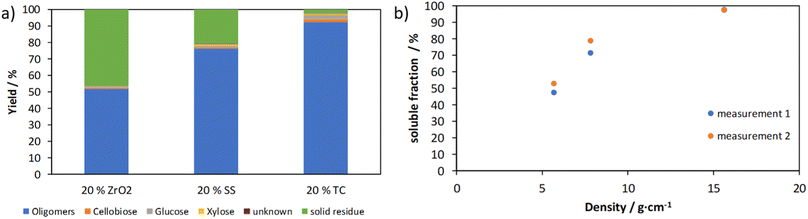
The grinding ball material and the grinding ball diameter influence the energy input per impact on the mechanocatalytic partial depolymerization. With increasing grinding ball density and grinding ball diameter, the energy input increases due to the kinetic energy of the grinding balls. The yields by varying the grinding ball material zirconia, stainless steel and tungsten carbide and the plot of the soluble fraction against the grinding ball density are shown in Fig. 8.As expected, the oligomer fraction increases with increasing density of the grinding ball material. When using zirconia with a density of 5.68 g cm−3 the oligomer fraction is about 50% and for tungsten carbide reaches almost 100% with a density of 15.8 g cm−3. The effect of density and the resulting mass on kinetic energy is proportional, suggesting a proportional increase in yield. This cannot be confirmed for the soluble fraction, but a clear increase can be seen. The reason for this is the preferential formation of soluble oligomers with a lower molecular weight with longer reaction times and higher grinding ball densities.
The weight average molecular weight (Table 2) for tungsten carbide decreases and is below that of zirconia and stainless steel. A correspondingly lower molecular weight with longer grinding times is expected for zirconia and stainless steel. While investigating the influence of the grinding ball material, the abrasion of the individual ball materials during the grinding process was examined and compared with the content of the components in untreated cellulose using XRF (Table 2). It can be seen that there is an increase of the respective metal in the product after mechanocatalytic treatment for all materials. Zirconia shows the smallest increase of about 10 ppm, while stainless steel and tungsten carbide show an increase of about 70 ppm and 65 ppm. In addition, when using stainless steel and tungsten carbide grinding balls, an increase in the zirconium content is found, which can be attributed to the abrasion of the grinding bowl. The increase by stainless steel can be explained by the lower hardness of the grinding material. In the case of tungsten carbide, the reason is the increased kinetic energy caused by the material density. This also leads to increased zirconia abrasion of the grinding bowl.
| Material | M w/Da | Content untreated | Content after mechanocatalysis |
|---|---|---|---|
| Zirconia | 4452 | 2.4 ppm Zr | 11.9 ppm Zr |
| Stainless steel | 4678 | 9.5 ppm Fe | 80.9 ppm Fe, 8.4 ppm Zr |
| Tungsten carbide | 2889 | 0.2 ppm W | 66.7 ppm W, 23,3 ppm Zr |
3.3. Influence of grinding ball diameter
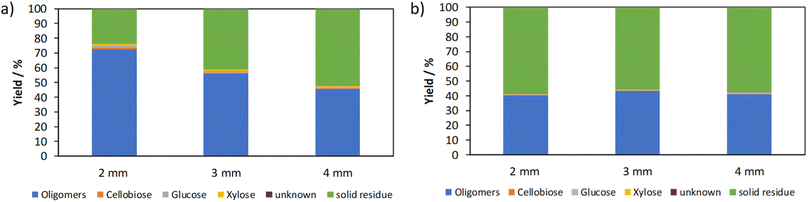
Fig. 9 shows the influence of the grinding ball diameter on the mechanocatalytic partial depolymerization of cellulose using two experiment series at rotational speeds of 350 rpm and 430 rpm.When comparing the two experiment series, two different trends can be observed. At a higher rotational speed of 430 rpm, the oligomer fraction decreases with increasing grinding ball diameter from 72% with 2 mm grinding balls to 45% with 4 mm grinding balls. At 350 rpm, on the other hand, the oligomer yield remains constant at around 40%. A possible explanation is the Arrhenius approach, in which both the number of collisions and the reaction probability are decisive. The pre-exponential factor A corresponds to the number of collisions and the second term with the activation energy Ea, the ideal gas constant R and the temperature T to the reaction probability in classical chemical reactions. For the following discussion, the two terms are considered as a whole and it is assumed that the pre-exponential factor A corresponds to the mechanical impacts of the grinding balls.
The number of impacts is increased with the 2 mm grinding balls due to the higher number of balls at same filling level. At 430 rpm, the grinding balls have a higher kinetic energy, which is crucial for a reaction to take place. The product of the number of impacts and the probability of reaction is highest for 2 mm grinding balls and decreases for larger grinding balls due to the large difference in the number of impacts. At 350 rpm, on the other hand, the probability of reaction decreases due to the reduced energy input. The 4 mm grinding balls have the highest kinetic energy, but the lowest number of impacts. The situation changes for 2 mm and 3 mm grinding balls, where the number of impacts increases but the probability of reaction decreases. The result is an approximately equal product of the number of collisions and the reaction probability, which leads to comparable oligomer fractions.
The lower weight average molecular weight of the experiments with 6% acid content at 350 rpm is striking (Table 3). Due to the increased acid content, the reaction rate also increases and shorter-chain oligomers are formed. The acidity has the greatest influence on the molecular weight here. In addition, the molecular weights of each experiment series are in a similar range and for each grinding ball material.
| Experiment | M w/Da |
|---|---|
| 2 mm, 430 rpm, 3% | 2026 |
| 3 mm, 430 rpm, 3% | 2095 |
| 4 mm, 430 rpm, 3% | 2219 |
| 2 mm, 350 rpm, 6% | 1352 |
| 3 mm, 350 rpm, 6% | 1610 |
| 4 mm, 350 rpm, 6% | 1575 |
3.4. Influence of rotational speed
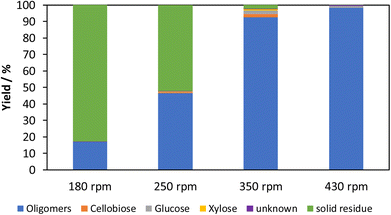
The influence of the rotational speed is determined with tungsten carbide grinding balls in the range between 180 rpm and 430 rpm. The results are shown in Fig. 10.At 430 rpm, the temperature after mechanochemical treatment is 115 °C and is thus above the maximum of 90 °C desired to prevent subsequent reactions. This led to a colour change of the product to dark grey, which is typical of the beginning of carbonization or a tleast subsequent condensation/humin-formation reactions of a sample. A rotational speed of 430 rpm is therefore not suitable despite the highest soluble content. At 180 rpm, the soluble fraction is 17% and consists almost exclusively of oligomers. As the rotational speed increases, the soluble fraction increases to around 48% at 250 rpm and 97% at 350 rpm. In the process, more glucose is formed, which means that the reaction approaches the equilibrium of polymerization and depolymerization.
The weight average molecular weights (Table 4) decrease from 4424 Da at 180 rpm to 2889 Da at 350 rpm, which is consistent with the progressive depolymerization towards the equilibrium of polymerization and depolymerization. At 430 rpm, on the other hand, a renewed increase to 4211 Da can be observed. In this test, the temperature is higher and is 115 °C. This leads to the beginning of carbonization which impacts the reaction. An interpretation of this experiment is therefore not possible.
| Rotational speed/rpm | M w/Da | T/°C |
|---|---|---|
| 180 | 4424 | 34 |
| 250 | 4939 | 48 |
| 350 | 2889 | 82 |
| 430 | 4211 | 115 |
3.5. Influence of filling level
The influence of the filling level is also examined with tungsten carbide grinding balls. The filling level is varied in 10% increments between 10% and 40%. The resulting product composition of the experiments is shown in Fig. 11.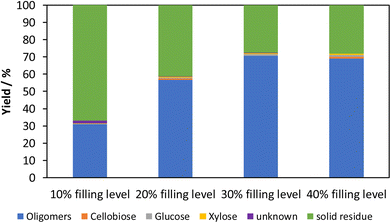 | ||
| Fig. 11 Product composition after mechanocatalytic partial depolymerization by varying the filling level with tungsten carbide grinding balls. Reaction conditions: 3% acid content, 250 rpm, 85 min. | ||
It can be seen that the filling level plays a crucial role in mechanocatalytic partial depolymerization. With a 10% filling level under the given reaction conditions, the soluble fraction is about 32% and increases to 59% at a 20% filling level up to 72% at 30% filling level. A further increase of the filling level up to 40% leads to an approximately constant soluble fraction. The reason for this is the limited freedom of movement as the filling level increases, which means that the grinding balls do not reach their maximum speed. This in turn reduces the kinetic energy and thus the energy input. If the filling level would be increased further, a decrease in the soluble fraction would be expected. This fact matches with results from literature, where too high filling levels reduced the energy input.29
In the relevant range of the filling level between 10% and 30%, no influence on the weight average molecular weight (Table 5) can be determined. As in the previous sections, the influence between polymerization and depolymerization is expected at high soluble fractions near equilibrium.
| Filling level/% | M w/Da |
|---|---|
| 10 | 4289 |
| 20 | 4423 |
| 30 | 4434 |
| 40 | 3268 |
4. Conclusion
Mechanocatalytic partial depolymerization of cellulose in a planetary ball mill is shown to be dependent on various reaction parameters. The reaction parameters investigated in this work are reaction time, acid content, grinding ball material and diameter as well as rotational speed and filling level. In general, higher glycan yields are observed with higher energy inputs during the reaction. Hereby the glycan yield is dependent on the impact number and the reaction probability which correlates with the kinetic energy of the grinding balls. Further it could be shown that the glycan yield and the glycan weight average molecular weight is dependent on all parameters. E.g. a higher acid content leads to a higher reaction rate and lower weight average molecular weight glycans, while varying the filling level influences the reaction rate and shows no influence towards the weight average molecular weight. For mechanocatalytic partial depolymerization at 350 rpm with 3% acid content, 20% filling level with tungsten carbide grinding balls a nearly fully soluble product, containing about 90% glycans with an weight average molecular weight of 2900 Da, is formed. Therefore the reaction parameters have to be adjusted depending on the favored product spectrum. Overall, the mechanocatalytic selective depolymerization of cellulose to oligomeric glycans can be well controlled regarding the product fraction. It depends on the most important milling parameters. Therefore, a suitable route for value-added products from cellulose as abundant biobased feedstock is enabled.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We gratefully acknowledge the German Federal Ministry of Education and Research and the Projektträger Jülich for the financial support (FKZ: 031B0905B). We thank Marion Trautmann from the working group of Prof. Biesalski (TU Darmstadt) for the GPC measurements.References
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- B. Kamm, P. R. Gruber and M. Kamm, Biorefineries-industrial processes and products, Wiley-VCH Weinheim, 2006, vol. 2 Search PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564–2601 CrossRef CAS PubMed.
- G. Li, W. Liu, C. Ye, X. Li and C.-L. Si, Int. J. Polym. Sci., 2018, 2018, 4723573 Search PubMed.
- R. Rinaldi, Angew. Chem., Int. Ed., 2014, 53, 8559–8560 CrossRef CAS PubMed.
- H. Lüers, Holz als Roh-und Werkstoff, 1937, 1, 35–40 CrossRef.
- J. L. Jones and K. T. Semrau, Biomass, 1984, 5, 109–135 CrossRef CAS.
- E. C. Gaudino, G. Cravotto, M. Manzoli and S. Tabasso, Chem. Soc. Rev., 2021, 50, 1785–1812 RSC.
- F. Gomollón-Bel, Chem. Int., 2019, 41, 12–17 Search PubMed.
- M. Käldström, N. Meine, C. Farès, F. Schüth and R. Rinaldi, Green Chem., 2014, 16, 3528–3538 RSC.
- M. Käldström, N. Meine, C. Farès, R. Rinaldi and F. Schüth, Green Chem., 2014, 16, 2454–2462 RSC.
- N. Meine, R. Rinaldi and F. Schüth, ChemSusChem, 2012, 5, 1449–1454 CrossRef CAS PubMed.
- G. Yang, E. Lam and A. Moores, ACS Sustainable Chem. Eng., 2023, 11, 7765–7774 CrossRef CAS.
- L. Schneider, J. Haverinen, M. Jaakkola and U. Lassi, Chem. Eng. J., 2017, 327, 898–905 CrossRef CAS.
- K. S. Guiao, C. Tzoganakis and T. H. Mekonnen, Chemosphere, 2022, 293, 133647 CrossRef CAS PubMed.
- K. Thielemans, Y. De Bondt, S. Van den Bosch, A. Bautil, C. Roye, A. Deneyer, C. M. Courtin and B. F. Sels, Carbohydr. Polym., 2022, 119764 CrossRef CAS PubMed.
- A. Karam, P. N. Amaniampong, J. M. Garcia Fernandez, C. Oldani, S. Marinkovic, B. Estrine, K. De Oliveira Vigier and F. Jérôme, Front. Chem., 2018, 6, 74 CrossRef PubMed.
- J. F. S. Cantor, K. D. O. Vigier, G. Labat, D. D. S. Perez and F. Jerome, RSC Sustainability, 2023, 1, 446–453 RSC.
- P. Chen, A. Shrotri and A. Fukuoka, Appl. Catal., A, 2021, 621, 118177 CrossRef CAS.
- M. Yabushita, H. Kobayashi, K. Hara and A. Fukuoka, Catal. Sci. Technol., 2014, 4, 2312–2317 RSC.
- S. M. Hick, C. Griebel, D. T. Restrepo, J. H. Truitt, E. J. Buker, C. Bylda and R. G. Blair, Green Chem., 2010, 12, 468–474 RSC.
- M. G. Davidson, S. Elgie, S. Parsons and T. J. Young, Green Chem., 2021, 23, 3154–3171 RSC.
- D.-Y. Kim, Y. Nishiyama, M. Wada and S. Kuga, Cellulose, 2001, 8, 29–33 CrossRef CAS.
- S. Julien, E. Chornet and R. Overend, J. Anal. Appl. Pyrolysis, 1993, 27, 25–43 CrossRef CAS.
- G. Meyer, M. Wolf, S. Hanstein and M. Rose, Sustainable Energy Fuels, 2023, 1870–1877 RSC.
- H. Zhao, J. H. Kwak, Z. C. Zhang, H. M. Brown, B. W. Arey and J. E. Holladay, Carbohydr. Polym., 2007, 68, 235–241 CrossRef CAS.
- S. I. Martins, W. M. Jongen and M. A. Van Boekel, Trends Food Sci. Technol., 2000, 11, 364–373 CrossRef CAS.
- C. F. Burmeister and A. Kwade, Chem. Soc. Rev., 2013, 42, 7660–7667 RSC.
| This journal is © The Royal Society of Chemistry 2024 |